Inhibition of Urban Particulate Matter-Induced Airway Inflammation by RIPK3 through the Regulation of Tight Junction Protein Production
Abstract
:1. Introduction
2. Results
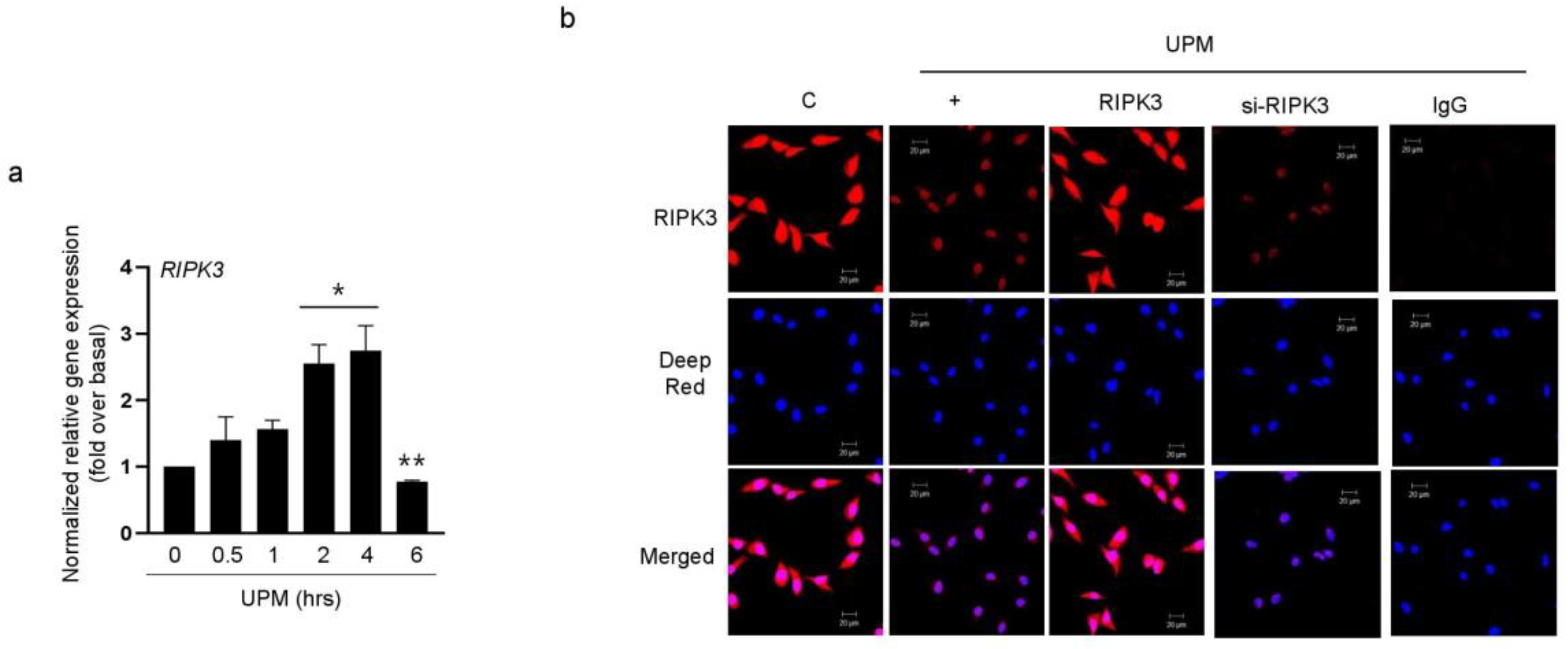
2.1. UPM Did Not Affect the Translocation from the Nucleus to the Cytoplasm in BEAS-2B Bronchial Epithelial Cells
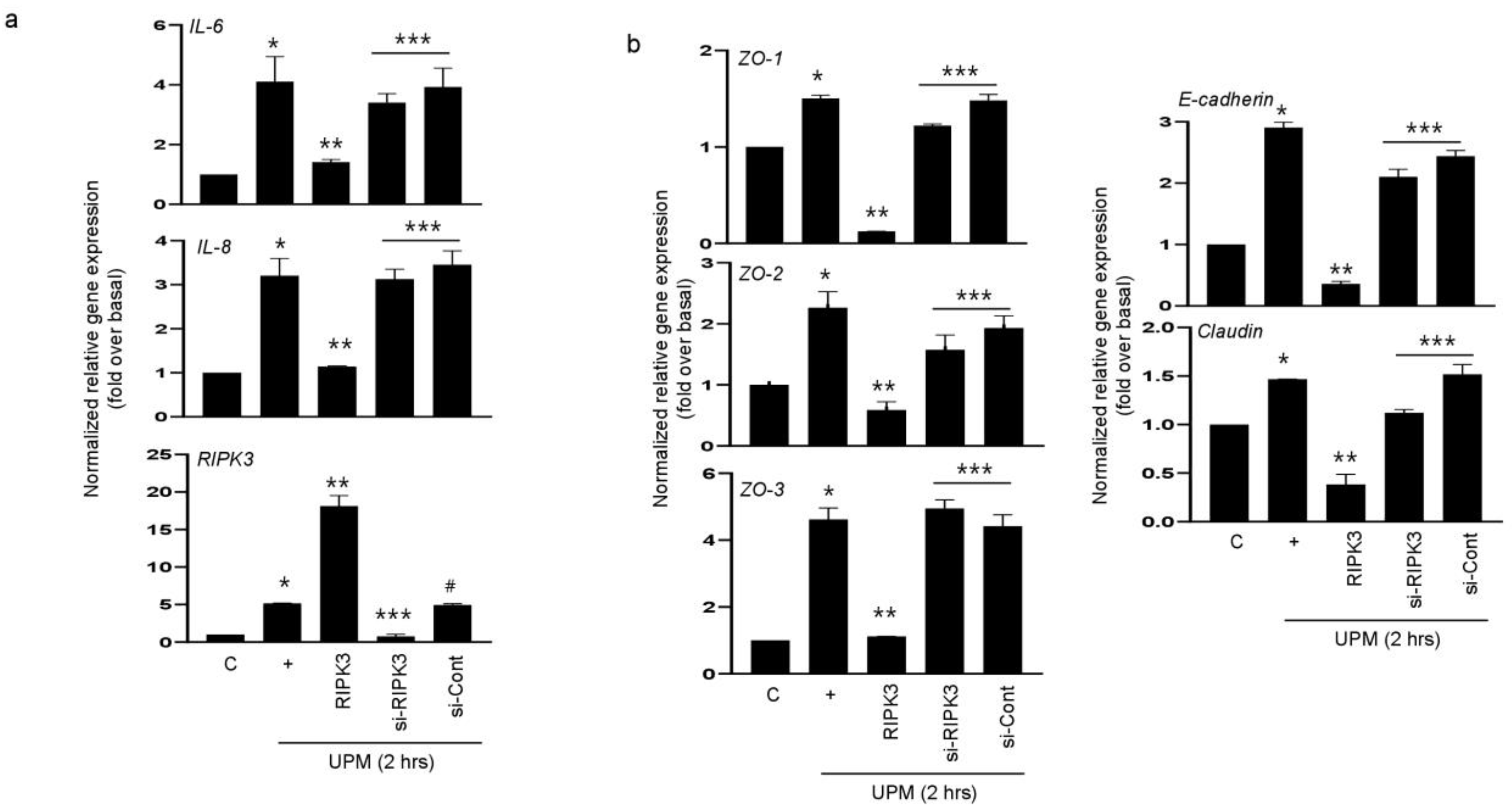
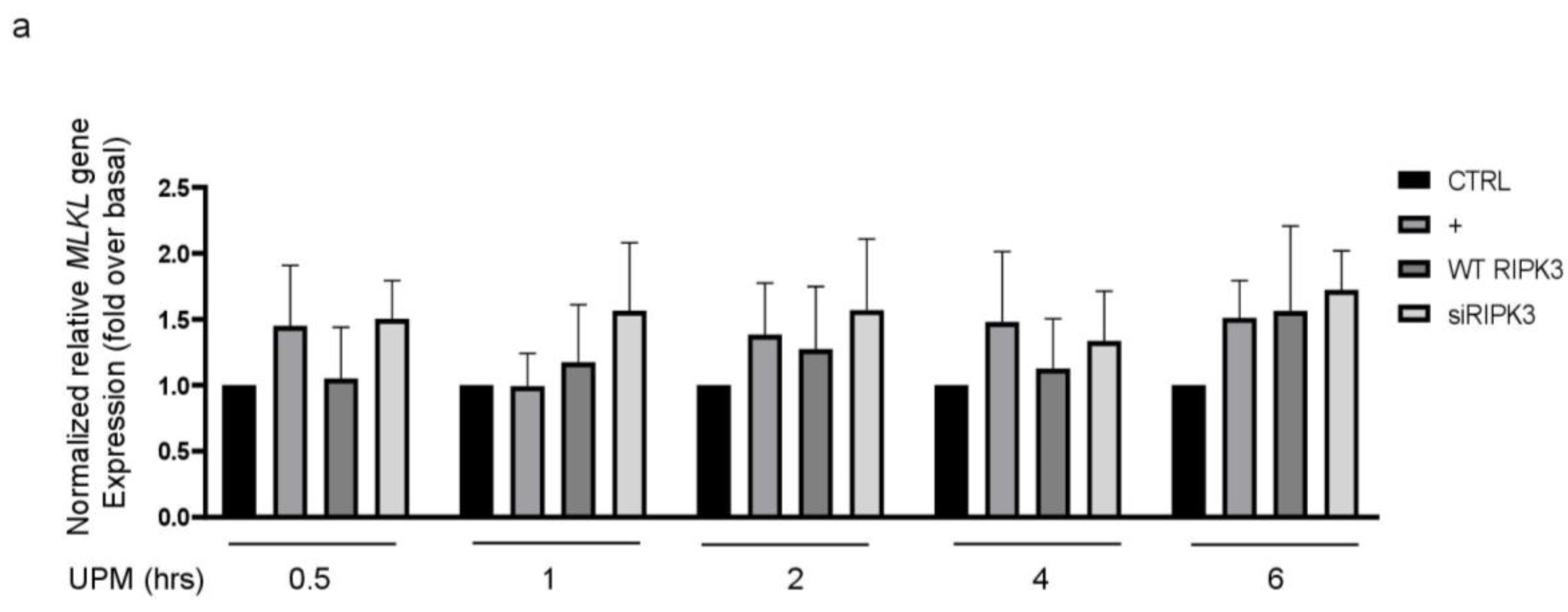
2.2. RIPK3 Overproduction Downregulates the UPM-Induced Gene Expression of Pro-Inflammatory Cytokines and Tight Junction Proteins
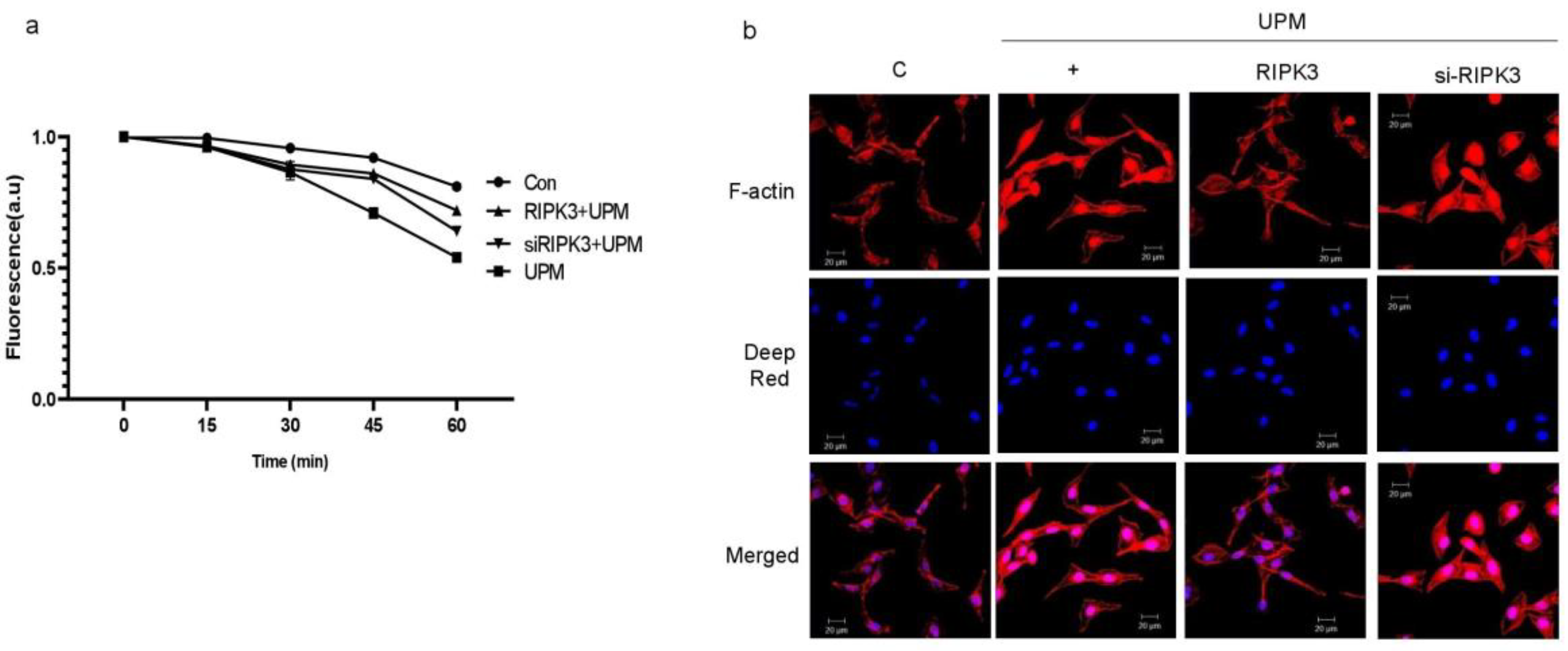
2.3. Overexpression of RIPK3 Regulates UPM-Induced TEER and F-Actin Production in Cells
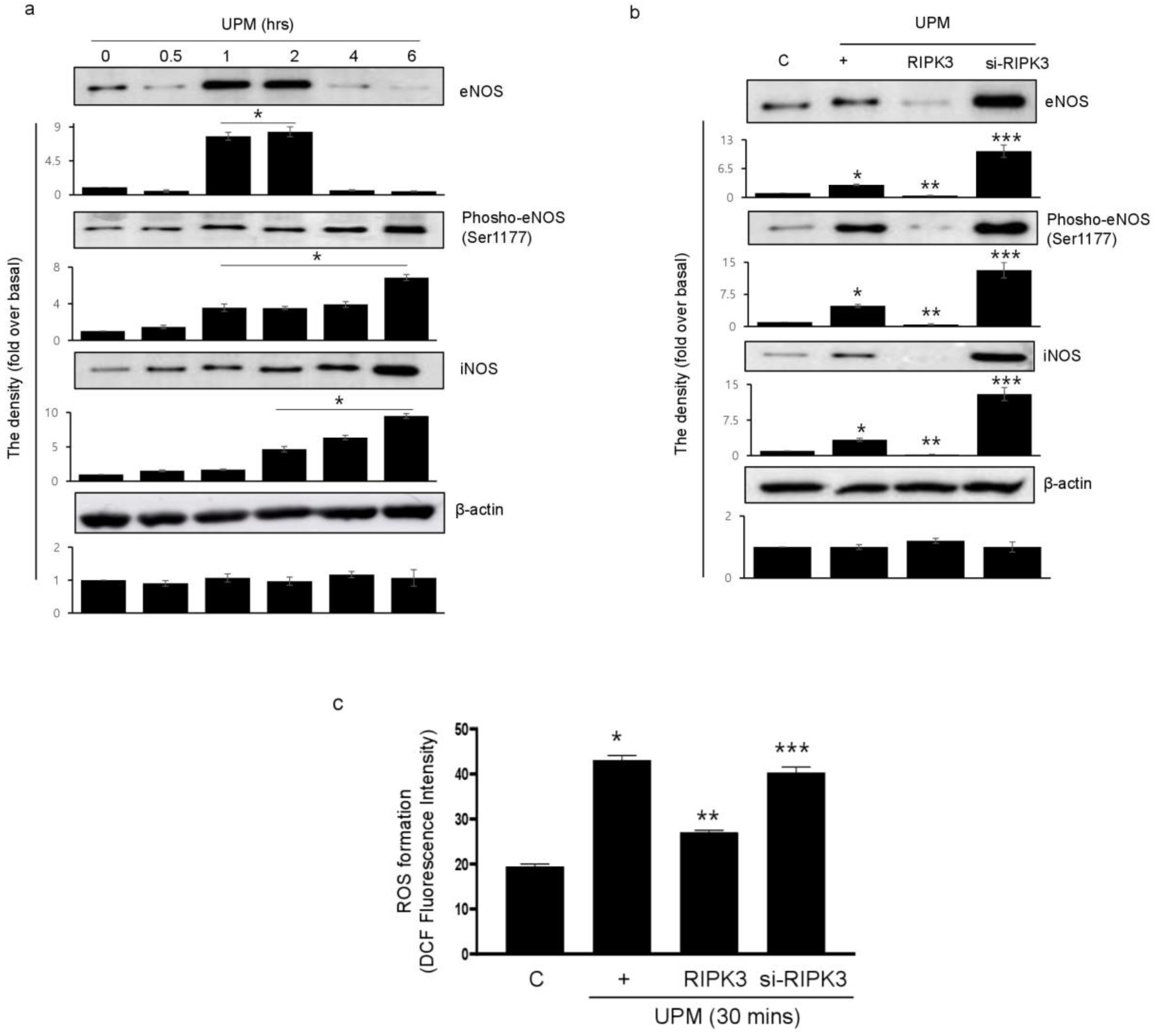
2.4. Overexpression of RIPK3 Decreases UPM-Induced ROS Production by Inhibiting Production of eNOS and iNOS
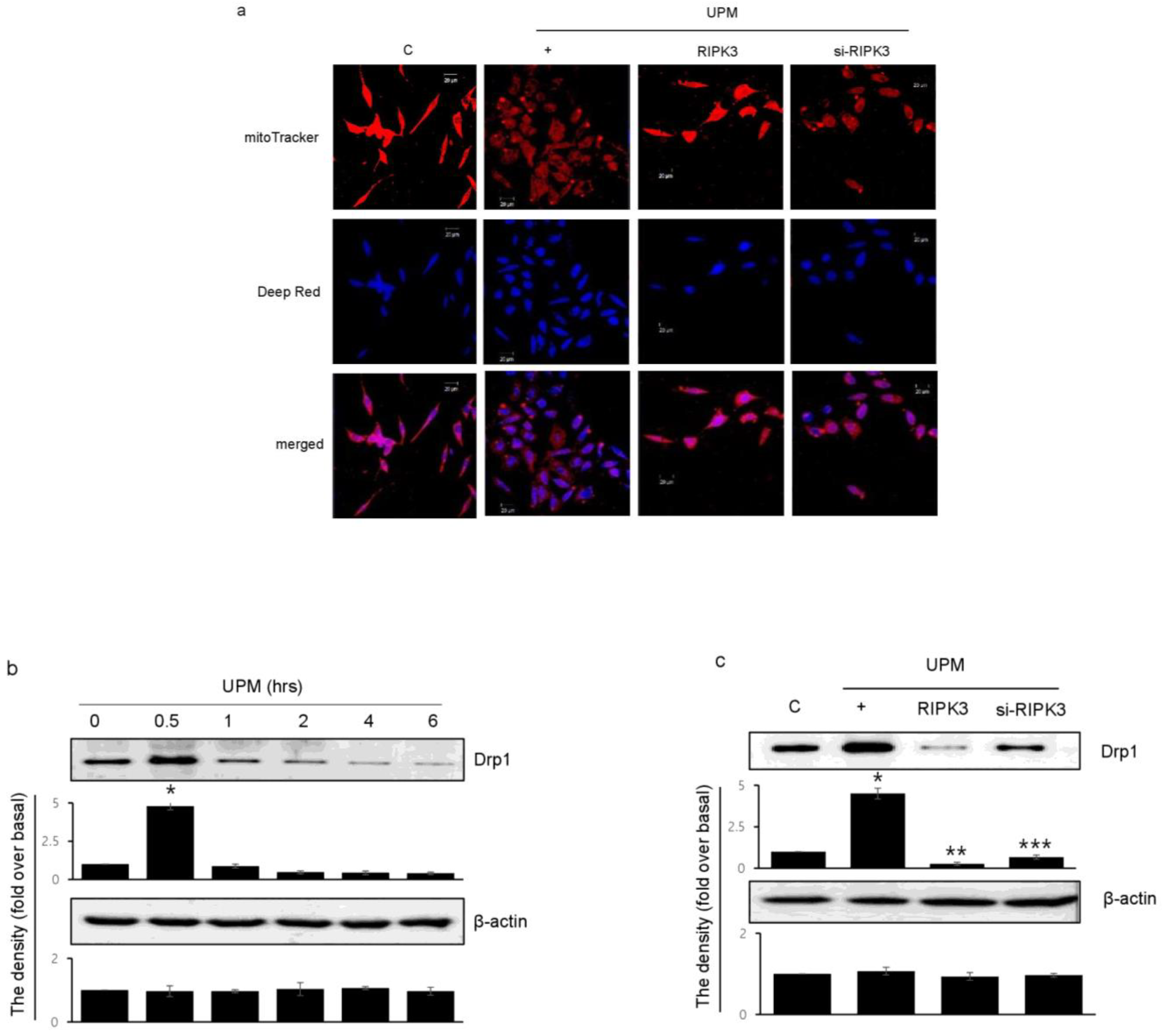
2.5. RIPK3 Overexpression Upregulates UPM-Induced Mitochondrial Membrane Integrity by Regulating Mitochondrial Fission
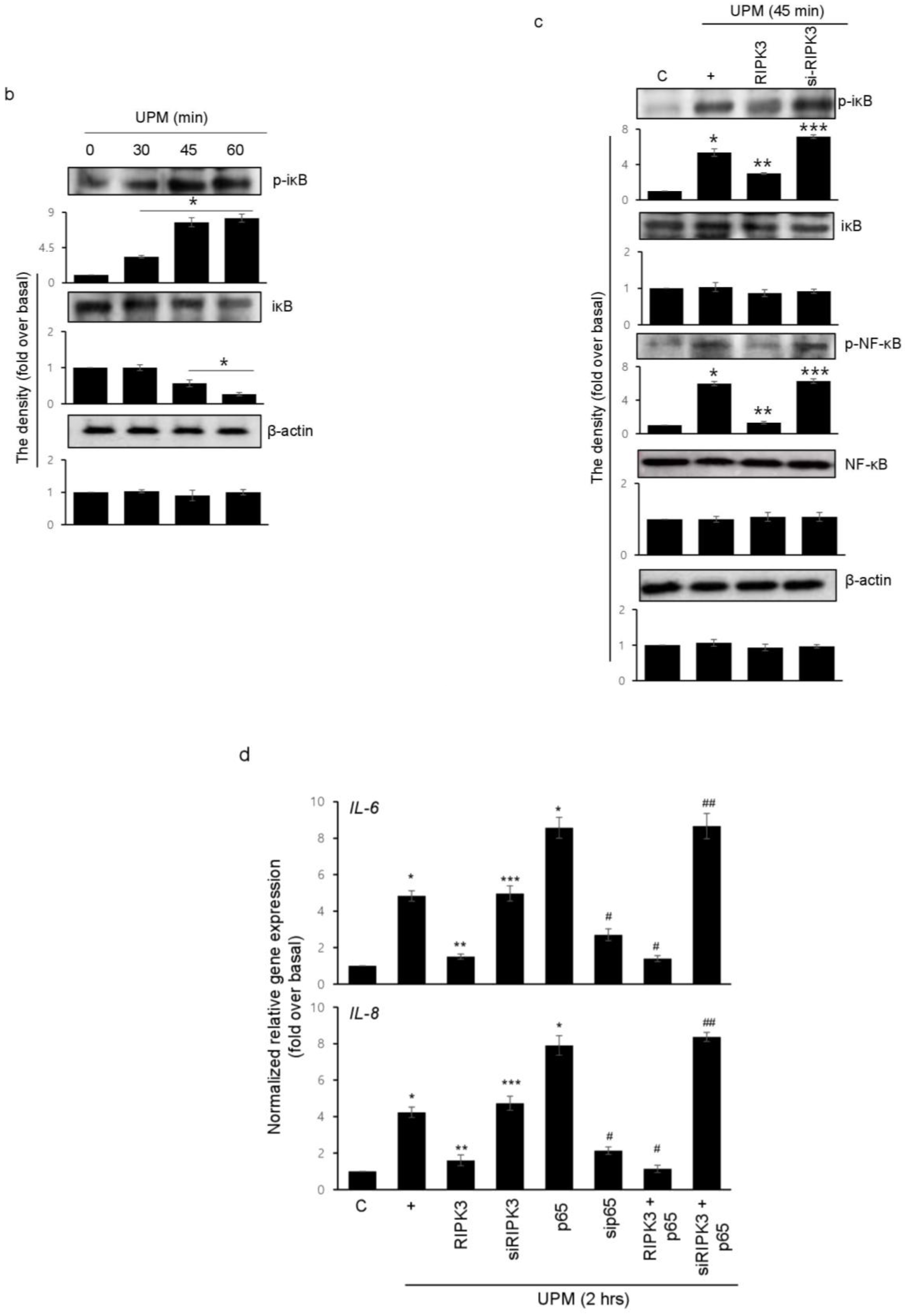
2.6. Overexpression of RIPK3 Diminishes UPM-Induced Pro-Inflammatory Cytokine Production by Inhibiting ikB Activation
3. Discussion
4. Materials and Methods
4.1. Materials and Cell Culture
4.2. Real-Time qRT-PCR
4.3. Immunocytochemistry
4.4. Trans-Epithelial Electrical Resistance (TEER)
4.5. F-Actin Staining
4.6. Western Blot Analysis
4.7. ROS Measurement
4.8. Analyses and Measurements of Mitochondrial Morphology
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alemayehu, Y.A.; Asfaw, S.L.; Terfie, T.A. Exposure to urban particulate matter and its association with human health risks. Environ. Sci. Pollut. Res. Int. 2020, 27, 27491–27506. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Fuller, G.W.; Anderson, R.A.; Harrison, R.M.; Armstrong, B. Urban ambient particle metrics and health: A time-series analysis. Epidemiology 2010, 21, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Ritz, B.; Hoffmann, B.; Peters, A. The Effects of Fine Dust, Ozone, and Nitrogen Dioxide on Health. Dtsch. Arztebl. Int. 2019, 23, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.S.; Choi, H.; Jang, G.; Lee, K.H.; Kim, E.; Kim, K.J.; Jeong, G.Y.; Kim, J.S.; Na, C.S.; Kim, S. Long-Term Exposure to Urban Particulate Matter on the Ocular Surface and the Incidence of Deleterious Changes in the Cornea, Conjunctiva and Retina in Rats. Int. J. Mol. Sci. 2020, 21, 4976. [Google Scholar] [CrossRef]
- Lee, T.G.; Hyun, S.W.; Jo, K.; Park, B.; Lee, I.S.; Song, S.J.; Kim, C.S. Achyranthis radix Extract Improves Urban Particulate Matter-Induced Dry Eye Disease. Int. J. Environ. Res. Public Health 2019, 16, 3229. [Google Scholar] [CrossRef]
- Morgan, M.J.; Kim, Y.S. Roles of RIPK3 in necroptosis, cell signaling, and disease. Exp. Mol. Med. 2022, 54, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Shlomovitz, I.; Zargrian, S.Z.; Gerlic, M. Mechanisms of RIPK3-induced inflammation. Immunol. Cell Biol. 2017, 95, 166–172. [Google Scholar] [CrossRef]
- Ge, C.; Hu, L.; Lou, D.; Li, Q.; Feng, J.; Wu, Y.; Tan, J.; Minxuan Xu, M. Nrf2 deficiency aggravates PM2.5-induced cardiomyopathy by enhancing oxidative stress, fibrosis and inflammation via RIPK3-regulated mitochondrial disorder. Aging 2020, 12, 4836–4865. [Google Scholar] [CrossRef]
- Sureshbabu, A.; Patino, E.; Ma, K.C.; Laursen, K.; Finkelsztein, E.J.; Akchurin, O.; Muthukumar, T.; Ryter, S.W.; Gudas, L.; Choi, A.M.K.; et al. RIPK3 promotes sepsis-induced acute kidney injury via mitochondrial dysfunction. JCI Insight 2018, 3, e98411. [Google Scholar] [CrossRef]
- Edelblum, K.L.; Turner, J.R. The tight junction in inflammatory disease: Communication breakdown. BMC Opin. Pharmacol. 2009, 9, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Weber, C.R.; Turner, J.R. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J. Cell Biol. 2008, 181, 683–695. [Google Scholar] [CrossRef]
- Sugita, K.; Kabashima, K. Tight junctions in the development of asthma, chronic rhinosinusitis, atopic dermatitis, eosinophilic esophagitis, and inflammatory bowel diseases. J. Leukoc. Biol. 2020, 107, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Izuhara, K. Barrier dysfunction in allergy. Allergol. Int. 2018, 67, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.; Park, S.H.; Kang, D.H.; Kim, J.W.; Kim, J.D.; Ryu, S.; Lee, J.; Jeong, H.M.; Hwang, H.R.; Song, K.S. Inhibition of Pseudomonas aeruginosa LPS-Induced airway inflammation by RIPK3 in human airway. J. Cell. Mol. Med. 2022, 26, 5506–5516. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fang, Y.; Wu, J.; Chen, H.; Zou, Z.; Zhang, X.; Shao, J.; Xu, Y. RIPK3-MLKL-mediated necroinflammation contributes to AKI progression to CKD. Cell Death Dis. 2018, 9, 878. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, W.; Guo, J.; Wang, X. Osmotic stress activates RIPK3/MLKL-mediated necroptosis by increasing cytosolic pH through a plasma membrane Na+/H+ exchanger. Sci. Signal. 2022, 15, eabn5881. [Google Scholar] [CrossRef]
- Yu, Z.; Jiang, N.; Su, W.; Zhuo, Y. Necroptosis: A Novel Pathway in Neuroinflammation. Front. Harmacol. 2021, 12, 701564. [Google Scholar] [CrossRef]
- Dhuriya, Y.K.; Sharma, D. Necroptosis: A regulated inflammatory mode of cell death. J. Neuroinflamm. 2018, 15, 199. [Google Scholar] [CrossRef]
- Kroemer, G.; El-Deiry, W.S.; Golstein, P.; Peter, M.E.; Vaux, D.; Vandenabeele, P.; Zhivotovsky, B.; Blagosklonny, M.V.; Malorni, W.; Knight, R.A.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005, 12, 1463–1467. [Google Scholar] [CrossRef]
- Fiers, W.; Beyaert, R.; Declercq, W.; Vandenabeele, P. More than one way to die: Apoptosis, necrosis and reactive oxygen damage. Oncogene 1999, 18, 7719–7730. [Google Scholar] [CrossRef] [PubMed]
- Heijink, I.H.; Brandenburg, S.M.; Noordhoek, J.A.; Postma, D.S.; Slebos, D.J.; van Oosterhout, A.J.M. Characterisation of cell adhesion in airway epithelial cell types using electric cell-substrate impedance sensing. Eur. Respir. J. 2010, 35, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Kenta Moriwaki, K.; Chan, F.K.M. RIP3: A molecular switch for necrosis and inflammation. Genes Dev. 2013, 27, 1640–1649. [Google Scholar] [CrossRef]
- Frank, D.; Garnish, S.E.; Sandow, J.J.; Weir, A.; Liu, L.; Clayer, E.; Meza, L.; Rashidi, M.; Cobbold, S.A.; Scutts, S.R.; et al. Ubiquitylation of RIPK3 beyond-the-RHIM can limit RIPK3 activity and cell death. iScience 2022, 25, 104632. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Park, H.H.; Kim, S.; Chung, J.M.; Noh, H.J.; Kim, S.K.; Song, H.K.; Lee, C.W.; Morgan, M.J.; Kang, H.C.; et al. PELI1 Selectively Targets Kinase-Active RIP3 for Ubiquitylation-Dependent Proteasomal Degradation. Mol. Cell 2018, 70, 920–935. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Feng, J.; Yu, J.; Chen, G. FKBP12 mediates necroptosis by initiating RIPK1-RIPK3-MLKL signal transduction in response to TNF receptor 1 ligation. J. Cell Sci. 2019, 132, jcs227777. [Google Scholar] [CrossRef]
- Mitic, L.L.; Anderson, J.M. Molecular architecture of tight junctions. Annu. Rev. Physiol. 1998, 60, 121–142. [Google Scholar] [CrossRef]
- Kim, J.S.; Oh, J.M.; Choi, H.; Kim, S.W.; Kim, S.; Kim, B.G.; Cho, J.H.; Lee, J.; Lee, D.C. Activation of the Nrf2/HO-1 pathway by curcumin inhibits oxidative stress in human nasal fibroblasts exposed to urban particulate matter. BMC Complement Med. Ther. 2020, 20, 101. [Google Scholar] [CrossRef]
- Wei, J.; Chen, L.; Wang, D.; Tang, L.; Xie, Z.; Chen, W.; Zhang, S.; Weng, G. Upregulation of RIP3 promotes necroptosis via a ROS-dependent NF-κB pathway to induce chronic inflammation in HK-2 cells. Mol. Med. Rep. 2021, 24, 783. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Zhang, Y.; He, X.; Zhong, C.Q.; Ni, H.; Chen, X.; Liang, Y.; Wu, J.; Zhao, S.; et al. RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis. Nat. Cell Biol. 2018, 20, 186–197. [Google Scholar] [CrossRef]
- Feng, Y.; Aliagan, A.I.; Tombo, N.; Draeger, D.; Bopassa, J.C. RIP3 Translocation into Mitochondria Promotes Mitofilin Degradation to Increase Inflammation and Kidney Injury after Renal Ischemia-Reperfusion. Cells 2022, 11, 1894. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhu, P.; Guo, J.; Hu, N.; Wang, S.; Li, D.; Hu, S.; Ren, J.; Cao, F.; Chen, Y. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox. Biol. 2017, 13, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Afonso, M.B.; Islam, T.; Magusto, J.; Amorim, R.; Lenoir, V.; Simões, R.F.; Teixeira, J.; Silva, L.C.; Wendum, D.; Jéru, I.; et al. RIPK3 dampens mitochondrial bioenergetics and lipid droplet dynamics in metabolic liver disease. Hepatology 2023, 77, 1319–1334. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Song, K.S. STAT3 inhibition decreases ATP-induced MUC8 gene expression in human airway epithelial cells. Kosin Med. J. 2022, 37, 134–139. [Google Scholar] [CrossRef]

| Gene | Sequence |
|---|---|
| GAPDH | F: CCACATGGCCTCCAAGGAGTAAGAC |
| R: AGGAGGGGAGATTCAGTGTGGTGGG | |
| IL-6 | F: AGACAGCCACTCACCTCTTCAG |
| R: TTCTGCCAGTGCCTCTTTGCTG | |
| IL-8 | F: GAGAGTGATTGAGAGTGGACCAC |
| R: CACAACCCTCTGCACCCAGTTT | |
| RIPK3 | F: GCTACGATGTGGCGGTCAAGA |
| R: TTGGCCCAGTTCACCTCTCG | |
| ZO-1 | F: GTCCAGAATCTCGGAAAAGTGCC |
| R: CTTTCAGCGCACCATACCAACC | |
| ZO-2 | F: ATTAGTGCGGGAGGATGCCGTT |
| R: TCTGCCACAAGCCAGGATGTCT | |
| ZO-3 | F: GCTTCCTCAAGGGCAAGAGCAT |
| R: CGTGTCAGGTTCTGGAATGGCA | |
| E-cadherin | F: GTCTGTAGGAAGGCACAGCC |
| R: TGCAACGTCGTTACGAGTCA | |
| Claudin | F: CAGCTGTTGGGCTTCATTCT |
| R: ATCACTCCCAGGAGGATGCC | |
| MLKL | F: TCACACTTGGCAAGCGCATGGT |
| R: GTAGCCTTGAGTTACCAGGAAGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-H.; Lee, H.-C.; Jeong, H.M.; Lee, J.-S.; Cha, H.-J.; Kim, C.H.; Kim, J.; Song, K.S. Inhibition of Urban Particulate Matter-Induced Airway Inflammation by RIPK3 through the Regulation of Tight Junction Protein Production. Int. J. Mol. Sci. 2023, 24, 13320. https://doi.org/10.3390/ijms241713320
Park S-H, Lee H-C, Jeong HM, Lee J-S, Cha H-J, Kim CH, Kim J, Song KS. Inhibition of Urban Particulate Matter-Induced Airway Inflammation by RIPK3 through the Regulation of Tight Junction Protein Production. International Journal of Molecular Sciences. 2023; 24(17):13320. https://doi.org/10.3390/ijms241713320
Chicago/Turabian StylePark, Sun-Hee, Hyun-Chae Lee, Hye Min Jeong, Jeong-Sang Lee, Hee-Jae Cha, Cheol Hong Kim, Jeongtae Kim, and Kyoung Seob Song. 2023. "Inhibition of Urban Particulate Matter-Induced Airway Inflammation by RIPK3 through the Regulation of Tight Junction Protein Production" International Journal of Molecular Sciences 24, no. 17: 13320. https://doi.org/10.3390/ijms241713320






