The Effect of Extremely Low-Frequency Electromagnetic Fields on Inflammation and Performance-Related Indices in Trained Athletes: A Double-Blinded Crossover Study
Abstract
:1. Introduction
2. Results
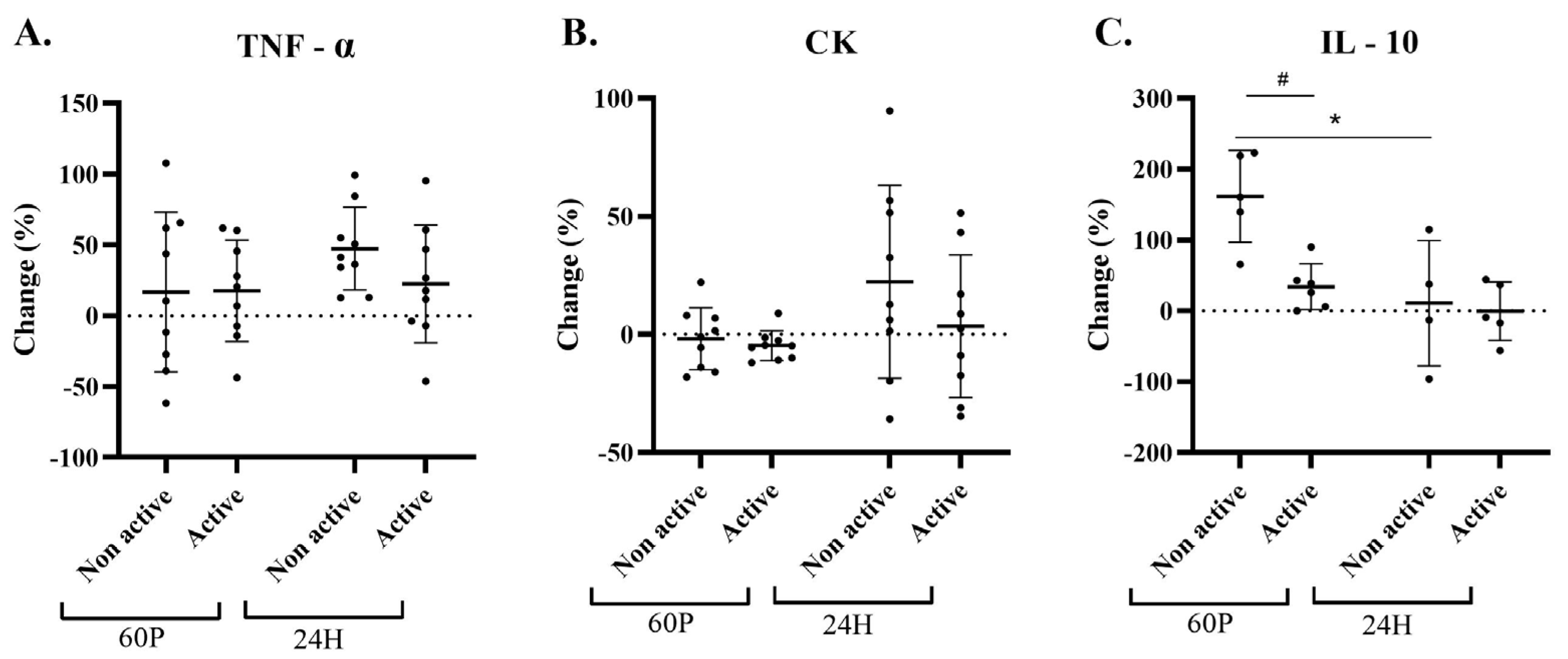
2.1. Blood Measurements
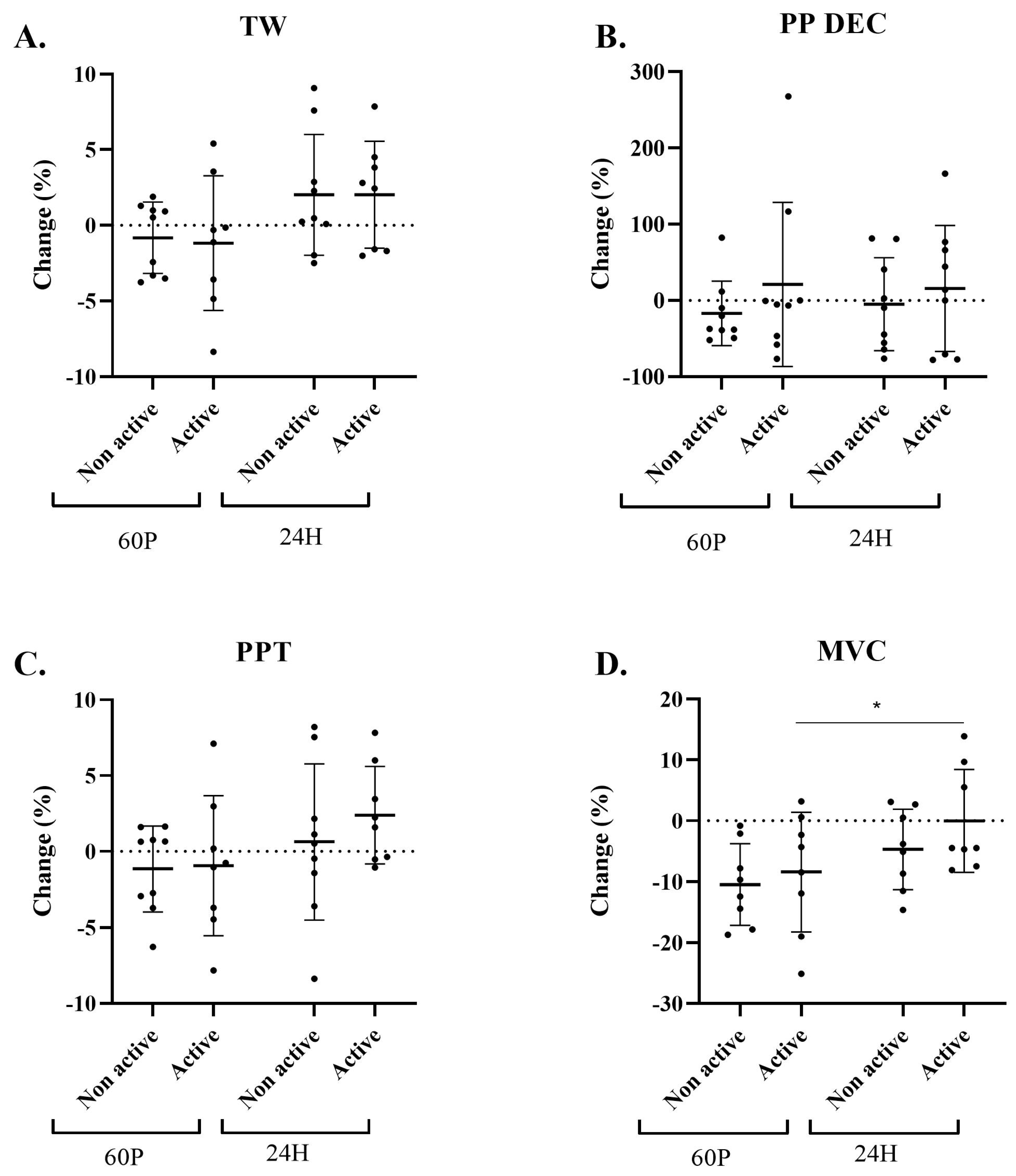
2.2. Performance-Indices Assessment
2.3. Subjective Measurements
2.4. Sensitivity Analysis
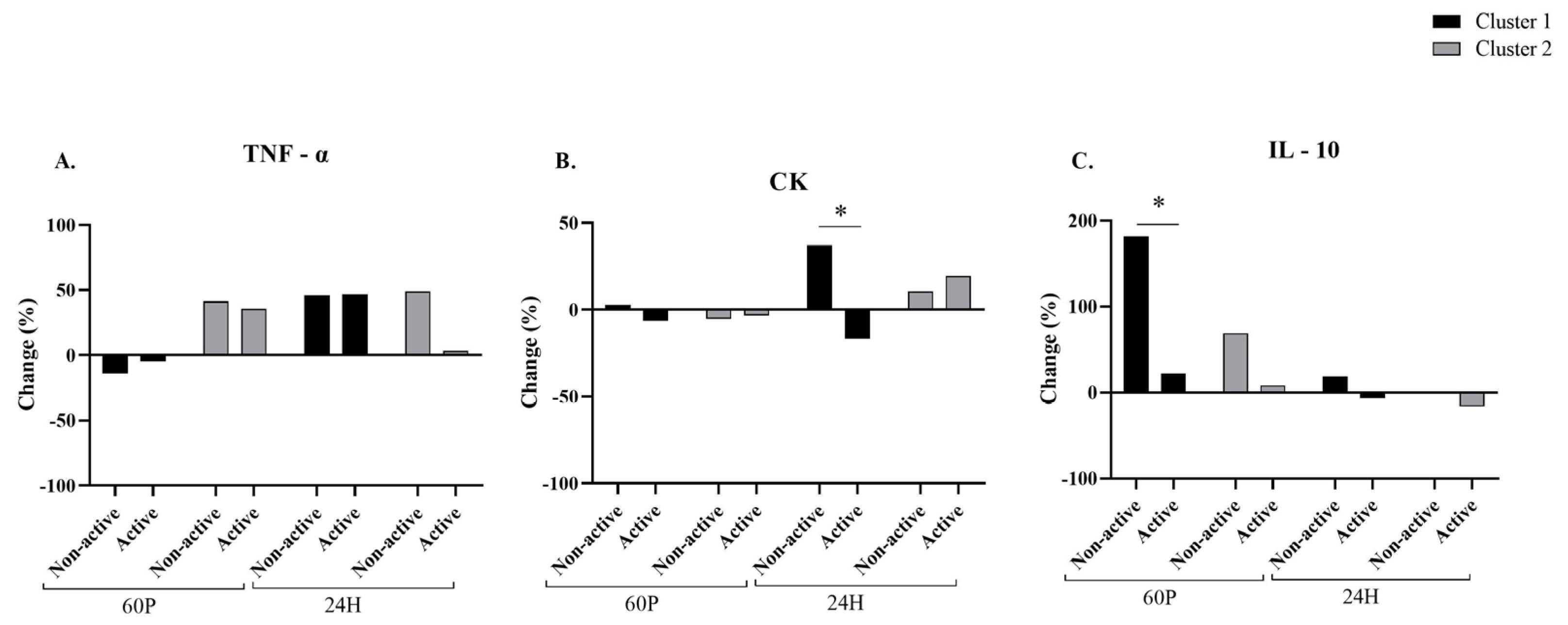
2.5. Blood Measurements Related to the Cluster Analysis
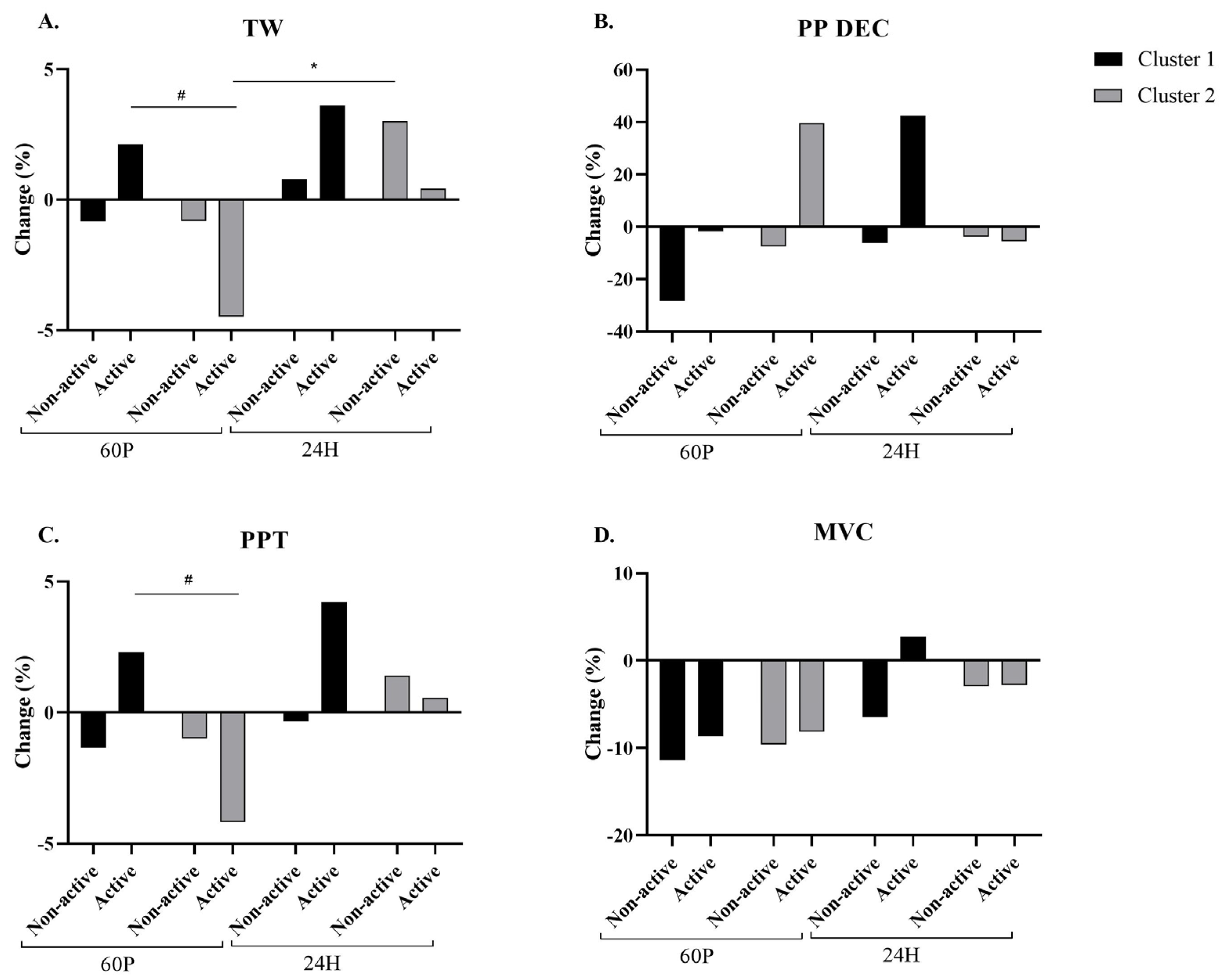
2.6. Performance Assessment
2.7. Subjective Measurements
3. Discussion
Limitations of the Study
4. Material and Methods
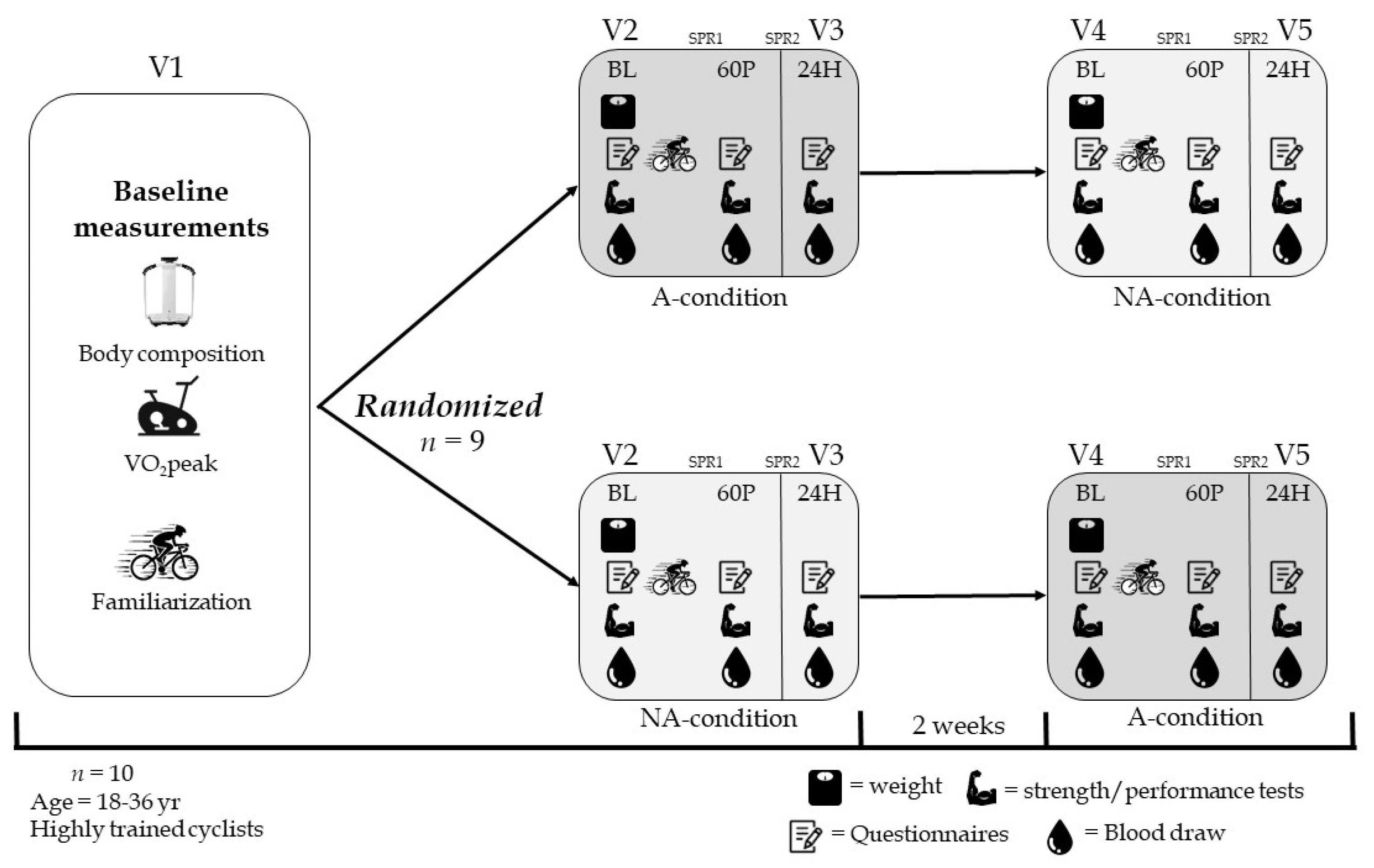
4.1. Study Population
4.2. Study Design
Questionnaires
4.3. Pulse Extremely Low Frequency—Electromagnetic Field (PELF-EMF)
4.4. Study Rationale and Operating Hypotheses
- 2 Hz affects inflammation by downregulating TNF-α and IL-1β;
- 4 Hz reduces oxidative stress;
- 12 Hz improves local microcirculation;
- 15 Hz increases alkaline phosphatase activity (ALP) and chondrogenesis;
- 30 Hz affects inflammation by downregulating IL-10;
- 50 Hz impacts inflammation by reducing chemokine production;
- 75 Hz upregulates A2A and A3 adenosine receptors and induces anti-inflammatory and antioxidant effects.
- (a)
- Concentrations of TNF-α and IL-1β, which are highly produced in the first hours after a workout, will be reduced using a frequency of 2 Hz.
- (b)
- Local oxidative stress, strictly linked with inflammation, will be reduced using a frequency of 4 Hz.
- (c)
- ALP activity will be stimulated using a frequency of 15 Hz.
- (a)
- Local inflammation will be modulated with frequencies of 75 and 50 Hz.
- (b)
- Local microcirculation will be stimulated using a frequency of 12 Hz.
- (c)
- Levels of cytokines TNF-α and IL-1β (using 2 Hz) and IL-10 (using 30 Hz) will be reduced.
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrero, A.; Schnell, F.; Carrault, G.; Kervio, G.; Matelot, D.; Carré, F.; Le Douairon Lahaye, S. Daily fatigue-recovery balance monitoring with heart rate variability in well-trained female cyclists on the Tour de France circuit. PLoS ONE 2019, 14, e0213472. [Google Scholar] [CrossRef]
- Markus, I.; Constantini, K.; Hoffman, J.R.; Bartolomei, S.; Gepner, Y. Exercise-induced muscle damage: Mechanism, assessment and nutritional factors to accelerate recovery. Eur. J. Appl. Physiol. 2021, 121, 969–992. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.Z.; Gregson, W.; Williams, K.; Robertson, C.; George, K. Muscle Contractile Function and Neural Control after Repetitive Endurance Cycling. Med. Sci. Sports Exerc. 2010, 42, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Cichoń, N.; Bijak, M.; Miller, E.; Saluk, J. Extremely Low Frequency Electromagnetic Field (ELF-EMF) Reduces Oxidative Stress and Improves Functional and Psychological Status in Ischemic Stroke Patients. Bioelectromagnetics 2017, 38, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Mahaki, H.; Tanzadehpanah, H.; Jabarivasal, N.; Sardanian, K.; Zamani, A. A Review on the Effects of Extremely Low Frequency Electromagnetic Field (ELF-EMF) on Cytokines of Innate and Adaptive Immunity. Electromagn. Biol. Med. 2019, 38, 84–95. [Google Scholar] [CrossRef]
- Ongaro, A.; Varani, K.; Masieri, F.F.; Pellati, A.; Massari, L.; Cadossi, R.; Vincenzi, F.; Borea, P.A.; Fini, M.; Caruso, A.; et al. Electromagnetic Fields (EMFs) and Adenosine Receptors Modulate Prostaglandin E2 and Cytokine Release in Human Osteoarthritic Synovial Fibroblasts. J. Cell. Physiol. 2012, 227, 2461–2469. [Google Scholar] [CrossRef]
- Kumar, S.; Dey, S.; Jain, S. Extremely Low-Frequency Electromagnetic Fields: A Possible Non-Invasive Therapeutic Tool for Spinal Cord Injury Rehabilitation. Electromagn. Biol. Med. 2017, 36, 88–101. [Google Scholar] [CrossRef]
- World Health Organization. Environmental Health Criteria 232. Static Fields; World Health Organization: Geneva, Switzerland, 2006.
- Patruno, A.; Ferrone, A.; Costantini, E.; Franceschelli, S.; Pesce, M.; Speranza, L.; Amerio, P.; D’Angelo, C.; Felaco, M.; Grilli, A.; et al. Extremely Low-Frequency Electromagnetic Fields Accelerates Wound Healing Modulating MMP-9 and Inflammatory Cytokines. Cell Prolif. 2018, 51, e12432. [Google Scholar] [CrossRef]
- Zou, J.; Chen, Y.; Qian, J.; Yang, H. Effect of a Low-Frequency Pulsed Electromagnetic Field on Expression and Secretion of IL-1β and TNF-α in Nucleus Pulposus Cells. J. Int. Med. Res. 2017, 45, 462–470. [Google Scholar] [CrossRef]
- Chiabrera, A.; Bianco, B.; Moggia, E.; Kaufman, J.J. Zeeman–Stark Modeling of the RF EMF Interaction with Ligand Binding. Bioelectromagnetics 2000, 21, 312–324. [Google Scholar] [CrossRef]
- Chou, C.K.; Bassen, H.; Osepchuk, J.; Balzano, Q.; Petersen, R.; Meltz, M.; Cleveland, R.; Lin, J.C.; Heynick, L. Radio Frequency Electromagnetic Exposure: Tutorial Review on Experimental Dosimetry. Bioelectromagnetics 1996, 17, 195–208. [Google Scholar] [CrossRef]
- D’Angelo, C.; Costantini, E.; Kamal, M.A.; Reale, M. Experimental Model for ELF-EMF Exposure: Concern for Human Health. Saudi J. Biol. Sci. 2015, 22, 75–84. [Google Scholar] [CrossRef]
- Patruno, A.; Pesce, M.; Grilli, A.; Speranza, L.; Franceschelli, S.; Lutiis, M.A.D.; Vianale, G.; Costantini, E.; Amerio, P.; Muraro, R.; et al. MTOR Activation by PI3K/Akt and ERK Signaling in Short ELF-EMF Exposed Human Keratinocytes. PLoS ONE 2015, 10, e0139644. [Google Scholar] [CrossRef] [PubMed]
- Trosko, J.E. Human Health Consequences of Environmentally-Modulated Gene Expression: Potential Roles of ELF–EMF Induced Epigenetic versus Mutagenic Mechanisms of Disease. Bioelectromagnetics 2000, 21, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Cichoń, N.; Rzeźnicka, P.; Bijak, M.; Miller, E.; Miller, S.; Saluk, J. Extremely Low Frequency Electromagnetic Field Reduces Oxidative Stress during the Rehabilitation of Post-Acute Stroke Patients. Adv. Clin. Exp. Med. Off. Organ Wroclaw Med. Univ. 2018, 27, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- De Mattei, M.; Pasello, M.; Pellati, A.; Stabellini, G.; Massari, L.; Gemmati, D.; Caruso, A. Effects of Electromagnetic Fields on Proteoglycan Metabolism of Bovine Articular Cartilage Explants. Connect. Tissue Res. 2003, 44, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Vincze, G.; Szasz, A.; Liboff, A.R. New Theoretical Treatment of Ion Resonance Phenomena. Bioelectromagnetics 2008, 29, 380–386. [Google Scholar] [CrossRef]
- Park, W.-H.; Soh, K.-S.; Lee, B.-C.; Pyo, M.-Y. 4 Hz Magnetic Field Decreases Oxidative Stress in Mouse Brain: A Chemiluminescence Study. Electromagn. Biol. Med. 2008, 27, 165–172. [Google Scholar] [CrossRef]
- Kwan, R.L.-C.; Wong, W.-C.; Yip, S.-L.; Chan, K.-L.; Zheng, Y.-P.; Cheing, G.L.-Y. Pulsed Electromagnetic Field Therapy Promotes Healing and Microcirculation of Chronic Diabetic Foot Ulcers: A Pilot Study. Adv. Skin Wound Care 2015, 28, 212–219. [Google Scholar] [CrossRef]
- Sun, L.-Y.; Hsieh, D.-K.; Lin, P.-C.; Chiu, H.-T.; Chiou, T.-W. Pulsed Electromagnetic Fields Accelerate Proliferation and Osteogenic Gene Expression in Human Bone Marrow Mesenchymal Stem Cells during Osteogenic Differentiation. Bioelectromagnetics 2010, 31, 209–219. [Google Scholar] [CrossRef]
- Ross, C.L.; Harrison, B.S. Effect of Time-Varied Magnetic Field on Inflammatory Response in Macrophage Cell Line RAW 264.7. Electromagn. Biol. Med. 2013, 32, 59–69. [Google Scholar] [CrossRef]
- Vianale, G.; Reale, M.; Amerio, P.; Stefanachi, M.; Di Luzio, S.; Muraro, R. Extremely Low Frequency Electromagnetic Field Enhances Human Keratinocyte Cell Growth and Decreases Proinflammatory Chemokine Production. Br. J. Dermatol. 2008, 158, 1189–1196. [Google Scholar] [CrossRef]
- Varani, K.; Vincenzi, F.; Ravani, A.; Pasquini, S.; Merighi, S.; Gessi, S.; Setti, S.; Cadossi, M.; Borea, P.A.; Cadossi, R. Adenosine Receptors as a Biological Pathway for the Anti-Inflammatory and Beneficial Effects of Low Frequency Low Energy Pulsed Electromagnetic Fields. Mediators Inflamm. 2017, 2017, 2740963. [Google Scholar] [CrossRef] [PubMed]
- Liboff, A.R. Geomagnetic Cyclotron Resonance in Living Cells. J. Biol. Phys. 1985, 13, 99–102. [Google Scholar] [CrossRef]
- Liboff, A.R. Electric-Field Ion Cyclotron Resonance. Bioelectromagnetics 1997, 18, 85–87. [Google Scholar] [CrossRef]
- Liboff, A.R. A Role for the Geomagnetic Field in Cell Regulation. Electromagn. Biol. Med. 2010, 29, 105–112. [Google Scholar] [CrossRef]
- Liboff, A.R. ION Cyclotron Resonance: Geomagnetic Strategy for Living Systems? Electromagn. Biol. Med. 2019, 38, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Zhadin, M.N.; Novikov, V.V.; Barnes, F.S.; Pergola, N.F. Combined Action of Static and Alternating Magnetic Fields on Ionic Current in Aqueous Glutamic Acid Solution. Bioelectromagnetics 1998, 19, 41–45. [Google Scholar] [CrossRef]
- Santalla, A.; Earnest, C.P.; Marroyo, J.A.; Lucia, A. The Tour de France: An updated physiological review. Int. J. Sports Physiol. Perform. 2012, 7, 200–209. [Google Scholar] [CrossRef]
- Moore, B.A.; Bemben, D.A.; Lein, D.H.; Bemben, M.G.; Singh, H. Fat Mass Is Negatively Associated with Muscle Strength and Jump Test Performance. J. Frailty Aging 2020, 9, 214–218. [Google Scholar] [CrossRef]
- Tanaka, H.; Seals, D.R. Endurance exercise performance in Masters athletes: Age-associated changes and underlying physiological mechanisms. J. Physiol. 2008, 586, 55–63. [Google Scholar] [CrossRef]
- Venugobal, S.; Tai, Y.K.; Goh, J.; Teh, S.; Wong, C.; Goh, I.; Maier, A.B.; Kennedy, B.K.; Franco-Obregón, A. Brief, weekly magnetic muscle therapy improves mobility and lean body mass in older adults: A Southeast Asia community case study. Aging 2023, 15, 1768–1790. [Google Scholar] [CrossRef] [PubMed]
- Howley, E.T.; Bassett, D.R.J.; Welch, H.G. Criteria for Maximal Oxygen Uptake: Review and Commentary. Med. Sci. Sports Exerc. 1995, 27, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Gaskill, S.; Ruby, B.; Walker, A.; Sanchez, O.; Serfass, R.; Leon, A. Validity and Reliability of Combining Three Methods to Determine Ventilatory Threshold. Med. Sci. Sports Exerc. 2001, 33, 1841–1848. [Google Scholar] [CrossRef]
- McGawley, K.; Bishop, D. Reliability of a 5 × 6-s Maximal Cycling Repeated-Sprint Test in Trained Female Team-Sport Athletes. Eur. J. Appl. Physiol. 2006, 98, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Micklewright, D.; St Clair Gibson, A.; Gladwell, V.; Al Salman, A. Development and Validity of the Rating-of-Fatigue Scale. Sports Med. Auckl. NZ 2017, 47, 2375–2393. [Google Scholar] [CrossRef]
- Zhadin, M.N. Combined Action of Static and Alternating Magnetic Fields on Ion Motion in a Macromolecule: Theoretical Aspects. Bioelectromagnetics 1998, 19, 279–292. [Google Scholar] [CrossRef]
| Characteristics | Findings (n = 9) |
|---|---|
| Age (y) | 26.7 ± 6.0 |
| Height (m) | 1.77 ± 0.06 |
| Weight (kg) | 69.6 ± 7.7 |
| Fat mass (%) | 13.4 ± 3.7 |
| Fat-free mass (kg) | 60.2 ± 5.7 |
| HRmax (bpm) | 184 ± 11 |
| VO2peak (mL/min) | 3965 ± 400 |
| VO2peak (mL/kg/min) | 57.3 ± 6.8 |
| Maximal power (W) | 400 ± 35 |
| Power at VT1 (W) | 216 ± 39 |
| Power at VT2 (W) | 281 ± 40 |
| Active (n = 9) | Non-Active (n = 9) | p Value | |||||
|---|---|---|---|---|---|---|---|
| BL | 60 P | 24 H | BL | 60 P | 24 H | ||
| MVC (Newton) | 467.5 ± 71.9 | 425.1 ± 58.3 | 464.2 ± 53.6 | 478.9 ± 67.9 | 425.1 ± 36.9 | 456.6 ± 77.4 | 0.951 |
| TW (Joule) | 22,034 ± 3103 | 21,706 ± 2631 | 22,427 ± 2845 | 21,705 ± 3404 | 21,508 ± 3236 | 22,164 ± 3711 | 0.998 |
| PPT (Watts) | 3904 ± 621 | 3857 ± 561 | 3989 ± 578 | 3944 ± 783 | 3889 ± 699 | 3955 ± 689 | 0.984 |
| PP DEC (%) | 3.69 ± 3.33 | 3.45 ± 2.91 | 3.22 ± 2.38 | 4.59 ± 2.04 | 3.58 ± 1.69 | 3.90 ± 2.46 | 0.898 |
| ROF (0–11 scale) | 2 ± 1 | 2 ± 1 | 2 ± 2 | 2 ± 1 | 2 ± 1 | 3 ± 2 | 0.925 |
| VAS (0–15 scale) | 4 ± 3 | 6 ± 4 | 4 ± 3 | 2 ± 2 | 4 ± 3 | 4 ± 2 | 0.838 |
| Step | Waveform | Frequency (Hz) | Intensity (%) | T-on (s) | T-off (s) | Duration (min) |
|---|---|---|---|---|---|---|
| (a) | ||||||
| 1 | 14 | 2 | 100 | 4 | 1 | 5 |
| 2 | 25 | 30 | 100 | 3 | 2 | 7 |
| 3 | 18 | 4 | 100 | 3 | 1 | 5 |
| 4 | 7 | 50 | 100 | 3 | 1 | 3 |
| 5 | 21 | 2 | 100 | 2 | 1 | 7 |
| 6 | 25 | 15 | 100 | 3 | 1 | 3 |
| 7 | 26 | 75 | 100 | 3 | 2 | 7 |
| 8 | 26 | 50 | 100 | 5 | 2 | 5 |
| 9 | 29 | 20 | 100 | 5 | 1 | 3 |
| (b) | ||||||
| 1 | 20 | 75 | 100 | 3 | 2 | 40 |
| 2 | 14 | 50 | 100 | 5 | 2 | 40 |
| 3 | 5 | 12 | 100 | 2 | 1 | 40 |
| 4 | 25 | 20 | 100 | 5 | 1 | 40 |
| 5 | 26 | 2 | 100 | 4 | 1 | 40 |
| 6 | 27 | 4 | 100 | 3 | 1 | 40 |
| 7 | 29 | 30 | 100 | 3 | 2 | 40 |
| 8 | 20 | 50 | 100 | 5 | 2 | 40 |
| 9 | 19 | 2 | 100 | 4 | 1 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markus, I.; Ohayon, E.; Constantini, K.; Geva-Kleinberger, K.; Ibrahim, R.; Ruban, A.; Gepner, Y. The Effect of Extremely Low-Frequency Electromagnetic Fields on Inflammation and Performance-Related Indices in Trained Athletes: A Double-Blinded Crossover Study. Int. J. Mol. Sci. 2023, 24, 13463. https://doi.org/10.3390/ijms241713463
Markus I, Ohayon E, Constantini K, Geva-Kleinberger K, Ibrahim R, Ruban A, Gepner Y. The Effect of Extremely Low-Frequency Electromagnetic Fields on Inflammation and Performance-Related Indices in Trained Athletes: A Double-Blinded Crossover Study. International Journal of Molecular Sciences. 2023; 24(17):13463. https://doi.org/10.3390/ijms241713463
Chicago/Turabian StyleMarkus, Irit, Evyatar Ohayon, Keren Constantini, Keren Geva-Kleinberger, Rawan Ibrahim, Angela Ruban, and Yftach Gepner. 2023. "The Effect of Extremely Low-Frequency Electromagnetic Fields on Inflammation and Performance-Related Indices in Trained Athletes: A Double-Blinded Crossover Study" International Journal of Molecular Sciences 24, no. 17: 13463. https://doi.org/10.3390/ijms241713463






