Sodium Ferrous Citrate and 5-Aminolevulinic Acid Exert a Therapeutic Effect on Endotoxin-Induced Uveitis in Rats
Abstract
:1. Introduction
2. Results
2.1. Therapeutic Effect of ALA/SFC on Clinical Scoring and the Number of Infiltrating Cells and Protein Levels in AqH
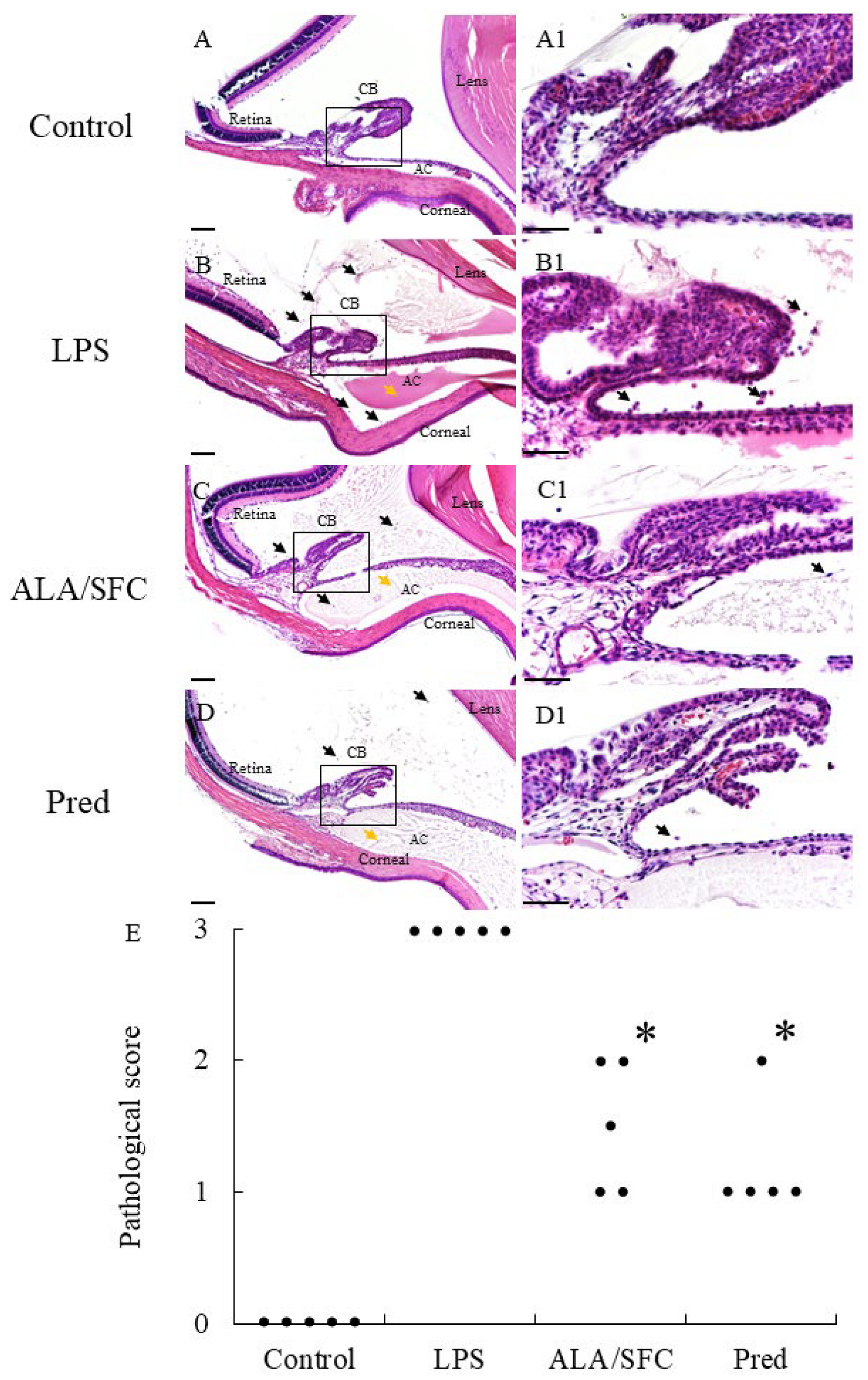
2.2. ALA/SFC Improves Histopathological Evaluation
2.3. Therapeutic Effect of ALA/SFC on TNF-α, IL-6, NO, and PGE2 Levels in AqH
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Endotoxin-Induced Uveitis
4.4. Evaluation of Clinical Scoring
4.5. Infiltrating Cell Count and Protein Level Measurement in AqH
4.6. Evaluation of Histopathologic Scores
4.7. Measurement of Cytokine Levels in AqH
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durrani, O.M.; Tehrani, N.N.; Marr, J.E.; Moradi, P.; Stavrou, P.; Murray, P.I. Degree, duration, and causes of visual loss in uveitis. Br. J. Ophthalmol. 2004, 88, 1159–1162. [Google Scholar] [CrossRef]
- Rothova, A.; Suttorp-van Schulten, M.S.; Treffers, W.F.; Kijlstra, A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br. J. Ophthalmol. 1996, 80, 332–336. [Google Scholar] [CrossRef]
- Tomkins-Netzer, O.; Talat, L.; Bar, A.; Lula, A.; Taylor, S.R.; Joshi, L.; Lightman, S. Long-term clinical outcome and causes of vision loss in patients with uveitis. Ophthalmology 2014, 121, 2387–2392. [Google Scholar] [CrossRef]
- Dick, A.D.; Tundia, N.; Sorg, R.; Zhao, C.; Chao, J.; Joshi, A.; Skup, M. Risk of Ocular Complications in Patients with Noninfectious Intermediate Uveitis, Posterior Uveitis, or Panuveitis. Ophthalmology 2016, 123, 655–662. [Google Scholar] [CrossRef] [PubMed]
- De Vos, A.F.; van Haren, M.A.; Verhagen, C.; Hoekzema, R.; Kijlstra, A. Kinetics of intraocular tumor necrosis factor and interleukin-6 in endotoxin-induced uveitis in the rat. Investig. Ophthalmol. Vis. Sci. 1994, 35, 1100–1106. [Google Scholar]
- Planck, S.R.; Huang, X.N.; Robertson, J.E.; Rosenbaum, J.T. Cytokine mRNA levels in rat ocular tissues after systemic endotoxin treatment. Investig. Ophthalmol. Vis. Sci. 1994, 35, 924–930. [Google Scholar]
- Tilton, R.G.; Chang, K.; Corbett, J.A.; Misko, T.P.; Currie, M.G.; Bora, N.S.; Kaplan, H.J.; Williamson, J.R. Endotoxin-induced uveitis in the rat is attenuated by inhibition of nitric oxide production. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3278–3288. [Google Scholar]
- Egwuagu, C.E.; Alhakeem, S.A.; Mbanefo, E.C. Uveitis: Molecular Pathogenesis and Emerging Therapies. Front. Immunol. 2021, 12, 623725. [Google Scholar] [CrossRef] [PubMed]
- Schwartzman, S. Advancements in the management of uveitis. Best. Pract. Res. Clin. Rheumatol. 2016, 30, 304–315. [Google Scholar] [CrossRef]
- Lee, F.F.; Foster, C.S. Pharmacotherapy of uveitis. Expert. Opin. Pharmacother. 2010, 11, 1135–1146. [Google Scholar] [CrossRef]
- Jabs, D.A.; Rosenbaum, J.T.; Foster, C.S.; Holland, G.N.; Jaffe, G.J.; Louie, J.S.; Nussenblatt, R.B.; Stiehm, E.R.; Tessler, H.; Van Gelder, R.N.V.; et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: Recommendations of an expert panel. Am. J. Ophthalmol. 2000, 130, 492–513. [Google Scholar] [CrossRef] [PubMed]
- De Smet, M.D.; Taylor, S.R.; Bodaghi, B.; Miserocchi, E.; Murray, P.I.; Pleyer, U.; Zierhut, M.; Barisani-Asenbauer, T.; LeHoang, P.; Lightman, S. Understanding uveitis: The impact of research on visual outcomes. Prog. Retin. Eye Res. 2011, 30, 452–470. [Google Scholar] [CrossRef] [PubMed]
- Thorne, J.E.; Woreta, F.A.; Dunn, J.P.; Jabs, D.A. Risk of cataract development among children with juvenile idiopathic arthritis-related uveitis treated with topical corticosteroids. Ophthalmology 2010, 117, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.T.; McDevitt, H.O.; Guss, R.B.; Egbert, P.R. Endotoxin-induced uveitis in rats as a model for human disease. Nature 1980, 286, 611–613. [Google Scholar] [CrossRef]
- Yang, P.; de Vos, A.F.; Kijlstra, A. Macrophages in the retina of normal Lewis rats and their dynamics after injection of lipopolysaccharide. Investig. Ophthalmol. Vis. Sci. 1996, 37, 77–85. [Google Scholar]
- Bhattacherjee, P.; Williams, R.N.; Eakins, K.E. An evaluation of ocular inflammation following the injection of bacterial endotoxin into the rat foot pad. Investig. Ophthalmol. Vis. Sci. 1983, 24, 196–202. [Google Scholar]
- Kanai, K.; Nagata, S.; Hatta, T.; Sugiura, Y.; Sato, K.; Yamashita, Y.; Kimura, Y.; Itoh, N. Therapeutic anti-inflammatory effects of luteolin on endotoxin-induced uveitis in Lewis rats. J. Vet. Med. Sci. 2016, 78, 1381–1384. [Google Scholar] [CrossRef]
- Rodrigues, G.B.; Passos, G.F.; Di Giunta, G.; Figueiredo, C.P.; Rodrigues, E.B.; Grumman, A., Jr.; Medeiros, R.; Calixto, J.B. Preventive and therapeutic anti-inflammatory effects of systemic and topical thalidomide on endotoxin-induced uveitis in rats. Exp. Eye Res. 2007, 84, 553–560. [Google Scholar] [CrossRef]
- Ishizuka, M.; Abe, F.; Sano, Y.; Takahashi, K.; Inoue, K.; Nakajima, M.; Kohda, T.; Komatsu, N.; Ogura, S.; Tanaka, T. Novel development of 5-aminolevurinic acid (ALA) in cancer diagnoses and therapy. Int. Immunopharmacol. 2011, 11, 358–365. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, J.; Zhou, J.; Qi, Q.; Du, G.; Chen, J. Recent advances in microbial production of δ-aminolevulinic acid and vitamin B12. Biotechnol. Adv. 2012, 30, 1533–1542. [Google Scholar] [CrossRef]
- Shemin, D.; Russell, C.S. δ-Aminolevulinic Acid, its Role in the Biosynthesis of Porphyrines and Purines. J. Am. Chem. Soc. 1953, 75, 4873–4874. [Google Scholar] [CrossRef]
- Martin, W.F.; Mentel, M. The Origin of Mitochondria. Nat. Educ. 2010, 3, 58. [Google Scholar]
- Döring, F.; Walter, J.; Will, J.; Föcking, M.; Boll, M.; Amasheh, S.; Clauss, W.; Daniel, H. Delta-aminolevulinic acid transport by intestinal and renal peptide transporters and its physiological and clinical implications. J. Clin. Investig. 1998, 101, 2761–2767. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Ito, K.; Hayashi, M.; Nakajima, M.; Tanaka, T.; Ogura, S. The effect of 5-aminolevulinic acid on cytochrome P450-mediated prodrug activation. PLoS ONE 2015, 10, e0131793. [Google Scholar] [CrossRef] [PubMed]
- Shimura, M.; Nozawa, N.; Ogawa-Tominaga, M.; Fushimi, T.; Tajika, M.; Ichimoto, K.; Matsunaga, A.; Tsuruoka, T.; Kishita, Y.; Ishii, T.; et al. Effects of 5-aminolevulinic acid and sodium ferrous citrate on fibroblasts from individuals with mitochondrial diseases. Sci. Rep. 2019, 9, 10549. [Google Scholar] [CrossRef]
- Nakamura, Y.; Haraguchi, A.; Horie, I.; Kawakami, A.; Abiru, N. Pilot Trial on the Effect of 5-Aminolevulinic Acid on Glucose Tolerance in Patients with Maternally Inherited Diabetes and Deafness. Diabetes Ther. 2023, 14, 447–459. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, P.; Fujino, M.; Zhu, S.; Ito, H.; Takahashi, K.; Nakajima, M.; Tanaka, T.; Zhuang, J.; Li, X.K. 5-ALA/SFC Attenuated Binge Alcohol-Induced Gut Leakiness and Inflammatory Liver Disease in HIV Transgenic Rats. Alcohol. Clin. Exp. Res. 2019, 43, 1651–1661. [Google Scholar] [CrossRef]
- Nakamura, Y.; Haraguchi, A.; Shigeno, R.; Ito, A.; Horie, I.; Kawakami, A.; Abiru, N. A single-arm, open-label, intervention study to investigate the improvement of glucose tolerance after administration of the 5-aminolevulinic acid (5-ALA) in the patients with mitochondrial diabetes mellitus. Medicine 2021, 100, e25100. [Google Scholar] [CrossRef]
- Ito, H.; Nishio, Y.; Hara, T.; Sugihara, H.; Tanaka, T.; Li, X.K. Oral administration of 5-aminolevulinic acid induces heme oxygenase-1 expression in peripheral blood mononuclear cells of healthy human subjects in combination with ferrous iron. Eur. J. Pharmacol. 2018, 833, 25–33. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Hu, X.; Ito, H.; Takahashi, K.; Nakajima, M.; Tanaka, T.; Zhu, P.; Li, X.K. 5-aminolevulinic acid combined with sodium ferrous citrate ameliorated lupus nephritis in a mouse chronic graft-versus-host disease model. Int. Immunopharmacol. 2021, 96, 107626. [Google Scholar] [CrossRef]
- Shinoda, Y.; Kato, D.; Ando, R.; Endo, H.; Takahashi, T.; Tsuneoka, Y.; Fujiwara, Y. Systematic Review and Meta-Analysis of In Vitro Anti-Human Cancer Experiments Investigating the Use of 5-Aminolevulinic Acid (5-ALA) for Photodynamic Therapy. Pharmaceuticals 2021, 14, 229. [Google Scholar] [CrossRef] [PubMed]
- Otaka, Y.; Kanai, K.; Mori, A.; Okada, D.; Nagai, N.; Yamashita, Y.; Ichikawa, Y.; Tajima, K. 5-ALA/SFC Ameliorates Endotoxin-Induced Ocular Inflammation in Rats by Inhibiting the NF-κB Signaling Pathway and Activating the HO-1/Nrf2 Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 8653. [Google Scholar] [CrossRef] [PubMed]
- Fujino, M.; Nishio, Y.; Ito, H.; Tanaka, T.; Li, X.K. 5-Aminolevulinic acid regulates the inflammatory response and alloimmune reaction. Int. Immunopharmacol. 2016, 37, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fujino, M.; Zhu, S.; Isaka, Y.; Ito, H.; Takahashi, K.; Nakajima, M.; Tanaka, T.; Zhu, P.; Li, X.K. 5-ALA/SFC enhances HO-1 expression through the MAPK/Nrf2 antioxidant pathway and attenuates murine tubular epithelial cell apoptosis. FEBS Open Bio 2019, 9, 1928–1938. [Google Scholar] [CrossRef]
- Smith, J.R.; Hart, P.H.; Williams, K.A. Basic pathogenic mechanisms operating in experimental models of acute anterior uveitis. Immunol. Cell. Biol. 1998, 76, 497–512. [Google Scholar] [CrossRef]
- Perez, M.H.; Rodriguez, B.L.; Shintani, T.T.; Watanabe, K.; Miyanari, S.; Harrigan, R.C. Shintani. 5-Aminolevulinic acid (5-ALA): Analysis of preclinical and safety literature. Food Nutr. Sci. 2013, 4, 1009–1013. [Google Scholar]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]

| Group | TNF-α (pg/mL) | IL-6 (pg/mL) | NO (µM) | PGE2 (pg/mL) |
|---|---|---|---|---|
| Control | 8.53 ± 5.29 | 16.79 ± 11.91 | 1.98 ± 4.43 | 197.33 ± 40.09 |
| LPS | 64.95 ± 9.09 | 86.28 ± 37.84 | 199.12 ± 35.31 | 1947.40 ± 219.27 |
| ALA/SFC | 23.27 ± 14.41 c | 24.67 ± 18.32 a | 149.21 ± 30.22 a | 1137.17 ± 100.40 c |
| Pred | 26.26 ± 9.62 c | 28.70 ± 25.18 a | 124.19 ± 12.00 b | 1021.47 ± 113.21 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otaka, Y.; Kanai, K.; Okada, D.; Nagai, N.; Yamashita, Y.; Ichikawa, Y.; Tajima, K. Sodium Ferrous Citrate and 5-Aminolevulinic Acid Exert a Therapeutic Effect on Endotoxin-Induced Uveitis in Rats. Int. J. Mol. Sci. 2023, 24, 13525. https://doi.org/10.3390/ijms241713525
Otaka Y, Kanai K, Okada D, Nagai N, Yamashita Y, Ichikawa Y, Tajima K. Sodium Ferrous Citrate and 5-Aminolevulinic Acid Exert a Therapeutic Effect on Endotoxin-Induced Uveitis in Rats. International Journal of Molecular Sciences. 2023; 24(17):13525. https://doi.org/10.3390/ijms241713525
Chicago/Turabian StyleOtaka, Yuya, Kazutaka Kanai, Daiki Okada, Noriaki Nagai, Yohei Yamashita, Yoichiro Ichikawa, and Kazuki Tajima. 2023. "Sodium Ferrous Citrate and 5-Aminolevulinic Acid Exert a Therapeutic Effect on Endotoxin-Induced Uveitis in Rats" International Journal of Molecular Sciences 24, no. 17: 13525. https://doi.org/10.3390/ijms241713525
APA StyleOtaka, Y., Kanai, K., Okada, D., Nagai, N., Yamashita, Y., Ichikawa, Y., & Tajima, K. (2023). Sodium Ferrous Citrate and 5-Aminolevulinic Acid Exert a Therapeutic Effect on Endotoxin-Induced Uveitis in Rats. International Journal of Molecular Sciences, 24(17), 13525. https://doi.org/10.3390/ijms241713525





