Can Nitric Oxide-Based Therapy Be Improved for the Treatment of Cancers? A Perspective
Abstract
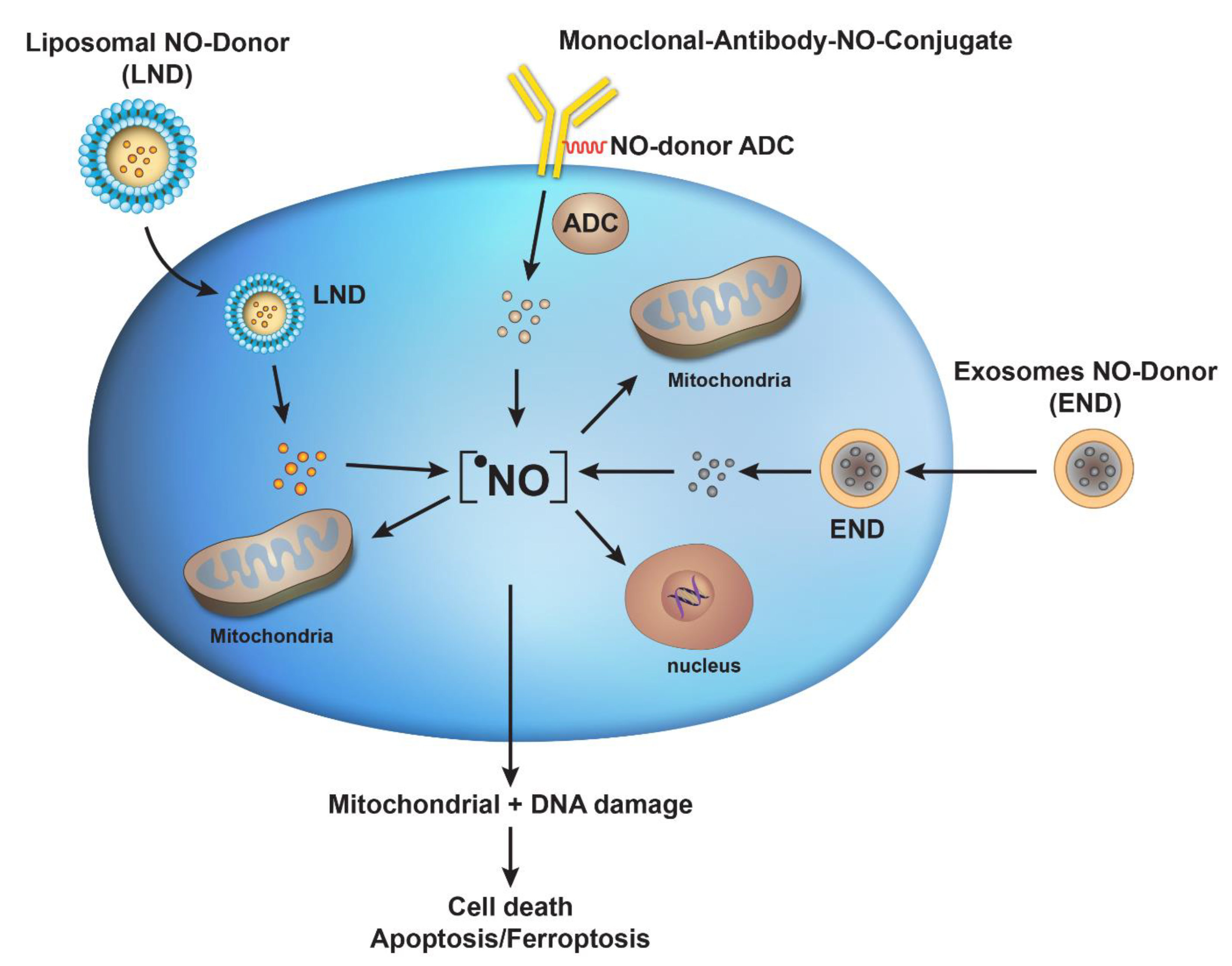
:1. Introduction
2. Prodrug Strategy for NO-Donors
3. Antibody–Drug Conjugates for Targeted Delivery of •NO in Tumors
4. Liposomes-Based Delivery of NO-Donors
5. Exosomes as a Delivery System for NO-Donors
6. Conclusions and Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Hirst, D.; Robson, T. Nitric oxide in cancer therapeutics: Interaction with cytotoxic chemotherapy. Curr. Pharm. Des. 2010, 16, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, S.K.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.K. Role of Oxygen and Nitrogen Radicals in the Mechanism of Anticancer Drug Cytotoxicity. J. Cancer Sci. Ther. 2020, 12, 10–18. [Google Scholar]
- Thomas, D.D.; Liu, X.; Kantrow, S.P.; Lancaster, J.R., Jr. The biological lifetime of nitric oxide: Implications for the perivascular dynamics of NO and O2. Proc. Natl. Acad. Sci. USA 2001, 98, 355–360. [Google Scholar] [CrossRef]
- Stamler, J.S.; Lamas, S.; Fang, F.C. Nitrosylation. the prototypic redox-based signaling mechanism. Cell 2001, 106, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Holotiuk, V.V.; Kryzhanivska, A.Y.; Churpiy, I.K.; Tataryn, B.B.; Ivasiutyn, D.Y. Role of nitric oxide in pathogenesis of tumor growth and its possible application in cancer treatment. Exp. Oncol. 2019, 41, 210–215. [Google Scholar] [CrossRef]
- Porrini, C.; Ramarao, N.; Tran, S.L. Dr. NO and Mr. Toxic—The versatile role of nitric oxide. Biol. Chem. 2020, 401, 547–572. [Google Scholar] [CrossRef]
- Burke, A.J.; Sullivan, F.J.; Giles, F.J.; Glynn, S.A. The yin and yang of nitric oxide in cancer progression. Carcinogenesis 2013, 34, 503–512. [Google Scholar] [CrossRef]
- Huang, Z.; Fu, J.; Zhang, Y. Nitric Oxide Donor-Based Cancer Therapy: Advances and Prospects. J. Med. Chem. 2017, 60, 7617–7635. [Google Scholar] [CrossRef]
- Huerta, S. Nitric oxide for cancer therapy. Future Sci. OA 2015, 1, FSO44. [Google Scholar] [CrossRef]
- Seabra, A.B.; de Lima, R.; Calderon, M. Nitric oxide releasing nanomaterials for cancer treatment: Current status and perspectives. Curr. Top. Med. Chem. 2015, 15, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Alimoradi, H.; Greish, K.; Gamble, A.B.; Giles, G.I. Controlled Delivery of Nitric Oxide for Cancer Therapy. Pharm. Nanotechnol. 2019, 7, 279–303. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yoon, B.; Dey, A.; Nguyen, V.Q.; Park, J.H. Recent progress in nitric oxide-generating nanomedicine for cancer therapy. J. Control. Release Off. J. Control. Release Soc. 2022, 352, 179–198. [Google Scholar] [CrossRef]
- Li, C.Y.; Anuraga, G.; Chang, C.P.; Weng, T.Y.; Hsu, H.P.; Ta, H.D.K.; Su, P.F.; Chiu, P.H.; Yang, S.J.; Chen, F.W.; et al. Repurposing nitric oxide donating drugs in cancer therapy through immune modulation. J. Exp. Clin. Cancer Res. 2023, 42, 22. [Google Scholar] [CrossRef]
- Pieretti, J.C.; Pelegrino, M.T.; Nascimento, M.H.M.; Tortella, G.R.; Rubilar, O.; Seabra, A.B. Small molecules for great solutions: Can nitric oxide-releasing nanomaterials overcome drug resistance in chemotherapy? Biochem. Pharmacol. 2020, 176, 113740. [Google Scholar] [CrossRef] [PubMed]
- Mintz, J.; Vedenko, A.; Rosete, O.; Shah, K.; Goldstein, G.; Hare, J.M.; Ramasamy, R.; Arora, H. Current Advances of Nitric Oxide in Cancer and Anticancer Therapeutics. Vaccines 2021, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Shami, P.J.; Saavedra, J.E.; Wang, L.Y.; Bonifant, C.L.; Diwan, B.A.; Singh, S.V.; Gu, Y.; Fox, S.D.; Buzard, G.S.; Citro, M.L.; et al. JS-K, a glutathione/glutathione S-transferase-activated nitric oxide donor of the diazeniumdiolate class with potent antineoplastic activity. Mol. Cancer Ther. 2003, 2, 409–417. [Google Scholar]
- Maciag, A.E.; Saavedra, J.E.; Chakrapani, H. The nitric oxide prodrug JS-K and its structural analogues as cancer therapeutic agents. Anticancer Agents Med. Chem. 2009, 9, 798–803. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Qu, W.; Leslie, E.; Bonifant, C.L.; Buzard, G.S.; Saavedra, J.E.; Keefer, L.K.; Waalkes, M.P. Nitric oxide prodrugs and metallochemotherapeutics: JS-K and CB-3-100 enhance arsenic and cisplatin cytolethality by increasing cellular accumulation. Mol. Cancer Ther. 2004, 3, 709–714. [Google Scholar] [CrossRef]
- Bonavida, B. Sensitizing activities of nitric oxide donors for cancer resistance to anticancer therapeutic drugs. Biochem. Pharmacol. 2020, 176, 113913. [Google Scholar] [CrossRef]
- Sinha, B.K.; Perera, L.; Cannon, R.E. Reversal of drug resistance by JS-K and nitric oxide in ABCB1- and ABCG2-expressing multi-drug resistant human tumor cells. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 120, 109468. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.K.; Perera, L.; Cannon, R.E. NCX-4040, a Unique Nitric Oxide Donor, Induces Reversal of Drug-Resistance in Both ABCB1- and ABCG2-Expressing Multidrug Human Cancer Cells. Cancers 2021, 13, 1680. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Feyzizadeh, M.; Barfar, A.; Nouri, Z.; Sarfraz, M.; Zakeri-Milani, P.; Valizadeh, H. Overcoming multidrug resistance through targeting ABC transporters: Lessons for drug discovery. Expert Opin. Drug Discov. 2022, 17, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.K.; Kumar, A.; Mason, R.P. Nitric oxide inhibits ATPase activity and induces resistance to topoisomerase II-poisons in human MCF-7 breast tumor cells. Biochem. Biophys. Rep. 2017, 10, 252–259. [Google Scholar] [CrossRef]
- Sinha, B.K.; Bortner, C.D.; Mason, R.P.; Cannon, R.E. Nitric oxide reverses drug resistance by inhibiting ATPase activity of p-glycoprotein in human multi-drug resistant cancer cells. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2806–2814. [Google Scholar] [CrossRef]
- Watanabe, H.; Kakihana, M.; Ohtsuka, S.; Sugishita, Y. Preventive effects of angiotensin-converting enzyme inhibitors on nitrate tolerance during continuous transdermal application of nitroglycerin in patients with chronic heart failure. Jpn. Circ. J. 1998, 62, 353–358. [Google Scholar] [CrossRef]
- Kruzliak, P.; Pechanova, O.; Kara, T. New perspectives of nitric oxide donors in cardiac arrest and cardiopulmonary resuscitation treatment. Heart Fail. Rev. 2014, 19, 383–390. [Google Scholar] [CrossRef]
- He, M.; Wang, D.; Xu, Y.; Jiang, F.; Zheng, J.; Feng, Y.; Cao, J.; Zhou, X. Nitric Oxide-Releasing Platforms for Treating Cardiovascular Disease. Pharmaceutics 2022, 14, 1345. [Google Scholar] [CrossRef]
- Yasuda, H.; Yamaya, M.; Nakayama, K.; Sasaki, T.; Ebihara, S.; Kanda, A.; Asada, M.; Inoue, D.; Suzuki, T.; Okazaki, T.; et al. Randomized phase II trial comparing nitroglycerin plus vinorelbine and cisplatin with vinorelbine and cisplatin alone in previously untreated stage IIIB/IV non-small-cell lung cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 688–694. [Google Scholar] [CrossRef]
- Siemens, D.R.; Heaton, J.P.; Adams, M.A.; Kawakami, J.; Graham, C.H. Phase II study of nitric oxide donor for men with increasing prostate-specific antigen level after surgery or radiotherapy for prostate cancer. Urology 2009, 74, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Heys, S.D.; Ogston, K.; Miller, I.; Hutcheon, A.W.; Walker, L.G.; Sarker, T.K.; Dewar, J.; Ah-See, A.K.; Eremin, O. Potentiation of the response to chemotherapy in patients with breast cancer by dietary supplementation with L-arginine: Results of a randomised controlled trial. Int. J. Oncol. 1998, 12, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Chakrapani, H. Site-directed delivery of nitric oxide to cancers. Nitric Oxide Biol. Chem./Off. J. Nitric Oxide Soc. 2014, 43, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; Blonquist, T.M.; Emadi, A.; Hasserjian, R.P.; Burke, M.; Lescinskas, C.; Neuberg, D.S.; Brunner, A.M.; Hobbs, G.; Hock, H.; et al. A phase 1 study of the antibody-drug conjugate brentuximab vedotin with re-induction chemotherapy in patients with CD30-expressing relapsed/refractory acute myeloid leukemia. Cancer 2020, 126, 1264–1273. [Google Scholar] [CrossRef]
- Sun, F.; Wang, Y.; Luo, X.; Ma, Z.; Xu, Y.; Zhang, X.; Lv, T.; Zhang, Y.; Wang, M.; Huang, Z.; et al. Anti-CD24 Antibody-Nitric Oxide Conjugate Selectively and Potently Suppresses Hepatic Carcinoma. Cancer Res. 2019, 79, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- Tfouni, E.; Truzzi, D.R.; Tavares, A.; Gomes, A.J.; Figueiredo, L.E.; Franco, D.W. Biological activity of ruthenium nitrosyl complexes. Nitric Oxide Biol. Chem./Off. J. Nitric Oxide Soc. 2012, 26, 38–53. [Google Scholar] [CrossRef]
- Ramos, L.C.B.; Rodrigues, F.P.; Biazzotto, J.C.; de Paula Machado, S.; Slep, L.D.; Hamblin, M.R.; da Silva, R.S. Targeting the mitochondrial VDAC in hepatocellular carcinoma using a polyclonal antibody-conjugated to a nitrosyl ruthenium complex. J. Biol. Inorg. Chem. 2018, 23, 903–916. [Google Scholar] [CrossRef]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- Kong, C.Y.; Guo, Z.; Song, P.; Zhang, X.; Yuan, Y.P.; Teng, T.; Yan, L.; Tang, Q.Z. Underlying the Mechanisms of Doxorubicin-Induced Acute Cardiotoxicity: Oxidative Stress and Cell Death. Int. J. Biol. Sci. 2022, 18, 760–770. [Google Scholar] [CrossRef]
- Postma, T.J.; Vermorken, J.B.; Liefting, A.J.; Pinedo, H.M.; Heimans, J.J. Paclitaxel-induced neuropathy. Ann. Oncol. 1995, 6, 489–494. [Google Scholar] [CrossRef]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Suchyta, D.J.; Schoenfisch, M.H. Controlled release of nitric oxide from liposomes. ACS Biomater. Sci. Eng. 2017, 3, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Suchyta, D.J.; Schoenfisch, M.H. Anticancer potency of nitric oxide-releasing liposomes. RSC Adv. 2017, 7, 53236–53246. [Google Scholar] [CrossRef] [PubMed]
- Suchyta, D.J.; Schoenfisch, M.H. Encapsulation of N-Diazeniumdiolates within Liposomes for Enhanced Nitric Oxide Donor Stability and Delivery. Mol. Pharm. 2015, 12, 3569–3574. [Google Scholar] [CrossRef]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, Biomedical Applications, and Stability Parameters with Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. [Google Scholar] [CrossRef]
- Kumar, D.N.; Chaudhuri, A.; Aqil, F.; Dehari, D.; Munagala, R.; Singh, S.; Gupta, R.C.; Agrawal, A.K. Exosomes as Emerging Drug Delivery and Diagnostic Modality for Breast Cancer: Recent Advances in Isolation and Application. Cancers 2022, 14, 1435. [Google Scholar] [CrossRef]
- Aqil, F.; Gupta, R.C. Exosomes in Cancer Therapy. Cancers 2022, 14, 500. [Google Scholar] [CrossRef]
- Chinnappan, M.; Srivastava, A.; Amreddy, N.; Razaq, M.; Pareek, V.; Ahmed, R.; Mehta, M.; Peterson, J.E.; Munshi, A.; Ramesh, R. Exosomes as drug delivery vehicle and contributor of resistance to anticancer drugs. Cancer Lett. 2020, 486, 18–28. [Google Scholar] [CrossRef]
- Gonzalez-Sarrias, A.; Iglesias-Aguirre, C.E.; Cortes-Martin, A.; Vallejo, F.; Cattivelli, A.; Del Pozo-Acebo, L.; Del Saz, A.; Lopez de Las Hazas, M.C.; Davalos, A.; Espin, J.C. Milk-Derived Exosomes as Nanocarriers to Deliver Curcumin and Resveratrol in Breast Tissue and Enhance Their Anticancer Activity. Int. J. Mol. Sci. 2022, 23, 2860. [Google Scholar] [CrossRef]
- Xi, X.M.; Xia, S.J.; Lu, R. Drug loading techniques for exosome-based drug delivery systems. Pharmazie 2021, 76, 61–67. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Zhu, L.; Xu, Z.; Liu, Y.; Li, Z.; Zhou, J.; Luo, F. Exosomes as Drug Carriers in Anti-Cancer Therapy. Front. Cell Dev. Biol. 2022, 10, 728616. [Google Scholar] [CrossRef]
- Eroy-Reveles, A.A.; Mascharak, P.K. Nitric oxide-donating materials and their potential in pharmacological applications for site-specific nitric oxide delivery. Future Med. Chem. 2009, 1, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Poh, W.H.; Rice, S.A. Recent Developments in Nitric Oxide Donors and Delivery for Antimicrobial and Anti-Biofilm Applications. Molecules 2022, 27, 674. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, M.J.; Kizhuveetil, U.; Johnson, A.; Balaji, G.; Nagarajan, R.; Muthuvijayan, V. Cancer nanomedicine: A review of nano-therapeutics and challenges ahead. RSC Adv. 2023, 13, 8606–8629. [Google Scholar] [CrossRef] [PubMed]
- Fulton, M.D.; Najahi-Missaoui, W. Liposomes in Cancer Therapy: How Did We Start and Where Are We Now. Int. J. Mol. Sci. 2023, 24, 6615. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.K.; Bortner, C.D.; Jarmusch, A.K.; Tokar, E.J.; Murphy, C.; Wu, X.; Winter, H.; Cannon, R.E. Ferroptosis-Mediated Cell Death Induced by NCX4040, The Non-Steroidal Nitric Oxide Donor, in Human Colorectal Cancer Cells: Implications in Therapy. Cells 2023, 12, 1626. [Google Scholar] [CrossRef]
- Benien, P.; Swami, A. 3D tumor models: History, advances and future perspectives. Future Oncol. 2014, 10, 1311–1327. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- Anderle, N.; Koch, A.; Gierke, B.; Keller, A.L.; Staebler, A.; Hartkopf, A.; Brucker, S.Y.; Pawlak, M.; Schenke-Layland, K.; Schmees, C. A Platform of Patient-Derived Microtumors Identifies Individual Treatment Responses and Therapeutic Vulnerabilities in Ovarian Cancer. Cancers 2022, 14, 2895. [Google Scholar] [CrossRef]
- Senkowski, W.; Gall-Mas, L.; Falco, M.M.; Li, Y.; Lavikka, K.; Kriegbaum, M.C.; Oikkonen, J.; Bulanova, D.; Pietras, E.J.; Vossgrone, K.; et al. A platform for efficient establishment and drug-response profiling of high-grade serous ovarian cancer organoids. Dev. Cell 2023, 58, 1106–1121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinha, B.K. Can Nitric Oxide-Based Therapy Be Improved for the Treatment of Cancers? A Perspective. Int. J. Mol. Sci. 2023, 24, 13611. https://doi.org/10.3390/ijms241713611
Sinha BK. Can Nitric Oxide-Based Therapy Be Improved for the Treatment of Cancers? A Perspective. International Journal of Molecular Sciences. 2023; 24(17):13611. https://doi.org/10.3390/ijms241713611
Chicago/Turabian StyleSinha, Birandra K. 2023. "Can Nitric Oxide-Based Therapy Be Improved for the Treatment of Cancers? A Perspective" International Journal of Molecular Sciences 24, no. 17: 13611. https://doi.org/10.3390/ijms241713611




