Ryanodine Receptor Staining Identifies Viable Cardiomyocytes in Human and Rabbit Cardiac Tissue Slices
Abstract
:1. Introduction
2. Results
2.1. Detection of RyR- and Dextran-Positive Myocytes
2.2. Dextran and RyR Staining in Rabbit Tissue
2.2.1. Complementary Signal of Dextran and RyR Staining
2.2.2. Artificial Permeabilization of the Membrane with Saponin as a Positive Control
2.3. Staining in Human Tissue
2.4. Correlation with Functional Data
2.5. Using RyR and Dextran to Compare Living and Dead Cells
2.6. Use of RyR Immunofluorescence without Dextran to Detect Viable CMs
2.7. RyR–Dextran Co-Staining in Fresh Cardiac Slices
2.8. Accuracy of RyR Immunofluorescence as a Test for Viable Myocytes
2.9. Changes in RyR and SERCA Regularity in Dying Myocytes
3. Discussion
3.1. RyR–Dextran Assay as a Suitable Live–Dead Staining Method in Cultured Cardiac Tissue Slices
3.2. RyR–Dextran Assay in Non-Cultured Cardiac Tissue Slices
3.3. Use of This Method to Compare the Structures of Viable and Dead Myocytes
3.4. RyR as a Myocyte Marker
3.5. Interrelation of the Staining Result with Functional Parameters
3.6. Presence and Influence of Non-Myocytes in the Culture System and In Vivo
3.7. Degradation of the SR as a Suggested Mechanism of RyR Staining Loss
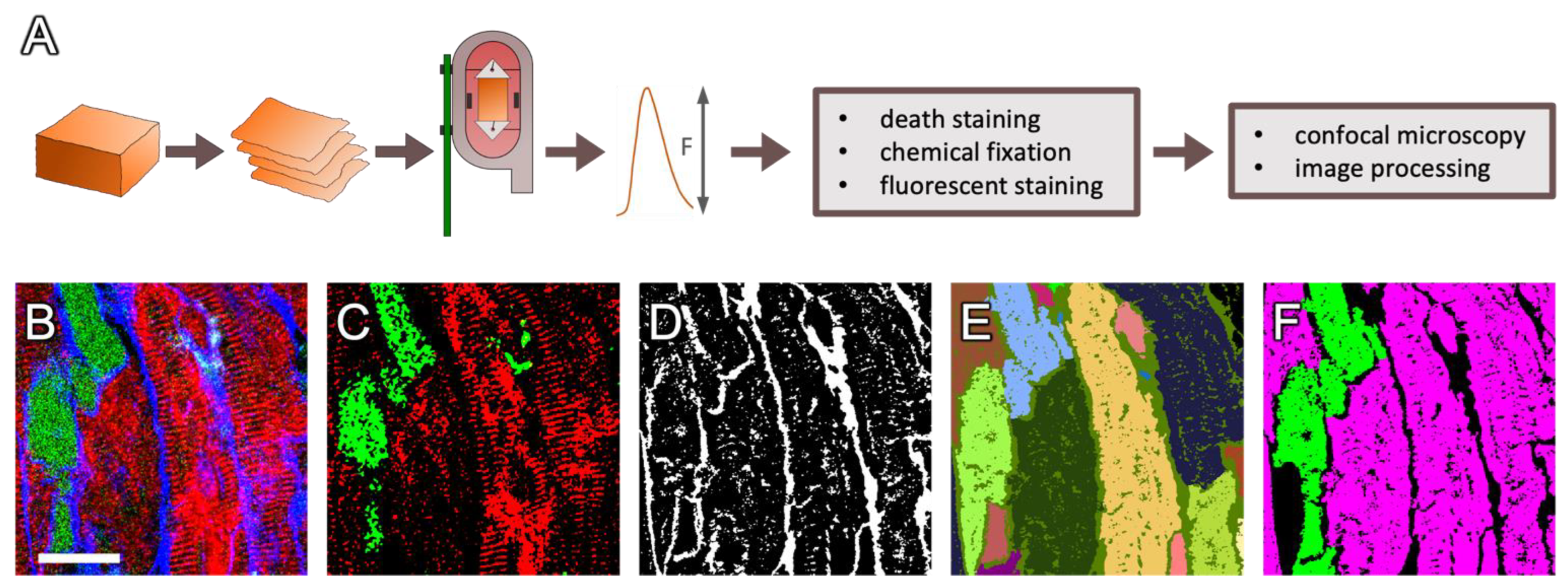
4. Materials and Methods
4.1. Cardiac Samples
4.2. Tissue Preparation and Cultivation
4.3. Dextran Assay and Immunostaining
4.4. Confocal Imaging
4.5. Image Processing
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fischer, C.; Milting, H.; Fein, E.; Reiser, E.; Lu, K.; Seidel, T.; Schinner, C.; Schwarzmayr, T.; Schramm, R.; Tomasi, R.; et al. Long-term functional and structural preservation of precision-cut human myocardium under continuous electromechanical stimulation in vitro. Nat. Commun. 2019, 10, 117. [Google Scholar] [CrossRef]
- Abu-Khousa, M.; Fiegle, D.J.; Sommer, S.T.; Minabari, G.; Milting, H.; Heim, C.; Weyand, M.; Tomasi, R.; Dendorfer, A.; Volk, T.; et al. The Degree of t-System Remodeling Predicts Negative Force-Frequency Relationship and Prolonged Relaxation Time in Failing Human Myocardium. Front. Physiol. 2020, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.A.; Duff, J.; Bardi, I.; Zabielska, M.; Atanur, S.S.; Jabbour, R.J.; Simon, A.; Tomas, A.; Smolenski, R.T.; Harding, S.E.; et al. Biomimetic electromechanical stimulation to maintain adult myocardial slices in vitro. Nat. Commun. 2019, 10, 2168. [Google Scholar] [CrossRef] [PubMed]
- Ou, Q.; Jacobson, Z.; Abouleisa, R.R.E.; Tang, X.-L.; Hindi, S.M.; Kumar, A.; Ivey, K.N.; Giridharan, G.; El-Baz, A.; Brittian, K.; et al. Physiological Biomimetic Culture System for Pig and Human Heart Slices. Circ. Res. 2019, 125, 628–642. [Google Scholar] [CrossRef] [PubMed]
- Lother, A.; Kohl, P. The heterocellular heart: Identities, interactions, and implications for cardiology. Basic Res. Cardiol. 2023, 118, 30. [Google Scholar] [CrossRef]
- Waleczek, F.J.G.; Sansonetti, M.; Xiao, K.; Jung, M.; Mitzka, S.; Dendorfer, A.; Weber, N.; Perbellini, F.; Thum, T. Chemical and mechanical activation of resident cardiac macrophages in the living myocardial slice ex vivo model. Basic Res. Cardiol. 2022, 117, 63. [Google Scholar] [CrossRef]
- Poch, C.M.; Foo, K.S.; de Angelis, M.T.; Jennbacken, K.; Santamaria, G.; Bähr, A.; Wang, Q.-D.; Reiter, F.; Hornaschewitz, N.; Zawada, D.; et al. Migratory and anti-fibrotic programmes define the regenerative potential of human cardiac progenitors. Nat. Cell Biol. 2022, 24, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Bojkova, D.; Wagner, J.U.G.; Shumliakivska, M.; Aslan, G.S.; Saleem, U.; Hansen, A.; Luxán, G.; Günther, S.; Pham, M.D.; Krishnan, J.; et al. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc. Res. 2020, 116, 2207–2215. [Google Scholar] [CrossRef] [PubMed]
- Pitoulis, F.; Perbellini, F.; Harding, S.E.; de Tombe, P.; Terracciano, C.M. P5373Mechanical heterogeneity across the left ventricular wall—A study using intact multicellular preparations. Eur. Heart J. 2019, 40, ehz746.0336. [Google Scholar] [CrossRef]
- Klumm, M.J.; Heim, C.; Fiegle, D.J.; Weyand, M.; Volk, T.; Seidel, T. Long-Term Cultivation of Human Atrial Myocardium. Front. Physiol. 2022, 13, 839139. [Google Scholar] [CrossRef]
- Esfandyari, D.; Idrissou, B.M.G.; Hennis, K.; Avramopoulos, P.; Dueck, A.; El-Battrawy, I.; Grüter, L.; Meier, M.A.; Näger, A.C.; Ramanujam, D.; et al. MicroRNA-365 regulates human cardiac action potential duration. Nat. Commun. 2022, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Perbellini, F.; Watson, S.A.; Scigliano, M.; Alayoubi, S.; Tkach, S.; Bardi, I.; Quaife, N.; Kane, C.; Dufton, N.P.; Simon, A.; et al. Investigation of cardiac fibroblasts using myocardial slices. Cardiovasc. Res. 2018, 114, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Toldra, R.; Kirwin, T.; Ferraro, E.; Pitoulis, F.G.; Nicastro, L.; Bardi, I.; Kit-Anan, W.; Gorelik, J.; Simon, A.R.; Terracciano, C.M. Mechanosensitive molecular mechanisms of myocardial fibrosis in living myocardial slices. ESC Heart Fail. 2022, 9, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.M.; Meki, M.H.; Ou, Q.; George, S.A.; Gams, A.; Abouleisa, R.R.E.; Tang, X.-L.; Ahern, B.M.; Giridharan, G.A.; El-Baz, A.; et al. Heart slice culture system reliably demonstrates clinical drug-related cardiotoxicity. Toxicol. Appl. Pharmacol. 2020, 406, 115213. [Google Scholar] [CrossRef] [PubMed]
- Piper, H.M.; Meuter, K.; Schäfer, C. Cellular mechanisms of ischemia-reperfusion injury. Ann. Thorac. Surg. 2003, 75, S644–S648. [Google Scholar] [CrossRef] [PubMed]
- Reimer, K.A.; Lowe, J.E.; Rasmussen, M.M.; Jennings, R.B. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs. duration of coronary occlusion in dogs. Circulation 1977, 56, 786–794. [Google Scholar] [CrossRef]
- Narula, J.; Haider, N.; Virmani, R.; DiSalvo, T.G.; Kolodgie, F.D.; Hajjar, R.J.; Schmidt, U.; Semigran, M.J.; Dec, G.W.; Khaw, B.A. Apoptosis in myocytes in end-stage heart failure. N. Engl. J. Med. 1996, 335, 1182–1189. [Google Scholar] [CrossRef]
- Gottlieb, R.A.; Burleson, K.O.; Kloner, R.A.; Babior, B.M.; Engler, R.L. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J. Clin. Investig. 1994, 94, 1621–1628. [Google Scholar] [CrossRef]
- Freude, B.; Masters, T.N.; Robicsek, F.; Fokin, A.; Kostin, S.; Zimmermann, R.; Ullmann, C.; Lorenz-Meyer, S.; Schaper, J. Apoptosis is initiated by myocardial ischemia and executed during reperfusion. J. Mol. Cell. Cardiol. 2000, 32, 197–208. [Google Scholar] [CrossRef]
- Maslov, L.N.; Popov, S.V.; Naryzhnaya, N.V.; Mukhomedzyanov, A.V.; Kurbatov, B.K.; Derkachev, I.A.; Boshchenko, A.A.; Khaliulin, I.; Prasad, N.R.; Singh, N.; et al. The regulation of necroptosis and perspectives for the development of new drugs preventing ischemic/reperfusion of cardiac injury. Apoptosis 2022, 27, 697–719. [Google Scholar] [CrossRef]
- Majno, G.; Joris, I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 1995, 146, 3–15. [Google Scholar] [PubMed]
- Levin, S. Apoptosis, necrosis, or oncosis: What is your diagnosis? A report from the Cell Death Nomenclature Committee of the Society of Toxicologic Pathologists. Toxicol. Sci. 1998, 41, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Levin, S.; Bucci, T.J.; Cohen, S.M.; Fix, A.S.; Hardisty, J.F.; LeGrand, E.K.; Maronpot, R.R.; Trump, B.F. The nomenclature of cell death: Recommendations of an ad hoc Committee of the Society of Toxicologic Pathologists. Toxicol. Pathol. 1999, 27, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S.A.; Dixon, D.; Hailey, J.R.; Harada, T.; Herbert, R.A.; Maronpot, R.R.; Nolte, T.; Rehg, J.E.; Rittinghausen, S.; Rosol, T.J.; et al. Recommendations from the INHAND Apoptosis/Necrosis Working Group. Toxicol. Pathol. 2016, 44, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.A.; Scigliano, M.; Bardi, I.; Ascione, R.; Terracciano, C.M.; Perbellini, F. Preparation of viable adult ventricular myocardial slices from large and small mammals. Nat. Protoc. 2017, 12, 2623–2639. [Google Scholar] [CrossRef]
- Coleman, J.; Liu, R.; Wang, K.; Kumar, A. Detecting Apoptosis, Autophagy, and Necrosis. In Apoptosis Methods in Toxicology; Muganda, P.M., Ed.; Springer: New York, NY, USA, 2016; pp. 77–92. ISBN 978-1-4939-3586-4. [Google Scholar]
- Miller, D.L.; Li, P.; Dou, C.; Armstrong, W.F.; Gordon, D. Evans blue staining of cardiomyocytes induced by myocardial contrast echocardiography in rats: Evidence for necrosis instead of apoptosis. Ultrasound Med. Biol. 2007, 33, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Ibla, J.C.; Khoury, J. Methods to Assess Tissue Permeability. In Cell-Cell Interactions; Humana Press: Totowa, NJ, USA, 2006; pp. 111–117. [Google Scholar]
- Carter, G.T.; Kikuchi, N.; Horasek, S.J.; Walsh, S.A. The use of fluorescent dextrans as a marker of sarcolemmal injury. Histol. Histopathol. 1994, 9, 443–447. [Google Scholar]
- McCormick, M.; Liu, X.; Jomier, J.; Marion, C.; Ibanez, L. ITK: Enabling reproducible research and open science. Front. Neuroinform. 2014, 8, 13. [Google Scholar] [CrossRef]
- Seidel, T.; Navankasattusas, S.; Ahmad, A.; Diakos, N.A.; Xu, W.D.; Tristani-Firouzi, M.; Bonios, M.J.; Taleb, I.; Li, D.Y.; Selzman, C.H.; et al. Sheet-Like Remodeling of the Transverse Tubular System in Human Heart Failure Impairs Excitation-Contraction Coupling and Functional Recovery by Mechanical Unloading. Circulation 2017, 135, 1632–1645. [Google Scholar] [CrossRef]
- Seidel, T.; Sankarankutty, A.C.; Sachse, F.B. Remodeling of the transverse tubular system after myocardial infarction in rabbit correlates with local fibrosis: A potential role of biomechanics. Prog. Biophys. Mol. Biol. 2017, 130, 302–314. [Google Scholar] [CrossRef]
- Lu, K.; Seidel, T.; Cao-Ehlker, X.; Dorn, T.; Batcha, A.M.N.; Schneider, C.M.; Semmler, M.; Volk, T.; Moretti, A.; Dendorfer, A.; et al. Progressive stretch enhances growth and maturation of 3D stem-cell-derived myocardium. Theranostics 2021, 11, 6138–6153. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Kwon, O.-S.; Smuder, A.J.; Wiggs, M.P.; Sollanek, K.J.; Christou, D.D.; Yoo, J.-K.; Hwang, M.-H.; Szeto, H.H.; Kavazis, A.N.; et al. Increased mitochondrial emission of reactive oxygen species and calpain activation are required for doxorubicin-induced cardiac and skeletal muscle myopathy. J. Physiol. 2015, 593, 2017–2036. [Google Scholar] [CrossRef] [PubMed]
- Lukyanenko, V.; Gyorke, S. Ca2+ sparks and Ca2+ waves in saponin-permeabilized rat ventricular myocytes. J. Physiol. 1999, 521 Pt 3, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Ferrantini, C.; Crocini, C.; Coppini, R.; Vanzi, F.; Tesi, C.; Cerbai, E.; Poggesi, C.; Pavone, F.S.; Sacconi, L. The transverse-axial tubular system of cardiomyocytes. Cell. Mol. Life Sci. 2013, 70, 4695–4710. [Google Scholar] [CrossRef] [PubMed]
- Seidel, T.; Fiegle, D.J.; Baur, T.J.; Ritzer, A.; Nay, S.; Heim, C.; Weyand, M.; Milting, H.; Oakley, R.H.; Cidlowski, J.A.; et al. Glucocorticoids preserve the t-tubular system in ventricular cardiomyocytes by upregulation of autophagic flux. Basic Res. Cardiol. 2019, 114, 47. [Google Scholar] [CrossRef]
- Takemura, G.; Fujiwara, H. Morphological aspects of apoptosis in heart diseases. J. Cell. Mol. Med. 2006, 10, 56–75. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, J.G.; Lopus, M. Cell death mechanisms in eukaryotes. Cell Biol. Toxicol. 2020, 36, 145–164. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Bergmann, O.; Zdunek, S.; Alkass, K.; Druid, H.; Bernard, S.; Frisén, J. Identification of cardiomyocyte nuclei and assessment of ploidy for the analysis of cell turnover. Exp. Cell Res. 2011, 317, 188–194. [Google Scholar] [CrossRef]
- Ruiz-Meana, M.; Garcia-Dorado, D.; Hofstaetter, B.; Piper, H.M.; Soler-Soler, J. Propagation of cardiomyocyte hypercontracture by passage of Na+ through gap junctions. Circ. Res. 1999, 85, 280–287. [Google Scholar] [CrossRef]
- Ruiz-Meana, M.; Abellán, A.; Miró-Casas, E.; Agulló, E.; Garcia-Dorado, D. Role of sarcoplasmic reticulum in mitochondrial permeability transition and cardiomyocyte death during reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1281–H1289. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Adameová, A.; Barile, L.; Cabrera-Fuentes, H.A.; Lazou, A.; Pagliaro, P.; Stensløkken, K.-O.; Garcia-Dorado, D. Mitochondrial and mitochondrial-independent pathways of myocardial cell death during ischaemia and reperfusion injury. J. Cell. Mol. Med. 2020, 24, 3795–3806. [Google Scholar] [CrossRef] [PubMed]
- Soonpaa, M.H.; Rubart, M.; Field, L.J. Challenges measuring cardiomyocyte renewal. Biochim. Biophys. Acta 2013, 1833, 799–803. [Google Scholar] [CrossRef]
- Crossman, D.J.; Shen, X.; Jüllig, M.; Munro, M.; Hou, Y.; Middleditch, M.; Shrestha, D.; Li, A.; Lal, S.; Dos Remedios, C.G.; et al. Increased collagen within the transverse tubules in human heart failure. Cardiovasc. Res. 2017, 113, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Crossman, D.J.; Ruygrok, P.R.; Soeller, C.; Cannell, M.B. Correction: Changes in the Organization of Excitation-Contraction Coupling Structures in Failing Human Heart. PLoS ONE 2011, 6, e17901. [Google Scholar] [CrossRef]
- Swift, F.; Franzini-Armstrong, C.; Øyehaug, L.; Enger, U.H.; Andersson, K.B.; Christensen, G.; Sejersted, O.M.; Louch, W.E. Extreme sarcoplasmic reticulum volume loss and compensatory T-tubule remodeling after Serca2 knockout. Proc. Natl. Acad. Sci. USA 2012, 109, 3997–4001. [Google Scholar] [CrossRef]
- Shen, X.; van den Brink, J.; Bergan-Dahl, A.; Kolstad, T.R.; Norden, E.S.; Hou, Y.; Laasmaa, M.; Aguilar-Sanchez, Y.; Quick, A.P.; Espe, E.K.S.; et al. Prolonged β-adrenergic stimulation disperses ryanodine receptor clusters in cardiomyocytes and has implications for heart failure. eLife 2022, 11, e77725. [Google Scholar] [CrossRef]
- Shen, X.; van den Brink, J.; Hou, Y.; Colli, D.; Le, C.; Kolstad, T.R.; MacQuaide, N.; Carlson, C.R.; Kekenes-Huskey, P.M.; Edwards, A.G.; et al. 3D dSTORM imaging reveals novel detail of ryanodine receptor localization in rat cardiac myocytes. J. Physiol. 2019, 597, 399–418. [Google Scholar] [CrossRef]
- Setterberg, I.E.; Le, C.; Frisk, M.; Li, J.; Louch, W.E. The Physiology and Pathophysiology of T-Tubules in the Heart. Front. Physiol. 2021, 12, 718404. [Google Scholar] [CrossRef]
- Kolstad, T.R.; van den Brink, J.; MacQuaide, N.; Lunde, P.K.; Frisk, M.; Aronsen, J.M.; Norden, E.S.; Cataliotti, A.; Sjaastad, I.; Sejersted, O.M.; et al. Ryanodine receptor dispersion disrupts Ca2+ release in failing cardiac myocytes. eLife 2018, 7, e39427. [Google Scholar] [CrossRef]
- Louch, W.E.; Mørk, H.K.; Sexton, J.; Strømme, T.A.; Laake, P.; Sjaastad, I.; Sejersted, O.M. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. J. Physiol. 2006, 574, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Gross, P.; Johnson, J.; Romero, C.M.; Eaton, D.M.; Poulet, C.; Sanchez-Alonso, J.; Lucarelli, C.; Ross, J.; Gibb, A.A.; Garbincius, J.F.; et al. Interaction of the Joining Region in Junctophilin-2 With the L-Type Ca2+ Channel Is Pivotal for Cardiac Dyad Assembly and Intracellular Ca2+ Dynamics. Circ. Res. 2021, 128, 92–114. [Google Scholar] [CrossRef] [PubMed]
- Rapundalo, S.T.; Briggs, F.N.; Feher, J.J. Effects of ischemia on the isolation and function of canine cardiac sarcoplasmic reticulum. J. Mol. Cell. Cardiol. 1986, 18, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Øyehaug, L.; Loose, K.Ø.; Jølle, G.F.; Røe, Å.T.; Sjaastad, I.; Christensen, G.; Sejersted, O.M.; Louch, W.E. Synchrony of cardiomyocyte Ca2+ release is controlled by T-tubule organization, SR Ca2+ content, and ryanodine receptor Ca2+ sensitivity. Biophys. J. 2013, 104, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, A.H. Cell Death. In Cytology and Cell Physiology, Supplement 17, 4th ed.; Jeon, K.W., Friedlander, M., Bourne, G.H., Eds.; Elsevier Science: Burlington, NJ, USA, 1987; pp. 755–785. ISBN 978-0-08-091882-2. [Google Scholar]
- Chien, K.R.; Peau, R.G.; Farber, J.L. Ischemic myocardial cell injury. Prevention by chlorpromazine of an accelerated phospholipid degradation and associated membrane dysfunction. Am. J. Pathol. 1979, 97, 505–529. [Google Scholar]
- Farber, J.L.; Chien, K.R.; Mittnacht, S. Myocardial ischemia: The pathogenesis of irreversible cell injury in ischemia. Am. J. Pathol. 1981, 102, 271–281. [Google Scholar]
- Chien, K.R.; Abrams, J.; Serroni, A.; Martin, J.T.; Farber, J.L. Accelerated phospholipid degradation and associated membrane dysfunction in irreversible, ischemic liver cell injury. J. Biol. Chem. 1978, 253, 4809–4817. [Google Scholar] [CrossRef]
- Croce, A.C.; Bottiroli, G. Autofluorescence spectroscopy and imaging: A tool for biomedical research and diagnosis. Eur. J. Histochem. 2014, 58, 2461. [Google Scholar] [CrossRef]
- Seidel, T.; Draebing, T.; Seemann, G.; Sachse, F.B. A Semi-automatic Approach for Segmentation of Three-Dimensional Microscopic Image Stacks of Cardiac Tissue. In Proceedings of the International Conference on Functional Imaging and Modeling of the Heart, London, UK, 20–22 June 2013; Springer: Berlin/Heidelberg, Germany, 2013; pp. 300–307. [Google Scholar]
- Seidel, T.; Edelmann, J.-C.; Sachse, F.B. Analyzing Remodeling of Cardiac Tissue: A Comprehensive Approach Based on Confocal Microscopy and 3D Reconstructions. Ann. Biomed. Eng. 2016, 44, 1436–1448. [Google Scholar] [CrossRef]









| Fraction of | Median | Mean | 95% Confidence Interval |
|---|---|---|---|
| Double-positive cells 1 | 3.4% | 6.1% | 3.5–8.6% |
| Double-positive pixels 2 | 1.2% | 2.5% | 1.3–3.7% |
| RyR in −dextran cells | 9.4% | 8.5% | 6.7–10.3% |
| RyR in +dextran cells | 1.4% | 3.6% | 2.1–5.1% |
| Dextran in −RyR cells | 30.1% | 34.0% | 28.6–39.3% |
| Dextran in +RyR cells | 4.1% | 8.5% | 5.2–12.0% |
| Fraction of | Median | Mean | 95% Confidence Interval |
|---|---|---|---|
| Double-positive cells 1 | 0.39% | 1.96% | 0.09–3.84% |
| Double-positive pixels 2 | 0.53% | 0.58% | 0.36–0.80% |
| RyR signal in −dextran cells | 1.4% | 2.8% | 6.7–10.3% |
| RyR signal in +dextran cells | 0.15% | 0.43% | 0.16–0.71% |
| Dextran signal in −RyR cells | 23.2% | 24.7% | 17.3–32.1% |
| Dextran signal in +RyR cells | 4.5% | 6.3% | 3.0–9.6% |
| Sensitivity | Specificity | NPV | PPV | |
|---|---|---|---|---|
| rabbit, non-cultured | 77.6% | 18.9% | 52.9% | 58.2% |
| rabbit, cultured | 69.1% | 80.3% | 75.2% | 75.0% |
| human, non-cultured | 69.5% | 66.8% | 30.6% | 91.2% |
| human, cultured | 45.0% | 83.6% | 48.1% | 81.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfeuffer, A.-K.M.; Küpfer, L.K.; Shankar, T.S.; Drakos, S.G.; Volk, T.; Seidel, T. Ryanodine Receptor Staining Identifies Viable Cardiomyocytes in Human and Rabbit Cardiac Tissue Slices. Int. J. Mol. Sci. 2023, 24, 13514. https://doi.org/10.3390/ijms241713514
Pfeuffer A-KM, Küpfer LK, Shankar TS, Drakos SG, Volk T, Seidel T. Ryanodine Receptor Staining Identifies Viable Cardiomyocytes in Human and Rabbit Cardiac Tissue Slices. International Journal of Molecular Sciences. 2023; 24(17):13514. https://doi.org/10.3390/ijms241713514
Chicago/Turabian StylePfeuffer, Ann-Katrin M., Linda K. Küpfer, Thirupura S. Shankar, Stavros G. Drakos, Tilmann Volk, and Thomas Seidel. 2023. "Ryanodine Receptor Staining Identifies Viable Cardiomyocytes in Human and Rabbit Cardiac Tissue Slices" International Journal of Molecular Sciences 24, no. 17: 13514. https://doi.org/10.3390/ijms241713514
APA StylePfeuffer, A.-K. M., Küpfer, L. K., Shankar, T. S., Drakos, S. G., Volk, T., & Seidel, T. (2023). Ryanodine Receptor Staining Identifies Viable Cardiomyocytes in Human and Rabbit Cardiac Tissue Slices. International Journal of Molecular Sciences, 24(17), 13514. https://doi.org/10.3390/ijms241713514






