Studying the Interaction between Bendamustine and DNA Molecule with SERS Based on AuNPs/ZnCl2/NpAA Solid-State Substrate
Abstract
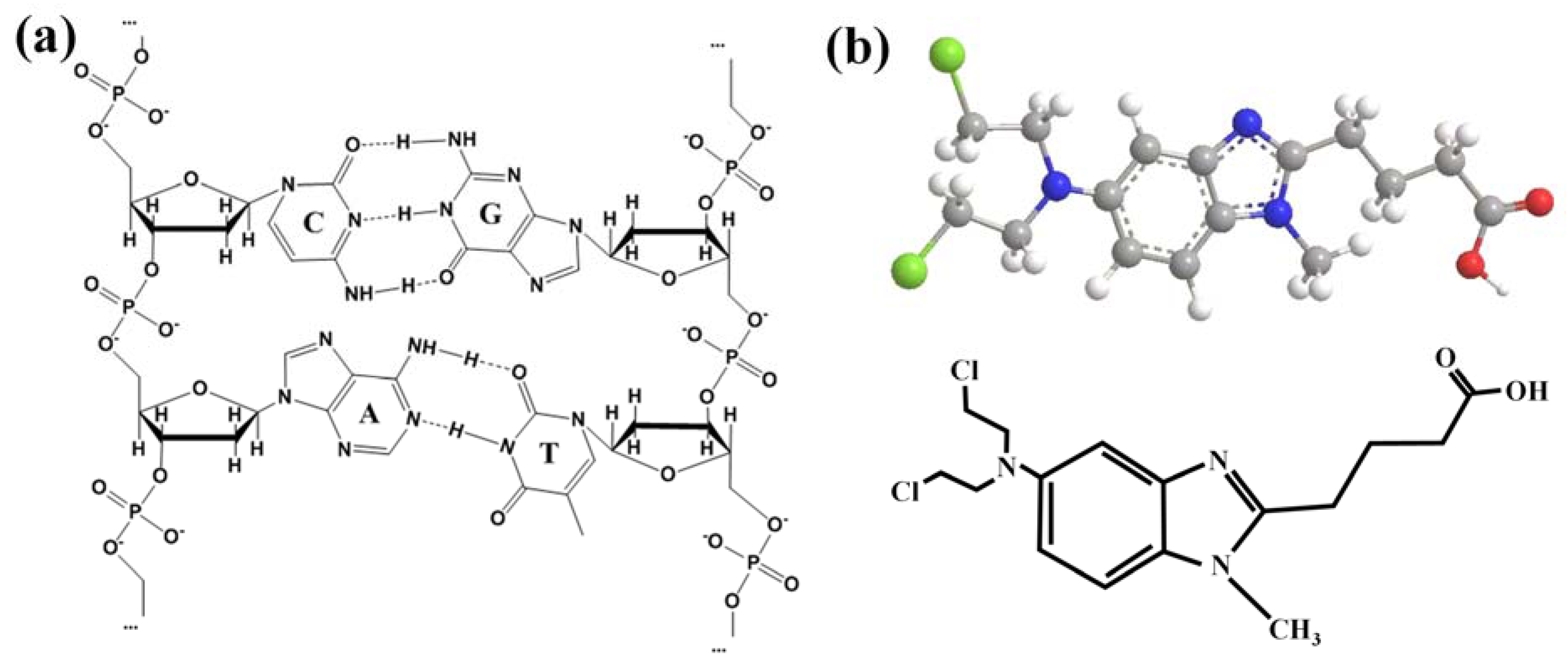
1. Introduction
2. Results and Discussion
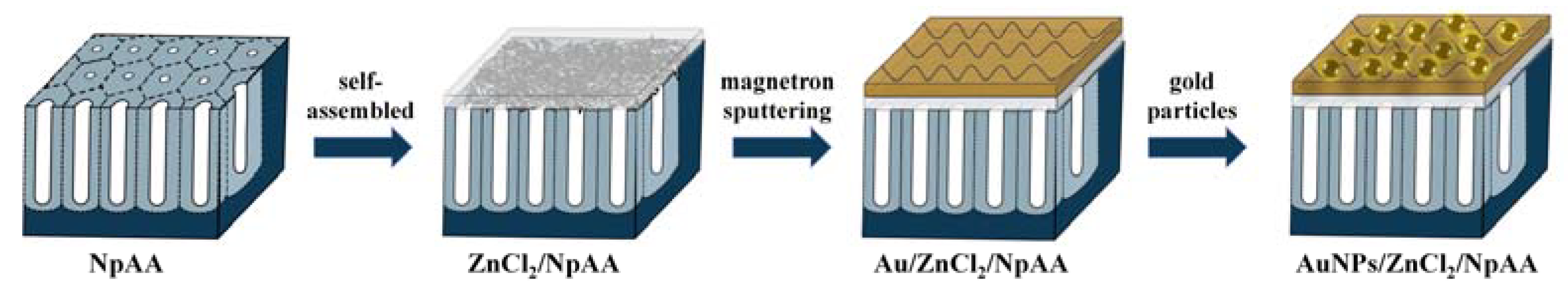
2.1. AuNPs/ZnCl2/NpAA Solid-State Substrate
2.1.1. Substrate Preparation
- The NpAA substrate was prepared by the standard two-step anodic oxidation method [26,27]. Add electrolyte into the self-made glass tank and put a magnet for magnetic stirring, place the glass tank in the low-temperature cooling tank, connect the external 40 V voltage regulator power supply, set the temperature of the low-temperature tank to 0 °C during anodic oxidation, the electrolyte added in the glass tank is 0.3 mol/L oxalic acid solution, the first oxidation time is 1 h, the second oxidation time is adjusted to 2 h;
- ZnCl2 layers were prepared on the surface of NpAA by self-assembly method. First, ZnCl2 solution with a concentration of 0.05 mol/L was prepared, and then the ZnCl2 solution was added to the NpAA substrate surface by drops until it was completely covered. Finally, the whole substrate, after being added with ZnCl2 solution, was placed in a closed room temperature environment for 7 days, and the surface would naturally form nanosheet structures;
- A layer of Au film of about 30 nm was plated on the surface of the substrate after the previous step by magnetron sputtering;
- Gold particles AuNPs with a particle size of 50 nm were used for modification on the uppermost layer of the substrate, which was first soaked in 2% PVP ethanol solution for 6 h, rinsed, and then transferred to AuNPs solution for 2 times, both for 6 h.
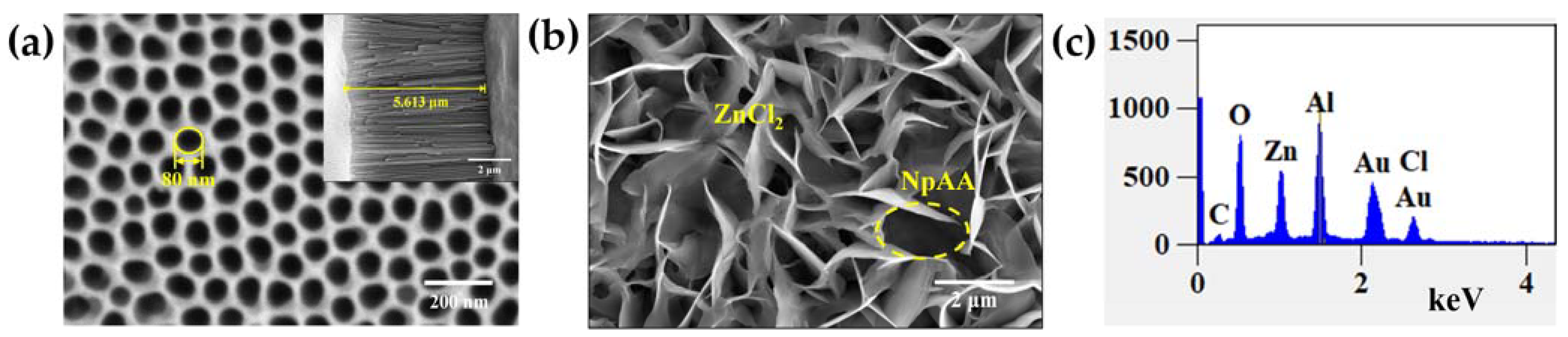
2.1.2. Substrate Characterization
2.1.3. Substrate Performance
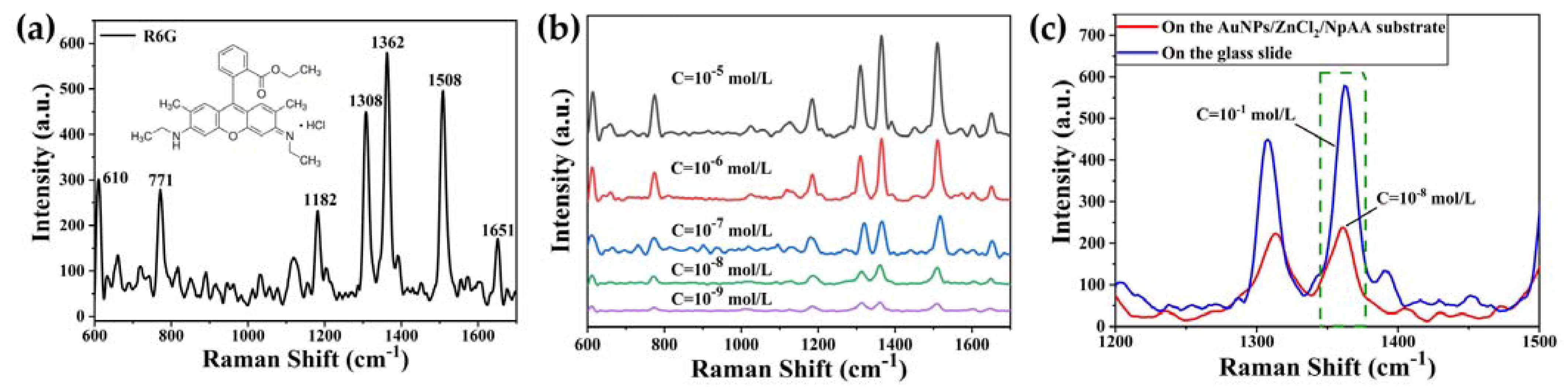
2.2. SERS Spectrum
2.2.1. SERS Spectrum of ctDNA
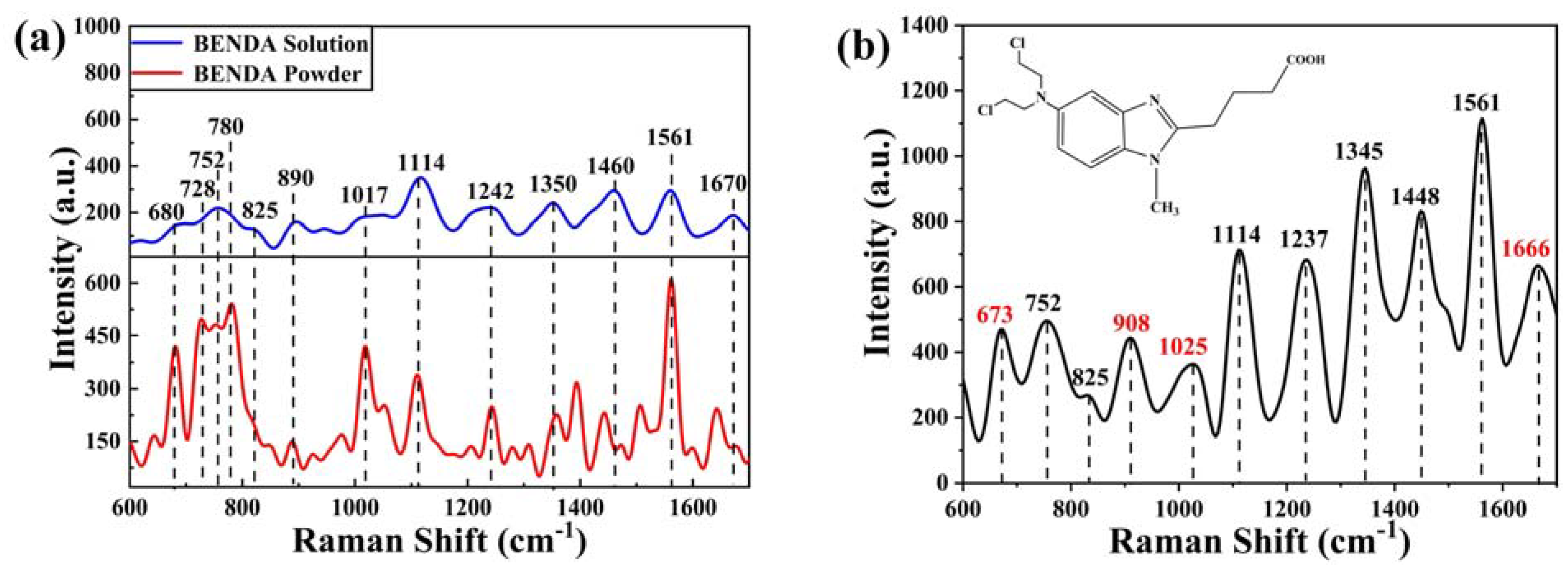
2.2.2. SERS Spectrum of BENDA
2.2.3. SERS Spectrum of BENDA-ctDNA
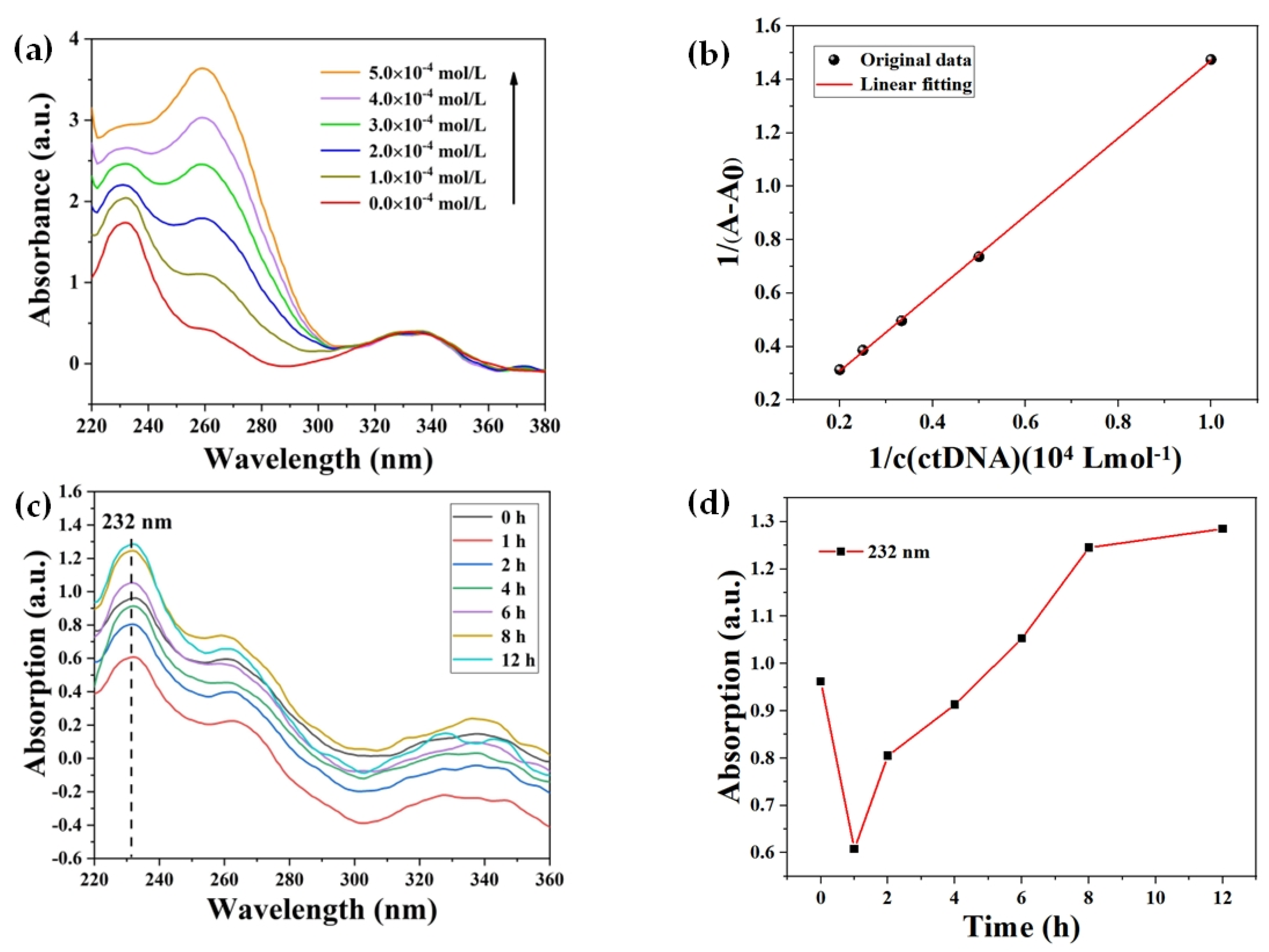
2.3. UV–Vis Absorption Spectroscopy
- ctDNA concentration
- Reaction time
2.4. Molecular Docking
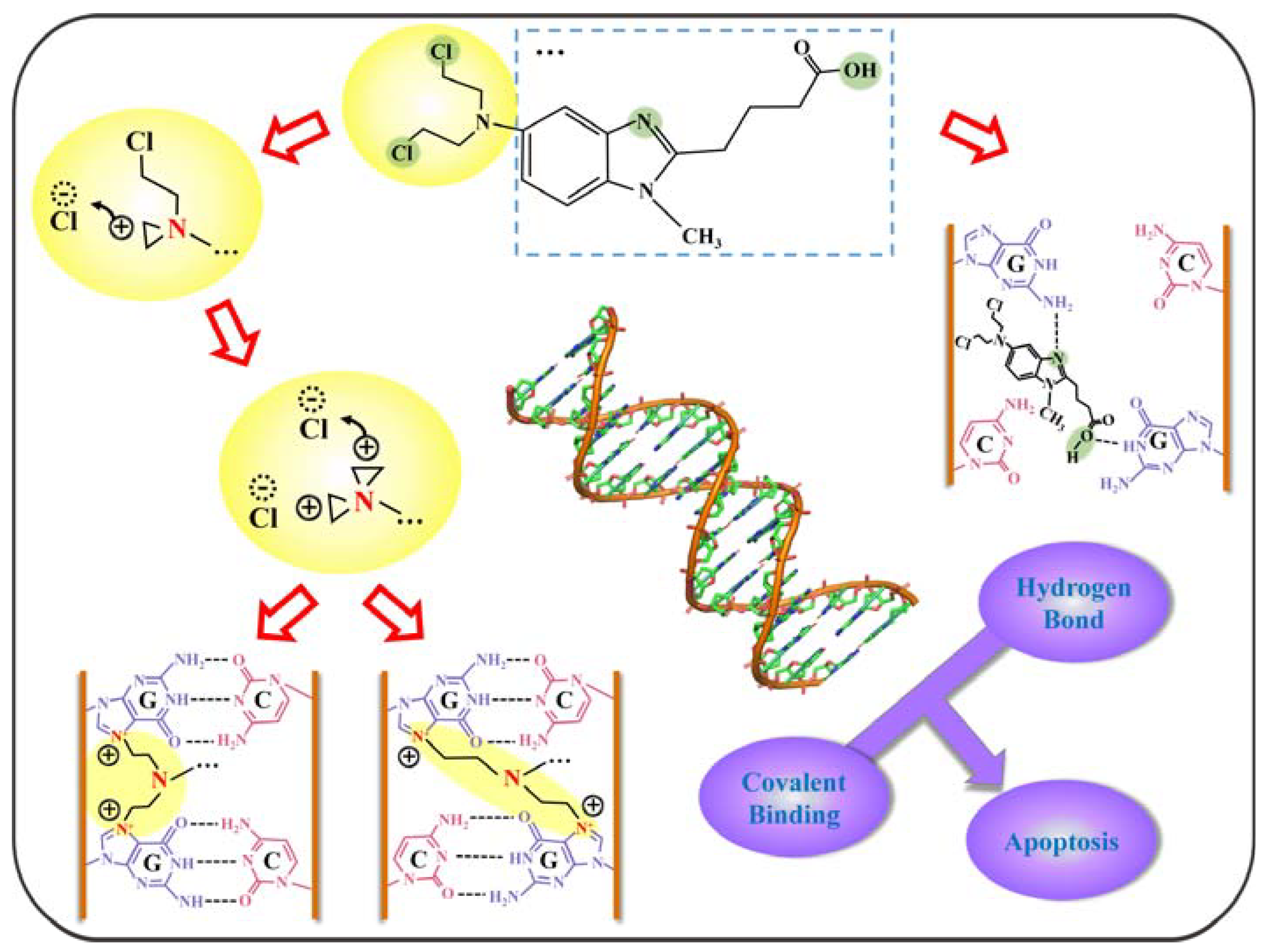
2.5. Mechanism of Action of BENDA and DNA
3. Materials and Methods
3.1. SERS
3.2. Scanning Electron Microscope
3.3. UV–Vis Absorption Spectroscopy
- ctDNA concentration
- reaction time
3.4. Molecular Docking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Yin, F.F.; Song, L.; Mao, X.H.; Li, F.; Fan, C.H.; Zuo, X.L.; Xia, Q. Nucleic Acid Tests for Clinical Translation. Chem. Rev. 2021, 121, 10469–10558. [Google Scholar] [CrossRef] [PubMed]
- Harding, S.E.; Channell, G.; Phillips-Jones, M.K. The discovery of hydrogen bonds in DNA and a re-evaluation of the 1948 Creeth two-chain model for its structure. Biochem. Soc. Trans. 2018, 46, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, W.S.; Saleh, E.M.; Menon, V.; Vazhappilly, C.G.; Abdu-Allah, H.; El-Shorbagi, A.; Mansour, W.; El-Awady, R. Induction of DNA damage, apoptosis and cell cycle perturbation mediate cytotoxic activity of new 5-aminosalicylate-4-thiazolinone hybrid derivatives. Biomed. Pharmacother. 2020, 131, 110571. [Google Scholar] [CrossRef] [PubMed]
- Suskiewicz, M.J.; Zobel, F.; Ogden, T.; Fontana, P.; Ariza, A.; Yang, J.C.; Zhu, K.; Bracken, L.; Hawthorne, W.J.; Ahel, D.; et al. HPF1 completes the PARP active site for DNA damage-induced ADP-ribosylation. Nature 2020, 579, 598–602. [Google Scholar] [CrossRef]
- Brown, H.S.; Ito, K.; Galetin, A.; Houston, J.B. Prediction of in vivo drug-drug interactions from in vitro data: Impact of incorporating parallel pathways of drug elimination and inhibitor absorption rate constant. Br. J. Clin. Pharmacol. 2005, 60, 508–518. [Google Scholar] [CrossRef]
- Wani, T.A.; Alsaif, N.; Bakheit, A.H.; Zargar, S.; Al-Mehizia, A.A.; Khan, A.A. Interaction of an abiraterone with calf thymus DNA: Investigation with spectroscopic technique and modelling studies. Bioorg. Chem. 2020, 100, 103957. [Google Scholar] [CrossRef]
- Tanzadehpanah, H.; Mahaki, H.; Moradi, M.; Afshar, S.; Moghadam, N.H.; Salehzadeh, S.; Najafi, R.; Amini, R.; Saidijam, M. The use of molecular docking and spectroscopic methods for investigation of the interaction between regorafenib with human serum albumin (HSA) and calf thymus DNA (ct-DNA) in the presence of different site markers. Protein Pept. Lett. 2021, 28, 290–303. [Google Scholar] [CrossRef]
- Brooks, N.; Hartley, J.A.; Simpson, J.E.J.; Wright, S.R.; Woo, S.; Centioni, S.; Fontaine, M.D.; McIntyre, T.E.; Lee, M. Structure-activity relationship of a series of C-terminus modified aminoalkyl, diaminoalkyl-and anilino-containing analogues of the benzoic acid mustard distamycin derivative tallimustine: Synthesis, DNA binding and cytotoxicity studies. Bioorg. Med. Chem. 1997, 5, 1497–1507. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Rich, J.; Hao, N.J.; Gu, Y.Y.; Chen, C.Y.; Yang, S.J.; Zhang, P.R.; Huang, T.J. Acoustofluidics for simultaneous nanoparticle-based drug loading and exosome encapsulation. Microsyst. Nanoeng. 2022, 8, 45. [Google Scholar] [CrossRef]
- Leoni, L.M. Bendamustine: Rescue of an Effective Antineoplastic Agent from the Mid-Twentieth Century. Semin. Hematol. 2011, 48, S4–S11. [Google Scholar] [CrossRef]
- Elefante, A.; Czuczman, M.S. Bendamustine for the treatment of indolent non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Am. J. Health-Syst. Pharm. 2010, 67, 713–723. [Google Scholar] [CrossRef]
- Hiraoka, N.; Kikuchi, J.; Yamauchi, T.; Koyama, D.; Wada, T.; Uesawa, M.; Akutsu, M.; Mori, S.; Nakamura, Y.; Ueda, T.; et al. Purine analog-like properties of bendamustine underlie rapid activation of DNA damage response and synergistic effects with pyrimidine analogues in lymphoid malignancies. PLoS ONE 2014, 9, e90675. [Google Scholar] [CrossRef]
- Strumberg, D.; Harstrick, A.; Doll, K.; Hoffmann, B.; Seeber, S. Bendamustine hydrochloride activity against doxorubicin-resistant human breast carcinoma cell lines. Anti-Cancer Drug 1996, 7, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Balfour, J.; Goa, K.L. Bendamustine. Drugs 2001, 61, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Leoni, L.M.; Bailey, B.; Niemeyer, C.C.; Reifert, J.; Bendall, H.; Dauffenbach, L.; Kerfoot, C. In vitro and ex vivo activity of SDX-105 (bendamustine) in drug-resistant lymphoma cells. Cancer Res. 2004, 64, 278. [Google Scholar]
- Leoni, L.M.; Bailey, B.; Reifert, J.; Bendall, H.H.; Zeller, R.W.; Corbeil, J.; Elliott, G.; Niemeyer, C.C. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin. Cancer Res. 2008, 14, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Leoni, L.M.; Hartley, J.A. Mechanism of Action: The Unique Pattern of Bendamustine-Induced Cytotoxicity. Semin. Hematol. 2011, 48, S12–S23. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Leoni, L. Bendamustine: Mechanism of action and clinical data. Clin. Adv. Hematol. Oncol. 2011, 9, 1–11. [Google Scholar]
- Rehman, S.U.; Sarwar, T.; Husain, M.A.; Ishqi, H.M.; Tabish, M. Studying non-covalent drug-DNA interactions. Arch. Biochem. Biophys. 2015, 576, 49–60. [Google Scholar] [CrossRef]
- Oliveira, S.; Chiorcea-Paquim, A.M.; Ribeiro, S.M.; Melo, A.; Vivan, M.; Oliveira-Brett, A.M. In situ electrochemical and AFM study of thalidomide-DNA interaction. Bioelectrochemistry 2009, 76, 201–207. [Google Scholar] [CrossRef]
- Saito, S.T.; Silva, G.; Pungartnik, C.; Brendel, M. Study of DNA-emodin interaction by FTIR and UV-vis spectroscopy. J. Photochem. Photobiol. B 2012, 111, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Barcelo, F.; Capo, D.; Portugal, J. Thermodynamic characterization of the multivalent binding of chartreusin to DNA. Nucleic Acids Res. 2002, 30, 4567–4573. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zong, C.; Xu, M.X.; Xu, L.J.; Wei, T.; Ma, X.; Zheng, X.S.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef] [PubMed]
- Ilkhani, H.; Hughes, T.; Li, J.; Zhong, C.J.; Hepel, M. Nanostructured SERS-electrochemical biosensors for testing of anticancer drug interactions with DNA. Biosens. Bioelectron. 2016, 80, 257–264. [Google Scholar] [CrossRef]
- Agarwal, S.; Ray, B.; Mehrotra, R. SERS as an advanced tool for investigating chloroethyl nitrosourea derivatives complexation with DNA. Int. J. Biol. Macromol. 2015, 81, 891–897. [Google Scholar] [CrossRef]
- Zhou, Y.K.; Dang, Y.; Wang, K.G.; Zhao, W.; Zhang, C.; Jiao, Y.; Feng, X.Q.; Wang, G.R.; Shen, T.H. A Stable NanoPAA-ZnO/ZnCl2 Composite with Variable 3D Structured Morphology and Sustained Superhydrophilicity. Langmuir 2021, 37, 5457–5463. [Google Scholar] [CrossRef]
- Gong, Z.Y.; Dang, Y.; Zhu, J.; Zheng, J.; Zhang, C.; Zhao, W.; Wang, K.G. Reflection Interference Spectroscopy Technology Monitoring the Synthesis of ZnCl2-ZnO Nanosheets on Nanoporous Anodic Alumina Substrate in Real Time. Photonics 2023, 10, 552. [Google Scholar] [CrossRef]
- Pilot, R.; Bozio, R. Validation of SERS enhancement factor measurements. J. Raman Spectrosc. 2018, 49, 462–471. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Li, S.; Wang, J.Y.; Shao, Y.P.; Mei, L.Y. A recyclable graphene/Ag/TiO2 SERS substrate with high stability and reproducibility for detection of dye molecules. New J. Chem. 2022, 46, 18787–18795. [Google Scholar] [CrossRef]
- Zhang, K.B.; Zeng, T.X.; Tan, X.L.; Wu, W.D.; Tang, Y.J.; Zhang, H.B. A facile surface-enhanced Raman scattering (SERS) detection of rhodamine 6G and crystal violet using Au nanoparticle substrates. Appl. Surf. Sci. 2015, 347, 569–573. [Google Scholar] [CrossRef]
- Hao, B.J.; Wang, K.G.; Zhou, Y.K.; Sui, C.F.; Wang, L.; Bai, R.; Yang, Z.J. Label-Free Detecting of the Compaction and Decompaction of ctDNA Molecules Induced by Surfactants with SERS Based on a nanoPAA-ZnCl2-AuLs Solid Substrate. ACS Omega 2020, 5, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Jangir, D.K.; Agarwal, S.; Ray, B.; Singh, P.; Srivastava, A.K. Interaction Studies of Anticancer Drug Lomustine with Calf Thymus DNA using Surface Enhanced Raman Spectroscopy. Mapan-J. Metrol. Soc. I 2013, 28, 273–277. [Google Scholar] [CrossRef]
- Zhang, R.H.; Zhu, J.; Sun, D.; Li, J.; Yao, L.N.; Meng, S.S.; Li, Y.; Dang, Y.; Wang, K.G. The Mechanism of Dynamic Interaction between Doxorubicin and Calf Thymus DNA at the Single-Molecule Level Based on Confocal Raman Spectroscopy. Micromachines 2022, 13, 940. [Google Scholar] [CrossRef] [PubMed]
- Kitt, J.P.; Bryce, D.A.; Minteer, S.D.; Harris, J.M. Raman Spectroscopy Reveals Selective Interactions of Cytochrome c with Cardiolipin That Correlate with Membrane Permeability. J. Am. Chem. Soc. 2017, 139, 3851–3860. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, B.B. Solid state linear dichroic infrared spectral analysis of benzimidazoles and their N-1-protonated salts. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 62, 58–65. [Google Scholar] [CrossRef]
- Ramana, P.V.; Sundius, T.; Muthu, S.; Mouli, K.C.; Krishna, Y.R.; Prasad, K.V.; Devi, R.N.; Irfan, A.; Santhamma, C. Spectroscopic, quantum mechanical, electronic excitation properties (Ethanol solvent), DFT investigations and molecular docking analysis of an anti-cancer drug Bendamustine. J. Mol. Struct. 2022, 1253, 132211. [Google Scholar] [CrossRef]
- Myres, G.J.; Harris, J.M. Nanomolar Binding of an Antibiotic Peptide to DNA Measured with Raman Spectroscopy. Langmuir 2023, 39, 4150–4160. [Google Scholar] [CrossRef]
- Aymen, S.; Nawaz, H.; Majeed, M.I.; Rashid, N.; Ehsan, U.; Shahzad, R.; Ali, M.Z.; Rimsha, G.; Fatima, R.; Meraj, L.; et al. Raman spectroscopy for the quantitative analysis of Lornoxicam in solid dosage forms. J. Raman Spectrosc. 2023, 54, 250–257. [Google Scholar] [CrossRef]
- Tong, C.L.; Hu, Z.; Wu, J.M. Interaction Between Methylene Blue and CalfThymus Deoxyribonucleic Acid by Spectroscopic Technologies. J. Fluoresc. 2010, 20, 261–267. [Google Scholar]
- Yao, L.N.; Zuo, Z.Z.; Gong, Z.Y.; Sun, D.; Feng, X.Q.; Wang, K.G. Interaction between anticancer drug bendamustine and calf thymus DNA with spectroscopy at the single-molecule level. In Proceedings of the 13th International Conference on Information Optics and Photonics (CIOP), Xi’an, China, 7–10 August 2022. [Google Scholar]
- Anjomshoa, M.; Fatemi, S.J.; Torkzadeh-Mahani, M.; Hadadzadeh, H. DNA-and BSA-binding studies and anticancer activity against human breast cancer cells (MCF-7) of the zinc (II) complex coordinated by 5,6-diphenyl-3-(2-pyridyl)-1,2,4-triazine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 127, 511–520. [Google Scholar] [CrossRef]
- Zhao, W.J.; Ma, Y.X.; Lu, K. Studies on the Interaction of l-thymidine with DNA. In Proceedings of the International Symposium on Biomedicine and Engineering (ISBE 2011), Bali Island, Indonesia, 4–5 August 2011. [Google Scholar]
- Ling, J.Y.; Yang, Q.Z.; Luo, S.S.; Li, Y.; Zhang, C.K. Preliminary study on cordycepin-DNA interaction by Raman spectroscopy. Chin. Chem. Lett. 2005, 16, 71–74. [Google Scholar]
- Li, J.F.; Shuang, S.M.; Dong, C. Study on the phosphorescence characterizations of palmatine chloride on the solid substrate and its interaction with ctDNA. Talanta 2009, 77, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lv, J.; Zhang, G.S.; Wang, G.K.; Liu, Q.F. Interaction of an anthracycline disaccharide with ctDNA: Investigation by spectroscopic technique and modeling studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 75, 1511–1515. [Google Scholar]

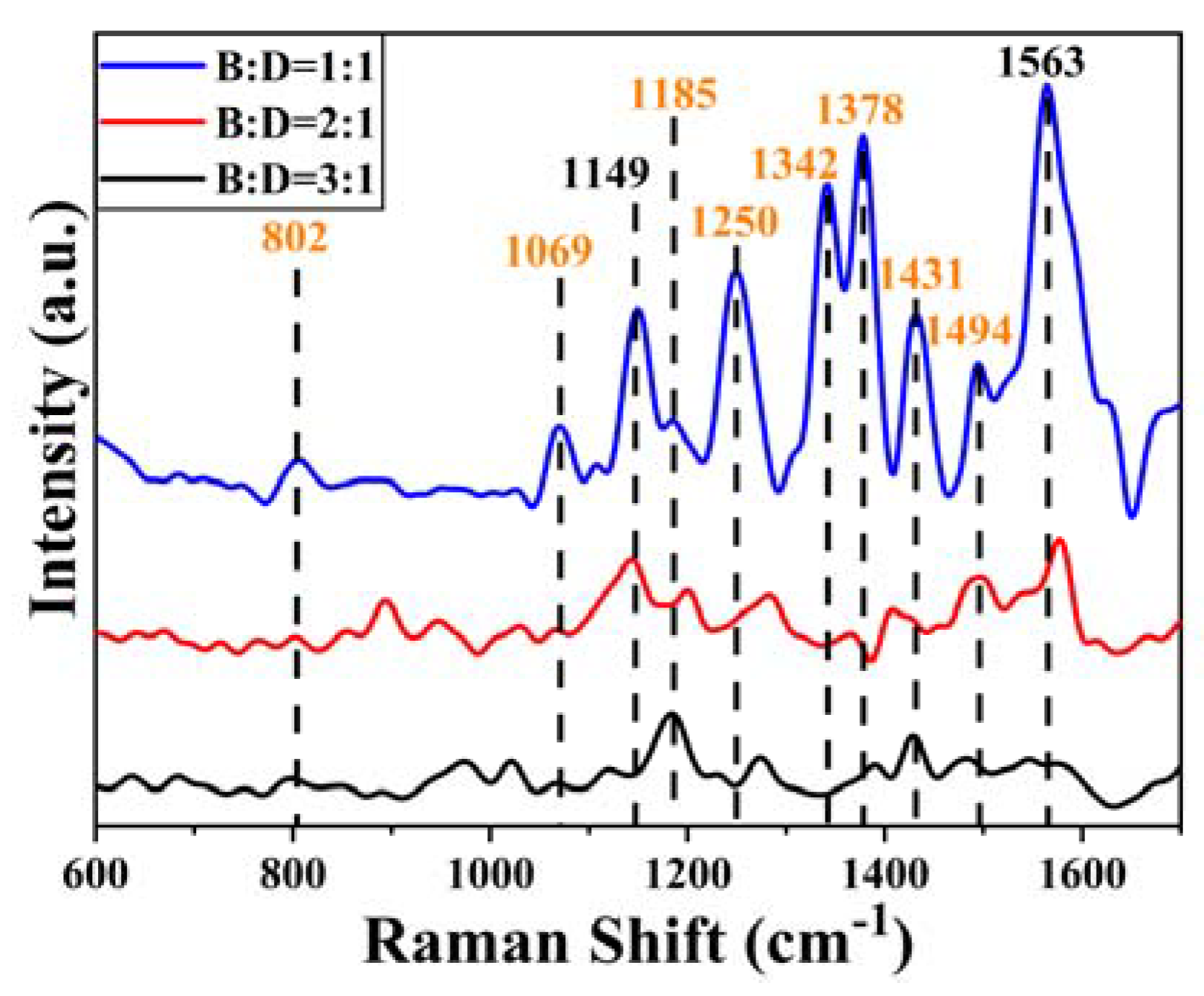

| BENDA-DNA/cm−1 | Attributed Species | Assignment |
|---|---|---|
| 802 | DNA | Phosphate backbone |
| 1069 | DNA | C-O stretch of deoxyribose |
| 1149 | BENDA | HCC, CC, CO |
| 1185 | DNA | extra-base C-N stretching |
| 1250 | DNA | A, C |
| 1342 | DNA | A |
| 1378 | DNA | T, A, G |
| 1431 | DNA | A |
| 1494 | DNA | G, A |
| 1563 | BENDA | C-C, H-C-H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Li, Y.; Zuo, Z.; Gong, Z.; Zhu, J.; Feng, X.; Sun, D.; Wang, K. Studying the Interaction between Bendamustine and DNA Molecule with SERS Based on AuNPs/ZnCl2/NpAA Solid-State Substrate. Int. J. Mol. Sci. 2023, 24, 13517. https://doi.org/10.3390/ijms241713517
Yao L, Li Y, Zuo Z, Gong Z, Zhu J, Feng X, Sun D, Wang K. Studying the Interaction between Bendamustine and DNA Molecule with SERS Based on AuNPs/ZnCl2/NpAA Solid-State Substrate. International Journal of Molecular Sciences. 2023; 24(17):13517. https://doi.org/10.3390/ijms241713517
Chicago/Turabian StyleYao, Lina, Yanjie Li, Zhenzhong Zuo, Ziyi Gong, Jie Zhu, Xiaoqiang Feng, Dan Sun, and Kaige Wang. 2023. "Studying the Interaction between Bendamustine and DNA Molecule with SERS Based on AuNPs/ZnCl2/NpAA Solid-State Substrate" International Journal of Molecular Sciences 24, no. 17: 13517. https://doi.org/10.3390/ijms241713517
APA StyleYao, L., Li, Y., Zuo, Z., Gong, Z., Zhu, J., Feng, X., Sun, D., & Wang, K. (2023). Studying the Interaction between Bendamustine and DNA Molecule with SERS Based on AuNPs/ZnCl2/NpAA Solid-State Substrate. International Journal of Molecular Sciences, 24(17), 13517. https://doi.org/10.3390/ijms241713517







