Chlorin E6-Curcumin-Mediated Photodynamic Therapy Promotes an Anti-Photoaging Effect in UVB-Irradiated Fibroblasts
Abstract
:1. Introduction
2. Results
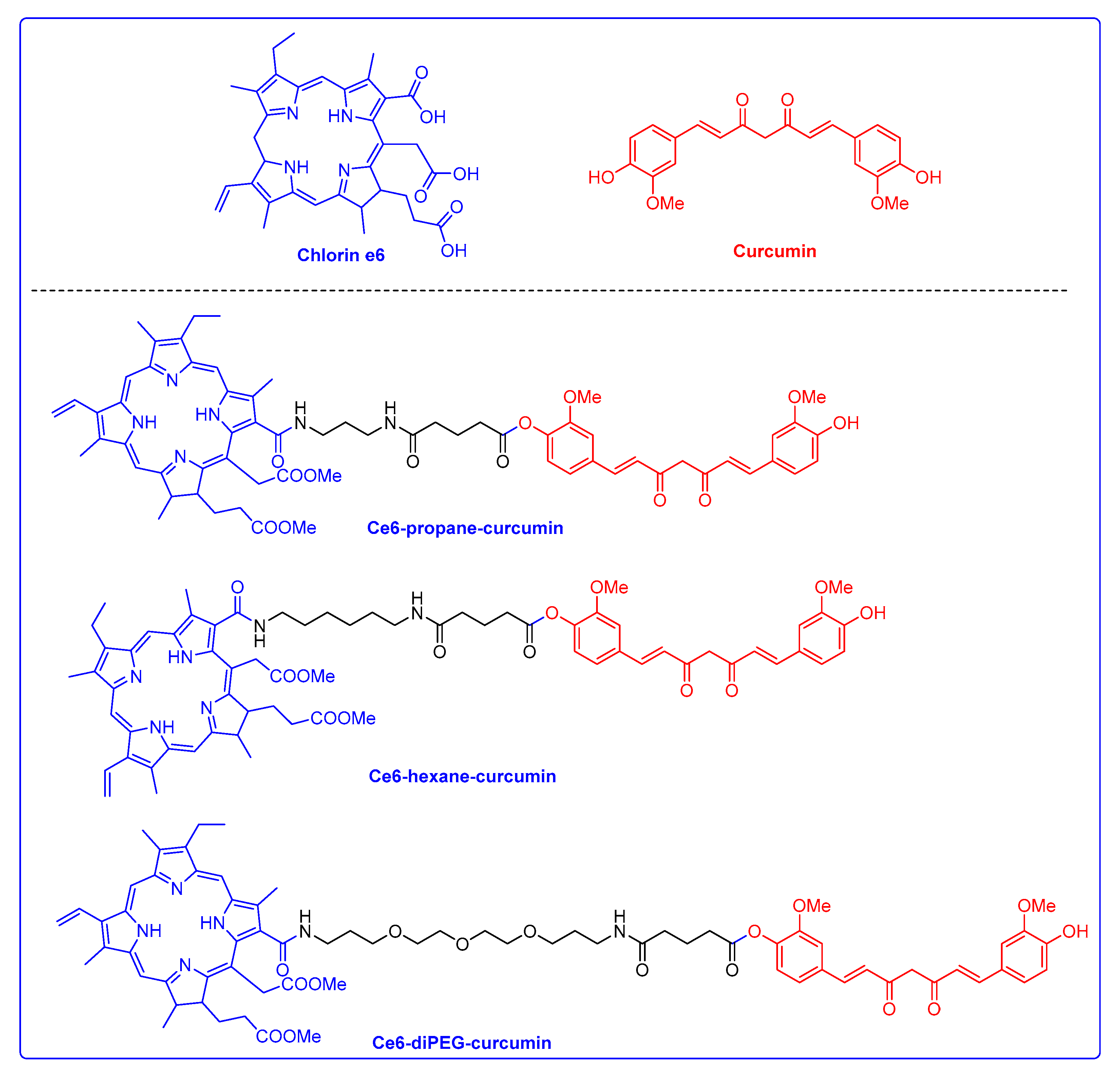
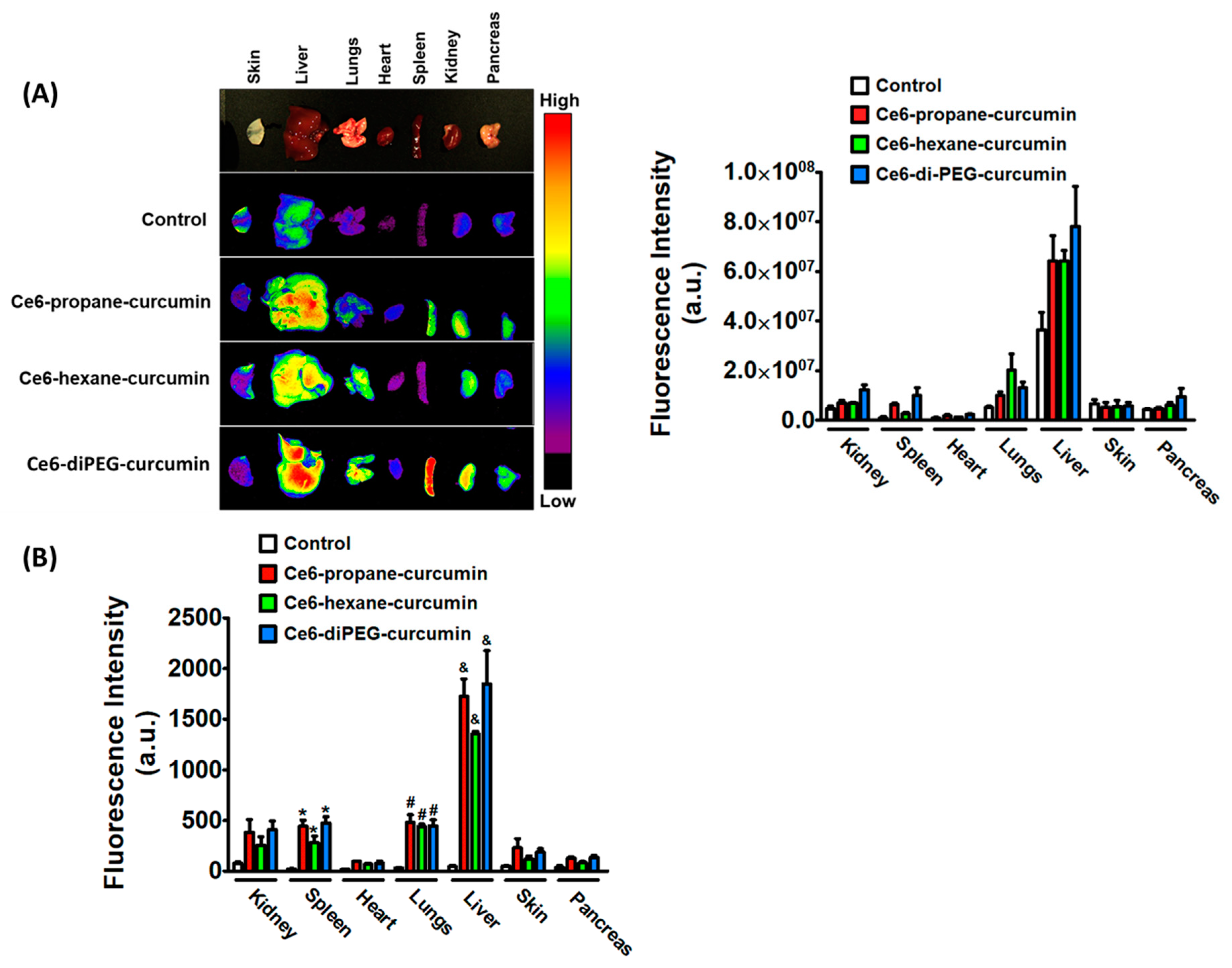
2.1. Photophysical Properties and Ex Vivo Distribution of Ce6-Curcumin Derivatives
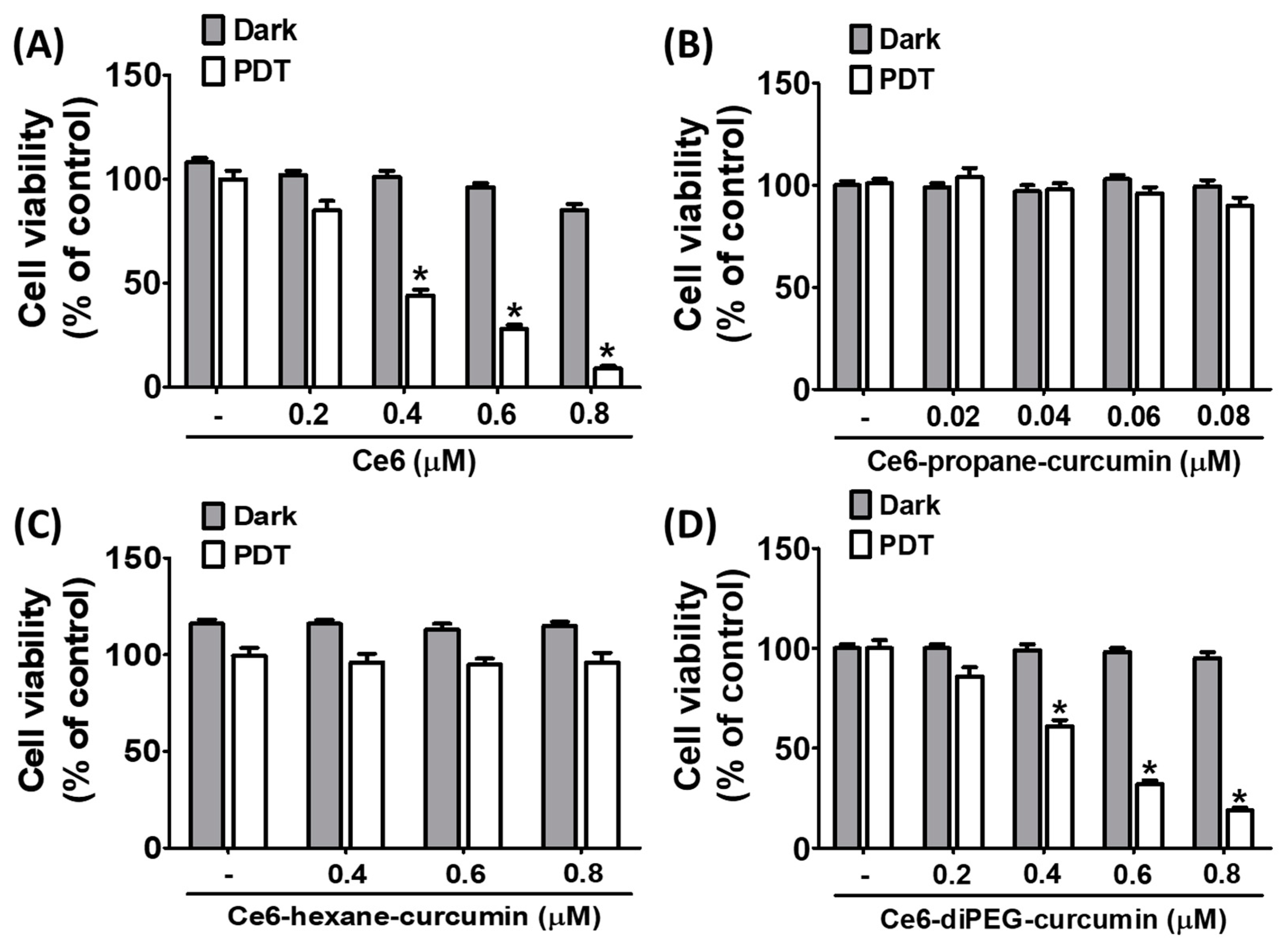
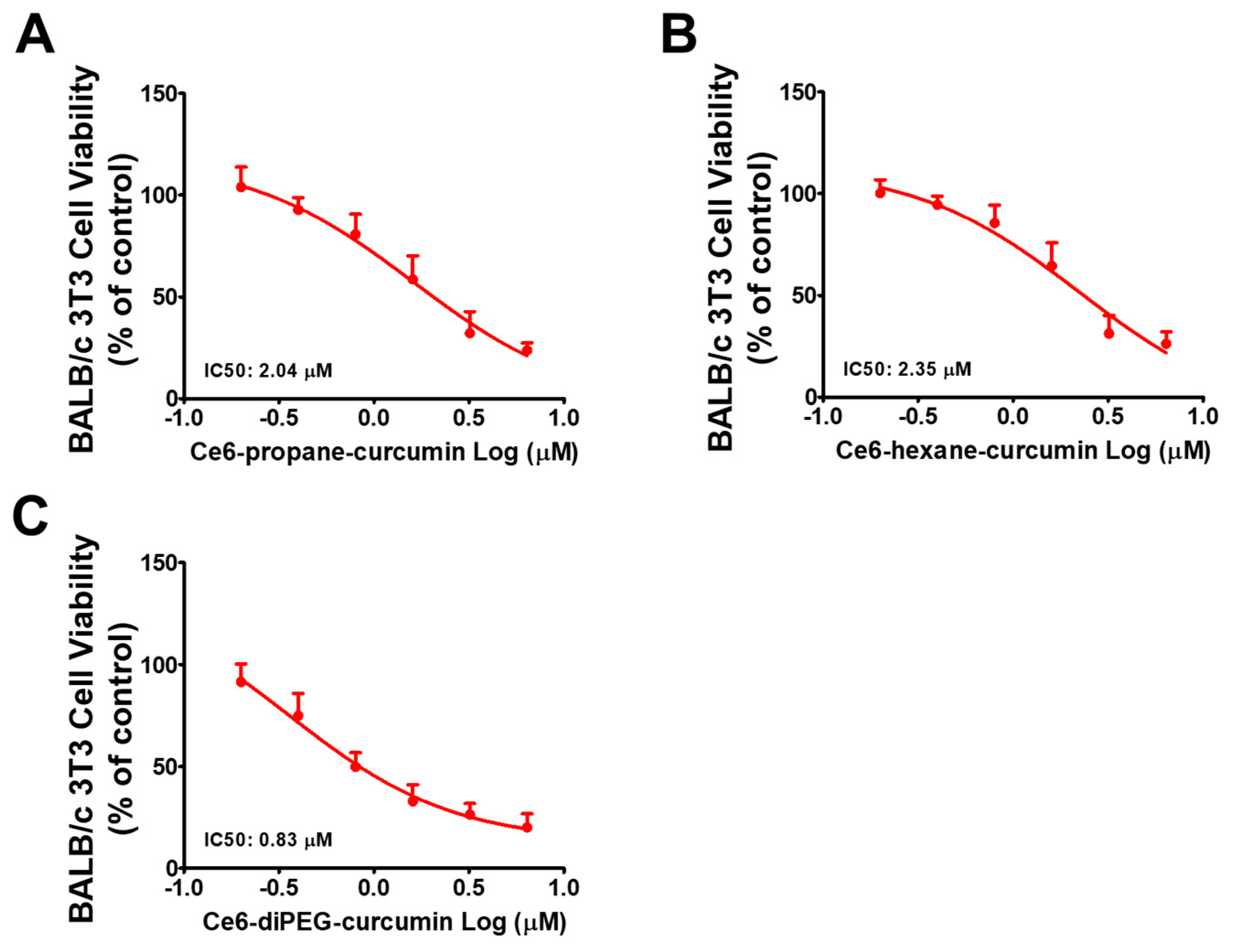
2.2. Effect of Ce6-Curcumin Derivatives in the Viability of Hs68 and BALB/c 3T3 Cells
2.3. Effect of UVB on BALB/c 3T3 Cell Viability
2.4. Antioxidant Potential of Ce6-Curcumin Derivativses
2.4.1. ABTS Radical Scavenging Activity
2.4.2. ORAC (Oxygen Radical Absorbance Capacity) Assay
2.5. Gelatin Zymography
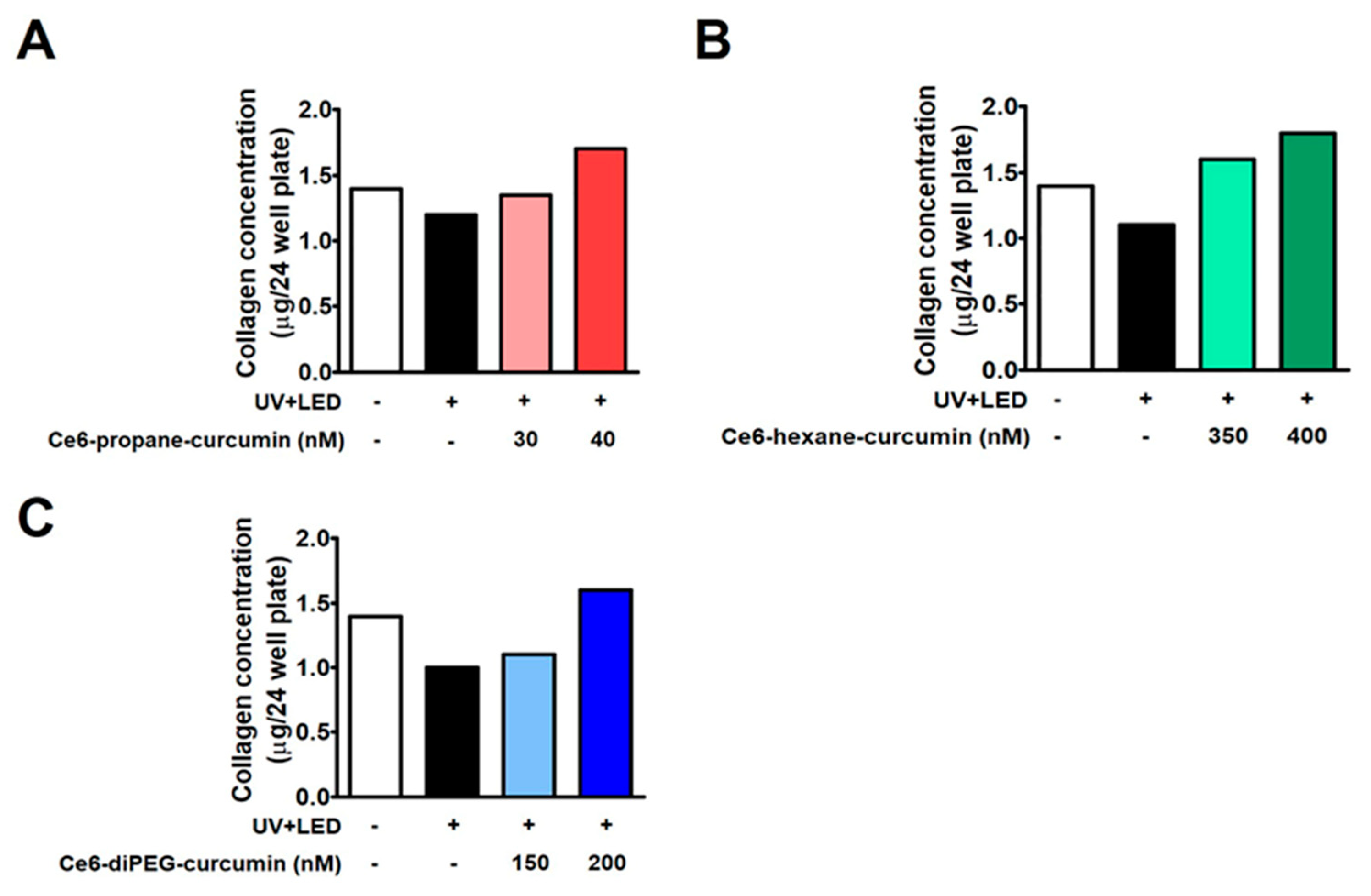
2.6. Collagen Assay
2.7. Effects of Ce6-Curcumin-Derivatives-PDT on MAPK, NF-κB and AP-1 Signaling in 3T3 Cells
2.8. Effects of Ce6-Curcumin-Derivatives-PDT on the Expressions of NF-κB-Dependent Genes
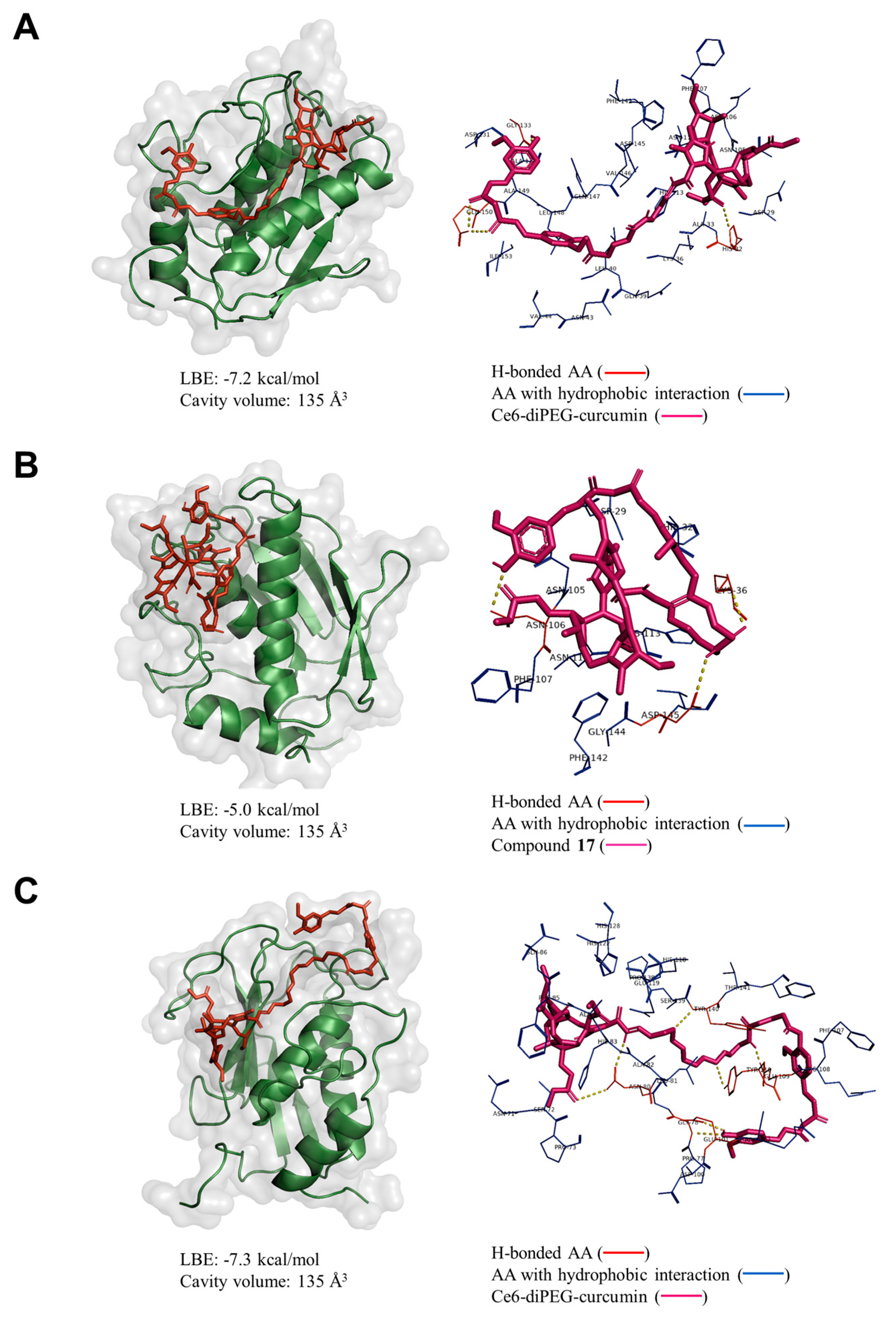
2.9. Molecular Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Synthetic Chemistry
4.2. Photophysical Properties and Comparative Biodistribution Study of Ce6-Curcumin Derivatives
4.3. Cell Culture
4.4. Cell Viability Assay (MTT Assay)
4.5. Cell Viability and UVB
4.6. Antioxidant Activity
4.6.1. ABTS Radical Scavenging Activity
4.6.2. ORAC (Oxygen Radical Absorbance Capacity) Assay
4.7. Gelatin Zymography
4.8. Collagen Assay
4.9. Western Blot
4.10. Animal Care
4.11. Ex Vivo Imaging of Ce6 and Ce6-Curcumin Conjugates in Mice
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, I.A.; Mikail, M.A.; Zamakshshari, N.; Abdullah, A.-S.H. Natural Anti-Aging Skincare: Role and Potential. Biogerontology 2020, 21, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.E.P.S.; Pedroso, D.M.M. UV Light and Skin Aging. Rev. Environ. Health 2014, 29, 243–254. [Google Scholar] [CrossRef]
- McDaniel, D.; Farris, P.; Valacchi, G. Atmospheric Skin Aging—Contributors and Inhibitors. J. Cosmet. Dermatol. 2018, 17, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kim, H.S.; Oh, J.Y.; Je, J.G.; Jeon, Y.-J.; Ryu, B. Protective Effect of Diphlorethohydroxycarmalol Isolated from Ishige Okamurae against UVB-Induced Damage in Vitro in Human Dermal Fibroblasts and in Vivo in Zebrafish. Food Chem. Toxicol. 2020, 136, 110963. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, Prevention and Therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.-Y.; Kim, Y.-S.; Lee, H.-G.; Lee, J.-S.; Jeon, Y.-J. Anti-Photoaging and Anti-Melanogenesis Effects of Fucoidan Isolated from Hizikia Fusiforme and Its Underlying Mechanisms. Mar. Drugs 2020, 18, 427. [Google Scholar] [CrossRef]
- Lorz, L.R.; Yoo, B.C.; Kim, M.-Y.; Cho, J.Y. Anti-Wrinkling and Anti-Melanogenic Effect of Pradosia Mutisii Methanol Extract. Int. J. Mol. Sci. 2019, 20, 1043. [Google Scholar] [CrossRef]
- Tan, C.Y.R.; Tan, C.L.; Chin, T.; Morenc, M.; Ho, C.Y.; Rovito, H.A.; Quek, L.S.; Soon, A.L.; Lim, J.S.Y.; Dreesen, O.; et al. Nicotinamide Prevents UVB- and Oxidative Stress—Induced Photoaging in Human Primary Keratinocytes. J. Invest. Dermatol. 2022, 142, 1670–1681.e12. [Google Scholar] [CrossRef]
- Kim, D.J.; Iwasaki, A.; Chien, A.L.; Kang, S. UVB-Mediated DNA Damage Induces Matrix Metalloproteinases to Promote Photoaging in an AhR- and SP1-Dependent Manner. JCI Insight 2022, 7, e156344. [Google Scholar] [CrossRef]
- Subedi, S.; Pyun, J.-Y. Practical Fingerprinting Localization for Indoor Positioning System by Using Beacons. J. Sens. 2017, 2017, e9742170. [Google Scholar] [CrossRef]
- Mu, J.; Ma, H.; Chen, H.; Zhang, X.; Ye, M. Luteolin Prevents UVB-Induced Skin Photoaging Damage by Modulating SIRT3/ROS/MAPK Signaling: An in Vitro and in Vivo Studies. Front. Pharmacol. 2021, 12, 728261. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Zhang, Z.; Zheng, R.; Huang, J.; Guo, L. N-acetyl Cysteine Inhibits Lipopolysaccharide-induced Apoptosis of Human Umbilical Vein Endothelial Cells via the P38MAPK Signaling Pathway. Mol. Med. Rep. 2019, 20, 2945–2953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, Z.; Wang, P.; Zhang, G.; Wang, B.; Shi, L.; Liu, X.; Zhou, Z.; Wang, X. Long-Term Improvement on Photoaging after ALA Photodynamic Therapy for Actinic Keratosis: A Retrospective Study. Photodiagnosis Photodyn. Ther. 2021, 33, 102181. [Google Scholar] [CrossRef]
- Oleinick, N.L.; Evans, H.H. The Photobiology of Photodynamic Therapy: Cellular Targets and Mechanisms. Radiat. Res. 1998, 150, S146–S156. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.A.; Correia, J.H. Enhanced Photodynamic Therapy: A Review of Combined Energy Sources. Cells 2022, 11, 3995. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Little, E.; Quan, H.; Voorhees, J.J.; Fisher, G.J. Elevated Matrix Metalloproteinases and Collagen Fragmentation in Photodamaged Human Skin: Impact of Altered Extracellular Matrix Microenvironment on Dermal Fibroblast Function. J. Invest. Dermatol. 2013, 133, 1362–1366. [Google Scholar] [CrossRef]
- Baez, F.; Reilly, L.R. The Use of Light-Emitting Diode Therapy in the Treatment of Photoaged Skin. J. Cosmet. Dermatol. 2007, 6, 189–194. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA. Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Imberti, C.; Zhang, P.; Huang, H.; Sadler, P.J. New Designs for Phototherapeutic Transition Metal Complexes. Angew. Chem. Int. Ed. 2020, 59, 61–73. [Google Scholar] [CrossRef]
- Chilakamarthi, U.; Giribabu, L. Photodynamic Therapy: Past, Present and Future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Ryu, A.-R.; Jin, S.; Jeon, Y.-M.; Lee, M.-Y. Chlorin E6-Mediated Photodynamic Therapy Suppresses P. Acnes-Induced Inflammatory Response via NFκB and MAPKs Signaling Pathway. PLoS ONE 2017, 12, e0170599. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Ryu, A.-R.; Han, C.-S.; Lee, M.-Y. A Comparison of the Anti-Bacterial and Anti-Inflammatory Effect between Two Forms of Chlorins. Toxicol. Environ. Health Sci. 2016, 8, 271–276. [Google Scholar] [CrossRef]
- Wu, M.-F.; Deichelbohrer, M.; Tschernig, T.; Laschke, M.W.; Szentmáry, N.; Hüttenberger, D.; Foth, H.-J.; Seitz, B.; Bischoff, M. Chlorin E6 Mediated Photodynamic Inactivation for Multidrug Resistant Pseudomonas Aeruginosa Keratitis in Mice in Vivo. Sci. Rep. 2017, 7, 44537. [Google Scholar] [CrossRef]
- Ryu, A.-R.; Kim, Y.-W.; Lee, M.-Y. Chlorin E6-Mediated Photodynamic Therapy Modulates Adipocyte Differentiation and Lipogenesis in 3T3-L1 Cells. Photod. Photodyn. Ther. 2020, 31, 101917. [Google Scholar] [CrossRef]
- Bielak-Zmijewska, A.; Grabowska, W.; Ciolko, A.; Bojko, A.; Mosieniak, G.; Bijoch, Ł.; Sikora, E. The Role of Curcumin in the Modulation of Ageing. Int. J. Mol. Sci. 2019, 20, 1239. [Google Scholar] [CrossRef] [PubMed]
- Benameur, T.; Soleti, R.; Panaro, M.A.; La Torre, M.E.; Monda, V.; Messina, G.; Porro, C. Curcumin as Prospective Anti-Aging Natural Compound: Focus on Brain. Molecules 2021, 26, 4794. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Shi, H.; Li, X.; Li, Y.; Taha, A.; Xu, C. Protective Effect of Curcumin against Ultraviolet A Irradiation-induced Photoaging in Human Dermal Fibroblasts. Mol. Med. Rep. 2018, 17, 7227–7237. [Google Scholar] [CrossRef]
- Zhang, L.; Gan, J.Q.; Wang, H. Neurocognitive Mechanisms of Mathematical Giftedness: A Literature Review. Appl. Neuropsychol. Child 2017, 6, 79–94. [Google Scholar] [CrossRef]
- Jalde, S.S.; Chauhan, A.K.; Lee, J.H.; Chaturvedi, P.K.; Park, J.-S.; Kim, Y.-W. Synthesis of Novel Chlorin E6-Curcumin Derivatives as Photosensitizers for Photodynamic Therapy against Pancreatic Carcinoma. Eur. J. Med. Chem. 2018, 147, 66–76. [Google Scholar] [CrossRef]
- Thapa Magar, T.B.; Lee, J.; Lee, J.H.; Jeon, J.; Gurung, P.; Lim, J.; Kim, Y.-W. Novel Chlorin E6-Curcumin Derivatives as a Potential Photosensitizer: Synthesis, Characterization, and Anticancer Activity. Pharmaceutics 2023, 15, 1577. [Google Scholar] [CrossRef]
- Charachit, N.; Sukhamwang, A.; Dejkriengkraikul, P.; Yodkeeree, S. Hyperoside and Quercitrin in Houttuynia Cordata Extract Attenuate UVB-Induced Human Keratinocyte Cell Damage and Oxidative Stress via Modulation of MAPKs and Akt Signaling Pathway. Antioxidants 2022, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.-X.; Cao, Y. CB-Dock2: Improved Protein–Ligand Blind Docking by Integrating Cavity Detection, Docking and Homologous Template Fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef]
- Lee, L.-Y.; Liu, S.-X. Pathogenesis of Photoaging in Human Dermal Fibroblasts. Int. J. Dermatol. Venereol. 2020, 3, 37–42. [Google Scholar] [CrossRef]
- Fernandez-Flores, A.; Saeb-Lima, M. Histopathology of Cutaneous Aging. Am. J. Dermatopathol. 2019, 41, 469. [Google Scholar] [CrossRef]
- Lv, T.; Huang, Z.-F.; Wang, H.-W.; Lin, J.-Q.; Chen, G.-N.; Chen, X.-W.; Chen, R.; Huang, Z.; Wang, X.-L. Evaluation of Collagen Alteration after Topical Photodynamic Therapy (PDT) Using Second Harmonic Generation (SHG) Microscopy—In Vivo Study in a Mouse Model. Photod. Photodyn. Ther. 2012, 9, 164–169. [Google Scholar] [CrossRef]
- Bagazgoitia, L.; Cuevas Santos, J.; Juarranz, Á.; Jaén, P. Photodynamic Therapy Reduces the Histological Features of Actinic Damage and the Expression of Early Oncogenic Markers. Br. J. Dermatol. 2011, 165, 144–151. [Google Scholar] [CrossRef]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in Photosensitizers and Light Delivery for Photodynamic Therapy. Clin. Endosc. 2013, 46, 7–23. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Hwang, B.-M.; Noh, E.-M.; Kim, J.-S.; Kim, J.-M.; You, Y.-O.; Hwang, J.-K.; Kwon, K.-B.; Lee, Y.-R. Curcumin Inhibits UVB-Induced Matrix Metalloproteinase-1/3 Expression by Suppressing the MAPK-P38/JNK Pathways in Human Dermal Fibroblasts. Exp. Dermatol. 2013, 22, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Kaur, I.P. Inhibitory Effect of Encapsulated Curcumin on Ultraviolet-Induced Photoaging in Mice. Rejuvenation Res. 2010, 13, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Hur, G.-H.; Ryu, A.-R.; Kim, Y.-W.; Lee, M.-Y. The Potential Anti-Photoaging Effect of Photodynamic Therapy Using Chlorin E6-Curcumin Conjugate in UVB-Irradiated Fibroblasts and Hairless Mice. Pharmaceutics 2022, 14, 968. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Bhat, S.S.; Singh, N.; Venkanna, B.U.; Mohamed, R.; Rao, R.P. Cell-Based Model Systems for Validation of Various Efficacy-Based Claims for Cosmetic Ingredients. Cosmetics 2022, 9, 107. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Sakurai, S.; Kikuchi, A.; Gotoh, H. Hydrophilic Oxygen Radical Absorbance Capacity Values of Low-Molecular-Weight Phenolic Compounds Containing Carbon, Hydrogen, and Oxygen. RSC Adv. 2022, 12, 4094–4100. [Google Scholar] [CrossRef] [PubMed]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Rodríguez, S.; Folgueras, A.R.; López-Otín, C. The Role of Matrix Metalloproteinases in Aging: Tissue Remodeling and Beyond. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Goldsberry, A.; Hanke, C.W. Photodynamic Therapy for Photoaging. Curr. Dermatol. Rep. 2014, 3, 122–126. [Google Scholar] [CrossRef]
- Han, S.H.; Ballinger, E.; Choung, S.-Y.; Kwon, J.Y. Anti-Photoaging Effect of Hydrolysates from Pacific Whiting Skin via MAPK/AP-1, NF-ΚB, TGF-β/Smad, and Nrf-2/HO-1 Signaling Pathway in UVB-Induced Human Dermal Fibroblasts. Mar. Drugs 2022, 20, 308. [Google Scholar] [CrossRef]
- Fang, M.; Lee, H.-M.; Oh, S.; Zheng, S.; Bellere, A.D.; Kim, M.; Choi, J.; Kim, M.; Yu, D.; Yi, T.-H. Rosa Davurica Inhibits Skin Photoaging via Regulating MAPK/AP-1, NF-ΚB, and Nrf2/HO-1 Signaling in UVB-Irradiated HaCaTs. Photochem. Photobiol. Sci. 2022, 21, 2217–2230. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Huang, R.; Liu, Y.; Liu, Z.; Zhao, Y. Q-Switched 1,064-Nm Neodymium-Doped Yttrium Aluminum Garnet Laser Irradiation Induces Collagen Remodeling in SKH-1 Hairl Ess Mice by Activating ERK1/2 and P38MAPK Signaling Pathway. Food Sci. Technol. 2021, 42, e43721. [Google Scholar] [CrossRef]
- Roy, H.S.; Dubey, G.; Sharma, V.K.; Bharatam, P.V.; Ghosh, D. Molecular docking and molecular dynamics to identify collagenase inhibitors as lead compounds to address osteoarthritis. J. Biomol. Struct. Dyn. 2022, 40, 2339–2351. [Google Scholar] [CrossRef]
- Ahmad, A.; Sayed, A.; Ginnebaugh, K.R.; Sharma, V.; Suri, A.; Saraph, A.; Padhye, S.; Sarkar, F.H. Molecular docking and inhibition of matrix metalloproteinase-2 by novel difluorinatedbenzylidene curcumin analog. Am. J. Transl. Res. 2015, 7, 298–308. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4399093 (accessed on 23 August 2023). [PubMed]
- Nitulescu, G.; Mihai, D.P.; Zanfirescu, A.; Stan, M.S.; Gradinaru, D.; Nitulescu, G.M. Discovery of New Microbial Collagenase Inhibitors. Life 2022, 12, 2114. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, R.; Haenen, G.R.M.M.; van den Berg, H.; Bast, A. Applicability of an Improved Trolox Equivalent Antioxidant Capacity (TEAC) Assay for Evaluation of Antioxidant Capacity Measurements of Mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.; Sohail, A.; Fridman, R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Metastasis Res. Protoc. 2012, 878, 121–135. [Google Scholar] [CrossRef]





| Compounds | Dark Toxicity (IC50, nM) | Phototoxicity (IC50, nM) |
|---|---|---|
| Ce6 | ≥1000 | 615 |
| Ce6-propane-curcumin | ≥1000 | ≥1000 |
| Ce6-hexane-curcumin | ≥1000 | ≥1000 |
| Ce6-diPEG-curcumin | ≥1000 | 692.6 |
| Compounds | Dark Condition (EC50, μM) | LED Condition (EC50, μM) |
|---|---|---|
| Ce6 | 13.58 ± 4.14 | 19.81 ± 1.57 |
| Ce6-propane-curcumin | 18.90 ± 3.04 | 34.92 ± 8.16 |
| Ce6-hexane-curcumin | 22.36 ± 4.66 | 36.34 ± 4.02 |
| Ce6-diPEG-curcumin | 15.51 ± 0.01 | 22.33 ± 2.62 |
| 5-ALA | 60.28 ± 9.51 | - |
| Curcumin | 9.62 ± 0.005 | - |
| Compounds | Dark Condition (TE, μM) | LED Condition (TE, μM) | ||
|---|---|---|---|---|
| 1 | 10 | 1 | 10 | |
| Ce6 | 18.5 | 47.72 | 22.11 | 53.85 |
| Ce6-propane-curcumin | 20.11 | 26.83 | 2.78 | 30.66 |
| Ce6-hexane-curcumin | 15.97 | 20.19 | 2.26 | 24.17 |
| Ce6-diPEG-curcumin | 20.85 | 30.32 | 0.85 | 27.98 |
| 5-ALA | ND | ND | - | - |
| Curcumin | 38.56 | 56.54 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa Magar, T.B.; Mallik, S.K.; Gurung, P.; Lim, J.; Kim, Y.-T.; Shrestha, R.; Kim, Y.-W. Chlorin E6-Curcumin-Mediated Photodynamic Therapy Promotes an Anti-Photoaging Effect in UVB-Irradiated Fibroblasts. Int. J. Mol. Sci. 2023, 24, 13468. https://doi.org/10.3390/ijms241713468
Thapa Magar TB, Mallik SK, Gurung P, Lim J, Kim Y-T, Shrestha R, Kim Y-W. Chlorin E6-Curcumin-Mediated Photodynamic Therapy Promotes an Anti-Photoaging Effect in UVB-Irradiated Fibroblasts. International Journal of Molecular Sciences. 2023; 24(17):13468. https://doi.org/10.3390/ijms241713468
Chicago/Turabian StyleThapa Magar, Til Bahadur, Shyam Kumar Mallik, Pallavi Gurung, Junmo Lim, Young-Tak Kim, Rajeev Shrestha, and Yong-Wan Kim. 2023. "Chlorin E6-Curcumin-Mediated Photodynamic Therapy Promotes an Anti-Photoaging Effect in UVB-Irradiated Fibroblasts" International Journal of Molecular Sciences 24, no. 17: 13468. https://doi.org/10.3390/ijms241713468
APA StyleThapa Magar, T. B., Mallik, S. K., Gurung, P., Lim, J., Kim, Y.-T., Shrestha, R., & Kim, Y.-W. (2023). Chlorin E6-Curcumin-Mediated Photodynamic Therapy Promotes an Anti-Photoaging Effect in UVB-Irradiated Fibroblasts. International Journal of Molecular Sciences, 24(17), 13468. https://doi.org/10.3390/ijms241713468







