Fibres and Colorectal Cancer: Clinical and Molecular Evidence
Abstract
:1. Introduction: Epidemiology and Molecular Basis of Colorectal Cancer
2. Dietary Fiber
| Soluble Fibers | Insoluble Fibers |
|---|---|
| Found in vegetables and fruits | Found in cereals |
| Dissolves in water and gastrointestinal fluids when it enters the stomach and intestines. It is transformed into a gel-like substance, which is digested by bacteria in the large intestine, releasing gases and a few calories. | Does not dissolve in water or gastrointestinal fluids and remains more or less unchanged as it moves through the digestive tract. Because it is not digested at all, insoluble fiber is not a source of calories. |
| Rapid fermentation | Slow fermentation |
| Low fat absorption and cholesterol levels, reducing the risk of cardiovascular disease | Prevent constipation, speeding up gut movements |
| Stabilize blood sugar levels | Reduce the risk of cardiovascular disease |
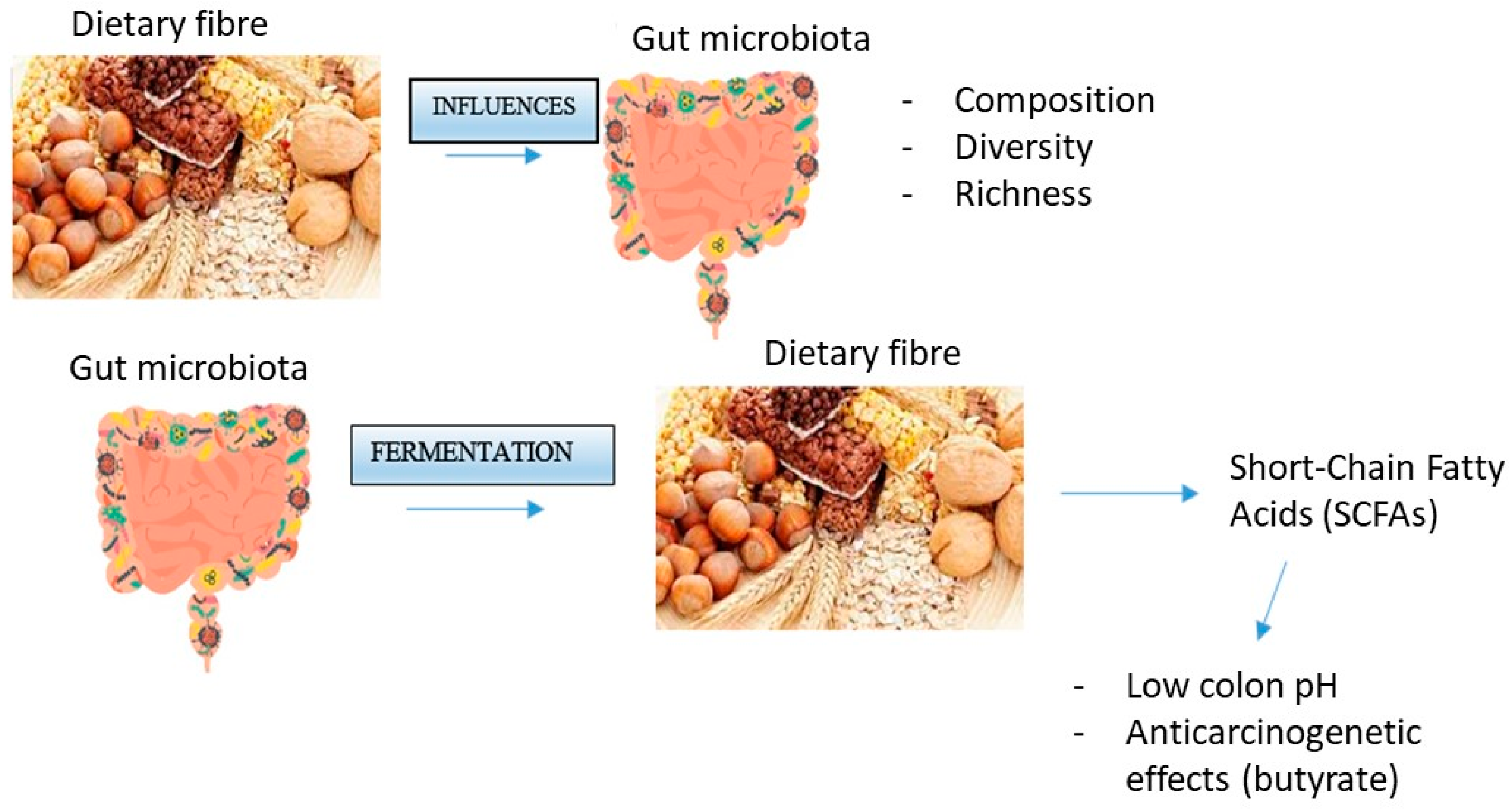
3. Interaction between Fiber and the Microbiota
4. Role of Butyrate in Colorectal Cancer Prevention
5. Interaction between Dietary Fiber and Bile Acids
6. Water-Holding Capacity of Fibers
7. Fiber in the Prevention of CRC
| Author, Year | Type of Trial | Results | Fibres |
|---|---|---|---|
| Alberts, 1996 [41] | Randomized, double-blinded trial | At 9 months, high-dose fiber supplementation caused 52% reduction (p = 0.001) in fecal concentrations of total bile acids | dietary wheat bran fiber (2.0 or 13.5 g/day) in the form of cereal and supplemental calcium carbonate (250 or 1500 mg/day elemental calcium) |
| Alberts, 2000 [42] | Randomized trial | OR (high-fiber group) = 0.88 (p = 0.28) OR (low-fiber group) = 0.99 (p = 0.93) | High amounts/low amounts of wheat bran fiber |
| Bonithon-Kopp, 2001 [43] | Randomized blinded, placebo-controlled trial | 45% increase in recurrent adenomas relative to the placebo group at 3 years (p = 0.04) | / |
| MacLennan, 1995 [44] | Randomized, partially double-blinded, placebo-controlled factorial trial | At 24 months, OR = 0.4 At 48 months, OR = 0.3 | 25 g of wheat bran daily and a capsule of beta carotene (20 mg daily) |
| Schatzkin, 2000 [45] | Randomized trial | In the control group, 39.7% and 39.5% had one recurrent adenoma (OR = 1) | High-fiber diet (18 g of dietary fiber per 1000 kcal) |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Màrmol, I.; Sànchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodruigez Yoldi, M.J. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W.; Chu, E. Biology of colorectal cancer. Cancer J. 2010, 16, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Cui, D.; Xiong, X.; Zhao, Y. The characteristics and roles of β-TrCP1/2 in carcinogenesis. FEBS J. 2020, 288, 3351–3374. [Google Scholar] [CrossRef] [PubMed]
- Young, G.P.; Hu, Y.; Le Leu, R.K.; Nyskohus, L. Dietary fibre and colorectal cancer: A model for environment—gene interactions. Mol. Nutr. Food Res. 2005, 49, 571–584. [Google Scholar] [CrossRef]
- Harris, P.J.; Ferguson, L.R. Dietary fibre: Its composition and role in protection against colorectal cancer. Mutat. Res./Fundam. Mol Mech. Mutagen. 1993, 290, 97–110. [Google Scholar] [CrossRef]
- Dai, F.; Chau, C. Classification and regulatory perspectives of dietary fiber. J. Food Drug Anal. 2017, 25, 37–42. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Maćkowiak, K.; Torlińska-Walkowiak, N.; Torlińska, B. Dietary fibre as an important constituent of the diet. Adv. Hyg. Exp. Med. 2016, 25, 104–109. [Google Scholar] [CrossRef]
- Oh, H.; Kim, H.; Hoon Lee, D.; Lee, A.; Giovannucci, E.L.; Kang, S.; Kerum, N. Different dietary fibre sources and risks of colorectal cancer and adenoma: A dose–response meta-analysis of prospective studies. Br. J. Nutr. 2019, 112, 6. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Preterre, A.L.; Iqbal, K.; Bechthold, A.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; et al. Food groups and risk of colorectal cancer. Int. J. Cancer. 2018, 142, 1748–1758. [Google Scholar] [CrossRef]
- Simpson, H.L.; Campbell, B.J. Review article: Dietary fibre-microbiota interactions. Aliment. Pharmacol. Ther. 2015, 42, 158–179. [Google Scholar] [CrossRef] [PubMed]
- Huizen, J. Soluble and insoluble fiber: What is the difference? Med. News Today 2017. Available online: https://www.medicalnewstoday.com/articles/319176 (accessed on 30 August 2023).
- Loo, Y.T.; Howell, K.; Suleria, H.; Zhang, P.; Liu, S.; Ng, K. Fibre fermentation and pig faecal microbiota composition are affected by the interaction between sugarcane fibre and (poly)phenols in vitro. Int. J. Food Sci. Nutr. 2023, 74, 219–233. [Google Scholar] [CrossRef]
- Manfredi, V.; Salvatori, T.; Villarini, M.; Moretti, M.; Nucci, D.; Realdon, S. Is dietary fibre truly protective against colon cancer? A systematic review and meta-analysis. Int. J. Food Sci. Nutr. 2018, 69, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liu, Z.; Li, K.; Bai, G.; Liu, L.; Zhong, R.; Chen, L.; Zhang, H. Time-course effects of different fiber-rich ingredients on energy values, microbiota composition and SCFA profile in growing pigs. Anim. Nutr. 2022, 12, 263–275. [Google Scholar] [CrossRef]
- Teigen, L.; Biruete, A.; Khoruts, A. Impact of diet on hydrogen sulfide production: Implications for gut health. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 55–58. [Google Scholar] [CrossRef]
- Kumar, R.; Maurya, A.K.; Parker, K.D.; Kant, R.; Ibrahim, H.; Kabir, M.I.; Kumar, D.; Weber, A.M.; Agarwal, R.; Kuhn, K.A.; et al. Gender-based effect of absence of gut microbiota on the protective efficacy of Bifidobacterium longum-fermented rice bran diet against inflammation-associated colon tumorigenesis. Mol. Carcinog. 2022, 61, 941–957. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Safratowich, B.D.; Wang, T.T.Y.; Hamlin, S.K.; Johnson, L.K. Butyrate Inhibits Deoxycholic-Acid-Resistant Colonic Cell Proliferation via Cell ycle Arrest and Apoptosis: A Potential Pathway Linking Dietary Fiber to Cancer Prevention. Mol. Nutr. Food Res. 2020, 64, e1901014. [Google Scholar] [CrossRef]
- Loke, Y.L.; Chew, M.T.; Ngeow, Y.F.; Lim, W.W.D.; Peh, S.C. Colon Carcinogenesis: The Interplay Between Diet and Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 603086. [Google Scholar] [CrossRef]
- Bultman, S.J. Molecular Pathways: Gene—environment interactions regulating dietary fiber induction of proliferation and apoptosis via butyrate for cancer prevention. Clin. Cancer Res. 2014, 20, 799–803. [Google Scholar] [CrossRef]
- Alvandi, E.; Wong, W.K.M.; Joglekar, M.V.; Spring, K.J.; Hardikar, A.A. Short-chain fatty acid concentrations in the incidence and risk-stratification of colorectal cancer: A systematic review and meta-analysis. BMC Med. 2022, 20, 323. [Google Scholar] [CrossRef] [PubMed]
- Shuwen, H.; Yangyangiu, W.; Jian, C.; Boyang, H.; Gong, C.; Jing, Z. Synergistic effect of sodium butyrate and oxaliplatin on colorectal cancer. Trans. Oncol. 2023, 27, 101598. [Google Scholar] [CrossRef]
- Kang, J.; Sun, M.; Chang, Y.; Chen, H.; Zhang, J.; Liang, X.; Xiao, T. Butyrate ameliorates colorectal cancer through regulating intestinal microecological disorders. Anticancer Drugs 2023, 34, 227–237. [Google Scholar] [CrossRef]
- Garavaglia, B.; Vallino, L.; Ferraresi, A.; Esposito, A.; Salwa, A.; Vidoni, C.; Gentili, S.; Isidoro, C. Butyrate Inhibits Colorectal Cancer Cell Proliferation through Autophagy Degradation of β-Catenin Regardless of APC and β-Catenin Mutational Status. Biomedicine 2022, 10, 1131. [Google Scholar] [CrossRef] [PubMed]
- Nagengast, F.M.; Grubben, M.J.A.L.; van Munster, I.P. Role of bile acids in colorectal carcinogenesis. Eur. J. Cancer. 1995, 31A, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.M.; Ablers, S.; Trautwein, C. Role of bile acids in the gut-liver axis. Hepatol. Snapshot 2018, 68, 1083–1085. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates The Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- World Cancer Research Fund; American Institute of Cancer Research. Continuous Update Project Report: Diet, Nutrition, Physical Activity and Colorectal Cancer. 2018. Available online: https://www.wcrf.org/wp-content/uploads/2021/02/Summary-of-Third-Expert-Report-2018.pdf (accessed on 30 August 2023).
- Burkitt, D.P. Epidemiology of cancer of the colon and rectum. Cancer 1971, 28, 3–13. [Google Scholar] [CrossRef]
- Norat, T.; Bingham, S.; Ferrari, P.; Slimani, N.; Jenab, M.; Mazuir, M.; Overvad, K.; Olsen, A.; Tjønneland, A.; Clavel, F.; et al. Meat, fish, and colorectal cancer risk: The European Prospective Investigation into cancer and nutrition. J. Natl. Cancer Inst. 2005, 97, 906–916. [Google Scholar] [CrossRef]
- Yu, Y.C.; Paragomi, P.; Jin, A.; Wang, R.; Schoen, R.E.; Koh, W.P.; Yuan, J.M.; Luu, H.N. Dietary Nonstarch Polysaccharide Intake and Risk of Colorectal Cancer: Findings from the Singapore Chinese Health Study. Cancer Res. Commun. 2022, 2, 1304–1311. [Google Scholar] [CrossRef]
- Byrd, D.A.; Gomez, M.; Hogue, S.; Murphy, G.; Sampson, J.N.; Vogtmann, E.; Albert, P.; Freedman, N.D.; Sinha, R.; Loftfield, E. Circulating Bile Acids and Adenoma Recurrence in the Context of Adherence to a High-Fiber, High-Fruit and Vegetable, and Low-Fat Dietary Intervention. Clin. Transl. Gastroenterol. 2022, 13, e00533. [Google Scholar] [CrossRef]
- Turati, F.; Concina, F.; Rossi, M.; Fiori, F.; Parpinel, M.; Taborelli, M.; Giacosa, A.; Crispo, A.; Pagan, E.; Rosato, V.; et al. Association of prebiotic fiber intake with colorectal cancer risk: The PrebiotiCa study. Eur. J. Nutr. 2023, 62, 455–464. [Google Scholar] [CrossRef]
- Mathers, J.C.; Elliott, F.; Macrae, F.; Mecklin, J.P.; Möslein, G.; McRonald, F.E.; Bertario, L.; Evans, D.G.; Gerdes, A.M.; Ho, J.W.C.; et al. Cancer Prevention with Resistant Starch in Lynch Syndrome Patients in the CAPP2-Randomized Placebo Controlled Trial: Planned 10-Year Follow-up. Cancer Prev. Res. 2022, 15, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Chan, A.T.; Sun, J. Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology 2020, 158, 322–340. [Google Scholar] [CrossRef]
- Aune, D.; Chan, D.S.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, M.; Zhou, L.; Ling, S.; Li, Y.; Kong, B.; Huang, P. Dietary fiber intake and risks of proximal and distal colon cancers: A meta-analysis. Medicine 2018, 97, 11678. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Suo, T.; Andersson, R.; Cao, Y.; Wang, C.; Lu, J.; Chui, E. Dietary fibre for the prevention of recurrent colorectal adenomas and carcinomas. Cochrane Database Syst. Rev. 2017, 1, CD003430. [Google Scholar] [CrossRef] [PubMed]
- Lanza, E.; Yu, B.; Murphy, G.; Albert, P.S.; Caan, B.; Marshall, J.R.; Lance, P.; Paskett, E.D.; Weissfeld, J.; Slattery, M.; et al. The Polyp Prevention Trial–Continued Follow-up Study: No Effect of a Low-Fat, High-Fiber, High-Fruit, and -Vegetable Diet on Adenoma Recurrence Eight Years after Randomization. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1745–1752. [Google Scholar] [CrossRef]
- Alberts, D.S.; Ritenbaugh, C.; Story, J.A.; Aickin, M.; Rees-McGee, S.; Buller, M.K.; Atwood, J.; Phelps, J.; Ramanujam, P.S.; Bellapravalu, S.; et al. Randomized, double-blinded, placebo-controlled study of effect of wheat bran fibre and calcium on fecal bile acids in patients with resected adenomatous colon polyps. J. Natl. Cancer Inst. 1996, 88, 81–92. [Google Scholar] [CrossRef]
- Alberts, D.S.; Martinez, M.E.; Roe, D.J.; Guillén-Rodríguez, J.M.; Marshall, J.R.; Van Leeuwen, J.B.; Reid, M.E.; Ritenbaugh, C.; Vargas, P.A.; Bhattacharyya, A.B.; et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians’ Network. New Engl. J. Med. 2000, 342, 1156–1162. [Google Scholar] [CrossRef]
- Bonithon-Kopp, C.; Kronborg, O.; Giacosa, A. Fibre supplementation increased the risk for recurrent adenomas, and calcium supplementation did not prevent recurrence. Evid. Based Med. 2001, 6, 90. [Google Scholar]
- MacLennan, R.; Macrae, F.; Bain, C.; Battistutta, D. Randomized trial of intake of fat, fiber, and beta carotene to prevent colorectal adenomas. J. Natl. Cancer Inst. 1995, 87, 1760–1766. [Google Scholar] [CrossRef]
- Schatzkin, A.; Lanza, E.; Corle, D.; Lance, P.; Iber, F.; Caan, B.; Shike, M.; Weissfeld, J.; Burt, R.; Cooper, M.R.; et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. New Engl. J. Med. 2000, 342, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, Y.J.; Abulimiti, A.; Huang, C.Y.; Liu, K.Y.; Chen, Y.M.; Zhang, C.X. Educational level and colorectal cancer risk: The mediating roles of lifestyle and dietary factors. Eur. J. Cancer Prev. 2022, 31, 137–144. [Google Scholar] [CrossRef]
- Arden, S.; Morelli, B.; Schoen, M.; Cashman, S.; Jahne, M.; Ma, X.; Garland, J. Human Health, Economic and Environmental Assessment of Onsite Non-Potable Water Reuse Systems for a Large, Mixed-Use Urban Building. Sustainability 2020, 12, 5459. [Google Scholar] [CrossRef] [PubMed]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives From Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef]
- Sorrentino, C.; Di Gisi, M.; Gentile, G.; Licitra, F.; D’Angiolo, R.; Giovannelli, P.; Migliaccio, A.; Castoria, G.; Di Donato, M. Agri-Food By-Products in Cancer: New Targets and Strategies. Cancers 2022, 14, 5517. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celiberto, F.; Aloisio, A.; Girardi, B.; Pricci, M.; Iannone, A.; Russo, F.; Riezzo, G.; D’Attoma, B.; Ierardi, E.; Losurdo, G.; et al. Fibres and Colorectal Cancer: Clinical and Molecular Evidence. Int. J. Mol. Sci. 2023, 24, 13501. https://doi.org/10.3390/ijms241713501
Celiberto F, Aloisio A, Girardi B, Pricci M, Iannone A, Russo F, Riezzo G, D’Attoma B, Ierardi E, Losurdo G, et al. Fibres and Colorectal Cancer: Clinical and Molecular Evidence. International Journal of Molecular Sciences. 2023; 24(17):13501. https://doi.org/10.3390/ijms241713501
Chicago/Turabian StyleCeliberto, Francesca, Adriana Aloisio, Bruna Girardi, Maria Pricci, Andrea Iannone, Francesco Russo, Giuseppe Riezzo, Benedetta D’Attoma, Enzo Ierardi, Giuseppe Losurdo, and et al. 2023. "Fibres and Colorectal Cancer: Clinical and Molecular Evidence" International Journal of Molecular Sciences 24, no. 17: 13501. https://doi.org/10.3390/ijms241713501










