Investigation of Potential cGMP-Specific PDE V and Aminopeptidase N Inhibitors of Allium ampeloprasum L. and Its Bioactive Components: Kinetic and Molecular Docking Studies
Abstract
:1. Introduction
2. Results
2.1. Effects of AAE, DATS, DADS, KAE, QUE, MT, and IA on PDE5 Activity
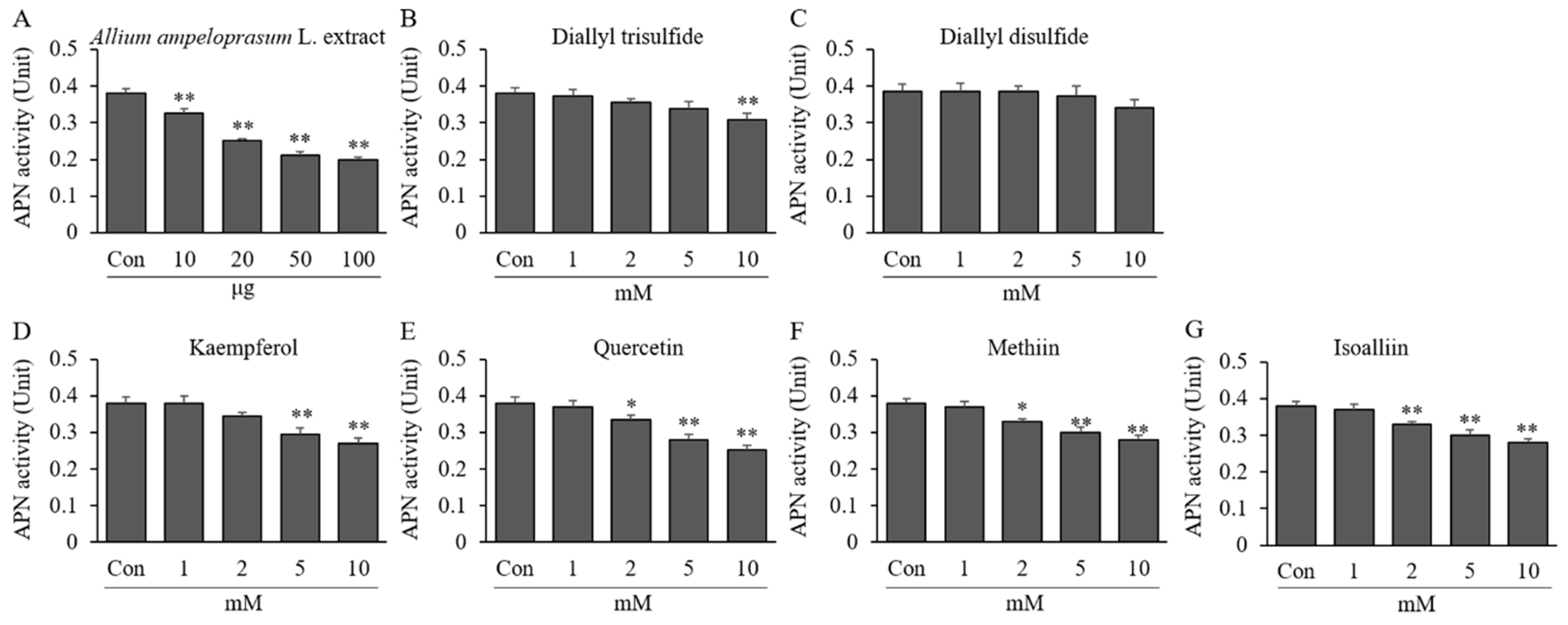
2.2. Effects of AAE, DATS, DADS, KAE, QUE, MT, and IA on APN Activity
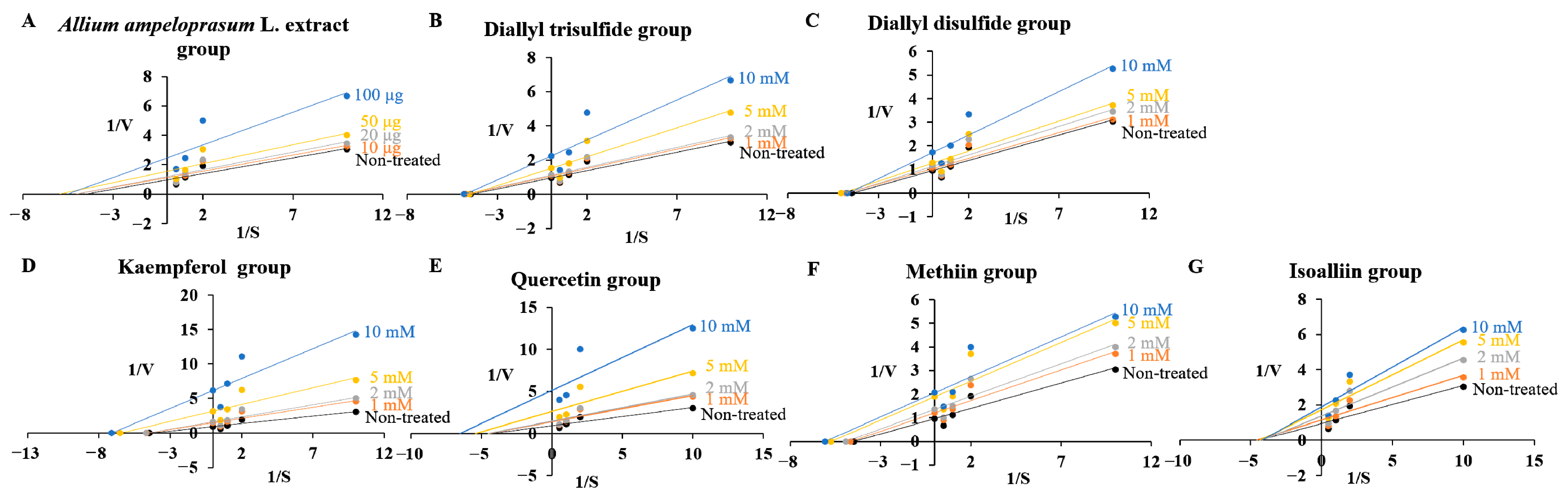
2.3. Effects of AAE, DATS, DADS, KAE, QUE, MT, and IA on Kinetic Parameters of Enzymes
2.4. In Silico Molecular Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction of Allium ampeloprasum L.
4.3. cGMP-Specific PDE V Assay
4.4. Aminopeptidase N Assay
4.5. Kinetic Assay
4.6. Molecular Docking
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Decaroli, M.C.; Rochira, V. Aging and sex hormones in males. Virulence 2017, 8, 545–570. [Google Scholar] [PubMed]
- Dudek, P.; Kozakowski, J.; Zgliczyński, W. Late-onset hypogonadism. Prz. Menopauzalny 2017, 16, 66–69. [Google Scholar] [PubMed]
- Schubert, M.; Jockenhövel, F. Late-onset hypogonadism in the aging male (LOH): Definition, diagnostic and clinical aspects. J. Endocrinol. Investig. 2005, 28, 23–27. [Google Scholar]
- Park, S.E.; Lee, H.J.; Jeong, I.S.; Kim, S. Effects of elephant garlic (Allium ampeloprasum) extract on testosterone synthesis in TM3 Leydig cells. Korean J. Food Preserv. 2022, 29, 790–799. [Google Scholar]
- Wang, C.; Nieschlag, E.; Swerdloff, R.; Behre, H.M.; Hellstrom, W.J.; Gooren, L.J.; Kaufman, J.M.; Legros, J.J.; Lunenfeld, B.; Morales, A.; et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur. J. Endocrinol. 2008, 159, 507–514. [Google Scholar] [PubMed]
- Singh, P. Andropause: Current concepts. Indian J. Endocrinol. Metab. 2013, 17, S621–S629. [Google Scholar]
- Sun, T.; Xu, W.; Tu, B.; Wang, T.; Liu, J.; Liu, K.; Luan, Y. Engineered adipose-derived stem cells overexpressing RXFP1 via CRISPR activation ameliorate erectile dysfunction in diabetic rats. Antioxidants 2023, 12, 171. [Google Scholar]
- Sert, S.; Karabay, E.; Gungor, B.; Yildirimturk, O. Effect of angiotensin receptor-neprilysin inhibitor treatment on erectile dysfunction in heart failure with a reduced ejection fraction. Marmara Med. J. 2023, 36, 99–104. [Google Scholar]
- Srivastava, H.; Pozzoli, M.; Lau, E. Defining the roles of cardiokines in human aging and age-associated diseases. Front. Aging 2022, 3, 884321. [Google Scholar]
- Lugnier, C.; Meyer, A.; Charloux, A.; Andrès, E.; Gény, B.; Talha, S. The endocrine function of the heart: Physiology and involvements of natriuretic peptides and cyclic nucleotide phosphodiesterases in heart failure. J. Clin. Med. 2019, 8, 1746. [Google Scholar]
- Boolell, M.; Allen, M.J.; Ballard, S.A.; Gepi-Attee, S.; Muirhead, G.J.; Naylor, A.M.; Osterloh, I.H.; Gingell, C. Sildenafil: An orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int. J. Impot. Res. 1996, 8, 47–52. [Google Scholar] [PubMed]
- Guazzi, M.; Vicenzi, M.; Arena, R.; Guazzi, M.D. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: Results of a 1-year, prospective, randomized, placebo-controlled study. Circ. Heart Fail. 2011, 4, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Klinger, J.R.; Thaker, S.; Houtchens, J.; Preston, I.R.; Hill, N.S.; Farber, H.W. Pulmonary hemodynamic responses to brain natriuretic peptide and sildenafil in patients with pulmonary arterial hypertension. Chest 2006, 129, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, K.H.; Yook, H.S. Analysis of active components of giant black garlic. J. Korean Soc. Food Sci. Nutr. 2015, 44, 1672–1681. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Antimicrobial activity of elephant garlic oil against Vibrio cholerae in vitro and in a food model. Biosci. Biotechnol. Biochem. 2009, 73, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitch, H.D.; Brewster, J.L. Onions and Allied Crops: Biochemistry Food Science Minor Crops; CRC Press: Boca Raton, FL, USA, 1989; pp. 20–22. [Google Scholar]
- Satyal, P.; Craft, J.D.; Dosoky, N.S.; Setzer, W.N. The chemical compositions of the volatile oils of garlic (Allium sativum) and wild garlic (Allium vineale). Foods 2017, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Shelke, P.A.; Rafiq, S.M.; Bhavesh, C.; Rafiq, S.I.; Swapnil, P.; Mushtaq, R. Leek (Allium ampeloprasum L.). In Antioxidants in Vegetables and Nuts—Properties and Health Benefits; Nayik, G.A., Gull, A., Eds.; Springer: Singapore, 2020; pp. 309–331. [Google Scholar]
- Polito, F.; Amato, G.; Caputo, L.; De Feo, V.; Fratianni, F.; Candido, V.; Nazzaro, F. Chemical composition and agronomic traits of Allium sativum and Allium ampeloprasum leaves and bulbs and their action against Listeria monocytogenes and other food pathogens. Foods 2022, 11, 995. [Google Scholar] [CrossRef]
- Lee, S.G.; Hahn, D.Y.; Kim, S.R.; Lee, W.Y.; Nam, J.O. Elephant garlic extracts inhibit adipogenesis in 3T3-L1 adipocytes. Microbiol. Biotechnol. Lett. 2020, 48, 383–388. [Google Scholar] [CrossRef]
- Chae, J.; Lee, E.; Oh, S.M.; Ryu, H.W.; Kim, S.; Nam, J.O. Aged black garlic (Allium sativum L.) and aged black elephant garlic (Allium ampeloprasum L.) alleviate obesity and attenuate obesity-induced muscle atrophy in diet-induced obese C57BL/6 mice. Biomed. Pharmacother. 2023, 163, 114810. [Google Scholar] [CrossRef]
- Dey, P.; Khaled, K.L. An extensive review on Allium ampeloprasum a magical herb. Int. J. Sci. Res. 2013, 4, 371–377. [Google Scholar]
- Jalilian, F.; Chahardoli, A.; Sadrjavadi, K.; Fattahi, A.; Shokoohinia, Y. Green synthesized silver nanoparticle from Allium ampeloprasum aqueous extract: Characterization, antioxidant activities, antibacterial and cytotoxicity effects. Adv. Powder Technol. 2020, 31, 1323–1332. [Google Scholar] [CrossRef]
- Zhang, F.; Jia, J.; Yao, X. Allium ampeloprasum leaf aqueous extract green-formulated Ag nanoparticles: Determination of anti-human lung cancer and antioxidant effects. J. Eng. Res. 2023, 100091, in press. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. A Review of Leek (A. ampeloprasum L.), an important vegetable and food ingredient with remarkable pharmaceutical activities. Pharmacogn. Commn. 2021, 11, 9–12. [Google Scholar] [CrossRef]
- Cho, Y.K.; Ann, S.W.; Jang, M.J.; Oh, T.S.; Oh, M.G.; Park, Y.J.; Kim, C.H. Analysis of biological activity by time of black garlic ripening in Seosan Yukjok garlic and elephant garlic. J. Environ. Sci. Int. 2020, 29, 469–477. [Google Scholar] [CrossRef]
- Yang, X.; Du, Z.; Pu, J.; Zhao, H.; Chen, H.; Liu, Y.; Li, Z.; Cheng, Z.; Zhong, H.; Liao, F. Classification of difference between inhibition constants of an inhibitor to facilitate identifying the inhibition type. J. Enzyme Inhib. Med. Chem. 2013, 28, 205–213. [Google Scholar] [CrossRef]
- Sung, B.J.; Hwang, K.Y.; Jeon, Y.H.; Lee, J.I.; Heo, Y.S.; Kim, J.H.; Moon, J.; Yoon, J.M.; Hyun, Y.L.; Kim, E.; et al. Structure of the catalytic domain of human phosphodiesterase 5 with bound drug molecules. Nature 2003, 425, 98–102. [Google Scholar] [CrossRef]
- Xu, R.X.; Hassell, A.M.; Vanderwall, D.; Lambert, M.H.; Holmes, W.D.; Luther, M.A.; Rocque, W.J.; Milburn, M.V.; Zhao, Y.; Ke, H.; et al. Atomic structure of PDE4: Insights into phosphodiesterase mechanism and specificity. Science 2000, 288, 1822–1825. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.H.; Bessay, E.P.; Kotera, J.; Grimes, K.A.; Liu, L.; Thompson, W.J.; Corbin, J.D. Phosphorylation of isolated human phosphodiesterase-5 regulatory domain induces an apparent conformational change and increases cGMP binding affinity. J. Biol. Chem. 2002, 277, 47581–47587. [Google Scholar] [CrossRef]
- Barren, B.; Gakhar, L.; Muradov, H.; Boyd, K.K.; Ramaswamy, S.; Artemyev, N.O. Structural basis of phosphodiesterase 6 inhibition by the C-terminal region of the gamma-subunit. EMBO J. 2009, 28, 3613–3622. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Xu, W. The structure and main functions of aminopeptidase N. Curr. Med. Chem. 2007, 14, 639–647. [Google Scholar] [CrossRef]
- Ganji, R.J.; Reddi, R.; Gumpena, R.; Marapaka, A.K.; Arya, T.; Sankoju, P.; Bhukya, S.; Addlagatta, A. Structural basis for the inhibition of M1 family aminopeptidases by the natural product actinonin: Crystal structure in complex with E. coli aminopeptidase N. Protein Sci. 2015, 24, 823–831. [Google Scholar] [CrossRef]
- Lei, Y.P.; Liu, C.T.; Sheen, L.Y.; Chen, H.W.; Lii, C.K. Diallyl disulfide and diallyl trisulfide protect endothelial nitric oxide synthase against damage by oxidized low-density lipoprotein. Mol. Nutr. Food Res. 2010, 54, S42–S52. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, G.K.; Shackelford, R.E.; Shen, X.; Dominic, P.; Kevil, C.G. Sulfide regulation of cardiovascular function in health and disease. Nat. Rev. Cardiol. 2023, 20, 109–125. [Google Scholar] [CrossRef]
- Sabphon, C.; Temkitthawon, P.; Ingkaninan, K.; Sawasdee, P. Phosphodiesterase inhibitory activity of the flavonoids and xanthones from Anaxagorea luzonensis. Nat. Prod. Commun. 2015, 10, 301–303. [Google Scholar] [CrossRef]
- Gaikwad, D.T.; Jadhav, N.R. Discovery of potential inhibitors for phosphodiesterase 5A, sodium-potassium pump and beta-adrenergic receptor from Terminalia arjuna: In silico approach. J. Biomol. Struct. Dyn. 2021, 39, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, A.A.; Priya, V.M.H.; Kumaran, A. Medicinal plants as a potential source of phosphodiesterase-5 inhibitors: A review. J. Ethnopharmacol. 2021, 267, 113536. [Google Scholar] [CrossRef]
- Parellada, J.; Guinea, M. Flavonoid inhibitors of trypsin and leucine aminopeptidase: A proposed mathematical model for IC50 estimation. J. Nat. Prod. 1995, 58, 823–829. [Google Scholar] [CrossRef]
- Bormann, H.; Melzig, M.F. Inhibition of metallopeptidases by flavonoids and related compounds. Pharmazie 2000, 55, 129–132. [Google Scholar] [PubMed]
- Manukyan, A.E.; Hovhannisyan, A.A. Podophyllotoxin and quercetin in silico derivatives docking analysis with cyclooxygenase-2 and aminopeptidase-N. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kim, J.J.; Moon, D.G. Past, present and future of PDE5 inhibitor. Korean J. Androl. 2008, 26, 49–60. [Google Scholar]
- Ojo, O.A.; Ojo, A.B.; Oyinloye, B.E.; Ajiboye, B.O.; Anifowose, O.O.; Akawa, A.; Olaiya, O.E.; Olasehinde, O.R.; Kappo, A.P. Ocimum gratissimum Linn. leaves reduce the key enzymes activities relevant to erectile dysfunction in isolated penile and testicular tissues of rats. BMC Complement. Altern. Med. 2019, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Melzig, M.F.; Bormann, H. Betulinic acid inhibits aminopeptidase N activity. Planta Med. 1998, 64, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, S. In vitro antithrombotic, hematological toxicity, and inhibitor studies of protocatechuic, isovanillic, and p-hydroxybenzoic acids from Maclura tricuspidata (Carr.) Bur. Molecules 2022, 27, 3496. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Sousa, S.F.; Fernandes, P.A.; Ramos, M.J. Protein-ligand docking: Current status and future challenges. Proteins 2006, 65, 15–26. [Google Scholar] [CrossRef]


| Compound | Km (mM) | Vmax (mU/min) | Kcat (k s−1) | Ki | Kik | Kiv | Kik/Kiv | |
|---|---|---|---|---|---|---|---|---|
| Non-treated | 0.223 ± 0.013 | 1.039 ± 0.052 | 7858 ± 393 | - | - | - | - | |
| AAE (mg) | 0.01 | 0.199 ± 0.011 | 0.899 ± 0.033 | 6795 ± 249 | 0.084 mg | 0.437 | 0.065 | 6.8 |
| 0.02 | 0.201 ± 0.015 | 0.849 ± 0.028 | 6415 ± 212 | |||||
| 0.05 | 0.168 ± 0.011 | 0.646 ± 0.021 | 4887 ± 159 | |||||
| 0.1 | 0.182 ± 0.012 | 0.408 ± 0.020 | 3085 ± 151 | |||||
| DATS (mM) | 1 | 0.211 ± 0.012 | 0.940 ± 0.026 | 7105 ± 197 | 7.53 mM | 133.4 | 7.51 | 17.8 |
| 2 | 0.206 ± 0.014 | 0.894 ± 0.032 | 6757 ± 242 | |||||
| 5 | 0.221 ± 0.011 | 0.655 ± 0.023 | 4955 ± 174 | |||||
| 10 | 0.208 ± 0.009 | 0.446 ± 0.019 | 3371 ± 144 | |||||
| DADS (mM) | 1 | 0.208 ± 0.010 | 0.959 ± 0.025 | 7253 ± 189 | 13.53 mM | 201.9 | 12.61 | 16.0 |
| 2 | 0.211 ± 0.013 | 0.876 ± 0.021 | 6625 ± 159 | |||||
| 5 | 0.198 ± 0.013 | 0.781 ± 0.011 | 5905 ± 83 | |||||
| 10 | 0.213 ± 0.012 | 0.580 ± 0.010 | 4383 ± 76 | |||||
| KAE (mM) | 1 | 0.215 ± 0.009 | 0.671 ± 0.013 | 5076 ± 98 | 3.61 mM | 16.92 | 1.85 | 9.1 |
| 2 | 0.214 ± 0.008 | 0.607 ± 0.010 | 4591 ± 76 | |||||
| 5 | 0.153 ± 0.011 | 0.316 ± 0.016 | 2390 ± 121 | |||||
| 10 | 0.140 ± 0.007 | 0.162 ± 0.009 | 1228 ± 69 | |||||
| QUE (mM) | 1 | 0.224 ± 0.016 | 0.724 ± 0.031 | 5473 ± 234 | 4.05 mM | 21.80 | 2.32 | 9.4 |
| 2 | 0.221 ± 0.011 | 0.686 ± 0.022 | 5187 ± 166 | |||||
| 5 | 0.184 ± 0.015 | 0.383 ± 0.008 | 2893 ± 60 | |||||
| 10 | 0.153 ± 0.009 | 0.195 ± 0.005 | 1478 ± 38 | |||||
| MT (mM) | 1 | 0.215 ± 0.010 | 0.828 ± 0.025 | 6261 ± 189 | 21.46 mM | 28.02 | 8.78 | 3.2 |
| 2 | 0.203 ± 0.014 | 0.738 ± 0.021 | 5580 ± 159 | |||||
| 5 | 0.174 ± 0.014 | 0.530 ± 0.013 | 4007 ± 98 | |||||
| 10 | 0.165 ± 0.011 | 0.486 ± 0.019 | 3673 ± 144 | |||||
| IA (mM) | 1 | 0.219 ± 0.012 | 0.871 ± 0.021 | 6587 ± 159 | 10.80 mM | 136.3 | 10.46 | 13.0 |
| 2 | 0.238 ± 0.015 | 0.726 ± 0.020 | 5489 ± 151 | |||||
| 5 | 0.230 ± 0.013 | 0.580 ± 0.017 | 4387 ± 129 | |||||
| 10 | 0.240 ± 0.011 | 0.531 ± 0.015 | 4018 ± 114 | |||||
| Compound | Km (mM) | Vmax (mU/min) | Kcat (s−1) | Ki | Kik | Kiv | Kik/Kiv | |
|---|---|---|---|---|---|---|---|---|
| Non-treated | 0.427 ± 0.017 | 0.316 ± 0.011 | 392.0 ± 13.6 | - | - | - | - | |
| AAE (mg) | 0.01 | 0.763 ± 0.026 | 0.254 ± 0.005 | 315.5 ± 6.2 | 0.057 mg | 0.108 | 0.066 | 1.6 |
| 0.02 | 0.884 ± 0.025 | 0.194 ± 0.006 | 240.3 ± 7.4 | |||||
| 0.05 | 0.840 ± 0.021 | 0.171 ± 0.003 | 212.4 ± 3.7 | |||||
| 0.1 | 0.822 ± 0.020 | 0.126 ± 0.003 | 156.3 ± 3.7 | |||||
| DATS (mM) | 1 | 0.422 ± 0.012 | 0.297 ± 0.006 | 368.9 ± 7.4 | 18.74 mM | 0.685 | 0.269 | 2.5 |
| 2 | 0.499 ± 0.014 | 0.294 ± 0.005 | 365.3 ± 6.2 | |||||
| 5 | 0.466 ± 0.015 | 0.249 ± 0.004 | 309.2 ± 5.0 | |||||
| 10 | 0.489 ± 0.011 | 0.230 ± 0.004 | 285.9 ± 5.0 | |||||
| DADS (mM) | 1 | 0.439 ± 0.013 | 0.318 ± 0.003 | 394.8 ± 3.7 | 65.71 mM | 1.369 | 1.250 | 1.1 |
| 2 | 0.437 ± 0.016 | 0.306 ± 0.006 | 379.5 ± 7.4 | |||||
| 5 | 0.440 ± 0.012 | 0.297 ± 0.005 | 368.8 ± 6.2 | |||||
| 10 | 0.461 ± 0.015 | 0.294 ± 0.004 | 364.9 ± 5.0 | |||||
| KAE (mM) | 1 | 0.441 ± 0.015 | 0.319 ± 0.006 | 396.3 ± 7.5 | 11.94 mM | 0.421 | 0.217 | 1.9 |
| 2 | 0.478 ± 0.011 | 0.284 ± 0.006 | 352.8 ± 7.4 | |||||
| 5 | 0.563 ± 0.013 | 0.248 ± 0.004 | 308.0 ± 5.0 | |||||
| 10 | 0.528 ± 0.014 | 0.216 ± 0.003 | 268.5 ± 3.7 | |||||
| QUE (mM) | 1 | 0.430 ± 0.011 | 0.297 ± 0.005 | 368.1 ± 6.2 | 13.19 mM | 3.272 | 0.118 | 27.7 |
| 2 | 0.442 ± 0.009 | 0.255 ± 0.006 | 316.6 ± 7.4 | |||||
| 5 | 0.415 ± 0.010 | 0.197 ± 0.003 | 244.1 ± 3.7 | |||||
| 10 | 0.414 ± 0.013 | 0.171 ± 0.002 | 212.3 ± 2.5 | |||||
| MT (mM) | 1 | 0.419 ± 0.012 | 0.296 ± 0.003 | 367.0 ± 3.7 | 13.43 mM | 3.428 | 0.126 | 27.1 |
| 2 | 0.411 ± 0.007 | 0.250 ± 0.005 | 310.6 ± 6.2 | |||||
| 5 | 0.407 ± 0.009 | 0.199 ± 0.003 | 246.8 ± 3.7 | |||||
| 10 | 0.415 ± 0.010 | 0.176 ± 0.002 | 218.9 ± 2.5 | |||||
| IA (mM) | 1 | 0.430 ± 0.011 | 0.307 ± 0.005 | 381.3 ± 6.2 | 33.08 mM | 1.142 | 0.237 | 4.8 |
| 2 | 0.395 ± 0.009 | 0.267 ± 0.006 | 331.6 ± 7.4 | |||||
| 5 | 0.391 ± 0.013 | 0.241 ± 0.004 | 298.9 ± 4.9 | |||||
| 10 | 0.392 ± 0.010 | 0.222 ± 0.003 | 275.8 ± 3.7 | |||||
| Ligand | Van der Waals | Hydrogen Bond | Electrostatic | Hydrophobic | ||
|---|---|---|---|---|---|---|
| DATS | LEU540, ARG597, LEU600, LYS604 | - | Attractive charge: GLU536, GLU539 | - | - | - |
| DADS | GLU536, LEU540, ARG597, LEU600, LYS604 | - | Attractive charge: GLU539 | - | - | - |
| KAE | GLU536, THR537, LEU540, PRO696 | Conventional: GLU539, ARG597 | - | - | - | Pi-Alkyl: ARG597, LEU600, LYS604 |
| QUE | ASN605 | Conventional: LYS603, LYS604, TYR606, LYS608 | - | Pi-Anion: GLU539, ASP568 | Pi-Cation: LYS608 | Pi-Alkyl: LYS604 |
| MT | GLU536, ARG597, LEU600, SER601, LYS604, ASN698 | Conventional: GLU539 | Attractive charge: GLU539 | - | - | - |
| IA | GLU536, LEU540, ARG597, LEU600, SER601, LYS604, ASN698 | - | Attractive charge: GLU539 | - | - | - |
| Ligand | Van der Waals | Hydrogen Bond | Unfavorable | Electrostatic | Hydrophobic | Miscellaneous | |||
|---|---|---|---|---|---|---|---|---|---|
| DATS | MET260 *, GLY261 *, ASN272, TYR275, ILE751, ASN780, SER784 | - | Positive–positive: LYS274, ARG783 | - | - | - | - | - | - |
| DADS | ARG349, LYS541, LEU628 | Conventional: GLU627 | - | Attractive charge: GLU543 | - | - | - | - | Pi-Sulfur: PHE353 |
| KAE | LEU289, ASN343, ASN344 | Conventional: LYS286, ARG293 * Carbon hydrogen: LYS286, ARG293 *, ASN343 | - | - | Pi-Anion: ASP290 | - | Pi-Alkyl: LYS286 | - | - |
| QUE | LEU289, ARG293 *, ASN344, ARG346, GLU382 | Conventional: LYS286, ASN343 Carbon hydrogen: LYS286, ARG293 *, ASN343 | - | - | Pi-Anion: ASP290 | Pi-Cation: LYS286 | - | Pi-Sigma: LYS286 | - |
| MT | VAL622, THR755 | - | Positive–positive: ARG641 | - | - | - | - | - | - |
| IA | ARG641, LEU752, THR755 | - | - | - | - | - | - | - | - |
| Ligand | Lowest Binding Energy (kcal/mol) | Average Docking Energy (kcal/mol) |
|---|---|---|
| DATS | −5.6 | −5.06 (n = 10) |
| DADS | −5.3 | −4.74 (n = 10) |
| KAE | −7.2 | −6.6 (n = 9) |
| QUE | −7.7 | −6.0 (n = 9) |
| MT | −5.2 | −4.75 (n = 10) |
| IA | −7.1 | −5.16 (n = 10) |
| SILD | −9.4 | −8.54 (n = 9) |
| Ligand | Lowest Binding Energy (kcal/mol) | Average Docking Energy (kcal/mol) |
|---|---|---|
| DATS | −5.4 | −4.59 (n = 10) |
| DADS | −5.0 | −4.50 (n = 10) |
| KAE | −8.1 | −7.59 (n = 9) |
| QUE | −8.3 | −7.71 (n = 9) |
| MT | −5.9 | −5.05 (n = 10) |
| IA | −6.6 | −5.22 (n = 10) |
| BEST | −8.3 | −7.99 (n = 9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-H.; Park, S.-M.; Kim, S. Investigation of Potential cGMP-Specific PDE V and Aminopeptidase N Inhibitors of Allium ampeloprasum L. and Its Bioactive Components: Kinetic and Molecular Docking Studies. Int. J. Mol. Sci. 2023, 24, 13319. https://doi.org/10.3390/ijms241713319
Choi J-H, Park S-M, Kim S. Investigation of Potential cGMP-Specific PDE V and Aminopeptidase N Inhibitors of Allium ampeloprasum L. and Its Bioactive Components: Kinetic and Molecular Docking Studies. International Journal of Molecular Sciences. 2023; 24(17):13319. https://doi.org/10.3390/ijms241713319
Chicago/Turabian StyleChoi, Jun-Hui, Seung-Man Park, and Seung Kim. 2023. "Investigation of Potential cGMP-Specific PDE V and Aminopeptidase N Inhibitors of Allium ampeloprasum L. and Its Bioactive Components: Kinetic and Molecular Docking Studies" International Journal of Molecular Sciences 24, no. 17: 13319. https://doi.org/10.3390/ijms241713319






