Degree of Hydrolysis Regulated by Enzyme Mediation of Wheat Gluten Fibrillation: Structural Characterization and Analysis of the Mechanism of Action
Abstract
:1. Introduction
2. Results
2.1. Optimal Enzyme–Substrate Ratio
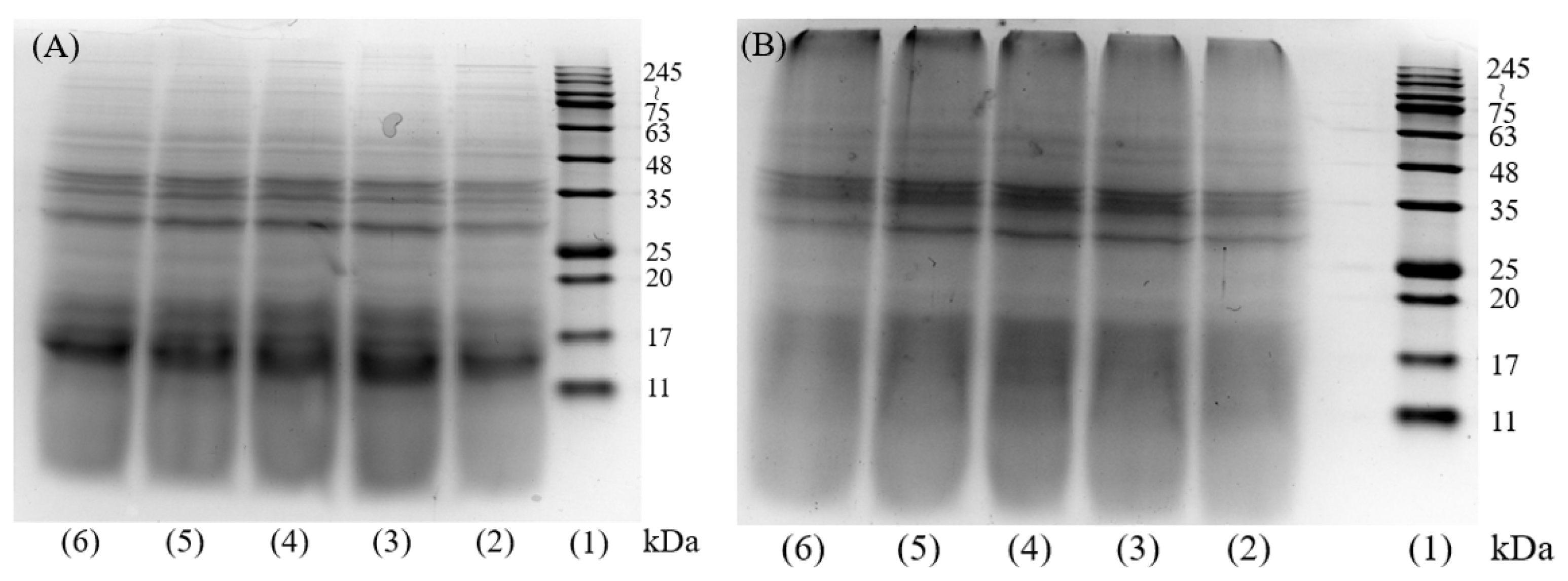
2.2. Analysis SDS-PAGE
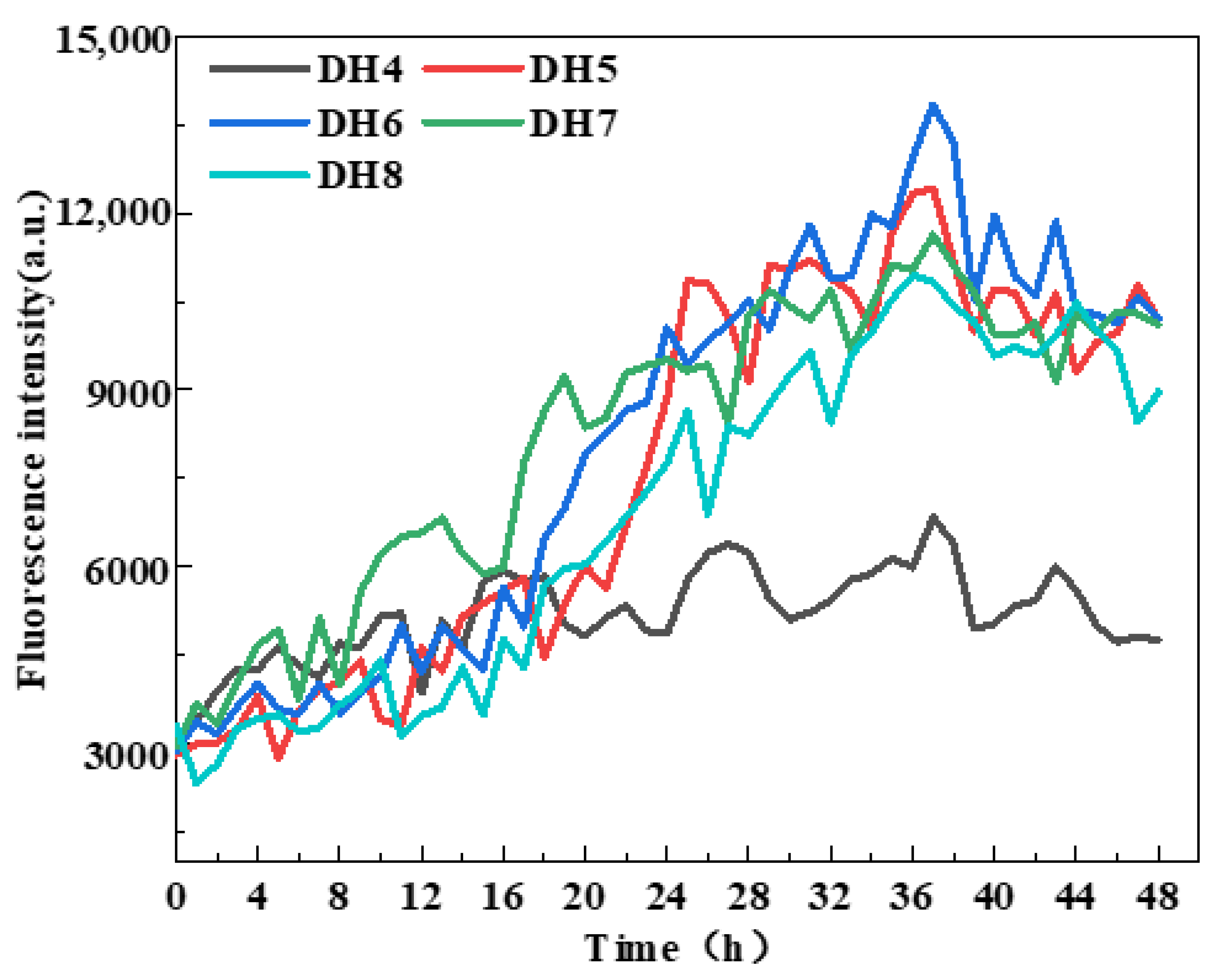
2.3. ThT Fluorescence Intensity
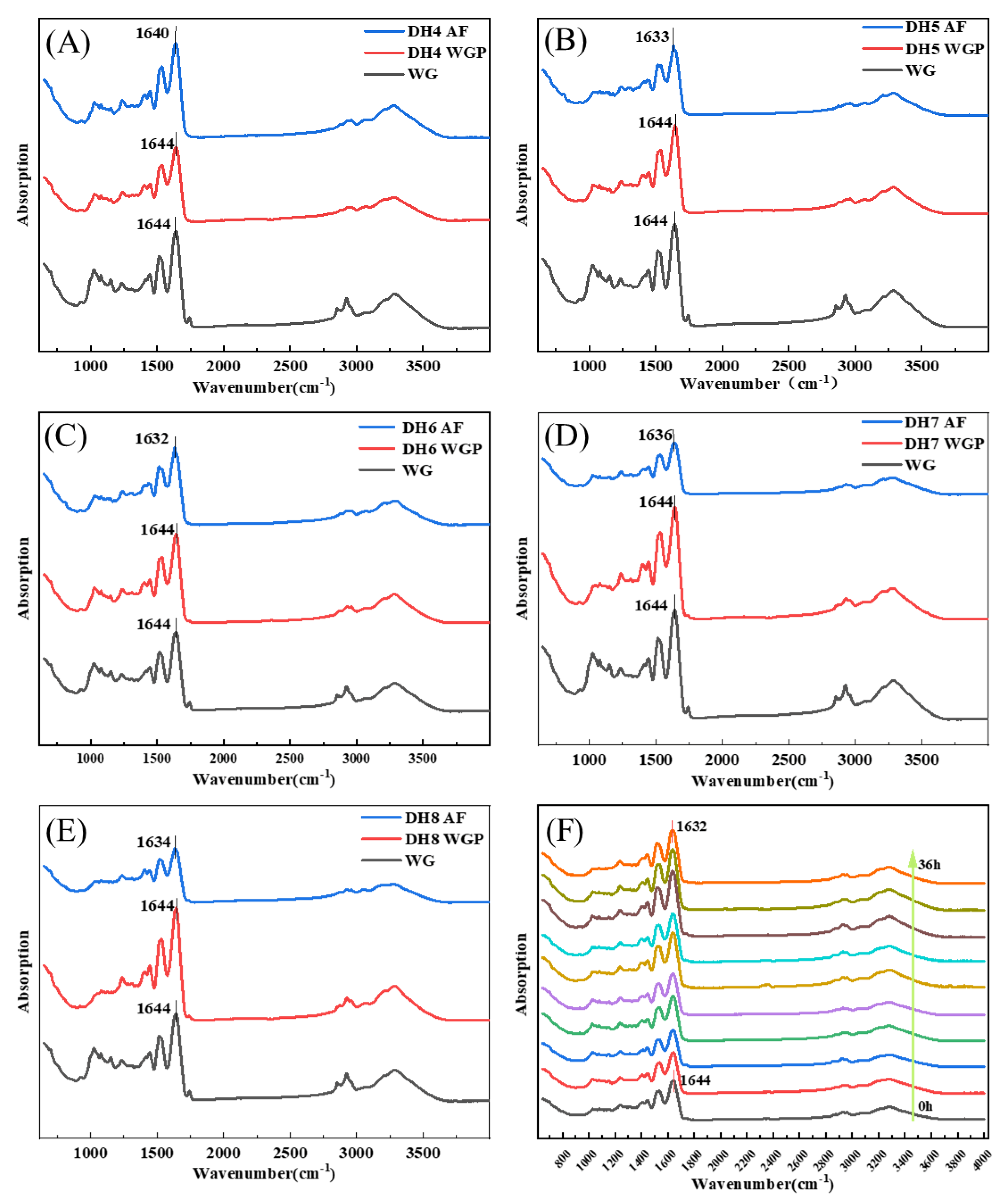
2.4. Analysis FTIR Absorption Spectra
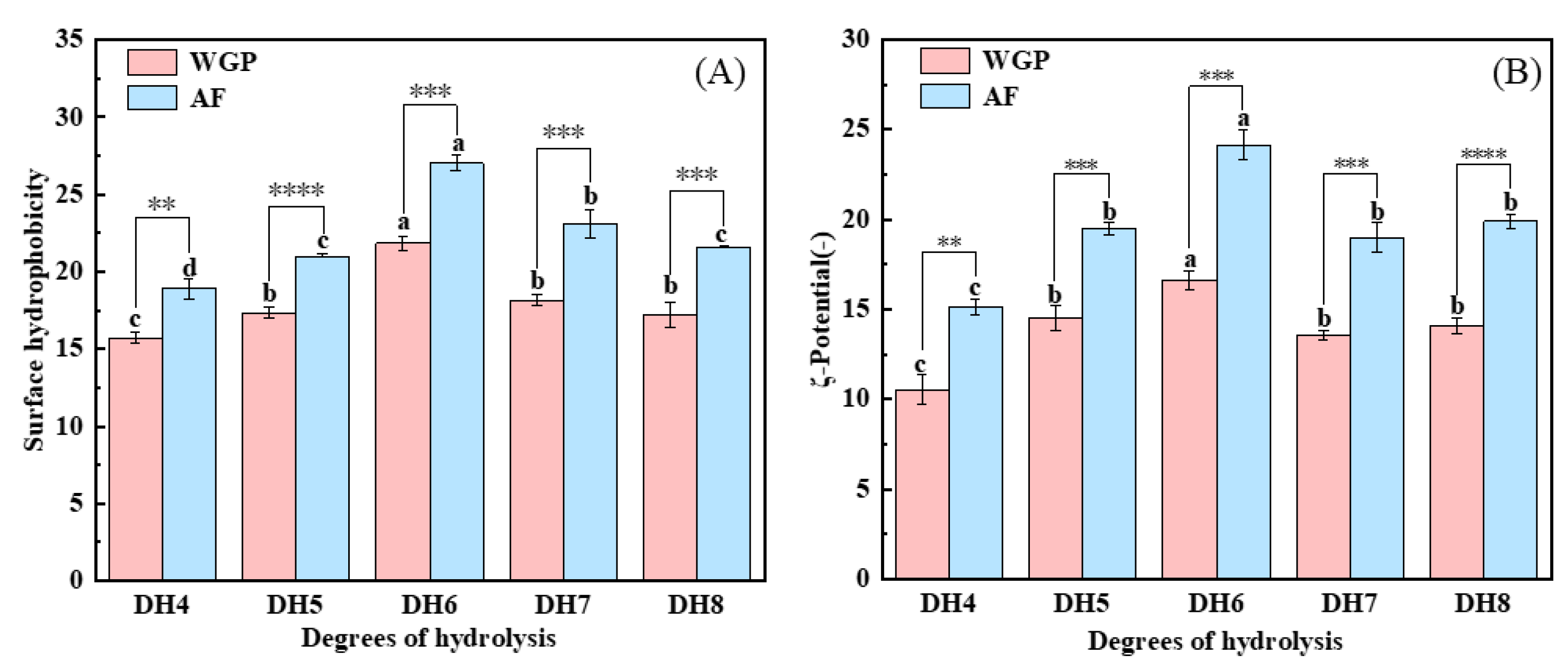
2.5. Analysis of Surface Hydrophobicity and ζ-Potential
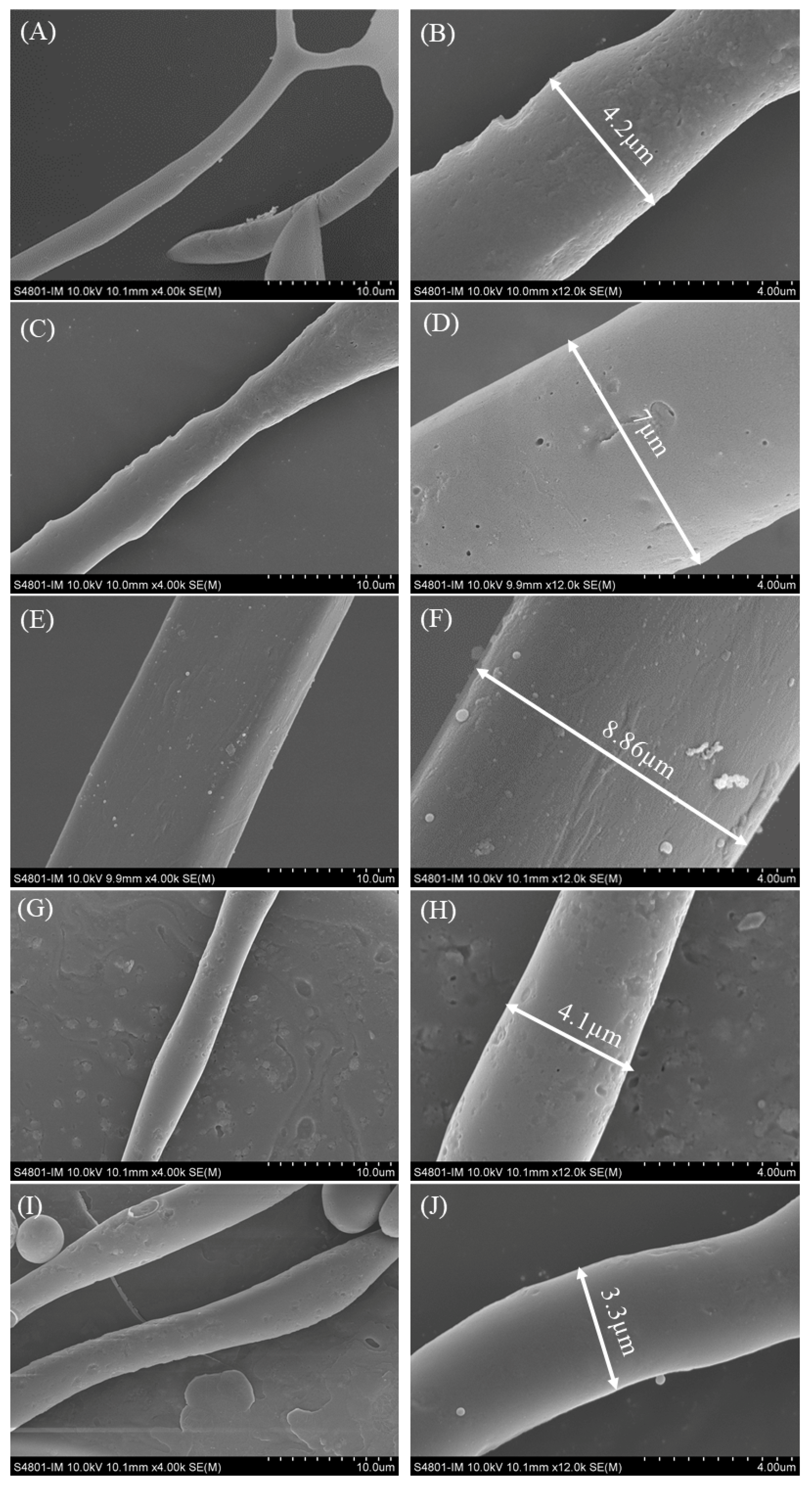
2.6. Analysis of SEM
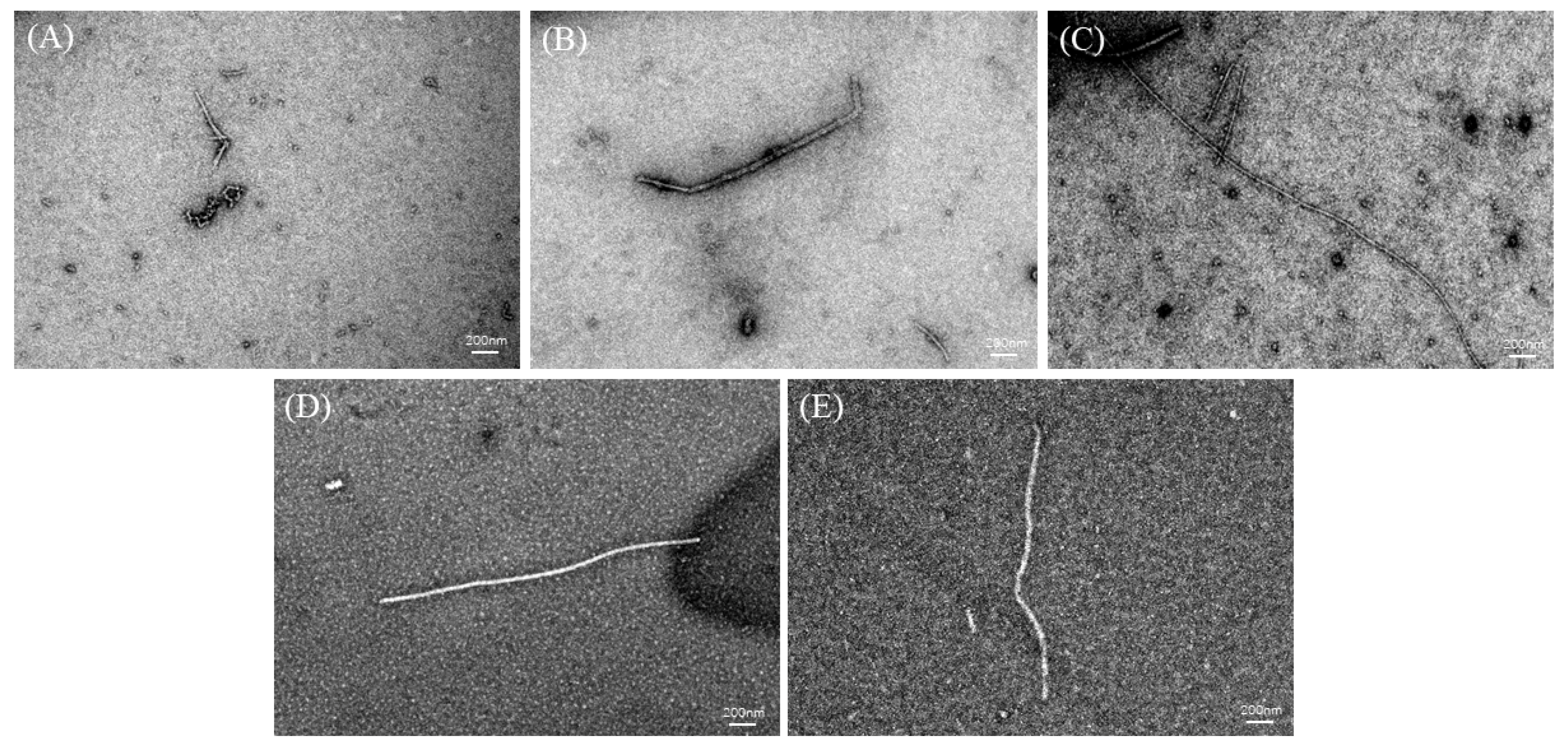
2.7. Analysis of TEM
3. Discussion
3.1. Possible Mechanism Analysis of AFs’ Formation Process of WG
3.2. Concluding Remarks and Future Perspectives
4. Materials and Methods
4.1. Materials
4.2. Hydrolysis of WG
4.3. Preparation of AFs
4.4. SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.5. ThT Fluorescence Assay
4.6. Surface Hydrophobicity Analysis
4.7. ζ-Potential Measurements
4.8. FTIR Spectroscopy
4.9. SEM
4.10. TEM
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, Y.P.; Mezzenga, R. Food protein amyloid fibrils: Origin, structure, formation, characterization, applications and health implications. Adv. Colloid Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef] [PubMed]
- Riek, R.; Eisenberg, D.S. The activities of amyloids from a structural perspective. Nature 2016, 539, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.S.; Sawaya, M.R. Structural Studies of Amyloid Proteins at the Molecular Level. Annu. Rev. Biochem. 2017, 86, 69–95. [Google Scholar] [CrossRef]
- Athamneh, A.I.; Barone, J.R. Enzyme-mediated self-assembly of highly ordered structures from disordered proteins. In Proceedings of the Conference on Smart Materials, Adaptive Structures and Intelligent Systems, Ellicott City, MD, USA, 28–30 October 2008. [Google Scholar] [CrossRef]
- Jansens, K.J.A.; Lambrecht, M.A.; Rombouts, I.; Monge Morera, M.; Brijs, K.; Rousseau, F.; Schymkowitz, J.; Delcour, J.A. Conditions Governing Food Protein Amyloid Fibril Formation-Part I: Egg and Cereal Proteins. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1256–1276. [Google Scholar] [CrossRef]
- Lambrecht, M.A.; Jansens, K.J.A.; Rombouts, I.; Brijs, K.; Rousseau, F.; Schymkowitz, J.; Delcour, J.A. Conditions Governing Food Protein Amyloid Fibril Formation. Part II: Milk and Legume Proteins. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1277–1291. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Halford, N.G.; Belton, P.S.; Tatham, A.S. The structure and properties of gluten: An elastic protein from wheat grain. Philos. Trans. R. Soc. B 2002, 357, 133–142. [Google Scholar] [CrossRef]
- Day, L.; Augustin, M.A.; Batey, I.L.; Wrigley, C.W. Wheat-gluten uses and industry needs. Trends Food Sci. Technol. 2006, 17, 82–90. [Google Scholar] [CrossRef]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef]
- Ma, W.J.; Yu, Z.T.; She, M.Y.; Zhao, Y.; Islam, S. Wheat gluten protein and its impacts on wheat processing quality. Front. Agric. Sci. Eng. 2019, 6, 279–287. [Google Scholar] [CrossRef]
- Chen, S.M.; Berthelier, V.; Hamilton, J.B.; O’Nuallain, B.; Wetzel, R. Amyloid-like Features of Polyglutamine Aggregates and Their Assembly Kinetics. Biochemistry 2002, 41, 7391–7399. [Google Scholar] [CrossRef]
- Ridgley, D.M.; Claunch, E.C.; Lee, P.W.; Barone, J.R. The role of protein hydrophobicity in conformation change and self-assembly into large amyloid fibers. Biomacromolecules 2014, 15, 1240–1247. [Google Scholar] [CrossRef]
- Ridgley, D.M.; Ebanks, K.C.; Barone, J.R. Peptide mixtures can self-assemble into large amyloid fibers of varying size and morphology. Biomacromolecules 2011, 12, 3770–3779. [Google Scholar] [CrossRef]
- Chen, Y.L.; Liu, Q.R.; Yang, F.W.; Yu, H.; Xie, Y.F.; Yao, W.R. Lysozyme amyloid fibril: Regulation, application, hazard analysis, and future perspectives. Int. J. Biol. Macromol. 2022, 200, 151–161. [Google Scholar] [CrossRef]
- Loveday, S.M.; Wang, X.L.; Rao, M.A.; Anema, S.G.; Singh, H. beta-Lactoglobulin nanofibrils: Effect of temperature on fibril formation kinetics, fibril morphology and the rheological properties of fibril dispersions. Food Hydrocoll. 2012, 27, 242–249. [Google Scholar] [CrossRef]
- Loveday, S.M.; Anema, S.G.; Singh, H. beta-Lactoglobulin nanofibrils: The long and the short of it. Int. Dairy J. 2017, 67, 35–45. [Google Scholar] [CrossRef]
- Vanik, V.; Bednarikova, Z.; Fabriciova, G.; Wang, S.S.S.; Gazova, Z.; Fedunova, D. Modulation of Insulin Amyloid Fibrillization in Imidazolium-Based Ionic Liquids with Hofmeister Series Anions. Int. J. Mol. Sci. 2023, 24, 9699. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Gao, Y.X.; Chang, Y.Y.; Sun, C.X.; Fang, Y.P. Concentration-Regulated Fibrillation of Soy Protein: Structure and In Vitro Digestion. J. Agric. Food Chem. 2023, 71, 11170–11179. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, L.; Geng, H.; Zhang, X.X.; Chen, Z.X. Formation, structural characteristics, foaming and emulsifying properties of rice glutelin fibrils. Food Chem. 2021, 354, 129554. [Google Scholar] [CrossRef]
- Zhou, J.T.; Li, T.; Peydayesh, M.; Usuelli, M.; Lutz-Bueno, V.; Teng, J.; Wang, L.; Mezzenga, R. Oat Plant Amyloids for Sustainable Functional Materials. Adv. Sci. 2022, 9, 2104445. [Google Scholar] [CrossRef] [PubMed]
- Monge-Morera, M.; Lambrecht, M.A.; Deleu, L.J.; Louros, N.N.; Rousseau, F.; Schymkowitz, J.; Delcour, J.A. Heating Wheat Gluten Promotes the Formation of Amyloid-like Fibrils. ACS Omega 2021, 6, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Ridgley, D.M.; Barone, J.R. Evolution of the amyloid fiber over multiple length scales. Acs Nano 2013, 7, 1006–1015. [Google Scholar] [CrossRef]
- Ridgley, D.M.; Claunch, E.C.; Barone, J.R. The effect of processing on large, self-assembled amyloid fibers. Soft Matter 2012, 8, 10298–10306. [Google Scholar] [CrossRef]
- Lambrecht, M.A.; Monge-Morera, M.; Godefroidt, T.; Vluymans, N.; Deleu, L.J.; Goos, P.; Schymkowitz, J.; Rousseau, F.; Delcour, J.A. Hydrothermal Treatments Cause Wheat Gluten-Derived Peptides to Form Amyloid-like Fibrils. J. Agric. Food Chem. 2021, 69, 1963–1974. [Google Scholar] [CrossRef]
- Wouters, A.G.B.; Rombouts, I.; Fierens, E.; Brijs, K.; Delcour, J.A. Relevance of the Functional Properties of Enzymatic Plant Protein Hydrolysates in Food Systems. Compr. Rev. Food Sci. Food Saf. 2016, 15, 786–800. [Google Scholar] [CrossRef] [PubMed]
- Wouters, A.G.B.; Rombouts, I.; Fierens, E.; Brijs, K.; Delcour, J.A. Enzymatically Hydrolyzed Wheat Gluten as a Foaming Agent in Food: Incorporation in a Meringue Recipe as a Proof-of-Concept. J. Food Sci. 2018, 83, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Athamneh, A.; Barone, J. Hierarchical Self-Assembly of Tryptic Peptides from Wheat Gluten. In Proceedings of the 2nd Annual ASME Conference on Smart Materials, Adaptive Structures and Intelligent Systems, Oxnard, CA, USA, 21–23 September 2009. [Google Scholar]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta 2010, 1804, 1405–1412. [Google Scholar] [CrossRef]
- Chen, X.Y.; Ru, Y.; Chen, F.L.; Wang, X.C.; Zhao, X.Y.; Ao, Q. FTIR spectroscopic characterization of soy proteins obtained through AOT reverse micelles. Food Hydrocoll. 2013, 31, 435–437. [Google Scholar] [CrossRef]
- Kannan, P.P.; Karthick, N.K.; Arivazhagan, G. Hydrogen bond interactions in the binary solutions of formamide with methanol: FTIR spectroscopic and theoretical studies. Spectrochim. Acta Part A 2020, 229, 117892. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiong, H.; Selomulya, C.; Chen, X.D.; Zhong, H.L.; Wang, S.Q.; Sun, W.J.; Zhou, Q. Enzymatic hydrolysis of rice dreg protein: Effects of enzyme type on the functional properties and antioxidant activities of recovered proteins. Food Chem. 2012, 134, 1360–1367. [Google Scholar] [CrossRef]
- Hu, Y.; He, C.X.; Jiang, C.J.; Liao, Y.; Xiong, H.; Zhao, Q. Complexation with whey protein fibrils and chitosan: A potential vehicle for curcumin with improved aqueous dispersion stability and enhanced antioxidant activity. Food Hydrocoll. 2020, 104, 105729. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, Y.Y.; Li, Z.Y.; Li, T.; Shi, Y.G.; Xie, H.J.; Li, Y.; Su, H.H.; Li, Z.P. Application of Whey Protein Isolate Fibrils in Encapsulation and Protection of β-carotene. Food Chem. 2020, 346, 128963. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, R.A.; Furtado, G.D.; Netto, F.M.; Cunha, R.L. Assessing the potential of whey protein fibril as emulsifier. J. Food Eng. 2018, 223, 99–108. [Google Scholar] [CrossRef]
- Cabuk, M.; Yavuz, M.; Unal, H.I. Electrokinetic, electrorheological and viscoelastic properties of Polythiophene- graft -Chitosan copolymer particles. Colloids Surf. A 2016, 510, 231–238. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Lin, Y.X.; Yagi, H.; Lee, Y.H.; Kitayama, H.; Sakurai, K.; So, M.; Ogi, H.; Naiki, H.; Goto, Y. Distinguishing crystal-like amyloid fibrils and glass-like amorphous aggregates from their kinetics of formation. Proc. Natl. Acad. Sci. USA 2012, 109, 14446–14451. [Google Scholar] [CrossRef] [PubMed]
- Ridgley, D.M.; Freedman, B.G.; Lee, P.W.; Barone, J.R. Genetically encoded self-assembly of large amyloid fibers. Biomater. Sci. 2014, 2, 560–566. [Google Scholar] [CrossRef]
- Zheng, L.L.; She, M.H.; Ai, B.L.; Yang, Y.; Zheng, X.Y.; Wang, S.W.; Xiao, D.; Jiang, Z.G.; Sheng, Z.W. Construction and properties of an amyloid fiber ferulic acid chitosan double network hydrogel and its inhibition of AGEs activity. Food Hydrocoll. 2023, 139, 108536. [Google Scholar] [CrossRef]
- Hu, B.; Shen, Y.; Adamcik, J.; Fischer, P.; Schneider, M.; Loessner, M.J.; Mezzenga, R. Polyphenol-Binding Amyloid Fibrils Self-Assemble into Reversible Hydrogels with Antibacterial Activity. ACS Nano 2018, 12, 3385–3396. [Google Scholar] [CrossRef]
- Monge-Morera, M.; Lambrecht, M.A.; Deleu, L.J.; Gallardo, R.; Louros, N.N.; De Vleeschouwer, M.; Rousseau, F.; Schymkowitz, J.; Delcour, J.A. Processing Induced Changes in Food Proteins: Amyloid Formation during Boiling of Hen Egg White. Biomacromolecules 2020, 21, 2218–2228. [Google Scholar] [CrossRef]
- Lambrecht, M.A.; Rombouts, I.; De Ketelaere, B.; Delcour, J.A. Prediction of heat-induced polymerization of different globular food proteins in mixtures with wheat gluten. Food Chem. 2017, 221, 1158–1167. [Google Scholar] [CrossRef]

| Degrees of Hydrolysis | Enzyme–Substrate Ratio | ||
|---|---|---|---|
| 1:100 | 1:500 | 1:1000 | |
| DH4 | 1.3 ± 0.1 (h) a | 2.2 ± 0.2 (h) a | 4.3 ± 0.2 (h) b |
| DH5 | 2.7 ± 0.15 (h) a | 3.5 ± 0.15 (h) a | 5.8 ± 0.32 (h) b |
| DH6 | 4.5 ± 0.25 (h) a | 4.5 ± 0.18 (h) a | 7 ± 0.31 (h) b |
| DH7 | 6.1 ± 0.25 (h) a | 7.5 ± 0.25 (h) a | 10 ± 0.25 (h) b |
| DH8 | 7.6 ± 0.15 (h) a | 8.5 ± 0.15 (h) a | 12 ± 0.31 (h) b |
| Degrees of Hydrolysis | Area (%) | ||||
|---|---|---|---|---|---|
| β-Sheet | Random Coil | α-Helix | Antiparallel β-Sheet | β-Turn | |
| DH4 | 30.7484 | 17.3761 | 34.5704 | 9.0298 | 12.2753 |
| DH5 | 31.9271 | 15.7266 | 33.3201 | 8.067 | 10.9592 |
| DH6 | 31.3268 | 14.8518 | 34.9059 | 8.1665 | 10.7489 |
| DH7 | 30.1374 | 16.1288 | 34.1976 | 8.2575 | 11.2787 |
| DH8 | 29.7842 | 17.635 | 31.6121 | 8.8613 | 12.1073 |
| Degrees of Hydrolysis | Area (%) | |||||
|---|---|---|---|---|---|---|
| Δβ-Sheet * | Β-Sheet | Random Coil | α-Helix | Antiparallel β-Sheet | β-Turn | |
| DH4 | 13.037 | 43.7854 | 25.1712 | 14.3445 | 5.6842 | 10.6739 |
| DH5 | 14.3558 | 46.2829 | 16.7061 | 17.7164 | 6.0611 | 12.9063 |
| DH6 | 19.1468 | 50.4736 | 21.5308 | 12.6679 | 5.7047 | 9.48678 |
| DH7 | 14.9632 | 45.1006 | 28.0488 | 10.3465 | 5.7071 | 10.5248 |
| DH8 | 14.6054 | 44.3896 | 15.5662 | 23.7324 | 6.1335 | 10.1332 |
| Amide I Structural Assignment | Wavenumber (cm−1) |
|---|---|
| cross-β | 1611–1637 |
| Random coil | 1637–1647 |
| α-helix | 1647–1662 |
| β-turn | 1662–1678; 1689–1699 |
| antiparallel β-sheet | 1679–1688 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Lv, S.; Ren, F.; Liu, J.; Wang, J. Degree of Hydrolysis Regulated by Enzyme Mediation of Wheat Gluten Fibrillation: Structural Characterization and Analysis of the Mechanism of Action. Int. J. Mol. Sci. 2023, 24, 13529. https://doi.org/10.3390/ijms241713529
Zhang H, Lv S, Ren F, Liu J, Wang J. Degree of Hydrolysis Regulated by Enzyme Mediation of Wheat Gluten Fibrillation: Structural Characterization and Analysis of the Mechanism of Action. International Journal of Molecular Sciences. 2023; 24(17):13529. https://doi.org/10.3390/ijms241713529
Chicago/Turabian StyleZhang, Huijuan, Shihao Lv, Feiyue Ren, Jie Liu, and Jing Wang. 2023. "Degree of Hydrolysis Regulated by Enzyme Mediation of Wheat Gluten Fibrillation: Structural Characterization and Analysis of the Mechanism of Action" International Journal of Molecular Sciences 24, no. 17: 13529. https://doi.org/10.3390/ijms241713529
APA StyleZhang, H., Lv, S., Ren, F., Liu, J., & Wang, J. (2023). Degree of Hydrolysis Regulated by Enzyme Mediation of Wheat Gluten Fibrillation: Structural Characterization and Analysis of the Mechanism of Action. International Journal of Molecular Sciences, 24(17), 13529. https://doi.org/10.3390/ijms241713529











