Impact of Classic Adrenal Secretagogues on mRNA Levels of Urotensin II and Its Receptor in Adrenal Gland of Rats
Abstract
:1. Introduction
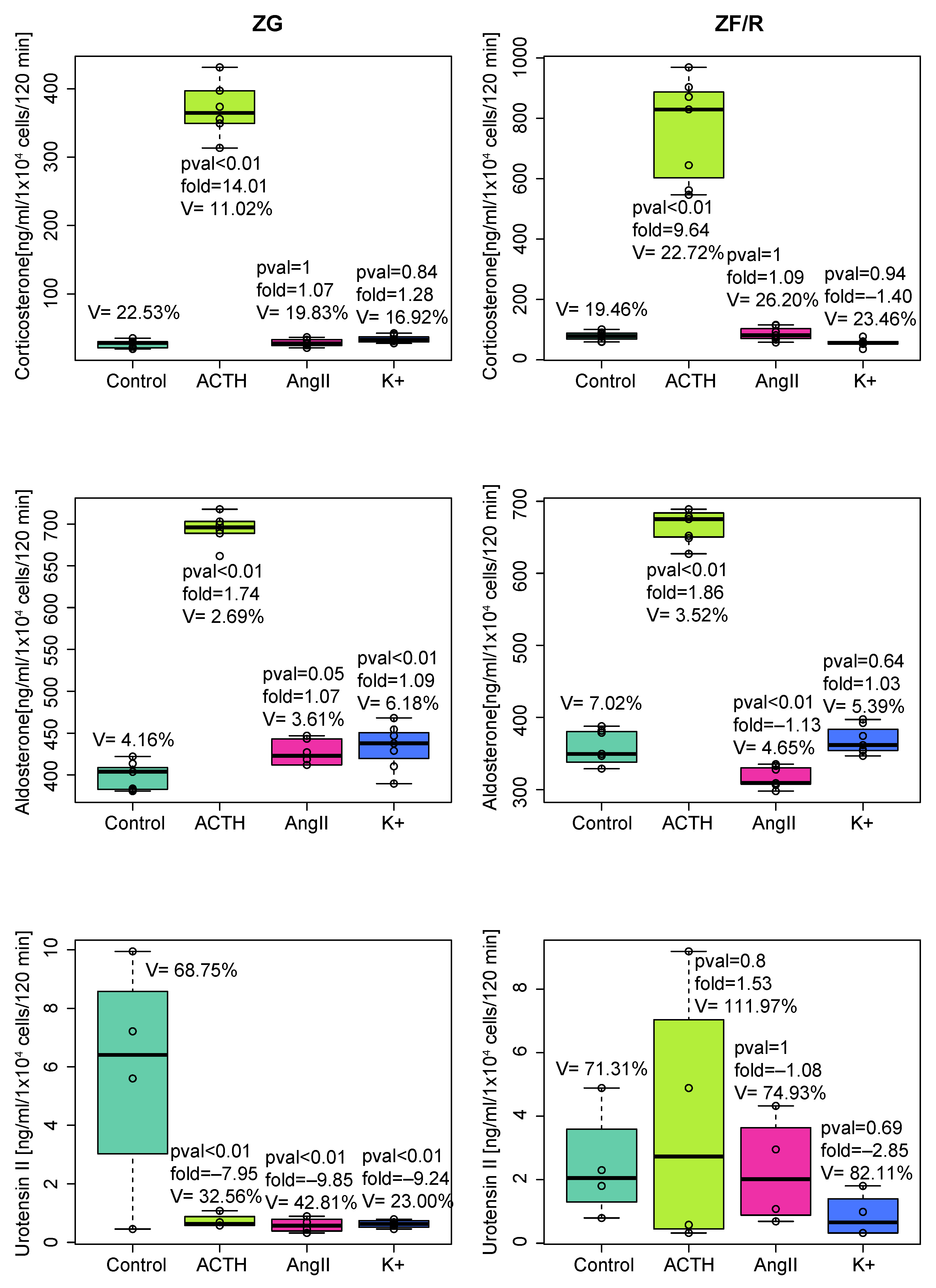
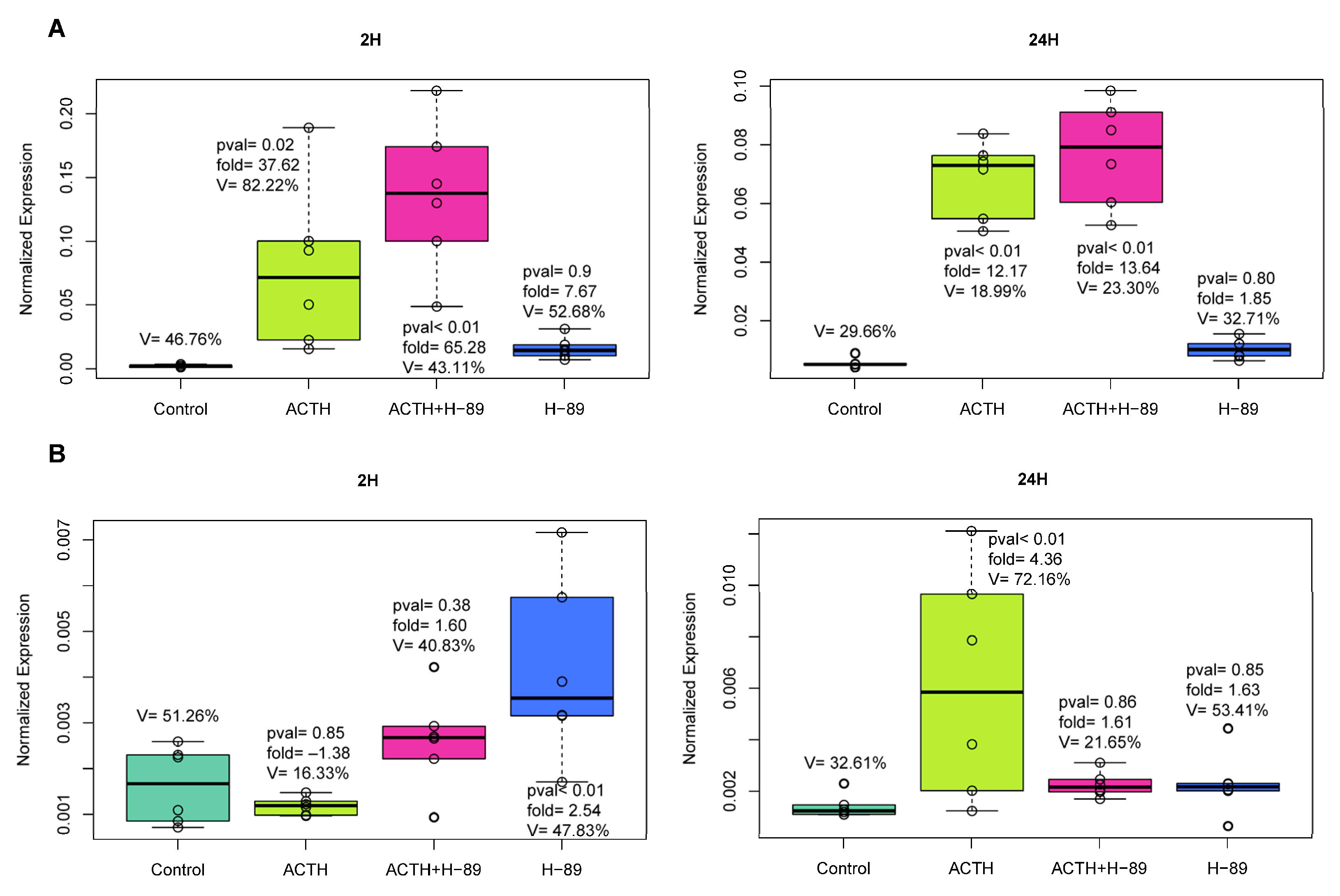
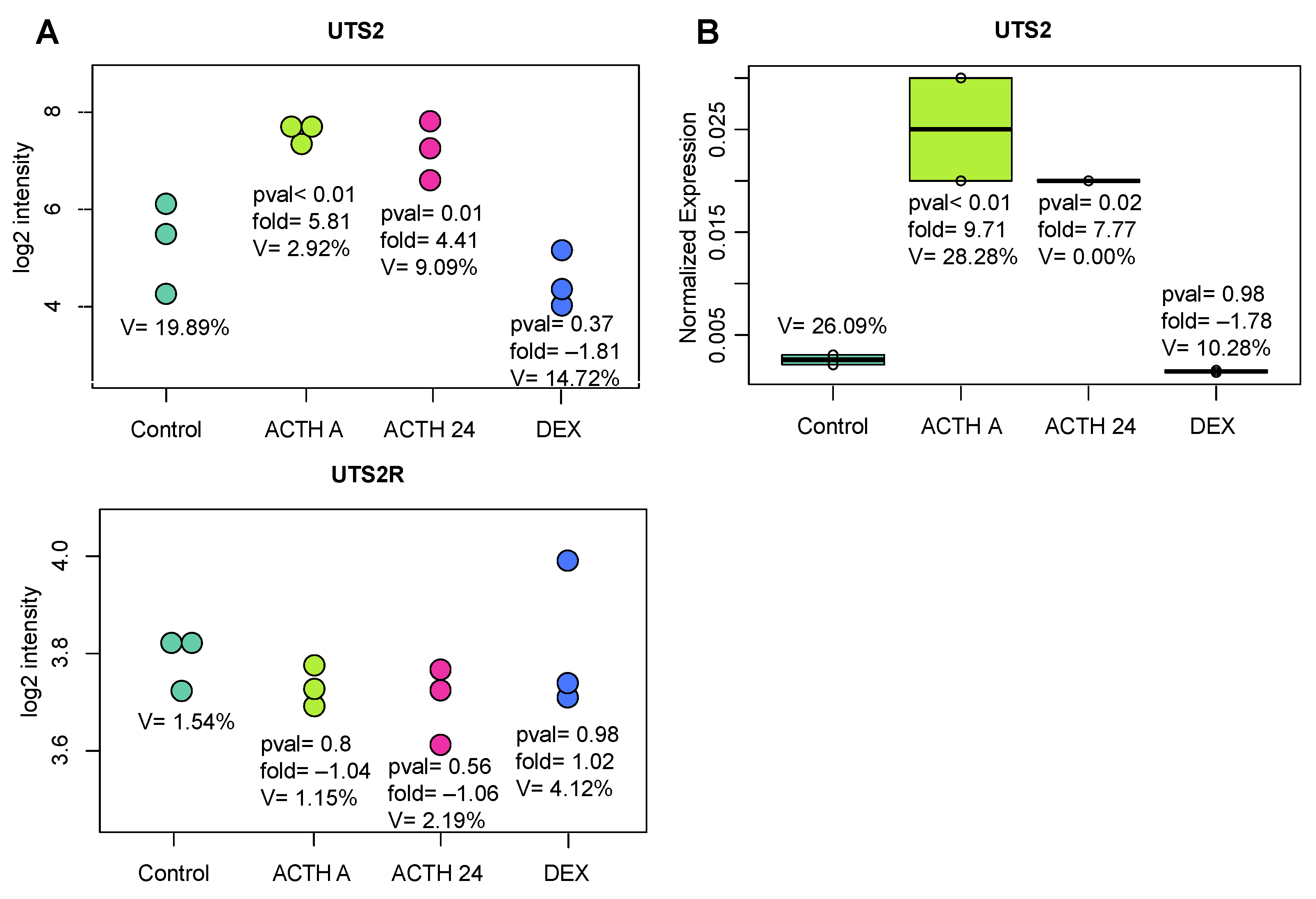
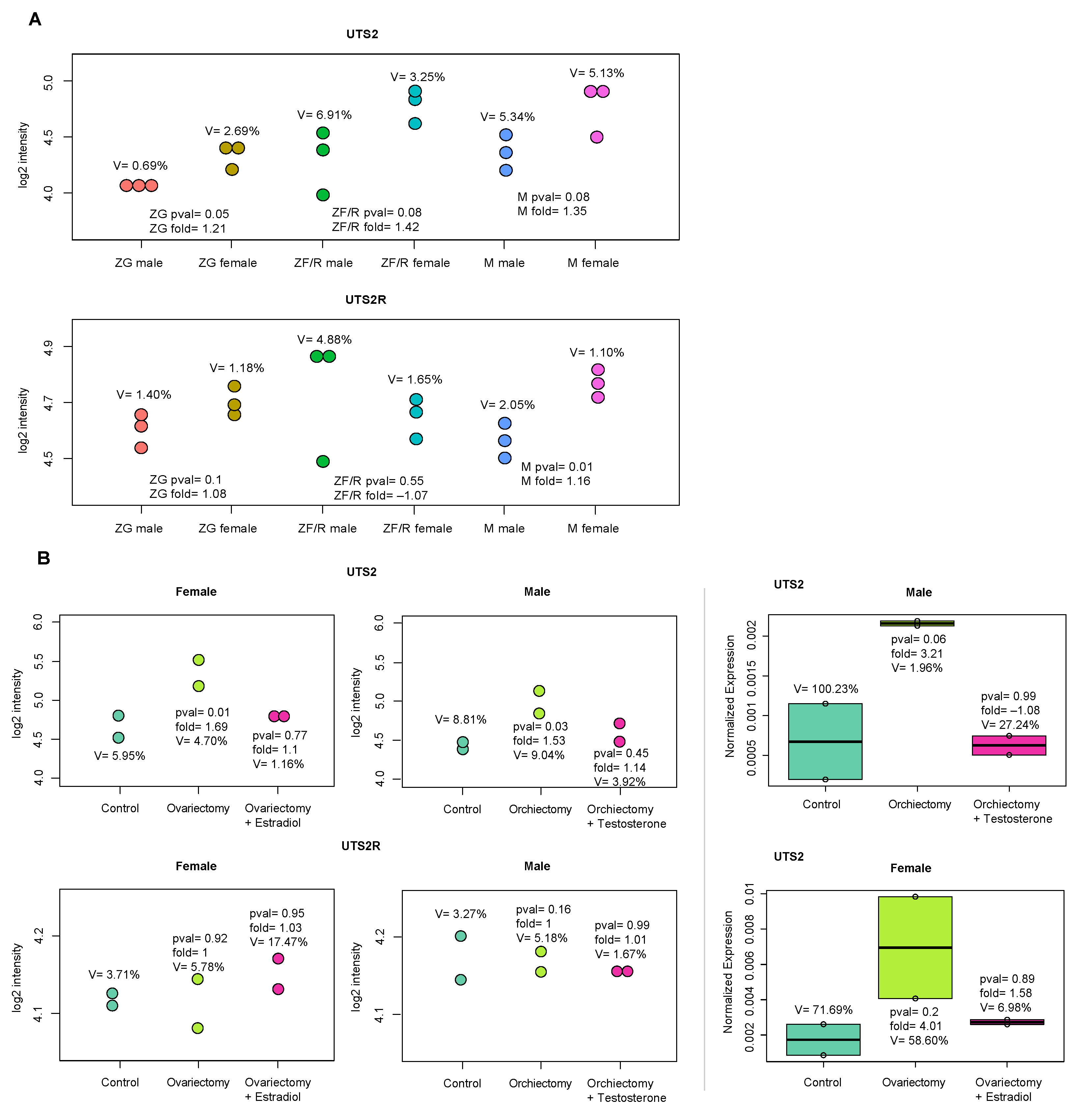
2. Results
2.1. In Vitro Experiments
2.2. In Vivo Experiments
3. Discussion
4. Materials and Methods
4.1. Animals and Reagents
4.2. In Vitro Experiments
4.2.1. Primary Adrenocortical Cell Culture
4.2.2. Freshly Isolated Rat Adrenal Cells
4.2.3. RNA Extraction
4.2.4. Reverse Transcription and qPCR
4.2.5. Microarray Expression Analysis
4.2.6. Corticosterone, Aldosterone, and Urotensin II Detection
4.3. In Vivo Experiments
4.3.1. The Effects of Acute and Prolonged ACTH Injections on Uts2 and Uts2r mRNA Levels in Rat Adrenal Glands Were Determined
4.3.2. Regeneration Model
4.3.3. Unilateral Adrenalectomy
4.3.4. Effects of Gonadectomy and Sex Hormones’ Supplementation on Uts2 and Uts2r Gene Expressions
4.4. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rooney, D.P.; Neely, R.D.; Cullen, C.; Ennis, C.N.; Sheridan, B.; Atkinson, A.B.; Trimble, E.R.; Bell, P.M. The effect of cortisol on glucose/glucose-6-phosphate cycle activity and insulin action. J. Clin. Endocrinol. Metab. 1993, 77, 1180–1183. [Google Scholar] [CrossRef] [PubMed]
- Rizza, R.A.; Mandarino, L.J.; Gerich, J.E. Cortisol-Induced Insulin Resistance in Man: Impaired Suppression of Glucose Production and Stimulation of Glucose Utilization due to a Postreceptor Defect of Insulin Action. J. Clin. Endocrinol. Metab. 1982, 54, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.; Seeley, R.J.; Green, P.K.; Wilkinson, C.W.; Schwartz, M.W.; Woods, S.C. Adrenalectomy increases sensitivity to central insulin. Physiol. Behav. 1997, 62, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Paschke, L.; Zemleduch, T.; Rucinski, M.; Ziolkowska, A.; Szyszka, M.; Malendowicz, L.K. Adiponectin and adiponectin receptor system in the rat adrenal gland: Ontogenetic and physiologic regulation, and its involvement in regulating adrenocortical growth and steroidogenesis. Peptides 2010, 31, 1715–1724. [Google Scholar] [CrossRef]
- Malendowicz, L.K.; Tortorella, C.; Nussdorfer, G.G. Orexins stimulate corticosterone secretion of rat adrenocortical cells, through the activation of the adenylate cyclase-dependent signaling cascade. J. Steroid Biochem. Mol. Biol. 1999, 70, 185–188. [Google Scholar] [CrossRef]
- Markowska, A.; Neri, G.; Hochol, A.; Nowak, M.; Nussdorfer, G.G.; Malendowicz, L.K. Effects of leptin and leptin fragments on steroid secretion and proliferative activity of regenerating rat adrenal cortex. Int. J. Mol. Med. 2004, 13, 139–141. [Google Scholar] [CrossRef]
- Ziolkowska, A.; Macchi, C.; Trejter, M.; Rucinski, M.; Nowak, M.; Nussdorfer, G.G.; Malendowicz, L.K. Effects of neuromedin-U on immature rat adrenocortical cells: In vitro and in vivo studies. Int. J. Mol. Med. 2008, 21, 303–307. [Google Scholar] [CrossRef]
- Albertin, G.; Casale, V.; Ziolkowska, A.; Spinazzi, R.; Malendowicz, L.K.; Rossi, G.P.; Nussdorfer, G.G. Urotensin-II and UII-receptor expression and function in the rat adrenal cortex. Int. J. Mol. Med. 2006, 17, 1111–1115. [Google Scholar] [CrossRef]
- Bern, H.A.; Pearson, D.; Larson, B.A.; Nishioka, R.S. Neurohormones from Fish Tails: The Caudal Neurosecretory System. In Recent Progress in Hormone Research; Elsevier: Berkeley, CA, USA, 1985; Volume 41, pp. 533–552. [Google Scholar] [CrossRef]
- Pearson, D.; Shively, J.E.; Clark, B.R.; Geschwind, I.I.; Barkley, M.; Nishioka, R.S.; Bern, H.A. Urotensin II: A somatostatin-like peptide in the caudal neurosecretory system of fishes. Proc. Natl. Acad. Sci. USA 1980, 77, 5021–5024. [Google Scholar] [CrossRef]
- Conlon, J.M.; Arnold-Reed, D.; Balment, R. Post-translational processing of prepro-urotensin II. FEBS Lett. 1990, 266, 37–40. [Google Scholar] [CrossRef]
- Coulouarn, Y.; Jégou, S.; Tostivint, H.; Vaudry, H.; Lihrmann, I. Cloning, sequence analysis and tissue distribution of the mouse and rat urotensin II precursors. FEBS Lett. 1999, 457, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Coulouarn, Y.; Lihrmann, I.; Jegou, S.; Anouar, Y.; Tostivint, H.; Beauvillain, J.C.; Conlon, J.M.; Bern, H.A.; Vaudry, H. Cloning of the cDNA encoding the urotensin II precursor in frog and human reveals intense expression of the urotensin II gene in motoneurons of the spinal cord. Proc. Natl. Acad. Sci. USA 1998, 95, 15803–15808. [Google Scholar] [CrossRef] [PubMed]
- Ames, R.S.; Sarau, H.M.; Chambers, J.K.; Willette, R.N.; Aiyar, N.V.; Romanic, A.M.; Louden, C.S.; Foley, J.J.; Sauermelch, C.F.; Coatney, R.W.; et al. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature 1999, 401, 282–286. [Google Scholar] [CrossRef]
- A Douglas, S.; Naselsky, D.; Ao, Z.; Disa, J.; Herold, C.L.; Lynch, F.; Aiyar, N.V. Identification and pharmacological characterization of native, functional human urotensin-II receptors in rhabdomyosarcoma cell lines: Urotensin receptors in skeletal muscle cell lines. Br. J. Pharmacol. 2004, 142, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Totsune, K.; Takahashi, K.; Arihara, Z.; Sone, M.; Satoh, F.; Ito, S.; Kimura, Y.; Sasano, H.; Murakami, O. Role of urotensin II in patients on dialysis. Lancet 2001, 358, 810–811. [Google Scholar] [CrossRef]
- Silvestre, R.A.; Rodríguez-Gallardo, J.; Egido, E.M.; Marco, J. Inhibition of Insulin Release by Urotensin II—A Study on the Perfused Rat Pancreas. Horm. Metab. Res. 2001, 33, 379–381. [Google Scholar] [CrossRef]
- Sugo, T.; Murakami, Y.; Shimomura, Y.; Harada, M.; Abe, M.; Ishibashi, Y.; Kitada, C.; Miyajima, N.; Suzuki, N.; Mori, M.; et al. Identification of urotensin II-related peptide as the urotensin II-immunoreactive molecule in the rat brain. Biochem. Biophys. Res. Commun. 2003, 310, 860–868. [Google Scholar] [CrossRef]
- A Douglas, S.; Tayara, L.; Ohlstein, E.H.; Halawa, N.; Giaid, A. Congestive heart failure and expression of myocardial urotensin II. Lancet 2002, 359, 1990–1997. [Google Scholar] [CrossRef]
- Takahashi, K.; Totsune, K.; Murakami, O.; Arihara, Z.; Noshiro, T.; Hayashi, Y.; Shibahara, S. Expression of urotensin II and its receptor in adrenal tumors and stimulation of proliferation of cultured tumor cells by urotensin II. Peptides 2003, 24, 301–306. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, G.; Li, H.; Fan, X.; Liu, D.; Tong, A.; Zheng, X.; Liu, C. The Effects of Urotensin-II on Proliferation of Pheochromocytoma Cells and mRNA Expression of Urotensin-II and Its Receptor in Pheochromocytoma Tissues. Ann. N. Y. Acad. Sci. 2006, 1073, 284–289. [Google Scholar] [CrossRef]
- Morimoto, R.; Satoh, F.; Murakami, O.; Totsune, K.; Arai, Y.; Suzuki, T.; Sasano, H.; Ito, S.; Takahashi, K. Immunolocalization of urotensin II and its receptor in human adrenal tumors and attached non-neoplastic adrenal tissues. Peptides 2008, 29, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Aita, Y.; Kasahara, T.; Isobe, K.; Kawakami, Y.; Takekoshi, K. Effect of Urotensin II on PC12 Rat Pheochromocytoma Cells. J. Neuroendocr. 2010, 22, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Totsune, K.; Murakami, O.; Shibahara, S. Expression of urotensin II and urotensin II receptor mRNAs in various human tumor cell lines and secretion of urotensin II-like immunoreactivity by SW-13 adrenocortical carcinoma cells. Peptides 2001, 22, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Shichiri, M.; Imai, T.; Iwashina, M.; Tanaka, H.; Takasu, N.; Hirata, Y. Co-expression of urotensin II and its receptor (GPR14) in human cardiovascular and renal tissues. J. Hypertens. 2001, 19, 2185–2190. [Google Scholar] [CrossRef]
- Hassan, G.S.; Douglas, S.A.; Ohlstein, E.H.; Giaid, A. Expression of urotensin-II in human coronary atherosclerosis. Peptides 2005, 26, 2464–2472. [Google Scholar] [CrossRef]
- Rakowski, E.; Hassan, G.; Dhanak, D.; Ohlstein, E.; Douglas, S.; Giaid, A. A role for urotensin II in restenosis following balloon angioplasty: Use of a selective UT receptor blocker. J. Mol. Cell Cardiol. 2005, 39, 785–791. [Google Scholar] [CrossRef]
- Totsune, K.; Takahashi, K.; Arihara, Z.; Sone, M.; Ito, S.; Murakami, O. Increased plasma urotensin II levels in patients with diabetes mellitus. Clin. Sci. 2003, 104, 1–5. [Google Scholar] [CrossRef]
- Opgaard, O.S.; Nothacker, H.-P.; Ehlert, F.J.; Krause, D.N. Human urotensin II mediates vasoconstriction via an increase in inositol phosphates. Eur. J. Pharmacol. 2000, 406, 265–271. [Google Scholar] [CrossRef]
- Hassan, G.S.; Chouiali, F.; Saito, T.; Hu, F.; Douglas, S.A.; Ao, Z.; Willette, R.N.; Ohlstein, E.H.; Giaid, A. Effect of human urotensin-II infusion on hemodynamics and cardiac function. Can. J. Physiol. Pharmacol. 2003, 81, 125–128. [Google Scholar] [CrossRef]
- Gibson, A.; Wallace, P.; Bern, H.A. Cardiovascular effects of urotensin II in anesthetized and pithed rats. Gen. Comp. Endocrinol. 1986, 64, 435–439. [Google Scholar] [CrossRef]
- Itoh, H.; Itoh, Y.; Rivier, J.; Lederis, K. Contraction of major artery segments of rat by fish neuropeptide urotensin II. Am. J. Physiol. Integr. Comp. Physiol. 1987, 252, R361–R366. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; McMaster, D.; Lederis, K. Functional receptors for fish neuropeptide urotensin II in major rat arteries. Eur. J. Pharmacol. 1988, 149, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.A.; Sulpizio, A.C.; Piercy, V.; Sarau, H.M.; Ames, R.S.; Aiyar, N.V.; Ohlstein, E.H.; Willette, R.N. Differential vasoconstrictor activity of human urotensin-II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey: Differential vascular reactivity and urotensin-II. Br. J. Pharmacol. 2000, 131, 1262–1274. [Google Scholar] [CrossRef]
- Castel, H.; Diallo, M.; Chatenet, D.; Leprince, J.; Desrues, L.; Schouft, M.; Fontaine, M.; Dubessy, C.; Lihrmann, I.; Scalbert, E.; et al. Biochemical and functional characterization of high-affinity urotensin II receptors in rat cortical astrocytes: High-affinity UII receptors in astrocytes. J. Neurochem. 2006, 99, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Ziltener, P.; Mueller, C.; Haenig, B.; Scherz, M.W.; Nayler, O. Urotensin II Mediates ERK1/2 Phosphorylation and Proliferation in Gpr14-Transfected Cell Lines. J. Recept. Signal Transduct. 2002, 22, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Morooka, T.; Nishida, E. Requirement of p38 Mitogen-activated Protein Kinase for Neuronal Differentiation in PC12 Cells. J. Biol. Chem. 1998, 273, 24285–24288. [Google Scholar] [CrossRef]
- Mazzocchi, G.; Neri, G.; Rucinski, M.; Rebuffat, P.; Spinazzi, R.; Malendowicz, L.K.; Nussdorfer, G.G. Ghrelin enhances the growth of cultured human adrenal zona glomerulosa cells by exerting MAPK-mediated proliferogenic and antiapoptotic effects. Peptides 2004, 25, 1269–1277. [Google Scholar] [CrossRef]
- Ho, W.-C.; Uniyal, S.; Meakin, S.O.; Morris, V.L.; Chan, B.M. A Differential Role of Extracellular Signal-Regulated Kinase in Stimulated PC12 Pheochromocytoma Cell Movement. Exp. Cell Res. 2001, 263, 254–264. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chen, Y.-F.; Wang, J.-Y.; Hsieh, M.-C.; Yeh, C.-S.; Lian, S.-T.; Shin, S.-J.; Lin, S.-R. Mutant K-ras oncogene regulates steroidogenesis of normal human adrenocortical cells by the RAF-MEK-MAPK pathway. Br. J. Cancer 2002, 87, 1000–1005. [Google Scholar] [CrossRef]
- Romero, D.G.; Plonczynski, M.W.; Welsh, B.L.; Gomez-Sanchez, C.E.; Zhou, M.Y.; Gomez-Sanchez, E.P. Gene expression profile in rat adrenal zona glomerulosa cells stimulated with aldosterone secretagogues. Physiol. Genom. 2007, 32, 117–127. [Google Scholar] [CrossRef]
- Nogueira, E.F.; Vargas, C.A.; Otis, M.; Gallo-Payet, N.; Bollag, W.B.; Rainey, W.E. Angiotensin-II acute regulation of rapid response genes in human, bovine, and rat adrenocortical cells. J. Mol. Endocrinol. 2007, 39, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Birker-Robaczewska, M.; Boukhadra, C.; Studer, R.; Mueller, C.; Binkert, C.; Nayler, O. The Expression of Urotensin II Receptor (U2R) is Up-regulated by Interferon-γ. J. Recept. Signal Transduct. 2003, 23, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Dallman, M.F. Control of Adrenocortical Growth in Vivo. Endocr. Res. 1984, 10, 213–242. [Google Scholar] [CrossRef]
- Tyczewska, M.; Rucinski, M.; Trejter, M.; Ziolkowska, A.; Szyszka, M.; Malendowicz, L.K. Angiogenesis in the course of enucleation-induced adrenal regeneration—Expression of selected genes and proteins involved in development of capillaries. Peptides 2012, 38, 404–413. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Engeland, W.C. Hyperinnervation during Adrenal Regeneration Influences the Rate of Functional Recovery. Neuroendocrinology 2000, 71, 107–123. [Google Scholar] [CrossRef]
- Jopek, K.; Tyczewska, M.; Celichowski, P.; Malendowicz, L.K.; Rucinski, M. Transcriptome Profile in Unilateral Adrenalectomy-Induced Compensatory Adrenal Growth in the Rat. Int. J. Mol. Sci. 2018, 19, 1111. [Google Scholar] [CrossRef] [PubMed]
- Malendowicz, L.K. Cytophysiology of the Mammalian Adrenal Cortex as Related to Sex, Gonadectomy and Gonadal Hormones; Wyd. Poznań. Tow. Przyjaciół Nauk: Poznań, Poland, 1994. [Google Scholar]
- Trejter, M.; Hochol, A.; Tyczewska, M.; Ziolkowska, A.; Jopek, K.; Szyszka, M.; Malendowicz, L.K.; Rucinski, M. Visinin-like peptide 1 in adrenal gland of the rat. Gene expression and its hormonal control. Peptides 2015, 63, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Trejter, M.; Hochol, A.; Tyczewska, M.; Ziolkowska, A.; Jopek, K.; Szyszka, M.; Malendowicz, L.K.; Rucinski, M. Sex-related gene expression profiles in the adrenal cortex in the mature rat: Microarray analysis with emphasis on genes involved in steroidogenesis. Int. J. Mol. Med. 2015, 35, 702–714. [Google Scholar] [CrossRef]
- Carlson, M. rat2302.db: Affymetrix Rat Genome 230 2.0 Array Annotation Data (chip rat2302), R package version 3.2.3. 2016. Available online: https://bioconductor.org/packages/release/data/annotation/html/rat2302.db.html(accessed on 1 July 2023).
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Ingle, D.J.; Higgins, G.M. Regeneration of the Adrenal Gland Following Enucleation. Am. J. Med. Sci. 1938, 196, 232–239. [Google Scholar] [CrossRef]
- MacKay, E.M.; MacKay, L.L. Compensatory Hypertrophy of the Adrenal Cortex. J. Exp. Med. 1926, 43, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Jopek, K.; Celichowski, P.; Szyszka, M.; Tyczewska, M.; Milecka, P.; Malendowicz, L.K.; Rucinski, M. Transcriptome Profile of Rat Adrenal Evoked by Gonadectomy and Testosterone or Estradiol Replacement. Front. Endocrinol. 2017, 8, 26. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jopek, K.; Tyczewska, M.; Szyszka, M.; Blatkiewicz, M.; Jopek, M.; Malendowicz, L.K.; Ruciński, M. Impact of Classic Adrenal Secretagogues on mRNA Levels of Urotensin II and Its Receptor in Adrenal Gland of Rats. Int. J. Mol. Sci. 2023, 24, 13412. https://doi.org/10.3390/ijms241713412
Jopek K, Tyczewska M, Szyszka M, Blatkiewicz M, Jopek M, Malendowicz LK, Ruciński M. Impact of Classic Adrenal Secretagogues on mRNA Levels of Urotensin II and Its Receptor in Adrenal Gland of Rats. International Journal of Molecular Sciences. 2023; 24(17):13412. https://doi.org/10.3390/ijms241713412
Chicago/Turabian StyleJopek, Karol, Marianna Tyczewska, Marta Szyszka, Małgorzata Blatkiewicz, Maria Jopek, Ludwik K. Malendowicz, and Marcin Ruciński. 2023. "Impact of Classic Adrenal Secretagogues on mRNA Levels of Urotensin II and Its Receptor in Adrenal Gland of Rats" International Journal of Molecular Sciences 24, no. 17: 13412. https://doi.org/10.3390/ijms241713412




