Characterization of Volatile Organic Compounds in Five Celery (Apium graveolens L.) Cultivars with Different Petiole Colors by HS-SPME-GC-MS
Abstract
:1. Introduction
2. Results
2.1. Total Ion Flow Diagram of Celery VOCs
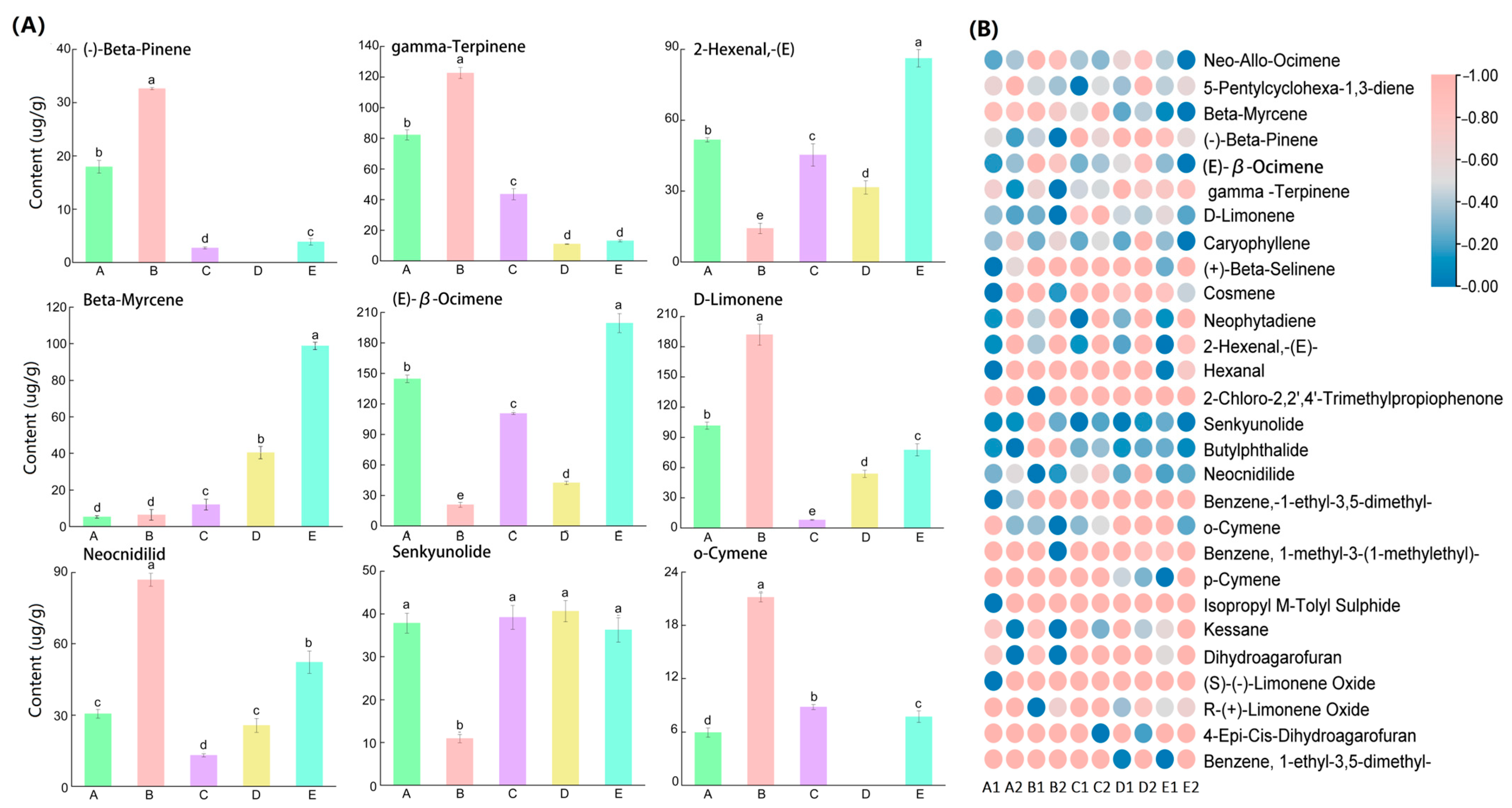
2.2. Composition and Content of VOCs
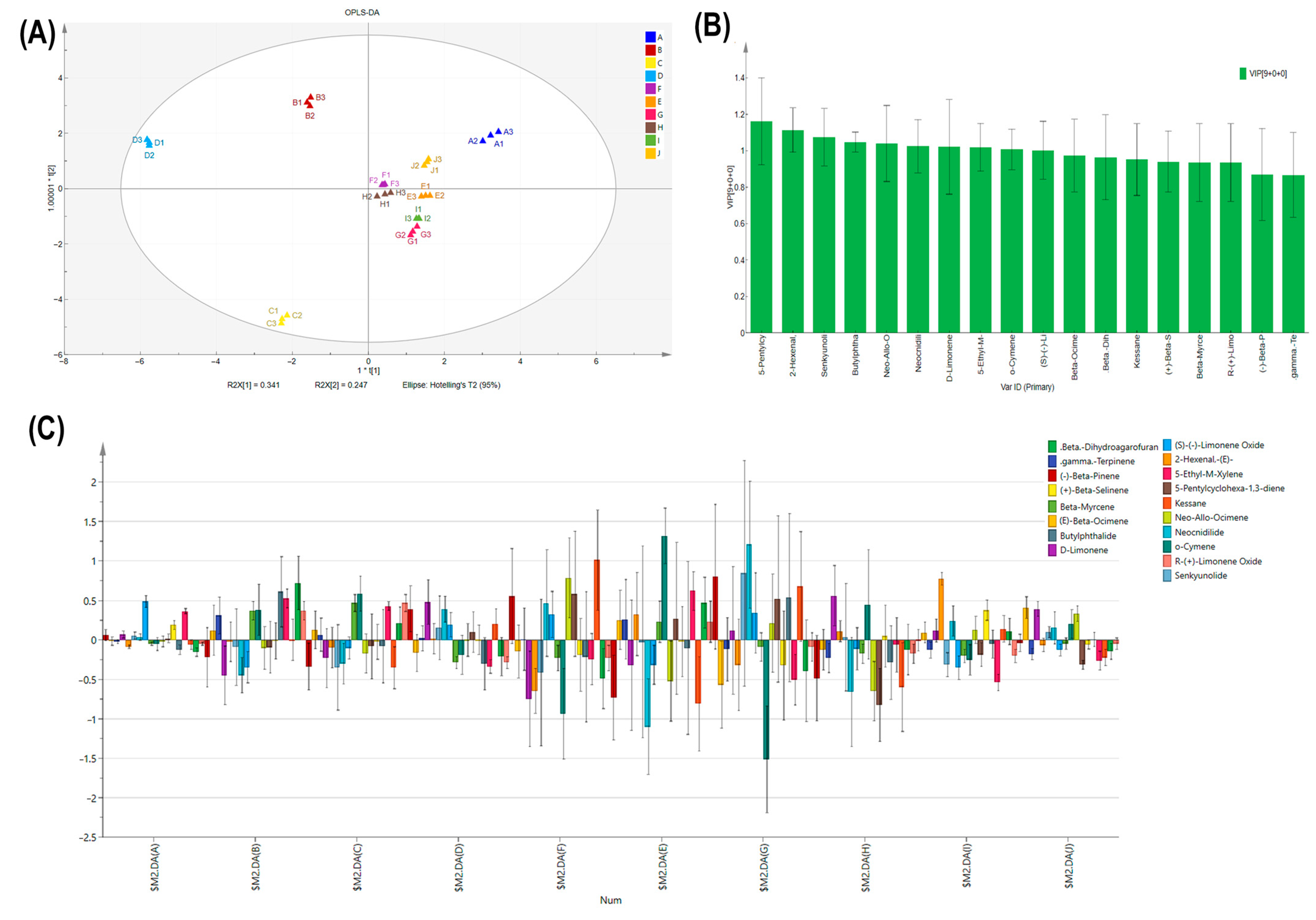
2.3. Comparison of Leaf Blade Petiole VOC Differentiation
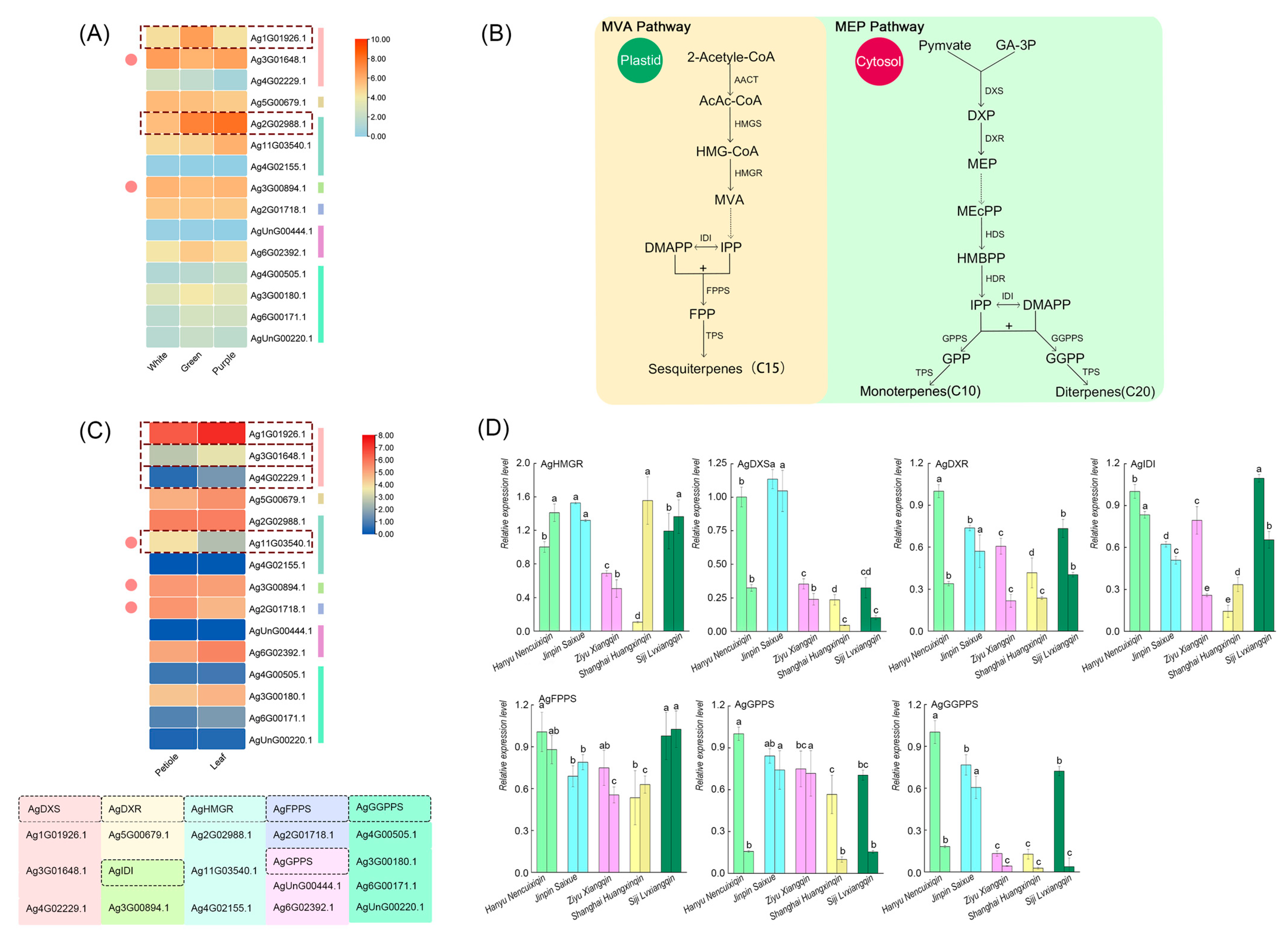
2.4. Validation of Key Regulatory Genes in the Terpenoid Synthesis Pathway
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Extraction of VOCs in Celery
4.3. Detection of VOCs by GC-MS Analysis Conditions
4.4. Analysis of VOCs and Gene Expressions
4.4.1. Qualitative and Quantitative Analysis of VOCs
4.4.2. Gene Expression Studies of Terpenoid Synthesis Pathways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.Y.; Feng, K.; Hou, X.L.; Jiang, Q.; Xu, Z.S.; Wang, G.L.; Liu, J.X.; Wang, F.; Xiong, A.S. The genome sequence of celery (Apium graveolens L.), an important leaf vegetable crop rich in apigenin in the Apiaceae family. Hortic. Res. 2020, 7, 223–253. [Google Scholar]
- Khairullah, A.R.; Solikhah, T.I.; Ansori, A.N.; Hidayatullah, A.R.; Hartadi, E.B.; Ramandinianto, S.C.; Fadholly, A. Review on the pharmacological and health aspects of Apium Graveolens or celery: An update. Syst. Rev. Pharm. 2021, 12, 606–612. [Google Scholar]
- Kooti, W.; Daraei, N. A review of the antioxidant activity of celery (Apium graveolens L.). Evid-Based. Compl. Alt. 2017, 22, 1029–1034. [Google Scholar]
- Tan, G.; Ma, J.; Zhang, X.; Xu, Z.; Xiong, A.S. AgFNS overexpression increase apigenin and decrease anthocyanins in petioles of transgenic celery. Plant Sci. 2017, 263, 31–38. [Google Scholar] [PubMed]
- Turner, L.; Lignou, S.; Gawthrop, F.; Wagstaff, C. Investigating the Relationship of Genotype and Climate Conditions on the Volatile Composition and Sensory Profile of Celery (Apium graveolens). Foods 2021, 10, 1335. [Google Scholar] [PubMed]
- Liscombe, D.K.; Kamiyoshihara, Y.; Ghironzi, J.; Kempthorne, C.J.; Hooton, K.; Bulot, B.; Kanellis, V.; McNulty, J.; Lam, N.B.; Nadeau, L.F.; et al. A flavin-dependent monooxygenase produces nitrogenous tomato aroma volatiles using cysteine as a nitrogen source. Proc. Natl. Acad. Sci. USA 2022, 119, e2118676119. [Google Scholar]
- Akogou, F.U.; Canoy, T.S.; Kayodé, A.P.; den Besten, H.M.; Linnemann, A.R.; Fogliano, V. Application of apigeninidin-rich red sorghum biocolorant in a fermented food improves product quality. J. Sci. Food Agric. 2019, 99, 2014–2020. [Google Scholar]
- Fincheira, P.; Quiroz, A. Microbial volatiles as plant growth inducers. Microbiol. Res. 2018, 208, 63–75. [Google Scholar]
- Abbas, F.; Zhou, Y.W.; O’Neill Rothenberg, D.; Alam, I.; Ke, Y.G.; Wang, H.C. Aroma Components in Horticultural Crops: Chemical Diversity and Usage of Metabolic Engineering for Industrial Applications. Plants 2023, 12, 1748. [Google Scholar]
- Farré-Armengol, G.; Filella, I.; Llusià, J.; Peñuelas, J. β-Ocimene, a key floral and foliar volatile involved in multiple interactions between plants and other organisms. Molecules 2017, 22, 1148–1156. [Google Scholar]
- Xu, L.; Zang, E.H.; Sun, S.Y.; Li, M.H. Main flavor compounds and molecular regulation mechanisms in fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2022, 1–21. [Google Scholar] [CrossRef]
- Yan, J.; Wang, H.; Wang, Y.; Xu, S.; Wang, Y.H.; He, L.F.; Yu, L.; Zhu, W.M. Integrated metabolome and transcriptome analysis reveals candidate genes involved in metabolism of terpenoids and phthalides in celery seeds. Ind. Crops Prod. 2021, 172, 114011. [Google Scholar]
- Khorramifar, A.; Karami, H.; Wilson, A.D.; Sayyah, A.H.A.; Shuba, A.; Lozano, J. Grape Cultivar Identification and Classification by Machine Olfaction Analysis of Leaf Volatiles. Chemosensors 2022, 10, 125. [Google Scholar]
- Madani, A.; Mazouni, N.; Nedjhioui, M. Plants’ Bioactive Metabolites and Extraction Methods. In Bioactive Compounds; Intech Open: London, UK, 2021; p. 3. [Google Scholar]
- Díaz-Maroto, M.C.; Guchu, E.; Castro-Vázquez, L.; Torres, C.D.; Pérez-Coello, M.S. Aroma-active compounds of American, French, Hungarian and Russian oak woods, studied by GC-MS and GC-O. Flavour Fragr. J. 2008, 23, 93–98. [Google Scholar]
- El, G.; Ahmed, H.; Fujioka, K.; Shibamoto, T. Determination of acrylamide formed in asparagine/d-Glucose maillard model systems by using gas chromatography with headspace solid-Phase microextraction. J. AOAC. Int. 2019, 89, 149–153. [Google Scholar]
- Gonzalez, A.; Benfodda, Z.; Bénimélis, D.; Fontaine, J.X.; Molinié, R.; Meffre, P. Extraction and identification of volatile organic compounds in scentless flowers of 14 Tillandsia species using HS-SPME/GC-MS. Metabolites 2022, 12, 628. [Google Scholar]
- Li, C.X.; Li, X.L.; Liang, G.L.; Xiang, S.Q.; Han, G.H. Volatile composition changes in lemon during fruit maturation by HS-SPME-GC-MS. J. Sci. Food Agric. 2022, 102, 3599–3606. [Google Scholar]
- Deng, C.H.; Song, G.X.; Hu, Y.M. Rapid determination of volatile compounds emitted from Chimonanthus praecox flowers by HS-SPME-GC-MS. Z. Für Naturforschung C J. Biosci. 2004, 59, 636–640. [Google Scholar]
- Ramya, M.; Kwon, O.K.; An, H.R.; Park, P.M.; Baek, Y.S.; Park, P.H. Floral scent: Regulation and role of MYB transcription factors. Phytochem. Lett. 2017, 19, 114–120. [Google Scholar]
- Imran, M.; Liu, Y.; Shafiq, S.; Abbas, F.; Ilahi, S.; Rehman, N.; Ahmar, S.; Fiaz, S.; Baran, N.; Pan, S.; et al. Transcriptional cascades in the regulation of 2-AP biosynthesis under Zn supply in fragrant rice. Physiol. Plant 2022, 174, e13721. [Google Scholar]
- Wang, X.; Zhang, C.; Miao, Y.; Deng, L.; Zhang, B.; Meng, J.; Wang, Y.; Pan, L.; Niu, L.; Liu, H.; et al. Interaction between PpERF5 and PpERF7 enhances peach fruit aroma by upregulating PpLOX4 expression. Plant Physiol. Biochem. 2022, 185, 378–389. [Google Scholar] [PubMed]
- Nair, A.R.; Divakaran, K.; Pillai, P. Evidence for Methylerythritol Pathway (MEP) Contributions to Zerumbone Biosynthesis as Revealed by Expression Analysis of Regulatory Genes and Metabolic Inhibitors Studies. Plant Mol. Biol. Rep. 2020, 38, 370–379. [Google Scholar]
- Silva, N.; Ivamoto-Suzuki, S.T.; Camargo, P.O.; Rosa, R.S.; Pereira, L.F.P.; Domingues, D.S. Low-Copy Genes in Terpenoid Metabolism: The Evolution and Expression of MVK and DXR Genes in Angiosperms. Plants 2020, 9, 525. [Google Scholar] [PubMed]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar]
- Srinath, M.; Bindu, B.B.V.; Shailaja, A.; Giri, C.C. Isolation, characterization and in silico analysis of 3-Hydroxy-3-methylglutaryl-coenzyme A reductase (HMGR) gene from Andrographis paniculate (Burm. f) Nees. Mol. Biol. Rep. 2020, 47, 639–654. [Google Scholar]
- Wei, H.; Xu, C.; Movahedi, A.; Sun, W.B.; Li, D.W.; Zhu, G.Q. Characterization and Function of 3-Hydroxy-3-Methylglutaryl-CoA Reductase in Populus trichocarpa: Overexpression of PtHMGR Enhances Terpenoids in Transgenic Poplar. Front. Plant Sci. 2019, 10, 1476. [Google Scholar]
- Neti, S.S.; Pan, J.J.; Poulter, C.D. Mechanistic Studies of the Protonation-Deprotonation Reactions for Type 1 and Type 2 Isopentenyl Diphosphate: Dimethylallyl Diphosphate Isomerase. J. Am. Chem. Soc. 2018, 140, 12900–12908. [Google Scholar]
- Liu, Z.; Tong, X.; Liu, R.; Zou, L. Metabolome and Transcriptome Profiling Reveal That Four Terpenoid Hormones Dominate the Growth and Development of Sanghuangporus baumii. J. Fungi 2022, 8, 648. [Google Scholar]
- Li, M.Y.; Li, X.Y.; Zhou, J.; Sun, Y.; Du, J.G.; Wang, Z.; Luo, Y.; Zhang, Y.; Chen, Q.; Wang, Y.; et al. Genome-wide identification and analysis of terpene synthase (TPS) genes in celery reveals their regulatory roles in terpenoid biosynthesis. Front. Plant Sci. 2022, 13, 1010780. [Google Scholar]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol. Biosyst. 2015, 11, 13–19. [Google Scholar]
- Robert, A.B.; Hoefsloot, H.C.J.; Westerhuis, J.A.; Westerhuis, J.A.; Smilde, A.K.; Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar]
- Tietel, Z.; Masaphy, S. Aroma-volatile profile of black morel (Morchella importuna) grown in Israel. J. Sci. Food Agric. 2018, 98, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, A.; Jurnatan, Y.; Fraatz, M.A.; Govori, S.; Haziri, A.; Millaku, F.; Zorn, H.; Zhang, Y. Aroma characterization of a wild plant (Sanguisorba albanica) from Kosovo using multiple headspace solid phase microextraction combined with gas chromatography-mass spectrometry-olfactometry. Food Res. Int. 2019, 120, 514–522. [Google Scholar] [CrossRef]
- Freitas, T.P.; Taver, I.B.; Spricigo, P.C.; do Amaral, L.B.; Purgatto, E.; Jacomino, A.P. Volatile Compounds and Physicochemical Quality of Four Jabuticabas (Plinia sp.). Molecules 2020, 25, 4543. [Google Scholar] [CrossRef]
- Lykomitros, D.; Fogliano, V.; Capuano, E. Flavor of roasted peanuts (Arachis hypogaea)-Part II: Correlation of volatile compounds to sensory characteristics. Food Res. Int. 2016, 89, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, J.; Tan, H.; Luo, Y.; Zhang, Y.; Chen, Q.; Wang, Y.; Lin, Y.; Zhang, Y.; Wang, X.; et al. Comparative metabolomics provides novel insights into the basis of petiole color differences in celery (Apium graveolens L.). J. Zhejiang Univ. Sci. B 2022, 23, 300–314. [Google Scholar] [CrossRef]
- Jia, X.L.; Wang, G.L.; Xiong, F.; Yu, X.R.; Xu, Z.S.; Wang, F.; Xiong, A.S. Anatomic Structure and Expression Profiles of Related Genes: Novel Insights into Leaf Development in Celery. Plant Growth Regul. 2015, 34, 519–531. [Google Scholar] [CrossRef]
- Lee, J.; Roux, S.; Descharles, N.; Bonazzi, C.; Rega, B. Quantitative determination of volatile compounds using TD-GC-MS and isotope standard addition for application to the heat treatment of food. Food Control 2021, 121, 107635. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Huang, P.W.; Wang, L.R.; Geng, S.S.; Ye, C.; Sun, X.M.; Huang, H. Strategies for enhancing terpenoids accumulation in microalgae. Appl. Microbiol. Biot. 2021, 105, 4919–4930. [Google Scholar] [CrossRef]
- Nagegowda, D.A. Plant volatile terpenoid metabolism: Biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. 2010, 584, 2965–2973. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.M.; Zhou, Y.; Zeng, L.T.; Dong, F.; Mei, X.; Liao, Y.Y.; Watanabe, N.H.; Yang, Z.Y. Analytical method for metabolites involved in biosynthesis of plant volatile compounds. RSC Adv. 2017, 7, 19363–19372. [Google Scholar] [CrossRef]
- Dwivedi, V.; Kumar, S.R.; Shilpashree, H.B.; Krishna, R.; Rao, S.; Shasany, A.K.; Olsson, S.B.; Nagegowda, D.A. An inducible potato (E,E)-farnesol synthase confers tolerance against bacterial pathogens in potato and tobacco. Plant J. 2020, 111, 1308. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Park, J.; Woo, H.M. Overexpression of the key enzymes in the methylerythritol 4-phosphate pathway in corynebacterium glutamicum for improving farnesyl diphosphate-derived terpene production. J. Agr. and Food Chem. 2020, 68, 10780–10786. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Liu, J.; Shi, T.; Chen, M.; Li, Y.; Du, J.; Jiang, H.; Yang, X.; Hu, H.; Wang, L. Integrating Transcriptomic and GC-MS Metabolomic Analysis to Characterize Color and Aroma Formation during Tepal Development in Lycoris longituba. Plants 2019, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, P.R.; Nunes, E.D.; Santos, F.N.; Bastos, L.P.; Costa, M.A.; Rodrigues, F.D.; Andrade, J.B. Discrimination of Eugenia uniflora L. biotypes based on volatile compounds in leaves using HS-SPME/GC–MS and chemometric analysis. Microchem. J. 2017, 130, 79–87. [Google Scholar] [CrossRef]
- Parmryd, I.; Shipton, A.S.; Andersson, B.; Dallner, G. Protein Prenylation in Spinach—Tissue Specificity and Greening-Induced Changes. Arch. Biochem. Biophys. 1997, 339, 73–78. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, H.Y.; Liu, Y.; Liu, Y.X.; Huang, L.Q. EVenn: Easy to create repeatable and editable Venn diagrams and Venn networks online. J. Genet. Genomics 2021, 48, 863–866. [Google Scholar] [CrossRef]



| Compounds (µg/g) | Hanyu Nencuixiqin | Jingpin Saixue | Ziyu Xiangqin | Shanghai Huangxinqin | Siji Lvxiangqin | CAS-No. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | Petiole | Leaf | Petiole | Leaf | Petiole | Leaf | Petiole | Leaf | Petiole | ||
| neo-allo-ocimene | 7.43 ± 1.44 b | 5.10 ± 0.97 c | 0.63 ± 0.03 e | 1.18 ± 0.04 de | 5.07 ± 0.70 c | 6.04 ± 0.75 bc | 2.77 ± 0.32 d | 1.36 ± 0.29 de | 4.77 ± 1.40 c | 12.87 ± 2.14 a | 007216-56-0 |
| 5-pentylcyclohexa-1,3-diene | 3.85 ± 0.62 d | 2.39 ± 0.54 e | 4.63 ± 0.41 bcd | 5.22 ± 0.04 b | 7.87 ± 0.66 a | 4.53 ± 0.23 cd | 5.30 ± 0.68 b | 2.54 ± 0.50 e | 4.86 ± 0.13 bc | 4.03 ± 0.12 cd | 056318-84-4 |
| β-myrcene | 2.46 ± 1.29 fg | 3.06 ± 0.73 fg | 2.04 ± 0.66 fg | 4.47 ± 2.19 f | 10.77 ± 2.41 e | 1.39 ± 0.51 g | 26.33 ± 3.16 c | 14.18 ± 0.26 d | 42.36 ± 1.16 b | 56.50 ± 0.82 a | 000123-35-3 |
| (-)-β-pinene | 3.75 ± 0.16 d | 14.20 ± 1.04 b | 5.39 ± 0.31 c | 27.24 ± 0.15 a | - | 2.72 ± 0.21 e | - | - | 0.68 ± 0.21 f | 3.17 ± 0.37 de | 018172-67-3 |
| (E)-β-ocimene | 92.75 ± 1.66 b | 51.85 ± 1.96 de | 7.12 ± 0.51 h | 13.68 ± 1.88 g | 62.85 ± 1.69 c | 47.66 ± 2.41 e | 32.15 ± 0.56 f | 10.22 ± 1.12 g | 54.84 ± 5.10 d | 144.59 ± 4.30 a | 003779-61-1 |
| γ-terpinene | 10.19 ± 1.26 de | 72.01 ± 1.99 b | 11.97 ± 0.89 d | 110.60 ± 2.69 a | 22.46 ± 2.30 c | 21.07 ± 1.30 c | 2.89 ± 0.57 g | 8.13 ± 0.35 ef | 7.49 ± 0.15 f | 5.61 ± 0.58 f | 000099-85-4 |
| d-limonene | 38.49 ± 0.83 d | 63.05 ± 2.83 b | 51.11 ± 2.73 c | 140.76 ± 7.81 a | 5.55 ± 0.11 g | 2.44 ± 0.40 g | 24.15 ± 0.14 e | 29.60 ± 3.78 e | 17.03 ± 1.66 f | 60.48 ± 4.38 b | 005989-27-5 |
| caryophyllene | 2.04 ± 0.03 c | 0.47 ± 0.39 e | 2.44 ± 0.21 c | 0.93 ± 0.06 de | 2.58 ± 0.59 bc | 1.31 ± 0.42 d | 2.64 ± 0.28 b | - | 2.07 ± 0.28 bc | 4.46 ± 0.17 a | 000087-44-5 |
| neophytadiene | 2.64 ± 0.03 b | - | 1.36 ± 0.03 c | - | 3.46 ± 0.20 a | - | 1.89 ± 0.69 c | - | 2.71 ± 0.33 b | - | 000504-96-1 |
| β-ocimene | - | 1.23 ± 0.23 a | 0.62 ± 0.08 c | 1.12 ± 0.01 a | - | 0.96 ± 0.17 ab | 0.69 ± 0.31 bc | - | - | 0.44 ± 0.03 c | 013877-91-3 |
| 2-hexenal,-(E)- | 51.70 ± 0.88 b | - | 14.19 ± 2.21 d | - | 45.28 ± 4.75 c | - | 31.35 ± 2.89 e | 0.19 ± 0.05 f | 85.18 ± 3.89 a | 1.00 ± 0.19 f | 006728-26-3 |
| senkyunolide | 20.40 ± 0.95 b | 17.46 ± 3.32 bc | - | 10.93 ± 0.97 d | 26.75 ± 3.67 a | 12.45 ± 0.51 d | 24.24 ± 0.92 a | 16.39 ± 3.20 c | 10.87 ± 0.46 d | 25.40 ± 0.42 a | 063038-10-8 |
| neocnidilide | 21.33 ± 1.16 d | 9.26 ± 0.77 e | 54.36 ± 5.02 a | 32.54 ± 2.32 b | 8.94 ± 0.76 e | 4.14 ± 0.13 g | 24.42 ± 2.81 cd | 1.26 ± 0.12 g | 27.73 ± 0.92 c | 24.44 ± 2.80 cd | 004567-33-3 |
| nonane | 0.15 ± 0.10 c | 1.54 ± 0.15 a | 0.31 ± 0.08 c | 1.55 ± 0.03 a | - | 0.79 ± 0.19 b | - | 0.94 ± 0.22 b | - | 0.84 ± 0.12 b | 000111-84-2 |
| cyclomethicone 5 | 0.38 ± 0.05 cde | 0.31 ± 0.02 cde | 0.42 ± 0.05 cd | 0.24 ± 0.01 de | 0.45 ± 0.12 c | 0.22 ± 0.01 e | 0.45 ± 0.10 c | 0.24 ± 0.04 de | 1.66 ± 0.28 a | 1.06 ± 0.02 b | 000541-02-6 |
| cyclohexasiloxane,-dodecamethyl- | 0.24 ± 0.11 e | 0.54 ± 0.120 bc | 0.86 ± 0.03 a | 0.64 ± 0.12 bc | 0.12 ± 0.02 e | 0.28 ± 0.06 de | 0.48 ± 0.22 cd | 0.28 ± 0.11 de | 0.75 ± 0.09 ab | 0.58 ± 0.06 bc | 000540-97-6 |
| cyclomethicone 7 | - | 0.23 ± 0.01 bc | 0.33 ± 0.04 b | 0.23 ± 0.05 bc | - | 0.15 ± 0.03 c | 0.31 ± 0.05 bc | - | 0.55 ± 0.21 a | - | 000107-50-6 |
| naphthalene | 1.70 ± 0.62 a | - | 1.07 ± 0.05 b | - | 0.45 ± 0.05 c | - | 0.39 ± 0.10 c | - | 0.69 ± 0.30 bc | - | 000091-20-3 |
| kessane | 0.54 ± 0.05 f | 5.85 ± 0.13 b | 0.35 ± 0.04 f | 6.27 ± 0.43 a | - | 3.14 ± 0.15 c | - | 2.20 ± 0.40 d | 1.33 ± 0.07 e | - | 003321-66-2 |
| Varieties | Hanyu Nencuixiqin | Jingpin Saixue | Ziyu Xiangqin | Shanghai Huangxinqin | Siji Lvxiangqin | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parts | Leaf | Petiole | Leaf | Petiole | Leaf | Petiole | Leaf | Petiole | Leaf | Petiole |
| Terpenes | 179.19 | 219.41 | 92.67 | 315.09 | 130.06 | 90.14 | 80.95 | 69.41 | 146.75 | 305.68 |
| Alcohols | 3.02 | 3.33 | 0.95 | 14.42 | 0.33 | 0.77 | 0.81 | 0.72 | 5.75 | 0.00 |
| Polymeric aldehyde | 62.41 | 0.00 | 14.96 | 0.40 | 47.23 | 0.00 | 32.20 | 0.43 | 97.50 | 2.11 |
| Aldehydes | 0.43 | 0.00 | 4.60 | 0.00 | 0.66 | 0.00 | 0.45 | 0.00 | 0.00 | 0.36 |
| Ester | 49.56 | 27.25 | 58.03 | 46.42 | 41.18 | 19.26 | 56.32 | 21.25 | 44.54 | 56.48 |
| Alkane | 4.12 | 4.83 | 2.77 | 2.66 | 1.86 | 1.95 | 2.05 | 2.80 | 6.60 | 5.01 |
| Aromatic compounds | 16.10 | 8.50 | 9.07 | 22.23 | 8.18 | 3.01 | 10.32 | 1.96 | 16.19 | 8.19 |
| Heterocyclic compounds | 21.80 | 12.09 | 16.62 | 13.83 | 0.99 | 6.53 | 5.68 | 5.28 | 7.57 | 2.04 |
| Unknow | 0.00 | 0.00 | 0.00 | 0.28 | 0.00 | 0.17 | 0.25 | 0.00 | 0.60 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Li, M.; Li, X.; Du, J.; Li, W.; Lin, Y.; Zhang, Y.; Wang, Y.; He, W.; Chen, Q.; et al. Characterization of Volatile Organic Compounds in Five Celery (Apium graveolens L.) Cultivars with Different Petiole Colors by HS-SPME-GC-MS. Int. J. Mol. Sci. 2023, 24, 13343. https://doi.org/10.3390/ijms241713343
Sun Y, Li M, Li X, Du J, Li W, Lin Y, Zhang Y, Wang Y, He W, Chen Q, et al. Characterization of Volatile Organic Compounds in Five Celery (Apium graveolens L.) Cultivars with Different Petiole Colors by HS-SPME-GC-MS. International Journal of Molecular Sciences. 2023; 24(17):13343. https://doi.org/10.3390/ijms241713343
Chicago/Turabian StyleSun, Yue, Mengyao Li, Xiaoyan Li, Jiageng Du, Weilong Li, Yuanxiu Lin, Yunting Zhang, Yan Wang, Wen He, Qing Chen, and et al. 2023. "Characterization of Volatile Organic Compounds in Five Celery (Apium graveolens L.) Cultivars with Different Petiole Colors by HS-SPME-GC-MS" International Journal of Molecular Sciences 24, no. 17: 13343. https://doi.org/10.3390/ijms241713343
APA StyleSun, Y., Li, M., Li, X., Du, J., Li, W., Lin, Y., Zhang, Y., Wang, Y., He, W., Chen, Q., Zhang, Y., Wang, X., Luo, Y., Xiong, A., & Tang, H. (2023). Characterization of Volatile Organic Compounds in Five Celery (Apium graveolens L.) Cultivars with Different Petiole Colors by HS-SPME-GC-MS. International Journal of Molecular Sciences, 24(17), 13343. https://doi.org/10.3390/ijms241713343









