Multivessel Coronary Artery Disease Complicated by Diabetes Mellitus Has a Relatively Small Effect on Endothelial and Lipoprotein Lipases Expression in the Human Atrial Myocardium and Coronary Perivascular Adipose Tissue
Abstract
:1. Introduction
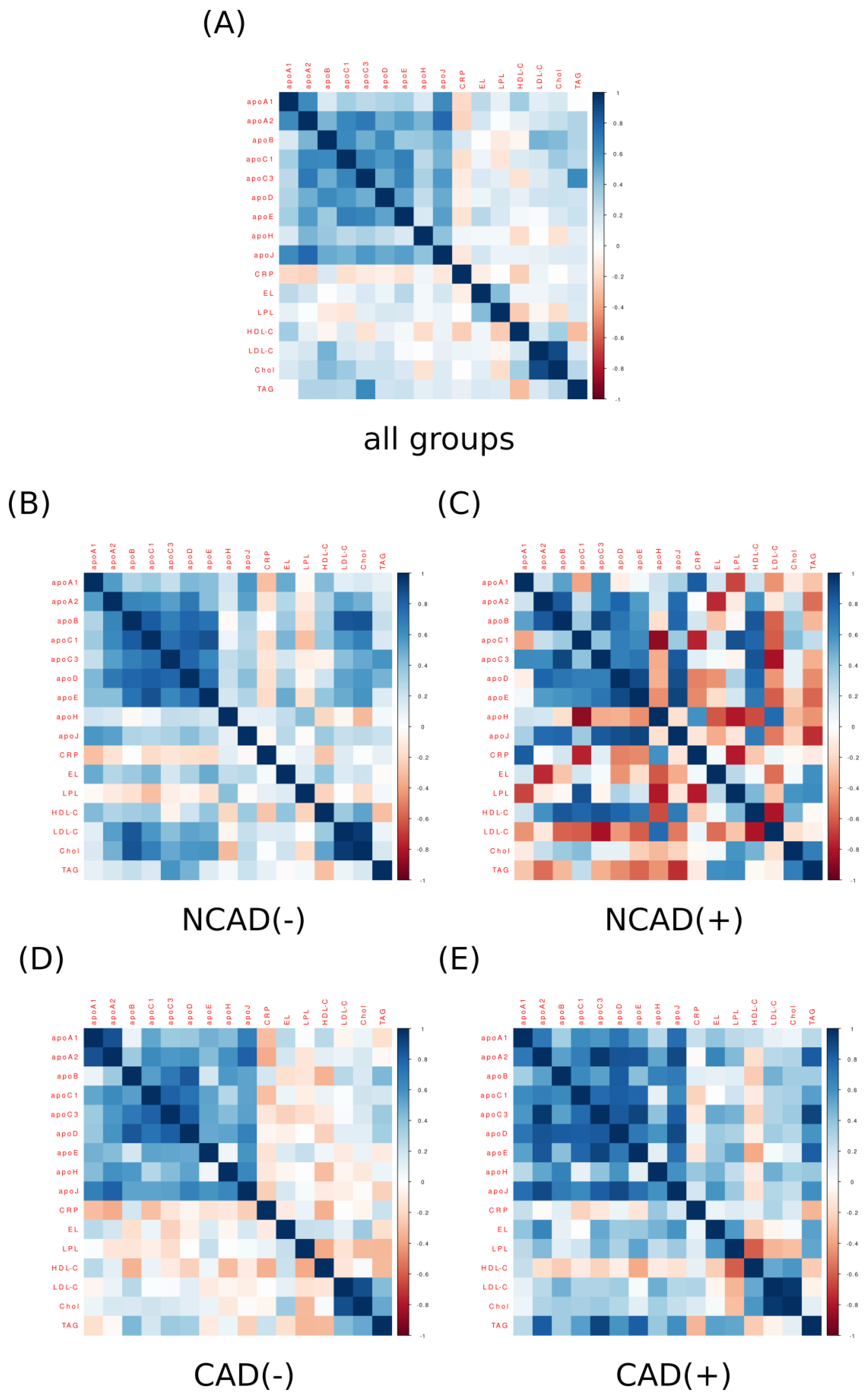
2. Results
2.1. Basic Characteristics
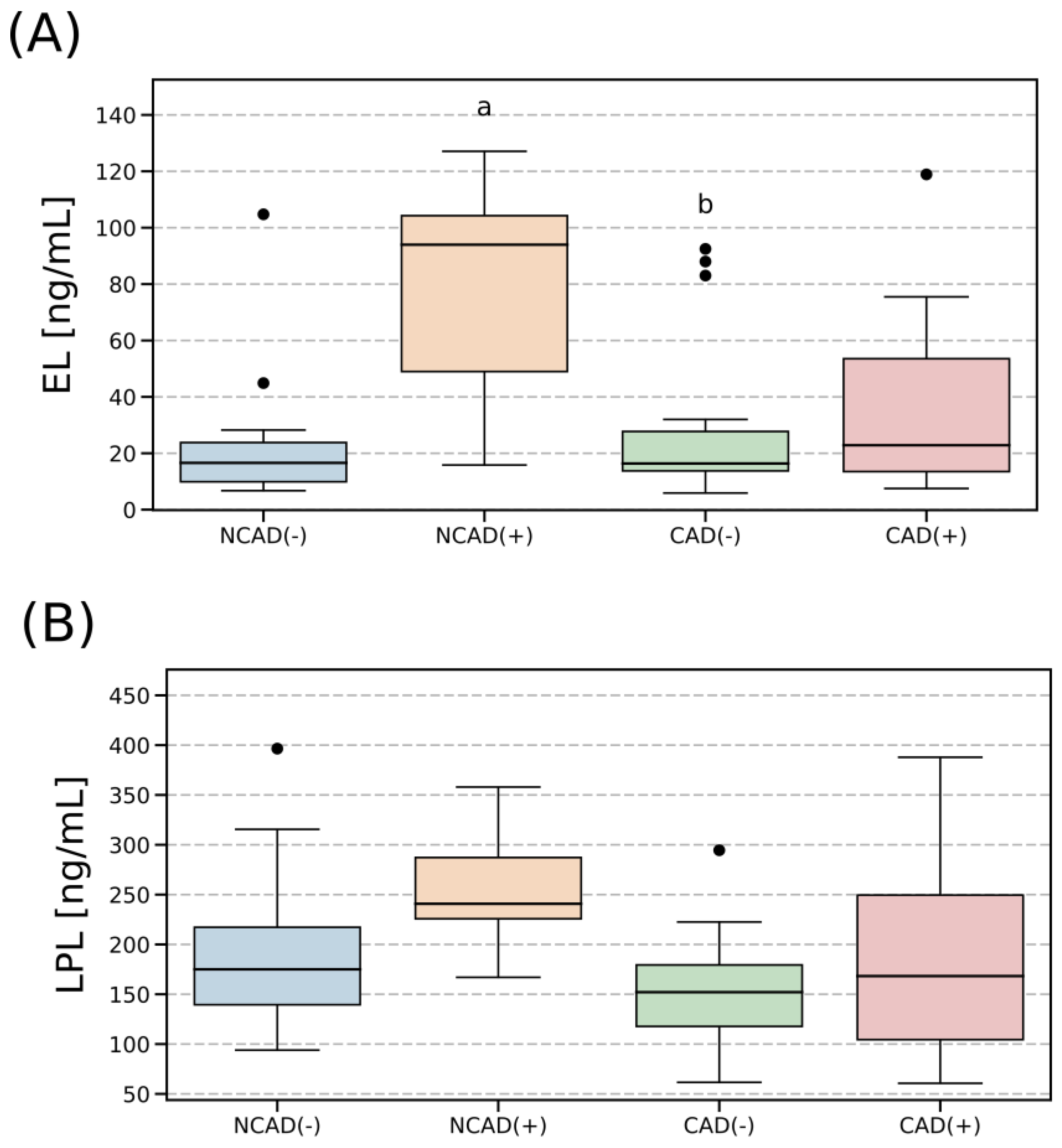
2.2. Plasma Concentration of EL and LPL
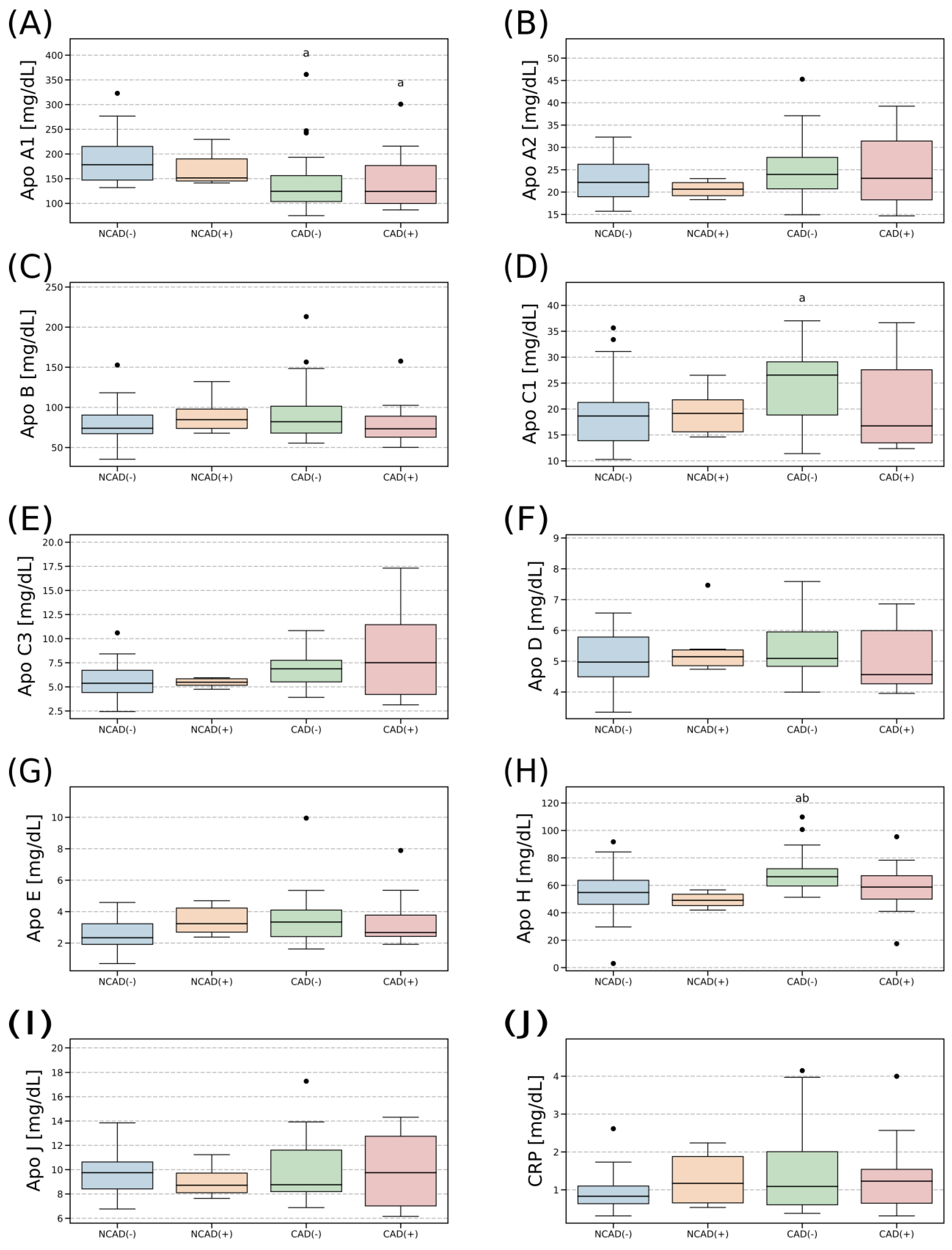
2.3. Plasma Concentration of Apolipoproteins
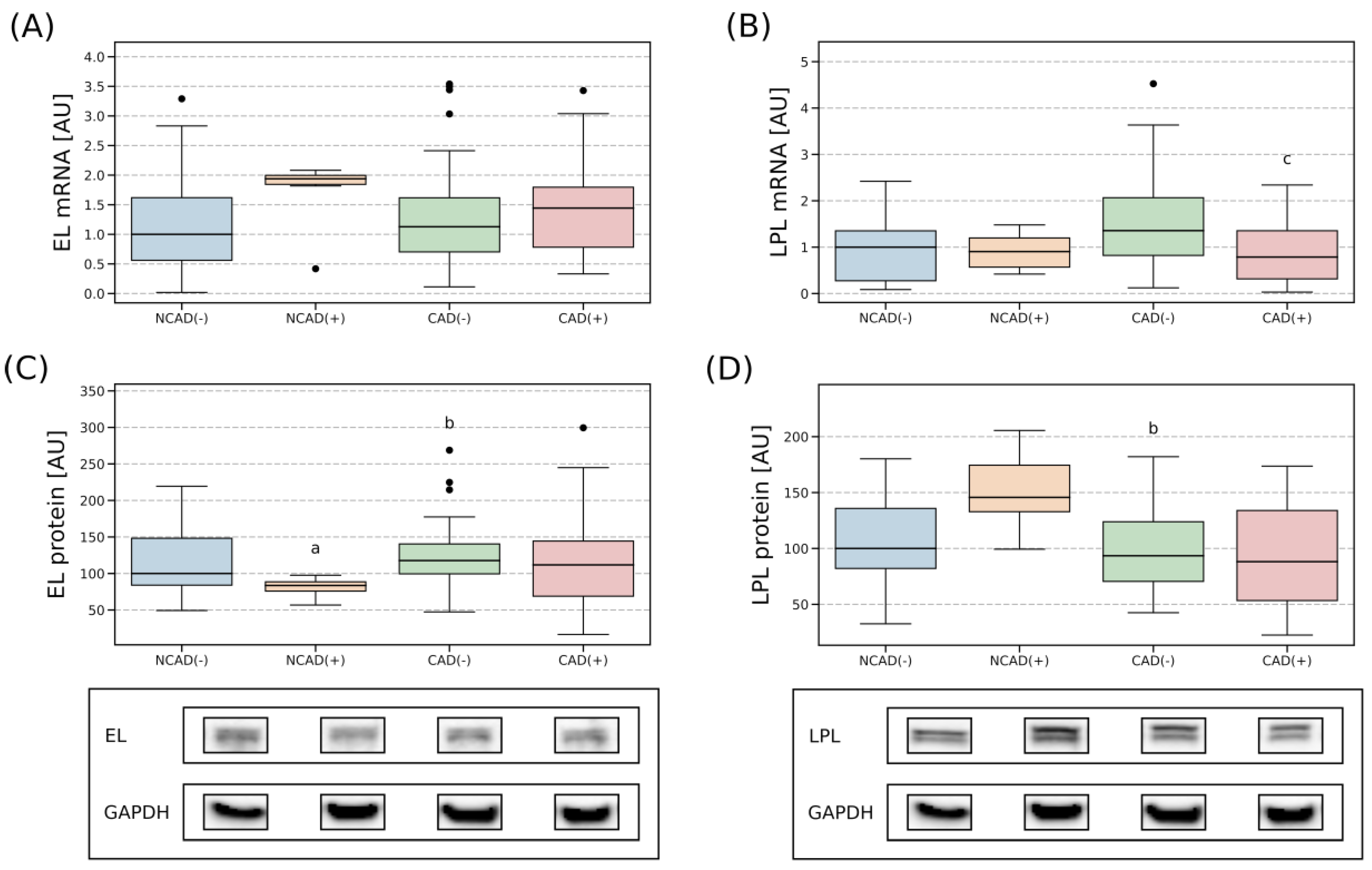
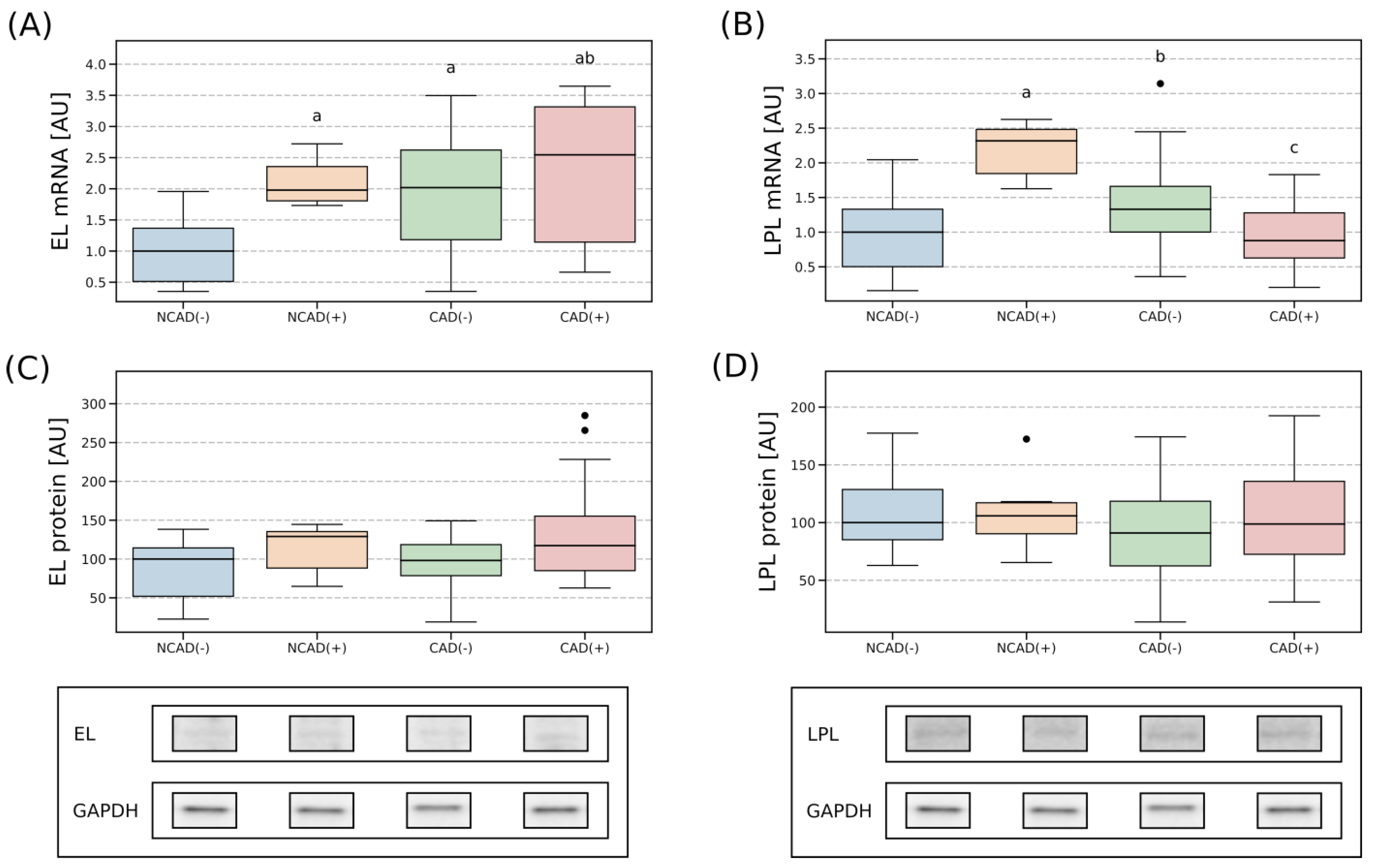
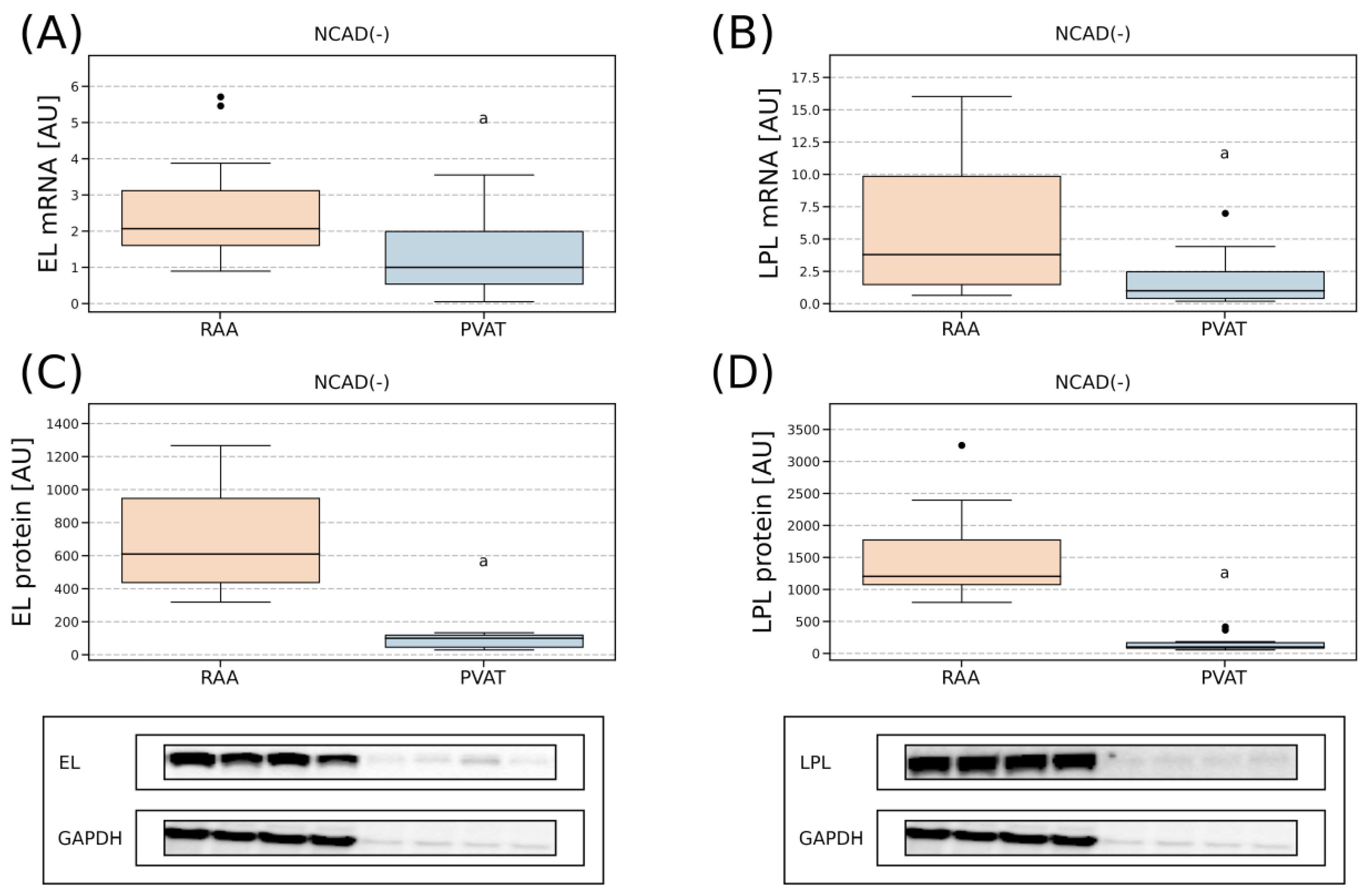
2.4. Tissue Expression of EL and LPL
2.4.1. Right Atrial Appendage
2.4.2. Perivascular Adipose Tissue
2.4.3. Comparison of Lipases Expression in the Myocardium and PVAT
3. Discussion
3.1. Tissue Expression of the EL and LPL in Patients with CAD
3.1.1. Atrial Myocardium
3.1.2. Perivascular Adipose Tissue
3.2. Comparison of the Lipases Expression in the Myocardium and PVAT
4. Materials and Methods
4.1. Patients
- Patients qualified for mitral or aortic valve replacement without angiographically confirmed coronary artery disease and without diagnosed type 2 diabetes mellitus (n = 28 patients, designation of the group: NCAD(−)).
- Patients qualified for mitral or aortic valve replacement without angiographically confirmed coronary artery disease and diagnosed with type 2 diabetes mellitus (n = 6 patients, designation of the group: NCAD(+)).
- Patients with multivessel coronary artery disease confirmed angiographically and qualified for coronary artery bypass grafting without diagnosed type 2 diabetes mellitus (n = 45 patients, designation of the group: CAD(−)).
- Patients with multivessel coronary artery disease confirmed angiographically and qualified for coronary artery bypass grafting with diagnosed type 2 diabetes mellitus (n = 23 patients, designation of the group: CAD(+)).
4.2. Blood Collection
4.3. Determination of the Apolipoprotein Concentrations
4.4. ELISA
4.5. Quantitative Real-Time PCR
4.6. Protein Extraction and Western Blot
4.7. Statistics
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Augustus, A.S.; Kako, Y.; Yagyu, H.; Goldberg, I.J. Routes of FA delivery to cardiac muscle: Modulation of lipoprotein lipolysis alters uptake of TG-derived FA. Am. J. Physiol. Endocrinol. Metab. 2003, 284, 331–339. [Google Scholar] [CrossRef]
- Agrawal, H.; Choy, H.H.K.; Liu, J.; Auyoung, M.; Albert, M.A. Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, E185–E192. [Google Scholar] [CrossRef] [PubMed]
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef] [PubMed]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef]
- Noyes, A.M. Cardiac adipose tissue and its relationship to diabetes mellitus and cardiovascular disease. World J. Diabetes 2014, 5, 868. [Google Scholar] [CrossRef] [PubMed]
- Nusca, A.; Tuccinardi, D.; Albano, M.; Cavallaro, C.; Ricottini, E.; Manfrini, S.; Pozzilli, P.; Di Sciascio, G. Glycemic variability in the development of cardiovascular complications in diabetes. Diabetes Metab. Res. Rev. 2018, 34, e3047. [Google Scholar] [CrossRef]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.A.; Thorpe, J.E.; Testa, R.; Boemi, M.; Giugliano, D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008, 57, 1349–1354. [Google Scholar] [CrossRef]
- Young, S.G.; Fong, L.G.; Beigneux, A.P.; Allan, C.M.; He, C.; Jiang, H.; Nakajima, K.; Meiyappan, M.; Birrane, G.; Ploug, M. GPIHBP1 and Lipoprotein Lipase, Partners in Plasma Triglyceride Metabolism. Cell Metab. 2019, 30, 51–65. [Google Scholar] [CrossRef]
- Kersten, S. Physiological regulation of lipoprotein lipase. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 919–933. [Google Scholar] [CrossRef]
- Hitsumoto, T.; Ohsawa, H.; Uchi, T.; Noike, H.; Kanai, M.; Yoshinuma, M.; Miyashita, Y.; Watanabe, H.; Shirai, K. Preheparin serum lipoprotein lipase mass is negatively related to coronary atherosclerosis. Atherosclerosis 2000, 153, 391–396. [Google Scholar] [CrossRef]
- Kobayashi, J.; Mabuchi, H. Lipoprotein lipase and atherosclerosis. Ann. Clin. Biochem. 2015, 52, 632–637. [Google Scholar] [CrossRef]
- Paradis, M.E.; Lamarche, B. Endothelial lipase: Its role in cardiovascular disease. Can. J. Cardiol. 2006, 22, 31B–34B. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Ishida, T.; Rader, D.J. Update on the role of endothelial lipase in high-density lipoprotein metabolism, reverse cholesterol transport, and atherosclerosis. Circ. J. 2010, 74, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.I.; Dichek, H.L.; Cioffi, J.A.; Choi, S.Y.; Leeper, N.J.; Quintana, L.; Kronmal, G.S.; Cooper, A.D.; Quertermous, T. Cloning of a unique lipase from endothelial cells extends the lipase gene family. J. Biol. Chem. 1999, 274, 14170–14175. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.E.; Han, S.-Y.; Wolfson, B.; Zhou, Q. The Role of Endothelial Lipase in Lipid Metabolism, Inflammation, and Cancer. Histol. Histopathol. 2018, 33, 1–10. [Google Scholar]
- Ishida, T.; Choi, S.; Kundu, R.K.; Hirata, K.; Rubin, E.M.; Cooper, A.D.; Quertermous, T. Endothelial lipase is a major determinant of HDL level. J. Clin. Investig. 2003, 111, 347–355. [Google Scholar] [CrossRef]
- Zvintzou, E.; Karampela, D.S.; Vakka, A.; Xepapadaki, E.; Karavia, E.A.; Hatziri, A.; Giannopoulou, P.C.; Kypreos, K.E. High density lipoprotein in atherosclerosis and coronary heart disease: Where do we stand today? Vascul. Pharmacol. 2021, 141, 106928. [Google Scholar] [CrossRef]
- Ko, D.T.; Alter, D.A.; Guo, H.; Koh, M.; Lau, G.; Austin, P.C.; Booth, G.L.; Hogg, W.; Jackevicius, C.A.; Lee, D.S.; et al. High-Density Lipoprotein Cholesterol and Cause-Specific Mortality in Individuals Without Previous Cardiovascular Conditions: The CANHEART Study. J. Am. Coll. Cardiol. 2016, 68, 2073–2083. [Google Scholar] [CrossRef]
- Sasso, F.C.; Pafundi, P.C.; Gelso, A.; Bono, V.; Costagliola, C.; Marfella, R.; Sardu, C.; Rinaldi, L.; Galiero, R.; Acierno, C.; et al. High HDL cholesterol: A risk factor for diabetic retinopathy? Findings from NO BLIND study. Diabetes Res. Clin. Pract. 2019, 150, 236–244. [Google Scholar] [CrossRef]
- Annema, W.; Tietge, U.J.F. Role of hepatic lipase and endothelial lipase in high-density lipoprotein-mediated reverse cholesterol transport. Curr. Atheroscler. Rep. 2011, 13, 257–265. [Google Scholar] [CrossRef]
- Sun, S.; Dean, R.; Jia, Q.; Zenova, A.; Zhong, J.; Grayson, C.; Xie, C.; Lindgren, A.; Samra, P.; Sojo, L.; et al. Discovery of XEN445: A potent and selective endothelial lipase inhibitor raises plasma HDL-cholesterol concentration in mice. Bioorganic Med. Chem. 2013, 21, 7724–7734. [Google Scholar] [CrossRef] [PubMed]
- Badellino, K.O.; Wolfe, M.L.; Reilly, M.P.; Rader, D.J. Endothelial lipase concentrations are increased in metabolic syndrome and associated with coronary atherosclerosis. PLoS Med. 2006, 3, 0245–0252. [Google Scholar] [CrossRef] [PubMed]
- Miksztowicz, V.; Schreier, L.; Mccoy, M.; Lucero, D.; Fassio, E.; Billheimer, J.; Rader, D.J.; Berg, G. Role of SN1 lipases on plasma lipids in metabolic syndrome and obesity. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.W.M.; Tan, K.C.B.; Huang, Y.; Wong, Y. Type 2 diabetes mellitus and endothelial lipase. Atherosclerosis 2008, 198, 441–447. [Google Scholar] [CrossRef]
- Yu, X.; Lu, J.; Li, J.; Guan, W.; Deng, S.; Deng, Q.; Ye, H.; Han, W.; Yu, Y.; Zhang, R. Serum triglyceride lipase concentrations are independent risk factors for coronary artery disease and in-stent restenosis. J. Atheroscler. Thromb. 2019, 26, 762–774. [Google Scholar] [CrossRef]
- Sun, L.; Ishida, T.; Miyashita, K.; Kinoshita, N.; Mori, K.; Yasuda, T.; Toh, R.; Nakajima, K.; Imamura, S.; Hirata, K.I. Plasma activity of endothelial lipase impacts high-density lipoprotein metabolism and coronary risk factors in humans. J. Atheroscler. Thromb. 2014, 21, 313–321. [Google Scholar] [CrossRef]
- Trbušić, M.; Potočnjak, I.; Tiran, B.; Bodrožić-Džakić, T.; Milošević, M.; Degoricija, V.; Frank, S. Endothelial lipase plasma levels are increased in both sexes in stable coronary artery disease and only in women with acute coronary syndrome but not associated with the severity of coronary artery disease. Croat. Med. J. 2016, 57, 482–492. [Google Scholar] [CrossRef]
- Nagao, M.; Nakajima, H.; Toh, R.; Hirata, K.I.; Ishida, T. Cardioprotective effects of high-density lipoprotein beyond its anti-atherogenic action. J. Atheroscler. Thromb. 2018, 25, 985–993. [Google Scholar] [CrossRef]
- Adachi, Y.; Ueda, K.; Takimoto, E. Perivascular adipose tissue in vascular pathologies—A novel therapeutic target for atherosclerotic disease? Front. Cardiovasc. Med. 2023, 10, 1151717. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Adhikari, B.K.; Chen, L.; Liu, W.; Wang, Y.; Zhang, H. The role of epicardial adipose tissue dysfunction in cardiovascular diseases: An overview of pathophysiology, evaluation, and management. Front. Endocrinol. 2023, 14, 1167952. [Google Scholar] [CrossRef]
- Takaoka, M.; Nagata, D.; Kihara, S.; Shimomura, I.; Kimura, Y.; Tabata, Y.; Saito, Y.; Nagai, R.; Sata, M. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ. Res. 2009, 105, 906–911. [Google Scholar] [CrossRef]
- Edmondson, A.C.; Brown, R.J.; Kathiresan, S.; Cupples, L.A.; Demissie, S.; Manning, A.K.; Jensen, M.K.; Rimm, E.B.; Wang, J.; Rodrigues, A.; et al. Loss-of-function variants in endothelial lipase are a cause of elevated HDL cholesterol in humans. J. Clin. Investig. 2009, 119, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, I.; Kern, S.; Hrzenjak, A.; Eichmann, T.O.; Stojakovic, T.; Scharnagl, H.; Duta-Mare, M.; Kratky, D.; Marsche, G.; Frank, S. Impact of Endothelial Lipase on Cholesterol Efflux Capacity of Serum and High-density Lipoprotein. Sci. Rep. 2017, 7, 12485. [Google Scholar] [CrossRef] [PubMed]
- Badellino, K.O.; Wolfe, M.L.; Reilly, M.P.; Rader, D.J. Endothelial lipase is increased in vivo by inflammation in humans. Circulation 2008, 117, 678–685. [Google Scholar] [CrossRef]
- Paradis, M.E.; Badellino, K.O.; Rader, D.J.; Deshaies, Y.; Couture, P.; Archer, W.R.; Bergeron, N.; Lamarche, B. Endothelial lipase is associated with inflammation in humans. J. Lipid Res. 2006, 47, 2808–2813. [Google Scholar] [CrossRef]
- Ishida, T.; Choi, S.Y.; Kundu, R.K.; Spin, J.; Yamashita, T.; Hirata, K.I.; Kojima, Y.; Yokoyama, M.; Cooper, A.D.; Quertermous, T. Endothelial lipase modulates susceptibility to atherosclerosis in apolipoprotein-E-deficient mice. J. Biol. Chem. 2004, 279, 45085–45092. [Google Scholar] [CrossRef]
- Wang, C.; Nishijima, K.; Kitajima, S.; Niimi, M.; Yan, H.; Chen, Y.; Ning, B.; Matsuhisa, F.; Liu, E.; Zhang, J.; et al. Increased Hepatic Expression of Endothelial Lipase Inhibits Cholesterol Diet-Induced Hypercholesterolemia and Atherosclerosis in Transgenic Rabbits. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.W.S.; Paul, A.; Ma, K.; Li, L.; Chan, L. Endothelial lipase modulates HDL but has no effect on atherosclerosis development in apoE−/− and LDLR−/− mice. J. Lipid Res. 2005, 46, 2586–2594. [Google Scholar] [CrossRef]
- Crowe, T.; Sasiela, W.J.; Tsai, J.; Orazem, J.; Magorien, R.D.; Shaughnessy, C.O. Statin Therapy, LDL Cholesterol, C-Reactive Protein, and Coronary Artery Disease. N. Engl. J. Med. 2005, 352, 29–38. [Google Scholar]
- Hughes, T.A.; Calderon, R.M.; Diaz, S.; Mendez, A.J.; Goldberg, R.B. Lipoprotein composition in patients with type 1 diabetes mellitus: Impact of lipases and adipokines. J. Diabetes Complications 2016, 30, 657–668. [Google Scholar] [CrossRef]
- Hanyu, O.; Miida, T.; Obayashi, K.; Ikarashi, T.; Soda, S.; Kaneko, S.; Hirayama, S.; Suzuki, K.; Nakamura, Y.; Yamatani, K.; et al. Lipoprotein lipase (LPL) mass in preheparin serum reflects insulin sensitivity. Atherosclerosis 2004, 174, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, T.; Nakajima, K.; Yatsuzuka, S.; Shimomura, Y.; Kobayashi, J.; Machida, T.; Sumino, H.; Murakami, M. The role of circulating lipoprotein lipase and adiponectin on the particle size of remnant lipoproteins in patients with diabetes mellitus and metabolic syndrome. Clin. Chim. Acta 2015, 440, 123–132. [Google Scholar] [CrossRef]
- Han, H.; Dai, D.; Wang, W.; Zhu, J.; Zhu, Z.; Lu, L.; Zhang, R. Impact of serum levels of lipoprotein lipase, hepatic lipase, and endothelial lipase on the progression of coronary artery disease. J. Interv. Med. 2019, 2, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Contois, J.H.; Langlois, M.R.; Cobbaert, C.; Sniderman, A.D. Standardization of Apolipoprotein B, LDL-Cholesterol, and Non-HDL-Cholesterol. J. Am. Heart Assoc. 2023, 12, e030405. [Google Scholar] [CrossRef]
- Cochran, B.J.; Ong, K.L.; Manandhar, B.; Rye, K.A. APOA1: A Protein with Multiple Therapeutic Functions. Curr. Atheroscler. Rep. 2021, 23, 11. [Google Scholar] [CrossRef]
- Sasso, F.C.; Simeon, V.; Galiero, R.; Caturano, A.; De Nicola, L.; Chiodini, P.; Rinaldi, L.; Salvatore, T.; Lettieri, M.; Nevola, R.; et al. The number of risk factors not at target is associated with cardiovascular risk in a type 2 diabetic population with albuminuria in primary cardiovascular prevention. Post-hoc analysis of the NID-2 trial. Cardiovasc. Diabetol. 2022, 21, 235. [Google Scholar] [CrossRef]
- Moore, R.E.; Navab, M.; Millar, J.S.; Zimetti, F.; Hama, S.; Rothblat, G.H.; Rader, D.J. Increased atherosclerosis in mice lacking apolipoprotein A-I attributable to both impaired reverse cholesterol transport and increased inflammation. Circ. Res. 2005, 97, 763–771. [Google Scholar] [CrossRef]
- Rubin, E.M.; Ishida, B.Y.; Clift, S.M.; Krauss, R.M. Expression of human apolipoprotein A-I in transgenic mice results in reduced plasma levels of murine apolipoprotein A-I and the appearance of two new high density lipoprotein size subclasses. Proc. Natl. Acad. Sci. USA 1991, 88, 434–438. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Puri, R.; Ballantyne, C.M.; Jukema, J.W.; Kastelein, J.J.P.; Koenig, W.; Wright, R.S.; Kallend, D.; Wijngaard, P.; Borgman, M.; et al. Effect of infusion of high-density lipoprotein mimetic containing recombinant apolipoprotein A-I Milano on coronary disease in patients with an acute coronary syndrome in the MILANO-PILOT trial: A randomized clinical trial. JAMA Cardiol. 2018, 3, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Rouland, A.; Masson, D.; Lagrost, L.; Vergès, B.; Gautier, T.; Bouillet, B. Role of apolipoprotein C1 in lipoprotein metabolism, atherosclerosis and diabetes: A systematic review. Cardiovasc. Diabetol. 2022, 21, 272. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, U. Inflammation and Coronary Artery Disease. Curr. Vasc. Pharmacol. 2005, 1, 65–70. [Google Scholar] [CrossRef]
- Vigelsø, A.; Prats, C.; Ploug, T.; Dela, F.; Helge, J.W. Human skeletal muscle perilipin 2 and 3 expression varies with insulin sensitivity. J. Biomed. Sci. Eng. 2013, 6, 65–72. [Google Scholar] [CrossRef]
- Rodrigues, B.; Cam, M.C.; Jian, K.; Lim, F.; Sambandam, N.; Shenherd, G. Differential effects of streptozotocin-induced diabetes on cardiac lipoprotein lipase activity. Diabetes 1997, 46, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Chiu, A.P.L.; Bierende, D.; Lal, N.; Wang, F.; Wan, A.; Vlodavsky, I.; Hussein, B.; Rodrigues, B. Dual effects of hyperglycemia on endothelial cells and cardiomyocytes to enhance coronary LPL activity. Am. J. Physiol. Hear. Circ. Physiol. 2018, 314, H82–H94. [Google Scholar] [CrossRef] [PubMed]
- Willecke, F.; Scerbo, D.; Nagareddy, P.; Obunike, J.C.; Barrett, T.J.; Abdillahi, M.L.; Trent, C.M.; Huggins, L.A.; Fisher, E.A.; Drosatos, K.; et al. Lipolysis, and not hepatic lipogenesis, is the primary modulator of triglyceride levels in streptozotocin-induced diabetic mice. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 102–110. [Google Scholar] [CrossRef]
- Barchuk, M.; Schreier, L.; López, G.; Cevey, A.; Baldi, J.; Fernandez Tomé, M.; Goren, N.; Rubio, M.; Miksztowicz, V.; Berg, G. Glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 and angiopoietin-like protein 4 are associated with the increase of lipoprotein lipase activity in epicardial adipose tissue from diabetic patients. Atherosclerosis 2019, 288, 51–59. [Google Scholar] [CrossRef]
- Escaned, J.; Flores, A.; García-Pavía, P.; Segovia, J.; Jimenez, J.; Aragoncillo, P.; Salas, C.; Alfonso, F.; Hernández, R.; Angiolillo, D.J.; et al. Assessment of microcirculatory remodeling with intracoronary flow velocity and pressure measurements: Validation with endomyocardial sampling in cardiac allografts. Circulation 2009, 120, 1561–1568. [Google Scholar] [CrossRef]
- Corradi, D.; Callegari, S.; Benussi, S.; Nascimbene, S.; Pastori, P.; Calvi, S.; Maestri, R.; Astorri, E.; Pappone, C.; Alfieri, O. Regional left atrial interstitial remodeling in patients with chronic atrial fibrillation undergoing mitral-valve surgery. Virchows Arch. 2004, 445, 498–505. [Google Scholar] [CrossRef]
- Kolahdouzi, S.; Talebi-Garakani, E.; Hamidian, G.; Safarzade, A. Exercise training prevents high-fat diet-induced adipose tissue remodeling by promoting capillary density and macrophage polarization. Life Sci. 2019, 220, 32–43. [Google Scholar] [CrossRef]
- Knapp, M.; Górski, J.; Lewkowicz, J.; Lisowska, A.; Gil, M.; Wójcik, B.; Hirnle, T.; Chabowski, A.; Mikłosz, A. The gene and protein expression of the main components of the lipolytic system in human myocardium and heart perivascular adipose tissue. Effect of coronary atherosclerosis. Int. J. Mol. Sci. 2020, 21, 737. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]

| NCAD(−) | NCAD(+) | CAD(−) | CAD(+) | |
|---|---|---|---|---|
| Age (years) | 61.6 ± 8.52 | 65.3 ± 7.12 | 63.6 ± 8.1 | 67.3 ± 7.95 |
| Gender (female/male) | 9/19 | 3/3 | 5/40 | 8/15 bc |
| DM (no/yes) | 28/0 | 0/6 a | 45/0 b | 0/23 ac |
| Glucose (mg/dL) | 99.4 ± 16.32 | 120.3 ± 16.99 a | 95.5 ± 14.32 | 134.4 ± 30.18 ac |
| GFR (mL/min) | 93.0 ± 16.26 | 76.3 ± 23.69 | 88.7 ± 18.55 | 78.5 ± 18.63 |
| Total cholesterol (mg/dL) | 193.0 ± 42.69 | 172.3 ± 21.8 | 183.4 ± 40.55 | 169.4 ± 47.58 |
| HDL-C (mg/dL) | 52.9 ± 10.81 | 42.2 ± 11.00 | 46.6 ± 11.09 | 41.4 ± 8.83 a |
| LDL-C (mg/dL) | 116.9 ± 37.01 | 117.5 ± 11.69 | 108.3 ± 36.73 | 105.4 ± 48.68 |
| TAG (mg/dL) | 126.0 ± 56.4 | 114.0 ± 40.71 | 146.3 ± 82.52 | 163.4 ± 91.65 |
| CRP (mg/dL) | 5.2 ± 5.6 | 3.5 ± 2.6 | 5.1 ± 9.34 | 4.2 ± 4.26 |
| EF (%) | 52.4 ± 6.88 | 43.0 ± 11.78 | 53.0 ± 8.63 | 52.8 ± 8.99 |
| BMI (kg/m2) | 28.5 ± 4.62 | 29.4 ± 5.34 | 27.4 ± 3.31 | 29.9 ± 4.57 |
| Hb (g/dL) | 13.9 ± 1.29 | 13.4 ± 1.48 | 13.6 ± 1.42 | 13.6 ± 1.34 |
| MI (no/yes) | 28/0 | 6/0 | 28/17 a | 16/7 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knapp, M.; Łukaszuk, B.; Lisowska, A.; Hirnle, T.; Górski, J.; Chabowski, A.; Mikłosz, A. Multivessel Coronary Artery Disease Complicated by Diabetes Mellitus Has a Relatively Small Effect on Endothelial and Lipoprotein Lipases Expression in the Human Atrial Myocardium and Coronary Perivascular Adipose Tissue. Int. J. Mol. Sci. 2023, 24, 13552. https://doi.org/10.3390/ijms241713552
Knapp M, Łukaszuk B, Lisowska A, Hirnle T, Górski J, Chabowski A, Mikłosz A. Multivessel Coronary Artery Disease Complicated by Diabetes Mellitus Has a Relatively Small Effect on Endothelial and Lipoprotein Lipases Expression in the Human Atrial Myocardium and Coronary Perivascular Adipose Tissue. International Journal of Molecular Sciences. 2023; 24(17):13552. https://doi.org/10.3390/ijms241713552
Chicago/Turabian StyleKnapp, Małgorzata, Bartłomiej Łukaszuk, Anna Lisowska, Tomasz Hirnle, Jan Górski, Adrian Chabowski, and Agnieszka Mikłosz. 2023. "Multivessel Coronary Artery Disease Complicated by Diabetes Mellitus Has a Relatively Small Effect on Endothelial and Lipoprotein Lipases Expression in the Human Atrial Myocardium and Coronary Perivascular Adipose Tissue" International Journal of Molecular Sciences 24, no. 17: 13552. https://doi.org/10.3390/ijms241713552





