Role of Bacteria-Derived Flavins in Plant Growth Promotion and Phytochemical Accumulation in Leafy Vegetables
Abstract
:1. Introduction
2. Results
2.1. Effect of Bacterial-Derived FLs in Kale and Lettuce Seedlings Growth Parameters
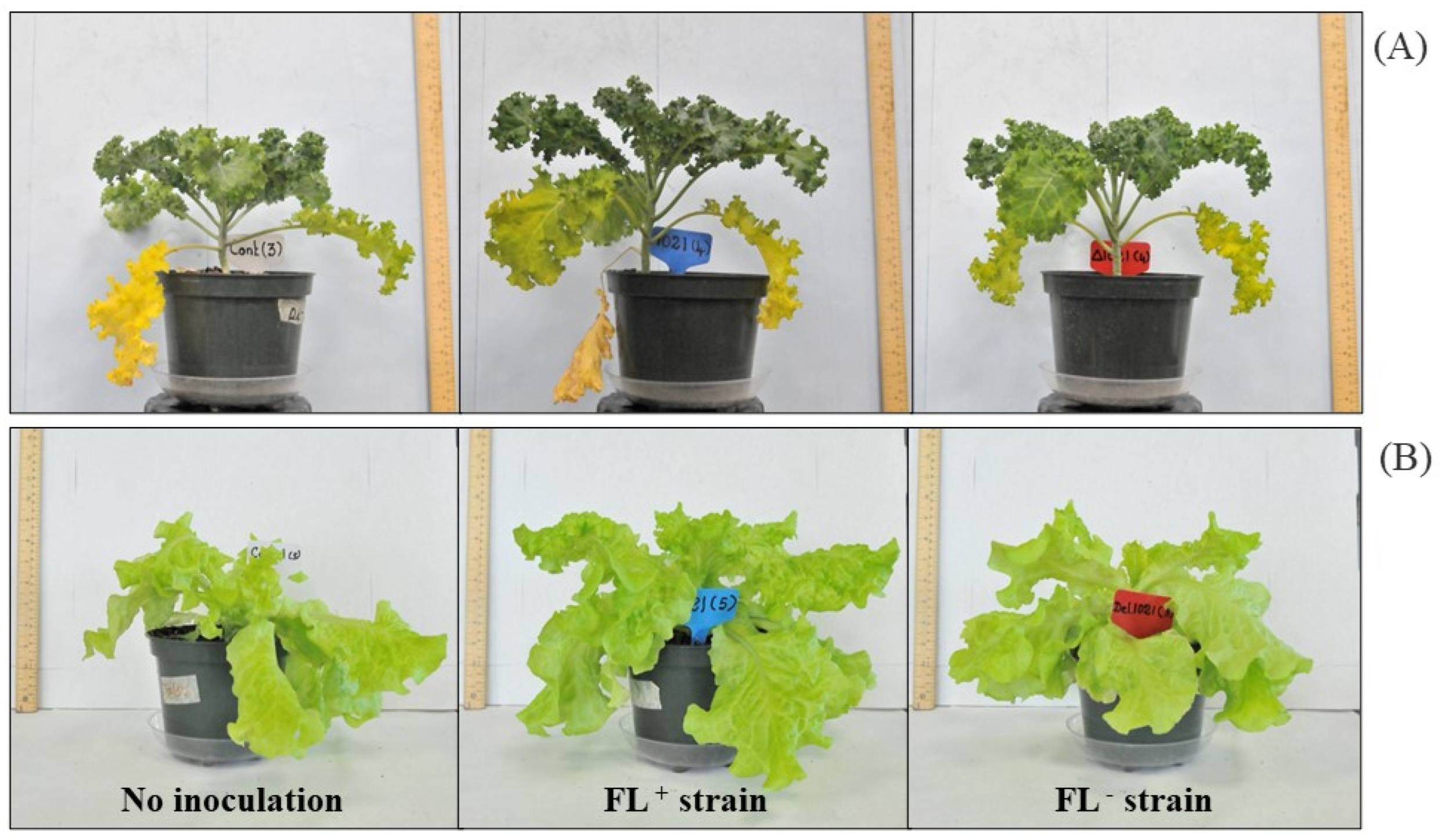
2.2. Plant Growth, Physiology, and Yield
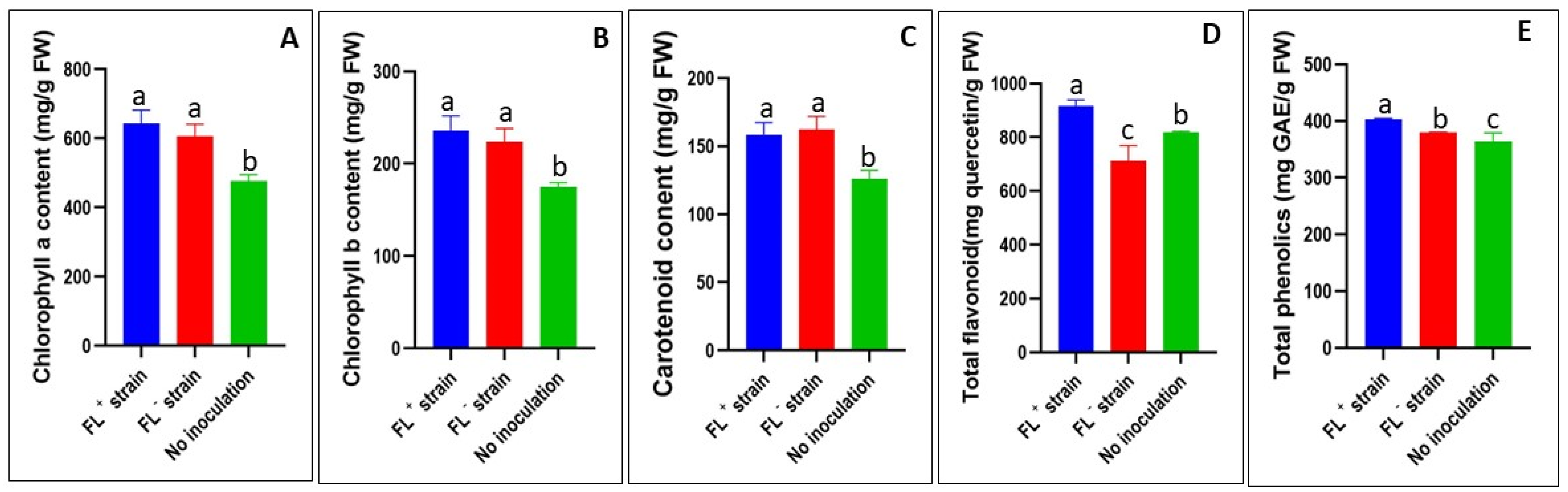
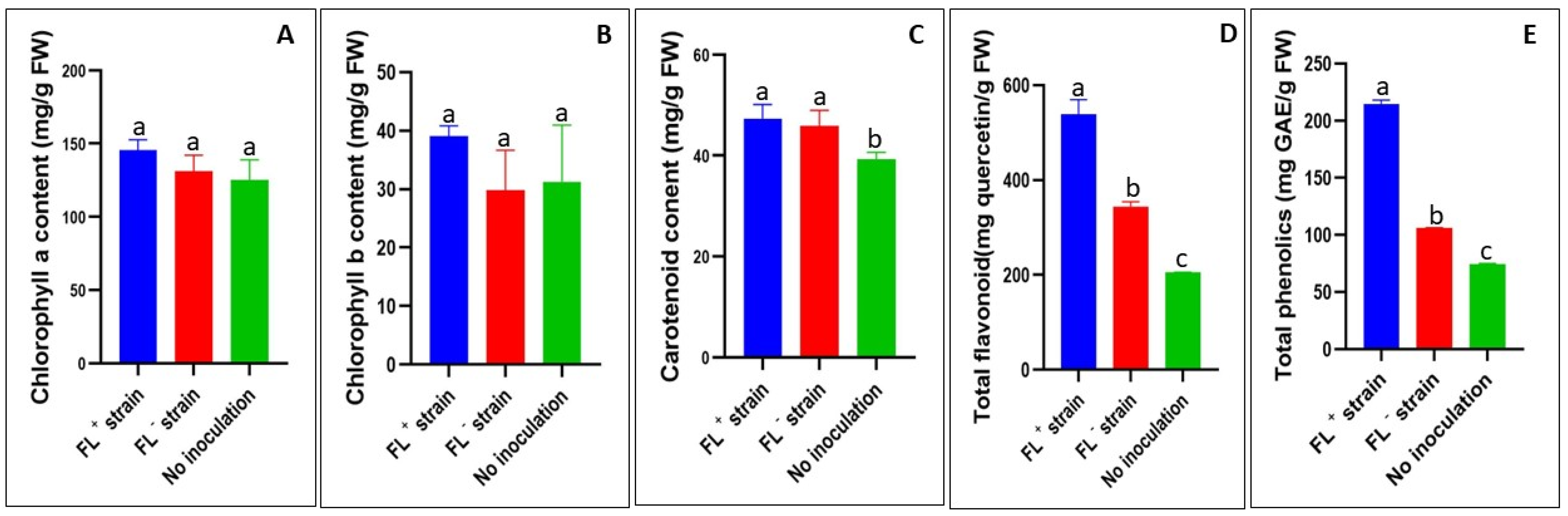
2.3. Leaf Tissue Phytochemical Analysis
2.4. Leaf Micro- and Macronutrient Elemental Compositions
3. Discussion
4. Materials and Methods
4.1. Germination Pouch Trial
4.1.1. Bacterial Strains and Media
4.1.2. Inoculum Preparation and Seed Treatment
4.1.3. Seedling Growth Components
4.1.4. Statistical Analysis
4.2. Greenhouse Potted-Plant Trial
4.2.1. Greenhouse Environment and Experimental Design
4.2.2. Plant Growth, Physiology, and Yield Parameters
4.2.3. Chlorophylls a and b, and Carotenoid Determination
4.2.4. Total Phenolics Determination
4.2.5. Total Flavonoid Determination
4.2.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandratos, N.; Bruinsma, J. World Agriculture Towards 2030/2050: The 2012 Revision. 2012. Available online: https://ageconsearch.umn.edu/record/288998 (accessed on 23 March 2023).
- Elferink, M.; Schierhorn, F. Global Demand for Food Is Rising. Can We Meet it? Harv. Bus Rev. 2016, 7, 2016. [Google Scholar]
- Kumar, R. The Impact of Chemical Fertilizers on Our Environment and Ecosystem. Chief Ed. 2019, 35, 69. [Google Scholar]
- Savci, S. Investigation of Effect of Chemical Fertilizers on Environment. APCBEE Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef]
- Bijay-Singh; Craswell, E. Fertilizers and Nitrate Pollution of Surface and Ground Water: An Increasingly Pervasive Global Problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Ansari, A.A.; Gill, S.S. Eutrophication: Causes, Consequences and Control; Springer: Dordrechet, The Netherlands, 2014; p. 2. ISBN 9789400778146. [Google Scholar]
- Xuejun, L.; Fusuo, Z. Nitrogen Fertilizer Induced Greenhouse Gas Emissions in China. Curr. Opin. Environ. Sustain. 2011, 3, 407–413. [Google Scholar] [CrossRef]
- Kundu, M.C.; Mandal, B.; Sarkar, D. Assessment of the Potential Hazards of Nitrate Contamination in Surface and Groundwater in a Heavily Fertilized and Intensively Cultivated District of India. Environ. Monit. Assess. 2008, 146, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil Beneficial Bacteria and Their Role in Plant Growth Promotion: A Review. Ann. Microbiol 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Mehmood, U.; Inam-Ul-Haq, M.; Saeed, M.; Altaf, A.; Azam, F.; Hayat, S. Plant Protection a Brief Review on Plant Growth Promoting Rhizobacteria (PGPR): A Key Role in Plant Growth Promotion. Plant Prot. 2018, 2, 77–82. [Google Scholar]
- Kumar Jha, C.; Saraf, M. Plant Growth Promoting Rhizobacteria (PGPR): A Review. EJARD 2015, 5, 108–0119. [Google Scholar]
- Billah, M.; Khan, M.; Bano, A.; Hassan, T.U.; Munir, A.; Gurmani, A.R. Phosphorus and Phosphate Solubilizing Bacteria: Keys for Sustainable Agriculture. Geomicrobiol. J. 2019, 36, 904–916. [Google Scholar] [CrossRef]
- Kishore, N.; Pindi, P.K.; Reddy, S.R. Phosphate-Solubilizing Microorganisms: A Critical Review. In Plant Biology and Biotechnology: Plant Diversity, Organization, Function and Improvement; Springer: New Delhi, India, 2015; Volume 1, pp. 307–333. ISBN 9788132222866. [Google Scholar]
- Shahzad, S.M.; Arif, M.S.; Riaz, M.; Iqbal, Z.; Ashraf, M. PGPR with Varied ACC-Deaminase Activity Induced Different Growth and Yield Response in Maize (Zea mays L.) under Fertilized Conditions. Eur. J. Soil. Biol 2013, 57, 27–34. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Methods for Isolating and Characterizing ACC Deaminase-Containing Plant Growth-Promoting Rhizobacteria. Physiol. Plant 2003, 118, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. New Perspectives and Approaches in Plant Growth-Promoting Rhizobacteria Research. Eur. J. Plant. Pathol. 2007, 119, 329–339. [Google Scholar] [CrossRef]
- Chuks Kenneth, O. Plant Growth Promoting Rhizobacteria (PGPR): A Bioprotectant Bioinoculant for Sustainable Agrobiology. A Review. Int. J. Adv. Res. Biol. Sci. 2017, 4, 123–142. [Google Scholar] [CrossRef]
- Tariq, M.; Noman, M.; Ahmed, T.; Hameed, A.; Hodowli, I.; Roslin, A. Antagonistic Features Displayed by Plant Growth Promoting Rhizobacteria (PGPR): A Review. J. Plant Sci. Phytopathol. 2017, 1, 038–043. [Google Scholar]
- Ahmed, A.; Shahida, H. Auxins as one of the factors of plant growth improvement by plant growth promoting rhizobacteria. Pol. J. Microbiol. 2014, 63, 261. [Google Scholar] [CrossRef] [PubMed]
- Dakora, F.D.; Matiru, V.N.; Kanu, A.S. Rhizosphere Ecology of Lumichrome and Riboflavin, Two Bacterial Signal Molecules Eliciting Developmental Changes in Plants. Front. Plant Sci. 2015, 6, 700. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.A.; Joseph, C.M.; Yang, G.-P.; Martinez-Romero, E.; Sanborn, J.R.; Volpin, H. Identification of Lumichrome as a Sinorhizobium Enhancer of Alfalfa Root Respiration and Shoot Growth. Proc. Natl. Acad. Sci. USA 1999, 96, 12275–12280. [Google Scholar] [CrossRef] [PubMed]
- Mansoorabadi, S.O.; Thibodeaux, C.J.; Liu, H.W. The Diverse Roles of Flavin Coenzymes-Nature’s Most Versatile Thespians. J. Org. Chem. 2007, 72, 6329–6342. [Google Scholar] [CrossRef]
- Roje, S. Vitamin B Biosynthesis in Plants. Phytoche 2007, 68, 1904–1921. [Google Scholar] [CrossRef]
- Yurgel, S.N.; Rice, J.; Domreis, E.; Lynch, J.; Sa, N.; Qamar, Z.; Rajamani, S.; Gao, M.; Roje, S.; Bauer, W.D. Sinorhizobium meliloti Flavin Secretion and Bacteria-Host Interaction: Role of the Bifunctional RibBA Protein. MPMI 2014, 27, 437–445. [Google Scholar] [CrossRef]
- Yurgel, S.N.; Ajeethan, N.; Smertenko, A. Response of Plant-Associated Microbiome to Plant Root Colonization by Exogenous Bacterial Endophyte in Perennial Crops. Front. Microbiol. 2022, 13, 863946. [Google Scholar] [CrossRef]
- Chi, F.; Yang, P.; Han, F.; Jing, Y.; Shen, S. Proteomic Analysis of Rice Seedlings Infected by Sinorhizobium meliloti 1021. Proteomics 2010, 10, 1861–1874. [Google Scholar] [CrossRef]
- Yanni, Y.G.; Dazzo, F.B. Enhancement of Rice Production Using Endophytic Strains of Rhizobium leguminosarum Bv. Trifolii in Extensive Field Inoculation Trials within the Egypt Nile Delta. Plant Soil 2010, 336, 129–142. [Google Scholar] [CrossRef]
- Jha, B.; Thakur, M.C.; Gontia, I.; Albrecht, V.; Stoffels, M.; Schmid, M.; Hartmann, A. Isolation, Partial Identification and Application of Diazotrophic Rhizobacteria from Traditional Indian Rice Cultivars. Eur. J. Soil Biol. 2009, 45, 62–72. [Google Scholar] [CrossRef]
- Canellas, L.P.; Balmori, D.M.; Médici, L.O.; Aguiar, N.O.; Campostrini, E.; Rosa, R.C.C.; Façanha, A.R.; Olivares, F.L. A Combination of Humic Substances and Herbaspirillum seropedicae Inoculation Enhances the Growth of Maize (Zea mays L.). Plant Soil 2013, 366, 119–132. [Google Scholar] [CrossRef]
- Yanni, Y.G.; Dazzo, F.B.; Squartini, A.; Zanardo, M.; Zidan, M.I.; Elsadany, A.E.Y. Assessment of the Natural Endophytic Association between Rhizobium and Wheat and Its Ability to Increase Wheat Production in the Nile Delta. Plant Soil 2016, 407, 367–383. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Clayton, G.W.; Hanson, K.G.; Rice, W.A.; Biederbeck, V.O. Endophytic Rhizobia in Barley, Wheat and Canola Roots. Can. J. Plant Sci. 2004, 84, 37–45. [Google Scholar] [CrossRef]
- Chabot, R.; Antoun, H.; Kloepper, J.W.; Beauchamp, C.J. Root Colonization of Maize and Lettuce by Bioluminescent Rhizobium Leguminosarum Biovar Phaseoli. AEM 1996, 62, 2767–2772. [Google Scholar] [CrossRef]
- Garcia-Fraile, P.; Carro, L.; Robledo, M.; Ramírez-Bahena, M.H.; Flores-Felix, J.D.; Fernandez, M.T.; Mateos, P.F.; Rivas, R.; Igual, J.M.; Martinez-Molina, E.; et al. Rhizobium Promotes Non-Legumes Growth and Quality in Several Production Steps: Towards a Biofertilization of Edible Raw Vegetables Healthy for Humans. PLoS ONE 2012, 7, e38122. [Google Scholar] [CrossRef]
- Alami, Y.; Achouak, W.; Marol, C.; Heulin, T. Rhizosphere Soil Aggregation and Plant Growth Promotion of Sunflowers by an Exopolysaccharide-Producing Rhizobium Sp. Strain Isolated from Sunflower Roots. Appl. Environ. Microbiol. 2000, 66, 3393–3398. [Google Scholar] [CrossRef]
- Biswas, J.C.; Ladha, J.K.; Dazzo, F.B. Rhizobia Inoculation Improves Nutrient Uptake and Growth of Lowland Rice. Soil Sci. Soc. Am. J. 2000, 64, 1644–1650. [Google Scholar] [CrossRef]
- Yanni, Y.G.; Rizk, R.Y.; Abd El-Fattah, F.K.; Squartini, A.; Corich, V.; Giacomini, A.; De Bruijn, F.; Rademaker, J.; Maya-Flores, J.; Ostrom, P.; et al. The Beneficial Plant Growth-Promoting Association of Rhizobium leguminosarum bv. Trifolii with Rice Roots. Austr. J. Plant Physiol 2001, 28, 845–870. [Google Scholar]
- Wu, Q.; Peng, X.; Yang, M.; Zhang, W.; Dazzo, F.B.; Uphoff, N.; Jing, Y.; Shen, S. Rhizobia Promote the Growth of Rice Shoots by Targeting Cell Signaling, Division and Expansion. Plant Mol. Biol. 2018, 97, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Chi, F.; Shen, S.H.; Cheng, H.P.; Jing, Y.X.; Yanni, Y.G.; Dazzo, F.B. Ascending Migration of Endophytic Rhizobia, from Roots to Leaves, inside Rice Plants and Assessment of Benefits to Rice Growth Physiology. Appl. Environ. Microbiol. 2005, 71, 7271–7278. [Google Scholar] [CrossRef]
- Biswas, J.C.; Ladha, J.K.; Dazzo, F.B.; Yanni, Y.G.; Rolfe, B.G. Rhizobial Inoculation Influences Seedling Vigor and Yield of Rice. Agron. J. 2000, 92, 880–886. [Google Scholar]
- Fassbinder, F.; Kist, M.; Bereswill, S. Structural and Functional Analysis of the Riboflavin Synthesis Genes Encoding GTP Cyclohydrolase II (RibA), DHBP Synthase (RibBA), Riboflavin Synthase (RibC), and Riboflavin Deaminase/Reductase (RibD) from Helicobacter pylori Strain P1. FEMS Microbiol. Lett. 2000, 191, 191–197. [Google Scholar] [CrossRef]
- Yang, G.; Bhuvaneswari, T.V.; Joseph, C.M.; King, M.D.; Phillips, D.A. Roles for Riboflavin in the Sinorhizobium-Alfalfa Association. MPMI 2002, 15, 456–462. [Google Scholar] [CrossRef]
- Gutierrez-Preciado, A.; Torres, A.G.; Merino, E.; Bonomi, H.R.; Goldbaum, F.A.; García-Angulo, V.A. Extensive Identification of Bacterial Riboflavin Transporters and Their Distribution across Bacterial Species. PLoS ONE 2015, 10, e0126124. [Google Scholar] [CrossRef]
- Matiru, V.N.; Dakora, F.D. The Rhizosphere Signal Molecule Lumichrome Alters Seedling Development in Both Legumes and Cereals. New Phytol. 2005, 166, 439–444. [Google Scholar] [CrossRef]
- Sandoval, F.J.; Zhang, Y.; Roje, S. Flavin Nucleotide Metabolism in Plants: Monofunctional Enzymes Synthesize FAD in Plastids. JBC 2008, 283, 30890–30900. [Google Scholar] [CrossRef]
- MacHeroux, P.; Kappes, B.; Ealick, S.E. Flavogenomics-A Genomic and Structural View of Flavin-Dependent Proteins. FEBS J. 2011, 278, 2625–2634. [Google Scholar]
- Joosten, V.; van Berkel, W.J. Flavoenzymes. Curr. Opin. Chem. Biol. 2007, 11, 195–202. [Google Scholar]
- Schall, P.; Marutschke, L.; Grimm, B. The Flavoproteome of the Model Plant Arabidopsis thaliana. Int. J. Mol. Sci. 2020, 21, 5371. [Google Scholar] [CrossRef]
- Camejo, D.; Guzman-Cedeno, A.; Moreno, A. Reactive Oxygen Species, Essential Molecules, during Plant-Pathogen Interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar]
- Ben Rejeb, I.; Pastor, V.; Mauch-Mani, B. Plant Responses to Simultaneous Biotic and Abiotic Stress: Molecular Mechanisms. Plants 2014, 3, 458–475. [Google Scholar]
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of Amine Oxidases in Plant Development and Defence. Trends Plant Sci. 2006, 11, 80–88. [Google Scholar]
- Maxwell1, K.; Johnson2, G.N. Chlorophyll Fluorescence-a Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar]
- Liu, C.; Liu, Y.; Lu, Y.; Liao, Y.; Nie, J.; Yuan, X.; Chen, F. Use of a Leaf Chlorophyll Content Index to Improve the Prediction of Above-Ground Biomass and Productivity. PeerJ 2019, 6, e6240. [Google Scholar] [CrossRef]
- Fromme, P.; Melkozernov, A.; Jordan, P.; Krauss, N. Structure and Function of Photosystem I: Interaction with Its Soluble Electron Carriers and External Antenna Systems. FEBS Lett. 2003, 555, 40–44. [Google Scholar]
- Tang, C.-J.; Luo, M.Z.; Zhang, S.; Jia, G.Q.; Tang, S.; Jia, Y.C.; Zhi, H.; Diao, X.-M. Variations in Chlorophyll Content, Stomatal Conductance, and Photosynthesis in Setaria EMS Mutants. J. Integr. Agric. 2023, 22, 1618–1630. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoid Oxidation Products as Stress Signals in Plants. Plant J. 2014, 79, 597–606. [Google Scholar]
- Havaux, M.; Dall’Osto, L.; Bassi, R. Zeaxanthin Has Enhanced Antioxidant Capacity with Respect to All Other Xanthophylls in Arabidopsis Leaves and Functions Independent of Binding to PSII Antennae. Plant Physiol. 2007, 145, 1506–1520. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar]
- Matusova, R.; Rani, K.; Verstappen, F.W.A.; Franssen, M.C.R.; Beale, M.H.; Bouwmeester, H.J. The Strigolactone Germination Stimulants of the Plant-Parasitic Striga and Orobanche Spp. Are Derived from the Carotenoid Pathway. Plant Physiol. 2005, 139, 920–934. [Google Scholar] [CrossRef]
- Clarkson, D.T.; Hanson, J.B. The mineral nutrition of higher plants. Annu. Rev. Plant Physiol. 1980, 31, 239–298. [Google Scholar]
- D’antuono, L.F.; Neri, R. Traditional Crop Revised: Yield and Quality of Palm-Tree Kale, grown as a Mechanised Industrial Crop, as a Function of Cutting Height. In Proceedings of the International Symposium on Sustainable Use of Plant Biodiversity to Promote New Opportunities for Horticultural Production, Antalya, Turkey, 6–9 November 2001; pp. 123–127. [Google Scholar]
- Qin, S.; Ding, Y.; Zhou, Z.; Zhou, M.; Wang, H.; Xu, F.; Yao, Q.; Lv, X.; Zhang, Z.; Zhang, L. Study on the Nitrogen Content Estimation Model of Cotton Leaves Based on “Image-Spectrum-Fluorescence” Data Fusion. Front. Plant Sci. 2023, 14, 1117277. [Google Scholar] [CrossRef]
- Korus, A. Effect of the Cultivar and Harvest Date of Kale (Brassica oleracea L. Var. Acephala) on Crop Yield and Plant Morphological Features. Veg. Crops Res. Bull. 2010, 73, 55–65. [Google Scholar] [CrossRef]
- Allison, J.C.S.; Williams, H.T.; Pammenter, N.W. Effect of Specific Leaf Nitrogen Content on Photosynthesis of Sugarcane. Ann. Appl. Biol. 1997, 131, 339–350. [Google Scholar] [CrossRef]
- Laza, R.C.; Bergman, B.; Vergara, B.S. Cultivar Differences in Growth and Chloroplast Ultrastructure in Rice as Affected by Nitrogen. J. Exp. Bot. 1993, 44, 1643–1648. [Google Scholar]
- Uchida, R. Essential Nutrients for Plant Growth: Nutrient Functions and Deficiency Symptoms; University of Hawaii: Manoa, HI, USA, 2000; pp. 31–55. [Google Scholar]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 171–190. ISBN 9789811090448. [Google Scholar]
- Fu, L.; Xu, B.T.; Gan, R.Y.; Zhang, Y.; Xu, X.R.; Xia, E.Q.; Li, H. Bin Total Phenolic Contents and Antioxidant Capacities of Herbal and Tea Infusions. Int. J. Mol. Sci. 2011, 12, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Olfat, N.; Ashoori, M.; Saedisomeolia, A. Riboflavin Is an Antioxidant: A Review Update. Br. J. Nutr. 2022, 128, 1887–1895. [Google Scholar] [CrossRef]
- Galibert, F.; Finan, T.M.; Long, S.R.; Pühler, A.; Abola, P.; Ampe, F.; Barloy-Hubler, F.; Barnet, M.J.; Becker, A.; Boistard, P.; et al. The Composite Genome of the Legume Symbiont Sinorhizobium meliloti. Science 2001, 293, 668–672. [Google Scholar] [CrossRef]
- Somerville, J.E.; Kahn, M.L. Cloning of the Glutamine Synthetase I Gene from Rhizobium meliloti. J. Bacteriol. 1983, 156, 168–176. [Google Scholar]
- Himmelbauer, M.L.; Loiskandl, W.; Kastanek, F. Estimating Length, Average Diameter and Surface Area of Roots Using Two Different Image Analyses Systems. Plant Soil 2004, 260, 111–120. [Google Scholar]
- Ofoe, R.; Gunupuru, L.R.; Wang-Pruski, G.; Fofana, B.; Thomas, R.H. Abbey, Lord Seed Priming with Pyroligneous Acid Mitigates Aluminum Stress, and Promotes Tomato Seed Germination and Seedling Growth. Plant Stress 2022, 4, 100083. [Google Scholar] [CrossRef]
- Qin, D.; He, Q.; Mousavi, S.M.N. Abbey, Lord Evaluation of Aging Methods on the Surface Characteristics of Hydrochar and Germination Indices for Kale Seeds. Horticulturae 2023, 9, 545. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Saleh, R.; Gunupuru, L.R.; Lada, R.; Nams, V.; Thomas, R.H. Abbey, Lord Growth and Biochemical Composition of Microgreens Grown in Different Formulated Soilless Media. Plants 2022, 11, 3546. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin-Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Chang, C.; Mang-Hua, Y.; Hwei-mei, W. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of food and drug analysis. JFDA 2002, 10, 178–182. [Google Scholar]
- Pourmorad, F.; Hosseinimehr, S.J.; Shahabimajd, N. Antioxidant Activity, Phenol and Flavonoid Contents of Some Selected Iranian Medicinal Plants. Afr. J. Biotechnol. 2006, 5, 1142–1145. [Google Scholar]

| Treatment | Total Root Length (cm) | Total Root Surface Area (cm2) | Total Hypocotyl Length (cm) | Total Hypocotyl Surface Area (cm2) |
|---|---|---|---|---|
| FL+ strain | 30.17 ± 2.56 a | 11.81 ± 1.26 a | 4.87 ± 0.86 a | 2.86 ± 0.24 a |
| FL− strain | 25.35 ± 2.41 b | 09.84 ± 1.52 b | 4.39 ± 0.44 ab | 2.58 ± 0.13 b |
| No inoculation | 20.56 ± 2.32 c | 10.38 ± 1.33 ab | 3.68 ± 0.50 b | 2.49 ± 0.14 b |
| Treatment | Total Root Length (cm) | Total Root Surface Area (cm2) | Total Shoot Length (cm) | Total Shoot Surface Area (cm2) |
|---|---|---|---|---|
| FL+ strain | 20.13 ± 0.83 a | 9.39 ± 0.38 a | 5.98 ± 0.75 a | 3.07 ± 0.22 a |
| FL− strain | 17.70 ± 0.79 b | 8.22 ± 0.85 b | 5.59 ± 0.61 a | 2.95 ± 0.18 a |
| No inoculation | 18.17 ± 0.50 ab | 8.32 ± 0.95 b | 5.15 ± 0.79 a | 2.92 ± 0.25 a |
| Kale | Lettuce | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | FL+ Strain | FL− Strain | No Inoculation | CV % | FL+ Strain | FL− Strain | No Inoculation | CV % | Reporting Limit |
| Nitrogen (mg/mL) | 19.70 | 20.80 | 19.20 | 4.113 | 14.00 | 12.80 | 14.10 | 5.306 | 00.20 |
| Calcium (mg/mL) | 16.79 | 16.37 | 16.85 | 1.569 | 10.79 | 10.62 | 10.26 | 2.563 | 00.02 |
| Potassium (mg/mL) | 21.82 | 21.64 | 20.37 | 3.715 | 22.29 | 23.34 | 23.64 | 3.070 | 00.15 |
| Magnesium (mg/mL) | 02.91 | 02.87 | 02.89 | 0.692 | 03.37 | 03.76 | 02.89 | 13.05 | 00.02 |
| Phosphorus (mg/mL) | 04.47 | 04.38 | 03.98 | 6.099 | 03.97 | 03.47 | 03.67 | 6.796 | 00.01 |
| Sodium (mg/mL) | 00.51 | 00.60 | 00.49 | 10.99 | 06.13 | 04.93 | 04.57 | 15.68 | 00.15 |
| Boron (mg/L) | 24.19 | 22.72 | 22.08 | 4.704 | 11.25 | 13.37 | 11.84 | 9.003 | 10.00 |
| Copper (mg/L) | 7.300 | 5.770 | ND | 16.56 | ND | ND | ND | NA | 5.000 |
| Iron (mg/L) | 50.53 | 50.14 | 43.11 | 8.713 | 41.20 | 45.00 | 37.43 | 9.185 | 5.000 |
| Manganese (mg/L) | 87.50 | 97.02 | 89.38 | 5.523 | 65.70 | 55.72 | 49.38 | 14.45 | 10.00 |
| Zinc (mg/L) | 51.87 | 47.45 | 53.03 | 5.798 | 33.81 | 32.94 | 34.87 | 2.853 | 2.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajeethan, N.; Yurgel, S.N.; Abbey, L. Role of Bacteria-Derived Flavins in Plant Growth Promotion and Phytochemical Accumulation in Leafy Vegetables. Int. J. Mol. Sci. 2023, 24, 13311. https://doi.org/10.3390/ijms241713311
Ajeethan N, Yurgel SN, Abbey L. Role of Bacteria-Derived Flavins in Plant Growth Promotion and Phytochemical Accumulation in Leafy Vegetables. International Journal of Molecular Sciences. 2023; 24(17):13311. https://doi.org/10.3390/ijms241713311
Chicago/Turabian StyleAjeethan, Nivethika, Svetlana N. Yurgel, and Lord Abbey. 2023. "Role of Bacteria-Derived Flavins in Plant Growth Promotion and Phytochemical Accumulation in Leafy Vegetables" International Journal of Molecular Sciences 24, no. 17: 13311. https://doi.org/10.3390/ijms241713311





