Gelatin Methacryloyl (GelMA) Hydrogel Scaffolds: Predicting Physical Properties Using an Experimental Design Approach
Abstract
:1. Introduction
2. Results and Discussion
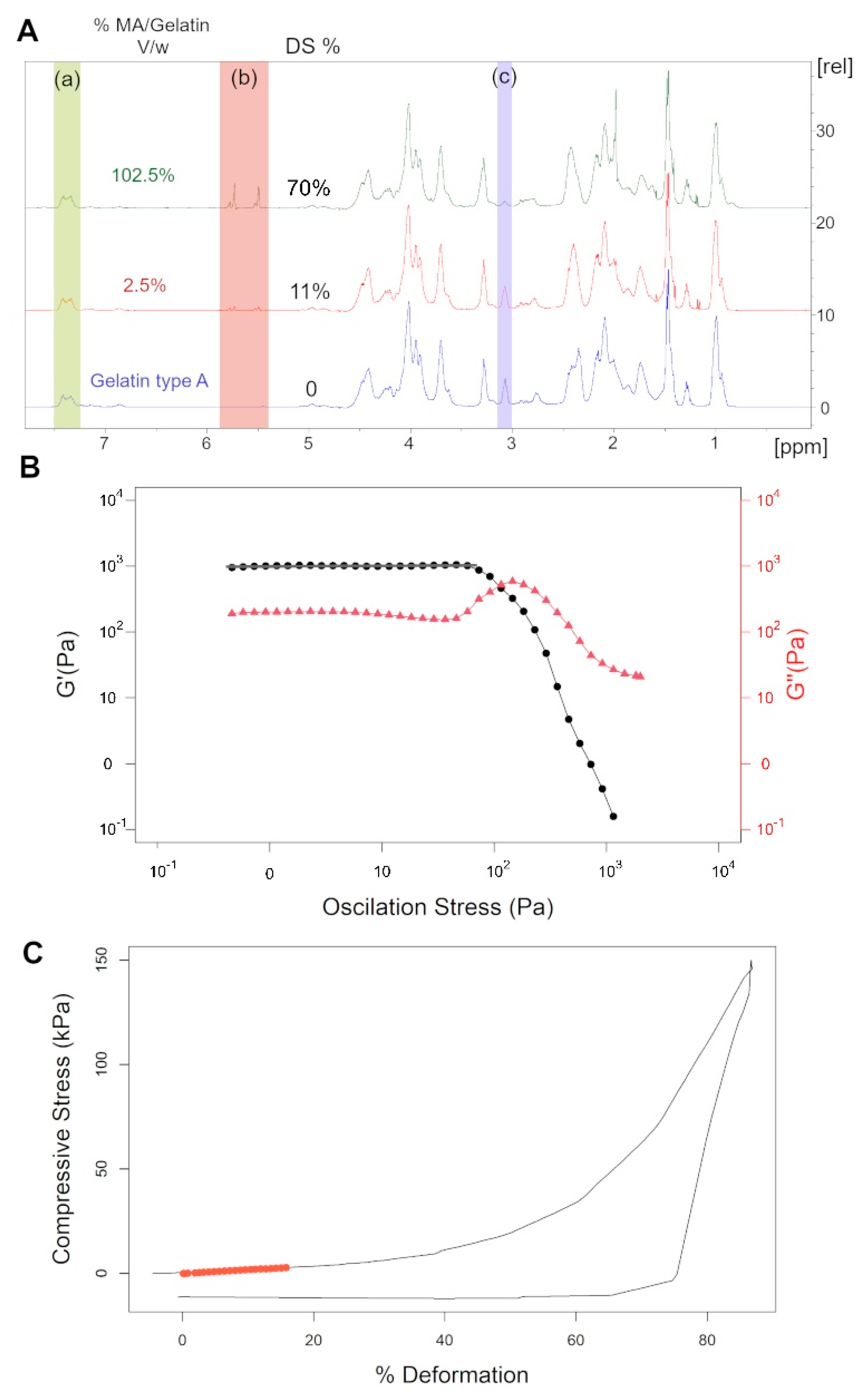
2.1. Hydrogel Crosslinking Conditions
2.2. NMR Analysis for GelMA Degree of Substitution and Synthesis Conditions
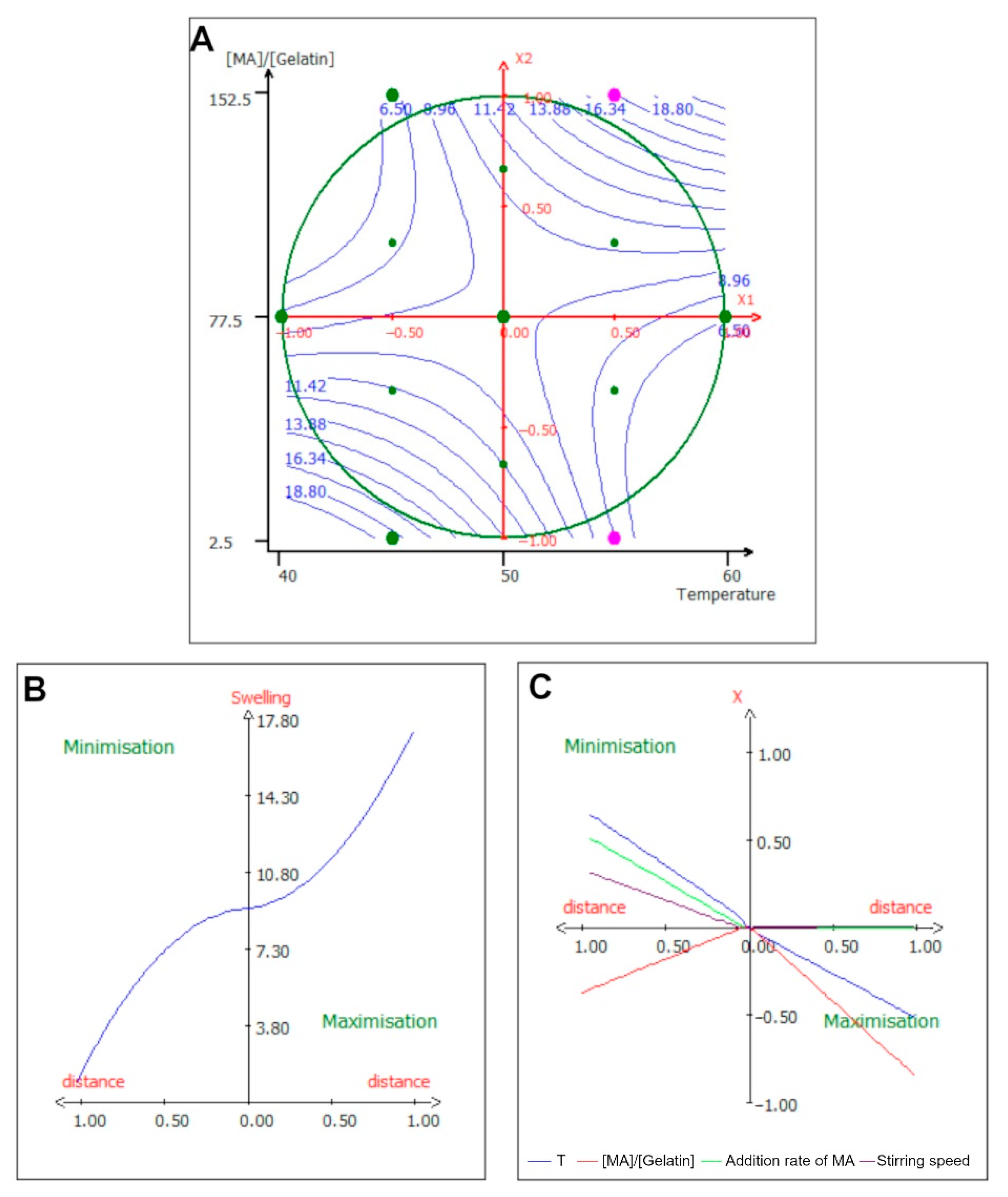
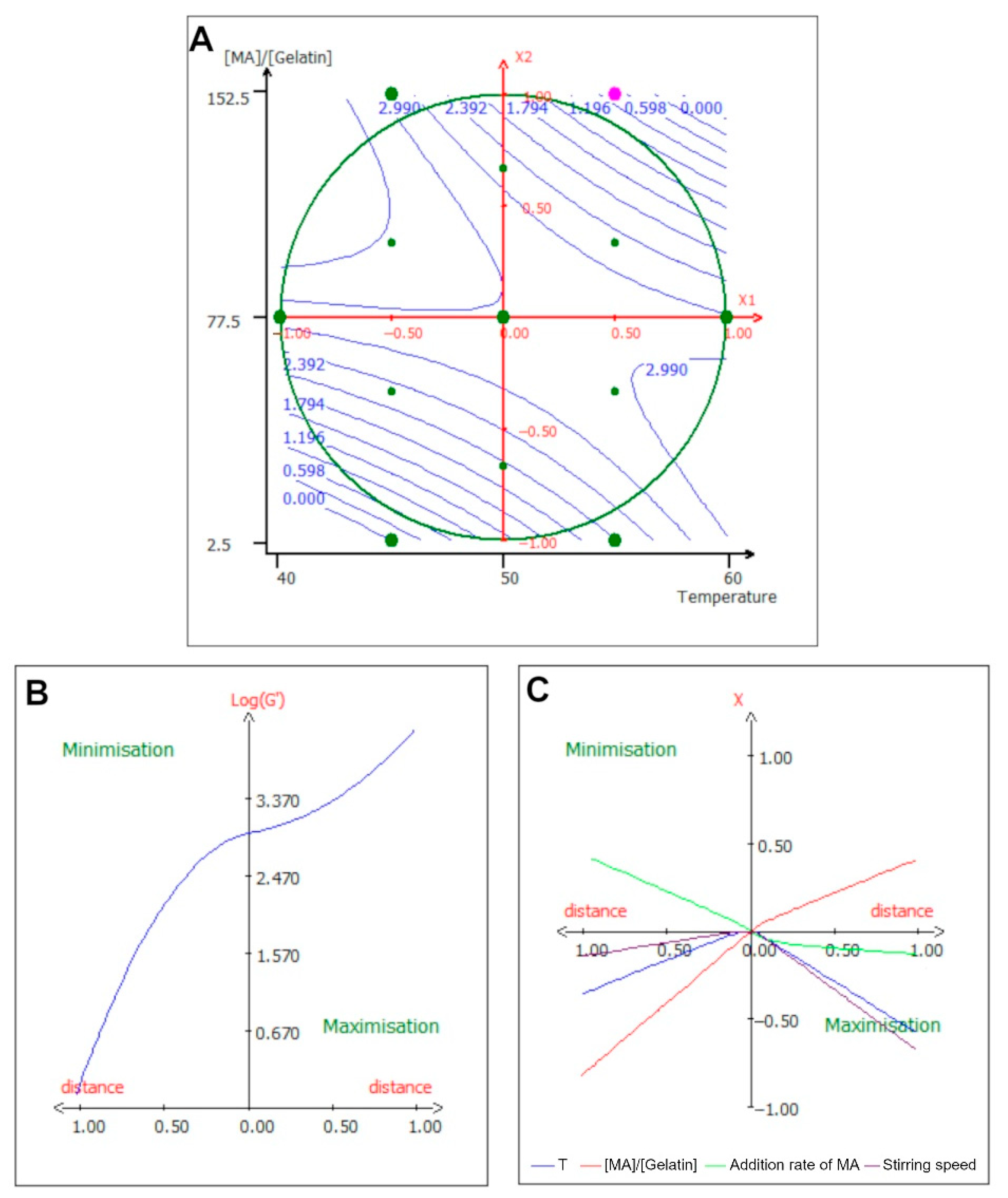
2.3. Surface Response Study
2.4. Effect of Experimental Parameters on Lysine Substitution Rate
2.5. Effect of Experimental Parameters on Swelling of the Gel
2.6. Effect of Experimental Parameters on the Storage Modulus (log(G’))
2.7. Effect of Experimental Parameters on the Compression Modulus at 15%
3. Materials and Methods
3.1. Reagents
3.2. GelMA
3.2.1. GelMA Synthesis
3.2.2. Hydrogel Preparation
3.3. Determination of GelMA Degree of Substitution
3.4. Rheological Testing
3.5. Mechanical Testing
3.6. Mass Swelling Ratio
3.7. Experimental Design
3.8. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Z.; Vizetto-Duarte, C.; Moay, Z.K.; Setyawati, M.I.; Rakshit, M.; Kathawala, M.H.; Ng, K.W. Composite Hydrogels in Three-Dimensional in Vitro Models. Front. Bioeng. Biotechnol. 2020, 8, 611. [Google Scholar] [CrossRef] [PubMed]
- Sart, S.; Tsai, A.-C.; Li, Y.; Ma, T. Three-Dimensional Aggregates of Mesenchymal Stem Cells: Cellular Mechanisms, Biological Properties, and Applications. Tissue Eng. Part B Rev. 2014, 20, 365–380. [Google Scholar] [CrossRef]
- Horrobin, D.F. Modern Biomedical Research: An Internally Self-Consistent Universe with Little Contact with Medical Reality? Nat. Rev. Drug Discov. 2003, 2, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Alhaque, S.; Themis, M.; Rashidi, H. Three-Dimensional Cell Culture: From Evolution to Revolution. Philos. Trans. R. Soc. B 2018, 373, 20170216. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.; Lee, H. Engineering Hydrogels for the Development of Three-Dimensional In Vitro Models. Int. J. Mol. Sci. 2022, 23, 2662. [Google Scholar] [CrossRef] [PubMed]
- Elkhoury, K.; Morsink, M.; Sanchez-Gonzalez, L.; Kahn, C.; Tamayol, A.; Arab-Tehrany, E. Biofabrication of Natural Hydrogels for Cardiac, Neural, and Bone Tissue Engineering Applications. Bioact. Mater. 2021, 6, 3904–3923. [Google Scholar] [CrossRef]
- Elkhoury, K.; Sanchez-Gonzalez, L.; Lavrador, P.; Almeida, R.; Gaspar, V.; Kahn, C.; Cleymand, F.; Arab-Tehrany, E.; Mano, J.F. Gelatin Methacryloyl (GelMA) Nanocomposite Hydrogels Embedding Bioactive Naringin Liposomes. Polymers 2020, 12, 2944. [Google Scholar] [CrossRef]
- Choi, Y.J.; Park, H.; Ha, D.H.; Yun, H.-S.; Yi, H.-G.; Lee, H. 3D Bioprinting of In Vitro Models Using Hydrogel-Based Bioinks. Polymers 2021, 13, 366. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish Gelatin: Properties, Challenges, and Prospects as an Alternative to Mammalian Gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Elkhoury, K.; Morsink, M.; Tahri, Y.; Kahn, C.; Cleymand, F.; Shin, S.R.; Arab-Tehrany, E.; Sanchez-Gonzalez, L. Synthesis and Characterization of C2C12-Laden Gelatin Methacryloyl (GelMA) from Marine and Mammalian Sources. Int. J. Biol. Macromol. 2021, 183, 918–926. [Google Scholar] [CrossRef]
- Vandooren, J.; Van Den Steen, P.E.; Opdenakker, G. Biochemistry and Molecular Biology of Gelatinase B or Matrix Metalloproteinase-9 (MMP-9): The next Decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-Laden Microengineered Gelatin Methacrylate Hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Aldana, A.; Malatto, L.; Rehman, M.; Boccaccini, A.; Abraham, G. Fabrication of Gelatin Methacrylate (GelMA) Scaffolds with Nano- and Micro-Topographical and Morphological Features. Nanomaterials 2019, 9, 120. [Google Scholar] [CrossRef]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef]
- Kurian, A.G.; Singh, R.K.; Patel, K.D.; Lee, J.-H.; Kim, H.-W. Multifunctional GelMA Platforms with Nanomaterials for Advanced Tissue Therapeutics. Bioact. Mater. 2022, 8, 267–295. [Google Scholar] [CrossRef]
- Elkhoury, K.; Koçak, P.; Kang, A.; Arab-Tehrany, E.; Ellis Ward, J.; Shin, S.R. Engineering Smart Targeting Nanovesicles and Their Combination with Hydrogels for Controlled Drug Delivery. Pharmaceutics 2020, 12, 849. [Google Scholar] [CrossRef]
- Quint, J.P.; Mostafavi, A.; Endo, Y.; Panayi, A.; Russell, C.S.; Nourmahnad, A.; Wiseman, C.; Abbasi, L.; Samandari, M.; Sheikhi, A.; et al. In Vivo Printing of Nanoenabled Scaffolds for the Treatment of Skeletal Muscle Injuries. Adv. Healthc. Mater. 2021, 10, 2002152. [Google Scholar] [CrossRef]
- Kadri, R.; Bacharouch, J.; Elkhoury, K.; Ben Messaoud, G.; Kahn, C.; Desobry, S.; Linder, M.; Tamayol, A.; Francius, G.; Mano, J.F.; et al. Role of Active Nanoliposomes in the Surface and Bulk Mechanical Properties of Hybrid Hydrogels. Mater. Today Bio 2020, 6, 100046. [Google Scholar] [CrossRef]
- Kumar, H.; Sakthivel, K.; Mohamed, M.G.A.; Boras, E.; Shin, S.R.; Kim, K. Designing Gelatin Methacryloyl (GelMA)-Based Bioinks for Visible Light Stereolithographic 3D Biofabrication. Macromol. Biosci. 2021, 21, 2000317. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, Z.; Menard, F.; Kim, K. Comparative study of gelatin methacrylate hydrogels from different sources for biofabrication applications. Biofabrication 2017, 9, 044101. [Google Scholar] [CrossRef]
- Elkhoury, K.; Chen, M.; Koçak, P.; Enciso-Martínez, E.; Bassous, N.J.; Lee, M.C.; Byambaa, B.; Rezaei, Z.; Li, Y.; Ubina López, M.E.; et al. Hybrid Extracellular Vesicles-Liposome Incorporated Advanced Bioink to Deliver MicroRNA. Biofabrication 2022, 14, 045008. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef]
- O’Connell, C.D.; Zhang, B.; Onofrillo, C.; Duchi, S.; Blanchard, R.; Quigley, A.; Bourke, J.; Gambhir, S.; Kapsa, R.; Di Bella, C.; et al. Tailoring the Mechanical Properties of Gelatin Methacryloyl Hydrogels through Manipulation of the Photocrosslinking Conditions. Soft Matter 2018, 14, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, S.; Janmaleki, M.; Riaz, S.; Sanati Nezhad, A.; Syed, N. A Tuned Gelatin Methacryloyl (GelMA) Hydrogel Facilitates Myelination of Dorsal Root Ganglia Neurons in Vitro. Mater. Sci. Eng. C 2021, 126, 112131. [Google Scholar] [CrossRef] [PubMed]
- Pepelanova, I.; Kruppa, K.; Scheper, T.; Lavrentieva, A. Gelatin-Methacryloyl (GelMA) Hydrogels with Defined Degree of Functionalization as a Versatile Toolkit for 3D Cell Culture and Extrusion Bioprinting. Bioengineering 2018, 5, 55. [Google Scholar] [CrossRef]
- Kim, C.; Young, J.L.; Holle, A.W.; Jeong, K.; Major, L.G.; Jeong, J.H.; Aman, Z.M.; Han, D.-W.; Hwang, Y.; Spatz, J.P.; et al. Stem Cell Mechanosensation on Gelatin Methacryloyl (GelMA) Stiffness Gradient Hydrogels. Ann. Biomed. Eng. 2020, 48, 893–902. [Google Scholar] [CrossRef]
- Billiet, T.; Gasse, B.V.; Gevaert, E.; Cornelissen, M.; Martins, J.C.; Dubruel, P. Quantitative Contrasts in the Photopolymerization of Acrylamide and Methacrylamide-Functionalized Gelatin Hydrogel Building Blocks. Macromol. Biosci. 2013, 13, 1531–1545. [Google Scholar] [CrossRef]
- Young, A.T.; White, O.C.; Daniele, M.A. Rheological Properties of Coordinated Physical Gelation and Chemical Crosslinking in Gelatin Methacryloyl (GelMA) Hydrogels. Macromol. Biosci. 2020, 20, 2000183. [Google Scholar] [CrossRef]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y.; Chalisserry, E.P.; Kim, M.H.; Kang, H.W.; Choi, I.-W.; Nam, S.Y. The Influence of Astaxanthin on the Proliferation of Adipose-Derived Mesenchymal Stem Cells in Gelatin-Methacryloyl (GelMA) Hydrogels. Materials 2019, 12, 2416. [Google Scholar] [CrossRef] [PubMed]
- Claaßen, C.; Claaßen, M.H.; Truffault, V.; Sewald, L.; Tovar, G.E.M.; Borchers, K.; Southan, A. Quantification of Substitution of Gelatin Methacryloyl: Best Practice and Current Pitfalls. Biomacromolecules 2018, 19, 42–52. [Google Scholar] [CrossRef]
- Martinez-Garcia, F.D.; Van Dongen, J.A.; Burgess, J.K.; Harmsen, M.C. Matrix Metalloproteases from Adipose Tissue-Derived Stromal Cells Are Spatiotemporally Regulated by Hydrogel Mechanics in a 3D Microenvironment. Bioengineering 2022, 9, 340. [Google Scholar] [CrossRef]
- Nazir, F.; Ashraf, I.; Iqbal, M.; Ahmad, T.; Anjum, S. 6-Deoxy-Aminocellulose Derivatives Embedded Soft Gelatin Methacryloyl (GelMA) Hydrogels for Improved Wound Healing Applications: In Vitro and in Vivo Studies. Int. J. Biol. Macromol. 2021, 185, 419–433. [Google Scholar] [CrossRef]
- Modaresifar, K.; Hadjizadeh, A.; Niknejad, H. Design and Fabrication of GelMA/Chitosan Nanoparticles Composite Hydrogel for Angiogenic Growth Factor Delivery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1799–1808. [Google Scholar] [CrossRef]
- Kulkarni, N.S.; Chauhan, G.; Goyal, M.; Sarvepalli, S.; Gupta, V. Development of Gelatin Methacrylate (GelMa) Hydrogels for Versatile Intracavitary Applications. Biomater. Sci. 2022, 10, 4492–4507. [Google Scholar] [CrossRef]
- Velasco-Rodriguez, B.; Diaz-Vidal, T.; Rosales-Rivera, L.C.; García-González, C.A.; Alvarez-Lorenzo, C.; Al-Modlej, A.; Domínguez-Arca, V.; Prieto, G.; Barbosa, S.; Soltero Martínez, J.F.A.; et al. Hybrid Methacrylated Gelatin and Hyaluronic Acid Hydrogel Scaffolds. Preparation and Systematic Characterization for Prospective Tissue Engineering Applications. Int. J. Mol. Sci. 2021, 22, 6758. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Shirahama, H.; Cho, N.-J.; Tan, L.P. Efficient and Controllable Synthesis of Highly Substituted Gelatin Methacrylamide for Mechanically Stiff Hydrogels. RSC Adv. 2015, 5, 106094–106097. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Li, J.; Wang, X.; Zhang, J.; Kawazoe, N.; Chen, G. 3D Culture of Chondrocytes in Gelatin Hydrogels with Different Stiffness. Polymers 2016, 8, 269. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.-J.; Lee, B.H. Gelatin Methacryloyl and Its Hydrogels with an Exceptional Degree of Controllability and Batch-to-Batch Consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef] [PubMed]
- Shirahama, H.; Lee, B.H.; Tan, L.P.; Cho, N.-J. Precise Tuning of Facile One-Pot Gelatin Methacryloyl (GelMA) Synthesis. Sci. Rep. 2016, 6, 31036. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Kawazoe, N.; Chen, G. Fabrication of Highly Crosslinked Gelatin Hydrogel and Its Influence on Chondrocyte Proliferation and Phenotype. Polymers 2017, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Lin, R.-Z.; Qi, H.; Yang, Y.; Bae, H.; Melero-Martin, J.M.; Khademhosseini, A. Functional Human Vascular Network Generated in Photocrosslinkable Gelatin Methacrylate Hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Noshadi, I.; Hong, S.; Sullivan, K.E.; Shirzaei Sani, E.; Portillo-Lara, R.; Tamayol, A.; Shin, S.R.; Gao, A.E.; Stoppel, W.L.; Black Iii, L.D.; et al. In Vitro and in Vivo Analysis of Visible Light Crosslinkable Gelatin Methacryloyl (GelMA) Hydrogels. Biomater. Sci. 2017, 5, 2093–2105. [Google Scholar] [CrossRef] [PubMed]


| Exp n° | Independent Variables | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 T | X2 [MA]/[Gelatin] | X3 Addition Rate of MA | X4 Stirring Speed | Y1 % Lysine Substitution | Y2 Swelling | Y3 Log(G’) | Y4 Compressive Modulus 15% | |
| °C | mL/g | mL/min | rpm | Pa | Pa | |||
| 1 | 60 | 77.5% | 1.75 | 550 | 54.18 | 7.14 | 3.05 | 313 |
| 2 | 40 | 77.5% | 1.75 | 550 | 71.76 | 7.55 | 2.41 | 204 |
| 3 | 55 | 152.5% | 1.75 | 550 | 67.92 | 6.98 | 3.12 | 155 |
| 4 | 45 | 2.5% | 1.75 | 550 | 12.33 | 19.28 | - | - |
| 5 | 55 | 2.5% | 1.75 | 550 | 12.11 | - | 1.75 | - |
| 6 | 45 | 152.5% | 1.75 | 550 | 68.65 | 7.29 | 3.21 | 174 |
| 7 | 55 | 102.5% | 2.5 | 550 | 67.47 | 7.62 | 2.27 | 156 |
| 8 | 45 | 52.5% | 1.0 | 550 | 50.75 | 8.05 | 3.19 | 147 |
| 9 | 55 | 52.5% | 1.0 | 550 | 58.06 | 7.65 | 3.21 | 139 |
| 10 | 50 | 127.5% | 1.0 | 550 | 70.79 | 6.78 | 1.81 | 188 |
| 11 | 45 | 102.5% | 2.5 | 550 | 58.22 | 6.90 | 2.81 | 110 |
| 12 | 50 | 27.5% | 2.5 | 550 | 46.68 | 8.19 | 2.67 | 196 |
| 13 | 55 | 102.5% | 2.0 | 650 | 70.35 | 7.59 | 2.84 | 199 |
| 14 | 45 | 52.5% | 1.5 | 450 | 52.86 | 9.87 | 2.91 | 130 |
| 15 | 55 | 52.5% | 1.5 | 450 | 60.72 | 6.58 | 2.71 | 272 |
| 16 | 50 | 127.5% | 1.5 | 450 | 68.80 | 8.00 | 2.97 | 183 |
| 17 | 50 | 77.5% | 2.25 | 450 | 64.98 | 8.39 | 2.93 | 153 |
| 18 | 45 | 102.5% | 2.0 | 650 | 56.84 | 9.49 | 3.00 | 126 |
| 19 | 50 | 27.5% | 2.0 | 650 | 45.77 | 8.52 | 2.93 | 130 |
| 20 | 50 | 77.5% | 1.25 | 650 | 61.11 | 8.67 | 2.36 | 155 |
| 21 | 50 | 77.5% | 1.75 | 550 | 63.75 | 9.09 | 2.96 | 174 |
| 22 | 50 | 77.5% | 1.75 | 550 | 64.99 | 8.73 | 2.96 | 184 |
| 23 | 50 | 77.5% | 1.75 | 550 | 63.71 | 9.05 | 2.98 | 181 |
| 24 | 50 | 77.5% | 1.75 | 550 | 66.26 | 9.72 | 2.97 | 251 |
| Name | % Lysine Substitution | Swelling | Log (G’) | Compressive Modulus 15% |
|---|---|---|---|---|
| b0 | 64.674 *** | 9.148 *** | 2.965 *** | 193.636 *** |
| b1 | 0.182 | −0.712 *** | −0.043 | 57.852 *** |
| b2 | 26.622 *** | −0.552 *** | 0.376 *** | 7.098 |
| b3 | −1.089 *** | 0.050 | −0.301 *** | −7.586 |
| b4 | −2.102 *** | 0.227 * | −0.064 ** | −9.142 |
| b1-1 | −1.704 *** | −1.799 *** | −0.206 *** | 73.530 *** |
| b2-2 | −31.995 *** | 4.235 *** | −1.497 *** | −97.782 *** |
| b3-3 | −0.594 | −3.393 *** | −0.495 *** | −59.137 *** |
| b4-4 | −0.33 | −1.36 *** | 0.32 *** | −36.369 * |
| b1-2 | −0.293 | 10.88 *** | −2.124 *** | −44.094 |
| b1-3 | 1.296 * | −3.76 *** | 0.521 *** | 50.292 * |
| b1-4 | 3.348 *** | −2.789 *** | 0.786 *** | −11.735 |
| b2-3 | −10.861 *** | 2.37 *** | 1.656 *** | −110.062 *** |
| b2-4 | −4.012 *** | 1.931 *** | −1.094 *** | −26.538 |
| b3-4 | 2.472 *** | −3.087 *** | 0.718 *** | 25.251 |
| R² | 0.896 *** | 0.947 *** | 0.878 *** | 0.720 *** |
| Factor | Name | Unit | Low Level Value | High Level Value | Level of Variation |
|---|---|---|---|---|---|
| X1 | Temperature | °C | 40 | 60 | 5 |
| X2 | Methacrylic anhydride over gelatin ratio | % | 2.5 | 152.5 | 7 |
| X3 | Methacrylic anhydride flow rate | mL·min−1 | 1 | 2.5 | 7 |
| X4 | Stirring speed | rpm | 450 | 650 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peyret, C.; Elkhoury, K.; Bouguet-Bonnet, S.; Poinsignon, S.; Boulogne, C.; Giraud, T.; Stefan, L.; Tahri, Y.; Sanchez-Gonzalez, L.; Linder, M.; et al. Gelatin Methacryloyl (GelMA) Hydrogel Scaffolds: Predicting Physical Properties Using an Experimental Design Approach. Int. J. Mol. Sci. 2023, 24, 13359. https://doi.org/10.3390/ijms241713359
Peyret C, Elkhoury K, Bouguet-Bonnet S, Poinsignon S, Boulogne C, Giraud T, Stefan L, Tahri Y, Sanchez-Gonzalez L, Linder M, et al. Gelatin Methacryloyl (GelMA) Hydrogel Scaffolds: Predicting Physical Properties Using an Experimental Design Approach. International Journal of Molecular Sciences. 2023; 24(17):13359. https://doi.org/10.3390/ijms241713359
Chicago/Turabian StylePeyret, Corentin, Kamil Elkhoury, Sabine Bouguet-Bonnet, Sophie Poinsignon, Corentin Boulogne, Tristan Giraud, Loïc Stefan, Yasmina Tahri, Laura Sanchez-Gonzalez, Michel Linder, and et al. 2023. "Gelatin Methacryloyl (GelMA) Hydrogel Scaffolds: Predicting Physical Properties Using an Experimental Design Approach" International Journal of Molecular Sciences 24, no. 17: 13359. https://doi.org/10.3390/ijms241713359
APA StylePeyret, C., Elkhoury, K., Bouguet-Bonnet, S., Poinsignon, S., Boulogne, C., Giraud, T., Stefan, L., Tahri, Y., Sanchez-Gonzalez, L., Linder, M., Tamayol, A., Kahn, C. J. F., & Arab-Tehrany, E. (2023). Gelatin Methacryloyl (GelMA) Hydrogel Scaffolds: Predicting Physical Properties Using an Experimental Design Approach. International Journal of Molecular Sciences, 24(17), 13359. https://doi.org/10.3390/ijms241713359









