Rapid Point-of-Care PCR Testing of Drug-Resistant Strains on Endotracheal Aspirate Samples: A Repurposed Effective Tool in the Stepwise Approach of Healthcare-Acquired Pneumonia—A Pilot Study
Abstract
:1. Introduction
- Pneumonia that develops in nonhospitalized patients in nursing homes and extended care facilities or in patients undergoing home infusion therapies, chronic dialysis, and other similar scenarios.
- Hospital-acquired pneumonia (HAP)—onset during hospitalization, after a minimum of 48 h from admission, on the regular ward.
2. Results
2.1. Baseline Characteristics
2.2. Infection Profile
2.3. Diagnostic Capabilities of the Sample-to-Answer PCR Test
2.4. The Potential Therapeutic Impact of the Rapid PCR Tests
3. Discussion
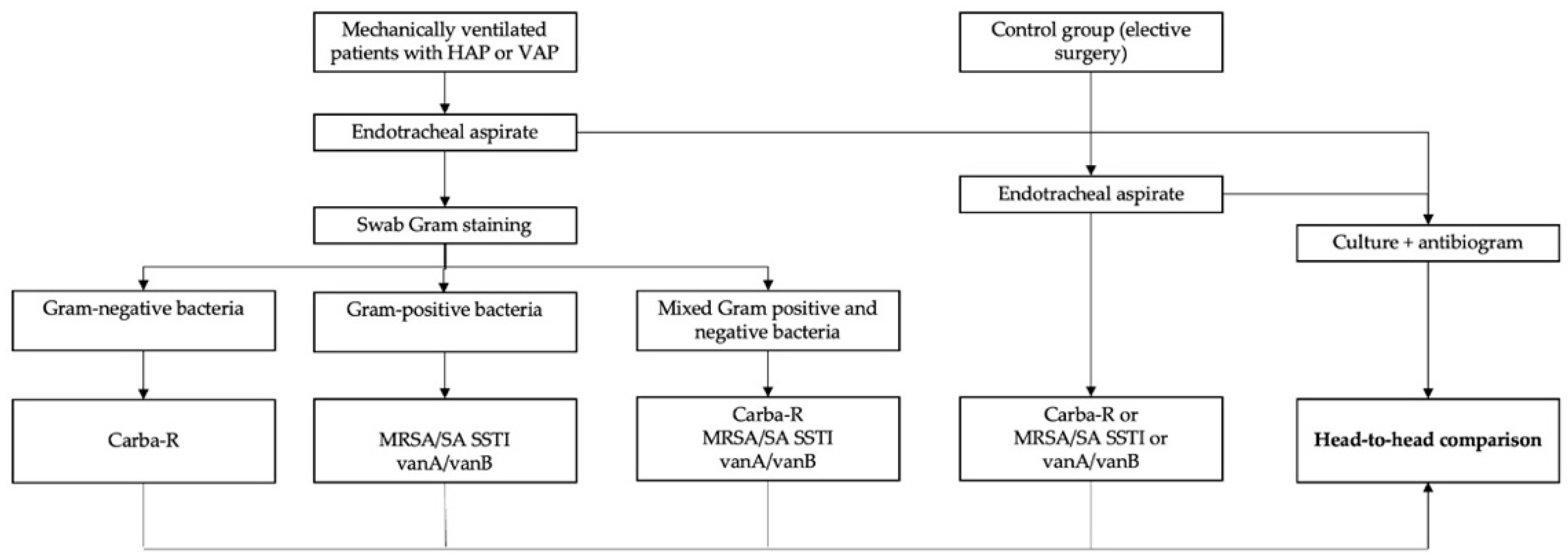
4. Materials and Methods
4.1. Study Design and Participants
4.1.1. The Study Group
- A minimum length of 48 h of hospital stay (HAP) or mechanical ventilation (VAP).
- Clinical suspicion of pneumonia based on the following:
- New or progressive lung consolidation on chest imaging;
- New onset of fever;
- Purulent respiratory secretions;
- New onset of leukocytosis or leukopenia;
- Worsening oxygenation;
- Surrogate criteria: hemodynamic status alterations, increase in other serum markers of systemic inflammation (C-reactive protein, procalcitonin, or presepsin).
- Mechanical ventilation at the time of inclusion.
4.1.2. The Control Group
- No clinical signs of respiratory tract or systemic infections at the time of admission (cough, shortness of breath, chest pain, sore throat, or fever);
- No prior history of chronic pulmonary disease (i.e., chronic bronchitis, chronic obstructive pulmonary disease, or bronchiectasis);
- No history of respiratory infections in the past four weeks;
- No history of antibiotic therapy in the past three months.
4.2. Study Protocol
4.2.1. Endotracheal Aspirate and Gram Staining
4.2.2. Bacterial Cultures
4.2.3. Identification of Bacteria or AMR Genes with GeneXpert
4.3. Statistical Analysis
4.4. Ethical Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rello, J.; Diaz, E. Pneumonia in the Intensive Care Unit. Crit. Care Med. 2003, 31, 2544–2551. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.S. Pneumonia—Overview. Ref. Module Biomed. Sci. 2020, 185–197. [Google Scholar] [CrossRef]
- Papazian, L.; Klompas, M.; Luyt, C.-E. Ventilator-Associated Pneumonia in Adults: A Narrative Review. Intensive Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Bassi, G.L.; Luna, C.M.; Martin-Loeches, I. International ERS/ESICM/ESCMID/ALAT Guidelines for the Management of Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia: Guidelines for the Management of Hospital-Acquired Pneumonia (HAP)/Ventilator-Associated Pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana Del Tórax (ALAT). Eur. Respir. J. 2017, 50, 1700582. [Google Scholar]
- Craven, D.E.; Hudcova, J.; Lei, Y. Diagnosis of Ventilator-Associated Respiratory Infections (VARI): Microbiologic Clues for Tracheobronchitis (VAT) and Pneumonia (VAP). Clin. Chest Med. 2011, 32, 547–557. [Google Scholar] [CrossRef]
- Hunter, J.D. Ventilator Associated Pneumonia. Bmj 2012, 344, e3325. [Google Scholar] [CrossRef]
- Ferrer, M.; Torres, A. Epidemiology of ICU-Acquired Pneumonia. Curr. Opin. Crit. Care 2018, 24, 325–331. [Google Scholar] [CrossRef]
- Saied, W.I.; Martin-Loeches, I.; Timsit, J.-F. What Is New in Non-Ventilated ICU-Acquired Pneumonia? Intensive Care Med. 2020, 46, 488–491. [Google Scholar] [CrossRef]
- Saied, W.I.; Mourvillier, B.; Cohen, Y.; Ruckly, S.; Reignier, J.; Marcotte, G.; Siami, S.; Bouadma, L.; Darmon, M.; de Montmollin, E. A Comparison of the Mortality Risk Associated with Ventilator-Acquired Bacterial Pneumonia and Nonventilator ICU-Acquired Bacterial Pneumonia. Crit. Care Med. 2019, 47, 345–352. [Google Scholar] [CrossRef]
- Melsen, W.G.; Rovers, M.M.; Groenwold, R.H.; Bergmans, D.C.; Camus, C.; Bauer, T.T.; Hanisch, E.W.; Klarin, B.; Koeman, M.; Krueger, W.A. Attributable Mortality of Ventilator-Associated Pneumonia: A Meta-Analysis of Individual Patient Data from Randomised Prevention Studies. Lancet Infect. Dis. 2013, 13, 665–671. [Google Scholar] [CrossRef]
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Menéndez, R.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Primer 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, K.K.; Baker, D.; Quinn, B. The Epidemiology of Nonventilator Hospital-Acquired Pneumonia in the United States. Am. J. Infect. Control 2018, 46, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Peiffer-Smadja, N.; Bouadma, L.; Mathy, V.; Allouche, K.; Patrier, J.; Reboul, M.; Montravers, P.; Timsit, J.-F.; Armand-Lefevre, L. Performance and Impact of a Multiplex PCR in ICU Patients with Ventilator-Associated Pneumonia or Ventilated Hospital-Acquired Pneumonia. Crit. Care 2020, 24, 366. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N. Microbial Etiologies of Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia. Clin. Infect. Dis. 2010, 51, S81–S87. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Bassetti, M.; Francois, B.; Burnham, J.; Dimopoulos, G.; Garnacho-Montero, J.; Lipman, J.; Luyt, C.-E.; Nicolau, D.P.; Postma, M.J. The Intensive Care Medicine Research Agenda on Multidrug-Resistant Bacteria, Antibiotics, and Stewardship. Intensive Care Med. 2017, 43, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ruan, S.-Y.; Pan, S.-C.; Lee, T.-F.; Chien, J.-Y.; Hsueh, P.-R. Performance of a Multiplex PCR Pneumonia Panel for the Identification of Respiratory Pathogens and the Main Determinants of Resistance from the Lower Respiratory Tract Specimens of Adult Patients in Intensive Care Units. J. Microbiol. Immunol. Infect. 2019, 52, 920–928. [Google Scholar] [CrossRef]
- Aliberti, S.; Cilloniz, C.; Chalmers, J.D.; Zanaboni, A.M.; Cosentini, R.; Tarsia, P.; Pesci, A.; Blasi, F.; Torres, A. Multidrug-Resistant Pathogens in Hospitalised Patients Coming from the Community with Pneumonia: A European Perspective. Thorax 2013, 68, 997–999. [Google Scholar] [CrossRef]
- Bassetti, M.; Kanj, S.S.; Kiratisin, P.; Rodrigues, C.; Van Duin, D.; Villegas, M.V.; Yu, Y. Early Appropriate Diagnostics and Treatment of MDR Gram-Negative Infections. JAC-Antimicrob. Resist. 2022, 4, dlac089. [Google Scholar] [CrossRef]
- Sfeir, M.M. Diagnosis of Multidrug-Resistant Pathogens of Pneumonia. Diagnostics 2021, 11, 2287. [Google Scholar] [CrossRef]
- Charles, M.P.; Kali, A.; Easow, J.M.; Joseph, N.M.; Ravishankar, M.; Srinivasan, S.; Kumar, S.; Umadevi, S. Ventilator-Associated Pneumonia. Australas. Med. J. 2014, 7, 334. [Google Scholar] [CrossRef]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Reed, C.; Grijalva, C.G.; Anderson, E.J.; Courtney, D.M. Community-Acquired Pneumonia Requiring Hospitalization among US Adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.U.; de Mello, A.J.; Manz, A. Chemical Amplification: Continuous-Flow PCR on a Chip. Science 1998, 280, 1046–1048. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, P.; Pipper, J.; Hsieh, T.M. Disposable Real-Time MicroPCR Device: Lab-on-a-Chip at a Low Cost. Mol. Biosyst. 2006, 2, 292–298. [Google Scholar] [CrossRef]
- Torres, A.; Lee, N.; Cilloniz, C.; Vila, J.; Van der Eerden, M. Laboratory Diagnosis of Pneumonia in the Molecular Age. Eur. Respir. J. 2016, 48, 1764–1778. [Google Scholar] [CrossRef]
- Marlowe, E.M.; Wolk, D.M. GeneXpert Testing: Applications for Clinical Microbiology, Part I. Clin. Microbiol. Newsl. 2008, 30, 175–179. [Google Scholar] [CrossRef]
- Xu, L.; Sun, X.; Ma, X. Systematic Review and Meta-Analysis of Mortality of Patients Infected with Carbapenem-Resistant Klebsiella Pneumoniae. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Gavaruzzi, F.; Ceccarelli, G.; Borrazzo, C.; Oliva, A.; Alessandri, F.; Magnanimi, E.; Pugliese, F.; Venditti, M. Multidrug-Resistant Acinetobacter Baumannii Infections in COVID-19 Patients Hospitalized in Intensive Care Unit. Infection 2022, 50, 83–92. [Google Scholar] [CrossRef]
- Čiginskienė, A.; Dambrauskienė, A.; Rello, J.; Adukauskienė, D. Ventilator-Associated Pneumonia Due to Drug-Resistant Acinetobacter Baumannii: Risk Factors and Mortality Relation with Resistance Profiles, and Independent Predictors of in-Hospital Mortality. Medicina 2019, 55, 49. [Google Scholar] [CrossRef]
- Satlin, M.J.; Chen, L.; Patel, G.; Gomez-Simmonds, A.; Weston, G.; Kim, A.C.; Seo, S.K.; Rosenthal, M.E.; Sperber, S.J.; Jenkins, S.G. Multicenter Clinical and Molecular Epidemiological Analysis of Bacteremia Due to Carbapenem-Resistant Enterobacteriaceae (CRE) in the CRE Epicenter of the United States. Antimicrob. Agents Chemother. 2017, 61, e02349-16. [Google Scholar] [CrossRef]
- Satlin, M.J.; Chen, L.; Gomez-Simmonds, A.; Marino, J.; Weston, G.; Bhowmick, T.; Seo, S.K.; Sperber, S.J.; Kim, A.C.; Eilertson, B. Impact of a Rapid Molecular Test for Klebsiella Pneumoniae Carbapenemase and Ceftazidime-Avibactam Use on Outcomes after Bacteremia Caused by Carbapenem-Resistant Enterobacterales. Clin. Infect. Dis. 2022, 75, 2066–2075. [Google Scholar] [CrossRef]
- Salimnia, H.; Fairfax, M.R.; Lephart, P.R.; Schreckenberger, P.; DesJarlais, S.M.; Johnson, J.K.; Robinson, G.; Carroll, K.C.; Greer, A.; Morgan, M. Evaluation of the FilmArray Blood Culture Identification Panel: Results of a Multicenter Controlled Trial. J. Clin. Microbiol. 2016, 54, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Tojo, M.; Fujita, T.; Ainoda, Y.; Nagamatsu, M.; Hayakawa, K.; Mezaki, K.; Sakurai, A.; Masui, Y.; Yazaki, H.; Takahashi, H. Evaluation of an Automated Rapid Diagnostic Assay for Detection of Gram-Negative Bacteria and Their Drug-Resistance Genes in Positive Blood Cultures. PLoS ONE 2014, 9, e94064. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. The Value of GeneXpert MTB/RIF for Detection in Tuberculosis: A Bibliometrics-Based Analysis and Review. J. Anal. Methods Chem. 2022, 2915018. [Google Scholar] [CrossRef] [PubMed]
- Zeka, A.N.; Tasbakan, S.; Cavusoglu, C. Evaluation of the GeneXpert MTB/RIF Assay for Rapid Diagnosis of Tuberculosis and Detection of Rifampin Resistance in Pulmonary and Extrapulmonary Specimens. J. Clin. Microbiol. 2011, 49, 4138–4141. [Google Scholar] [CrossRef]
- Evans, C.A. GeneXpert—A Game-Changer for Tuberculosis Control? PLoS Med. 2011, 8, e1001064. [Google Scholar] [CrossRef]
- Dortet, L.; Fusaro, M.; Naas, T. Improvement of the Xpert Carba-R Kit for the Detection of Carbapenemase-Producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2016, 60, 3832–3837. [Google Scholar] [CrossRef]
- Cortegiani, A.; Russotto, V.; Graziano, G.; Geraci, D.; Saporito, L.; Cocorullo, G.; Raineri, S.M.; Mammina, C.; Giarratano, A. Use of Cepheid Xpert Carba-R® for Rapid Detection of Carbapenemase-Producing Bacteria in Abdominal Septic Patients Admitted to Intensive Care Unit. PLoS ONE 2016, 11, e0160643. [Google Scholar] [CrossRef]
- Jin, S.; Lee, J.Y.; Park, J.Y.; Jeon, M.J. Xpert Carba-R Assay for Detection of Carbapenemase-Producing Organisms in Patients Admitted to Emergency Rooms. Medicine 2020, 99, E23410. [Google Scholar] [CrossRef]
- Baeza, L.L.; Pfennigwerth, N.; Greissl, C.; Göttig, S.; Saleh, A.; Stelzer, Y.; Gatermann, S.G.; Hamprecht, A. Comparison of Five Methods for Detection of Carbapenemases in Enterobacterales with Proposal of a New Algorithm. Clin. Microbiol. Infect. 2019, 25, 1286.e9–1286.e15. [Google Scholar] [CrossRef]
- Herráez, Ó.; Asencio-Egea, M.Á.; Huertas-Vaquero, M.; Carranza-González, R.; Castellanos-Monedero, J.; Franco-Huerta, M.; Barberá-Farré, J.R.; Tenías-Burillo, J.M. Estudio de Coste-Efectividad Del Diagnóstico Microbiológico de Tuberculosis Mediante GeneXpert MTB/RIF®. Enferm. Infecc. Microbiol. Clin. 2017, 35, 403–410. [Google Scholar] [CrossRef]
- Ejalu, D.L.; Irioko, A.; Kirabo, R.; Mukose, A.D.; Ekirapa, E.; Kagaayi, J.; Namutundu, J. Cost-Effectiveness of GeneXpert Omni Compared with GeneXpert MTB/Rif for Point-of-Care Diagnosis of Tuberculosis in a Low-Resource, High-Burden Setting in Eastern Uganda: A Cost-Effectiveness Analysis Based on Decision Analytical Modelling. BMJ Open 2022, 12, e059823. [Google Scholar] [CrossRef] [PubMed]
- Kaso, A.W.; Hailu, A. Costs and Cost-Effectiveness of Gene Xpert Compared to Smear Microscopy for the Diagnosis of Pulmonary Tuberculosis Using Real-World Data from Arsi Zone, Ethiopia. PLoS ONE 2021, 16, e0259056. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J. Management of Adults with Hospital-Acquired and Ventilator-Associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef] [PubMed]
- Gadsby, N.J.; Musher, D.M. The Microbial Etiology of Community-Acquired Pneumonia in Adults: From Classical Bacteriology to Host Transcriptional Signatures. Clin. Microbiol. Rev. 2022, 35, e00015-22. [Google Scholar] [CrossRef]
- Bartlett, J.G.; Finegold, S.M. Bacteriology of Expectorated Sputum with Quantitative Culture and Wash Technique Compared to Transtracheal Aspirates. Am. Rev. Respir. Dis. 1978, 117, 1019–1027. [Google Scholar]
- Ioanas, M.; Ferrer, R.; Angrill, J.; Ferrer, M.; Torres, A. Torres Microbial Investigation in Ventilator-Associated Pneumonia. Eur. Respir. J. 2001, 17, 791. [Google Scholar] [CrossRef]
- Schytz, H.W.; Hvedstrup, J. Evaluating Headache and Facial Pain in a Headache Diagnostic Laboratory: Experiences from the Danish Headache Center. Diagnostics 2023, 13, 2671. [Google Scholar] [CrossRef]
- McHugh, M.P.; Parcell, B.J.; MacKenzie, F.M.; Templeton, K.E.; Scottish Microbiology and Virology Network (SMVN) Molecular Diagnostics Evaluation Group. Rapid Molecular Testing for Staphylococcus Aureus Bacteraemia Improves Clinical Management. J. Med. Microbiol. 2020, 69, 552–557. [Google Scholar] [CrossRef]
- Wolk, D.; Struelens, M.; Pancholi, P.; Davis, T.; Della-Latta, P.; Fuller, D.; Picton, E.; Dickenson, R.; Denis, O.; Johnson, D. Rapid Detection of Staphylococcus Aureus and Methicillin-Resistant S. Aureus (MRSA) in Wound Specimens and Blood Cultures: Multicenter Preclinical Evaluation of the Cepheid Xpert MRSA/SA Skin and Soft Tissue and Blood Culture Assays. J. Clin. Microbiol. 2009, 47, 823–826. [Google Scholar] [CrossRef]
- Spencer, D.H.; Sellenriek, P.; Burnham, C.-A.D. Validation and Implementation of the GeneXpert MRSA/SA Blood Culture Assay in a Pediatric Setting. Am. J. Clin. Pathol. 2011, 136, 690–694. [Google Scholar] [CrossRef]
- Laurent, C.; Bogaerts, P.; Schoevaerdts, D.; Denis, O.; Deplano, A.; Swine, C.; Struelens, M.; Glupczynski, Y. Evaluation of the Xpert MRSA Assay for Rapid Detection of Methicillin-Resistant Staphylococcus Aureus from Nares Swabs of Geriatric Hospitalized Patients and Failure to Detect a Specific SCC Mec Type IV Variant. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Noto, M.J.; Kreiswirth, B.N.; Monk, A.B.; Archer, G.L. Gene Acquisition at the Insertion Site for SCC Mec, the Genomic Island Conferring Methicillin Resistance in Staphylococcus Aureus. J. Bacteriol. 2008, 190, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Francois, P.; Bento, M.; Renzi, G.; Harbarth, S.; Pittet, D.; Schrenzel, J. Evaluation of Three Molecular Assays for Rapid Identification of Methicillin-Resistant Staphylococcus Aureus. J. Clin. Microbiol. 2007, 45, 2011–2013. [Google Scholar] [CrossRef] [PubMed]
- Gazin, M.; Lammens, C.; Goossens, H.; Malhotra-Kumar, S.; MOSAR WP2 Study Team. Evaluation of GeneOhm VanR and Xpert VanA/VanB Molecular Assays for the Rapid Detection of Vancomycin-Resistant Enterococci. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Yağci, S.; Hatipoğlu, Ç.A.; Altun, Ş.; Bulut, C.; Tufan, Z.K.; Altun, H.U.; Ertem, G.; Erdinç, F.Ş. Evaluation of GeneXpert VanA/VanB Assay for the Detection of Vancomycin-Resistant Enterococci in Patients Newly Admitted to Intensive Care Units. Turk. J. Med. Sci. 2013, 43, 1008–1012. [Google Scholar] [CrossRef]
- Cantón, R.; Akóva, M.; Carmeli, Y.; Giske, C.G.; Glupczynski, Y.; Gniadkowski, M.; Livermore, D.M.; Miriagou, V.; Naas, T.; Rossolini, G. Rapid Evolution and Spread of Carbapenemases among Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 2012, 18, 413–431. [Google Scholar] [CrossRef]
| VAP/HAP Group (n = 29) | Control Group (n = 28) | p Value | |
|---|---|---|---|
| Gender—male (n, %) | 24 (82.75) | 15 (53.57) | 0.018 |
| Age (years) | 61.55 ± 18.42 | 59.07 ± 16.05 | 0.566 |
| Length of ICU stay (days) | 28 (14–48.5) | N/A | |
| Length of mechanical ventilation (hours) | 336 (168–800) | N/A | |
| Rationale for ICU admission Aspiration pneumonia (n, %) Septic shock (n, %) Trauma (n, %) Other (n, %) | 14 (48.27) 10 (34.48) 6 (20.68) 14 (48.27) | N/A | |
| SOFA | 9.28 ± 3.99 | N/A | |
| APACHE II | 19.21 ± 7.52 | N/A | |
| SAPS II | 46.59 ± 14.31 | N/A | |
| CPIS | 6.45 ± 1.20 | N/A | |
| White blood cell count (/mm3) | 13,270 (10,660–18,910) | 7700 (6210–11,230) | <0.001 |
| Neutrophil count (/mm3) | 12,700 (8650–22,350) | 6700 (3560–8560) | <0.001 |
| C-reactive protein (mg/dL) | 19.49 ± 12.46 | 4.26 (1.23–9.39) | 0.008 |
| Presepsin (ng/L) | 899 (218–1925) | N/A | |
| Procalcitonin (ng/mL) | 1.31 (0.12–4.68) | 0.46 (0.07–7.31) | 0.006 |
| Fibrinogen (mg/dL) | 525 (381–650) | N/A |
| Primary Isolate | n = 29 (100%) |
|---|---|
| Acinetobacter baumanii | |
| MDR | 6 (20.68%) |
| XDR | 10 (34.48%) |
| Klebsiella pneumoniae | |
| MDR | 2 (6.89%) |
| XDR (including CRE+) | 2 (6.89%) |
| Methicillin-resistant Staphylococcus aureus | |
| MDR | 2 (6.89%) |
| XDR (MSLb +) | 1 (3.44%) |
| Pseudomonas aeruginosa | |
| MDR | 4 (13.79%) |
| Providencia stuartii | |
| MDR | 1 (3.44%) |
| XDR | 1 (3.44%) |
| Secondary isolate | N = 5 (17.24%) |
| Klebsiella pneumoniae | |
| XDR (including CRE+) | 4 (13.79%) |
| Proteus spp. | |
| MDR | 1 (3.44%) |
| All PCR Tests (n = 81) | Carbapenem-Resistance Kit (n = 47) | MRSA—Kit (n = 18) | |
|---|---|---|---|
| Sensitivity | 92.31% (79.13–98.38) | 90.32% (74.25–97.96) | 100% (54.07–100) |
| Specificity | 97.67% (87.71–99.94) | 95.65% (78.05–99.89) | 91.67 (61.52–99.79) |
| Positive likelihood ratio | 39.69 (5.71–275.99) | 20.77 (3.04–141.75) | 12 (1.84–78.37) |
| Negative likelihood ratio | 0.08 (0.03–0.23) | 0.10 (0.03–0.30) | 0 |
| Positive predictive value | 97.30% (85.84—99.93) | 96.55% (82.24–99.91) | 85.71 (42.13–99.64) |
| Negative predictive value | 93.33% (81.73–98.60) | 88.00% (68.78–97.45) | 100% (71.51–100) |
| Accuracy | 95.12% (87.98–98.66) | 92.59% (82.11–97.94) | 94.44% (72.71–99.86%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bălan, A.-M.; Bodolea, C.; Nemes, A.; Crăciun, R.; Hagău, N. Rapid Point-of-Care PCR Testing of Drug-Resistant Strains on Endotracheal Aspirate Samples: A Repurposed Effective Tool in the Stepwise Approach of Healthcare-Acquired Pneumonia—A Pilot Study. Int. J. Mol. Sci. 2023, 24, 13393. https://doi.org/10.3390/ijms241713393
Bălan A-M, Bodolea C, Nemes A, Crăciun R, Hagău N. Rapid Point-of-Care PCR Testing of Drug-Resistant Strains on Endotracheal Aspirate Samples: A Repurposed Effective Tool in the Stepwise Approach of Healthcare-Acquired Pneumonia—A Pilot Study. International Journal of Molecular Sciences. 2023; 24(17):13393. https://doi.org/10.3390/ijms241713393
Chicago/Turabian StyleBălan, Andrei-Mihai, Constantin Bodolea, Andrada Nemes, Rareș Crăciun, and Natalia Hagău. 2023. "Rapid Point-of-Care PCR Testing of Drug-Resistant Strains on Endotracheal Aspirate Samples: A Repurposed Effective Tool in the Stepwise Approach of Healthcare-Acquired Pneumonia—A Pilot Study" International Journal of Molecular Sciences 24, no. 17: 13393. https://doi.org/10.3390/ijms241713393







