Novel Correlation between TGF-β1/-β3 and Hormone Receptors in the Human Corneal Stroma
Abstract
:1. Introduction
2. Results
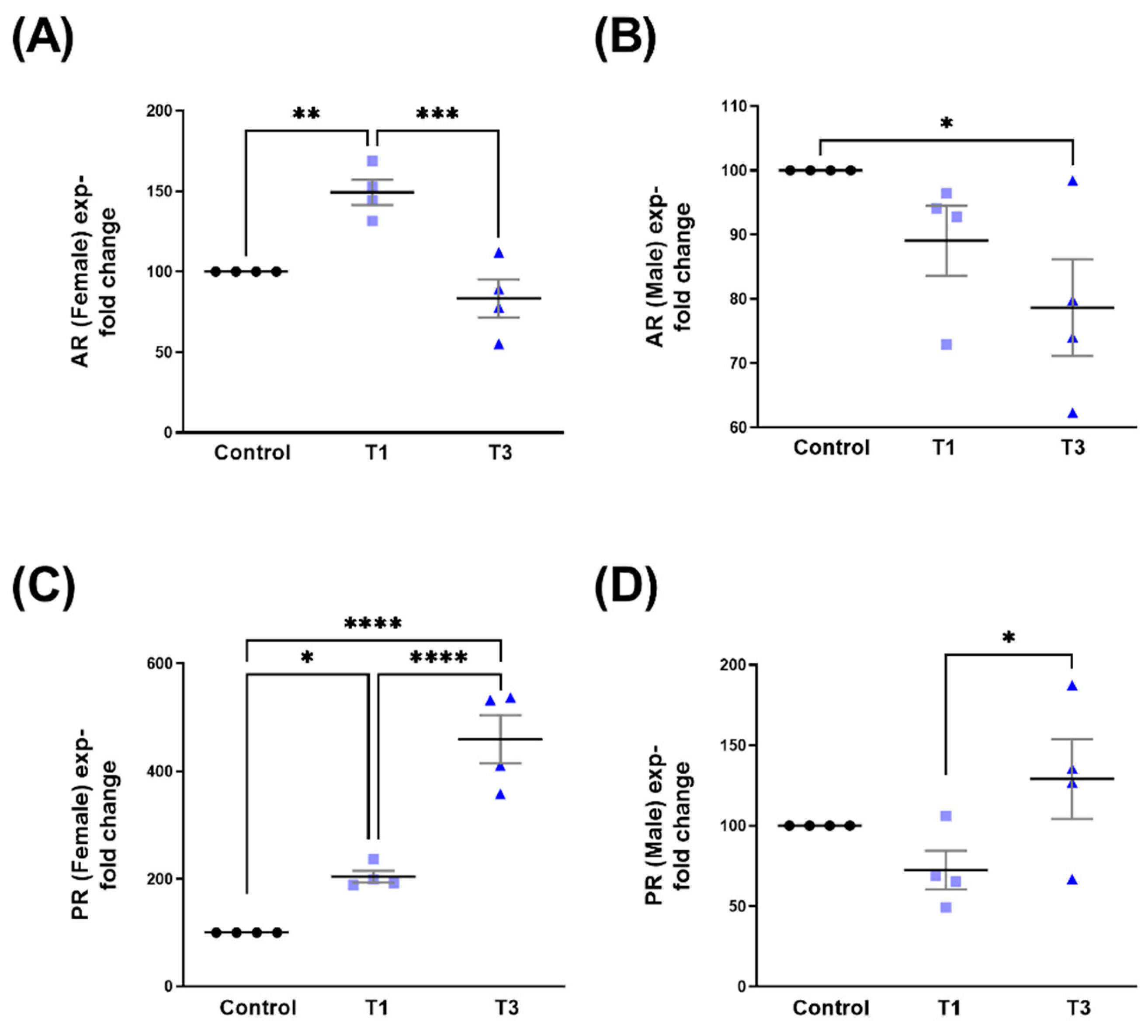
2.1. Androgen Receptor (AR) and Progesterone Receptor (PR)
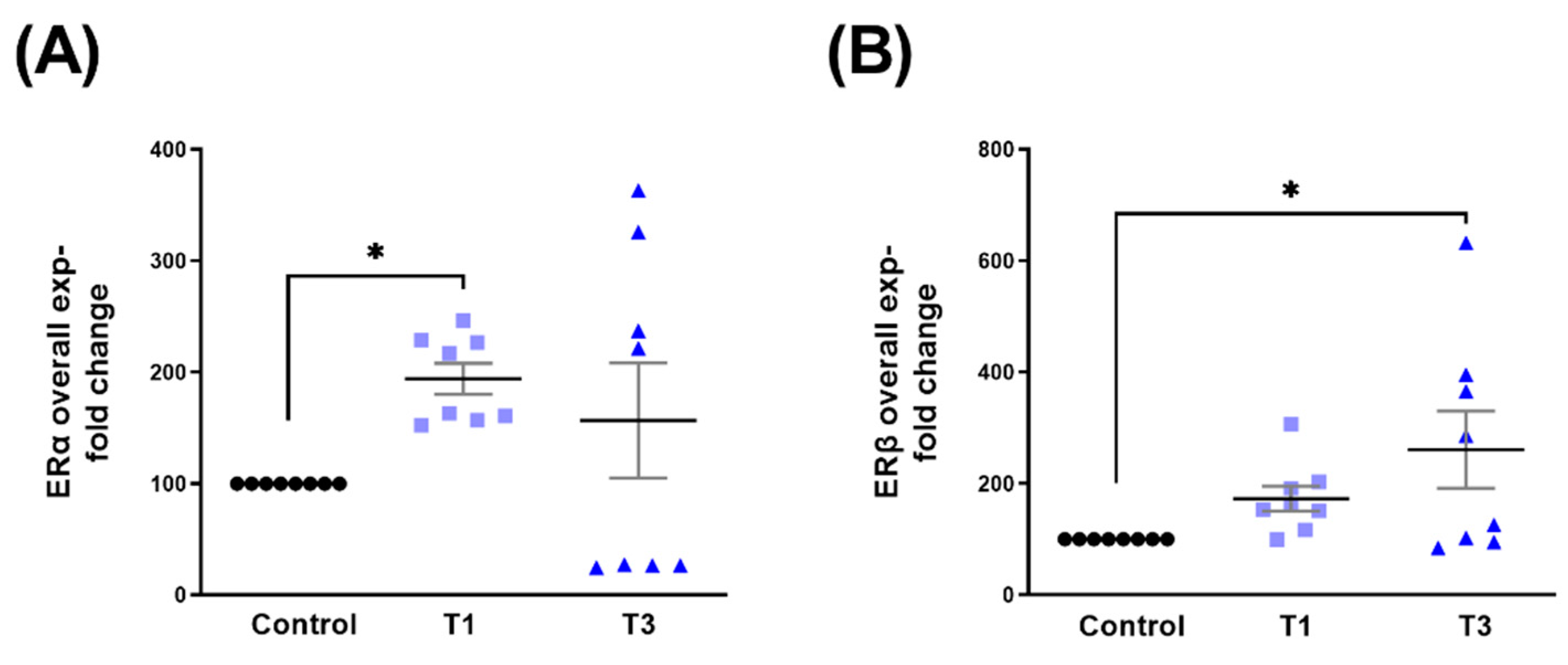
2.2. Estrogen Receptor Alpha (ERα) and Estrogen Receptor Beta (ERβ)
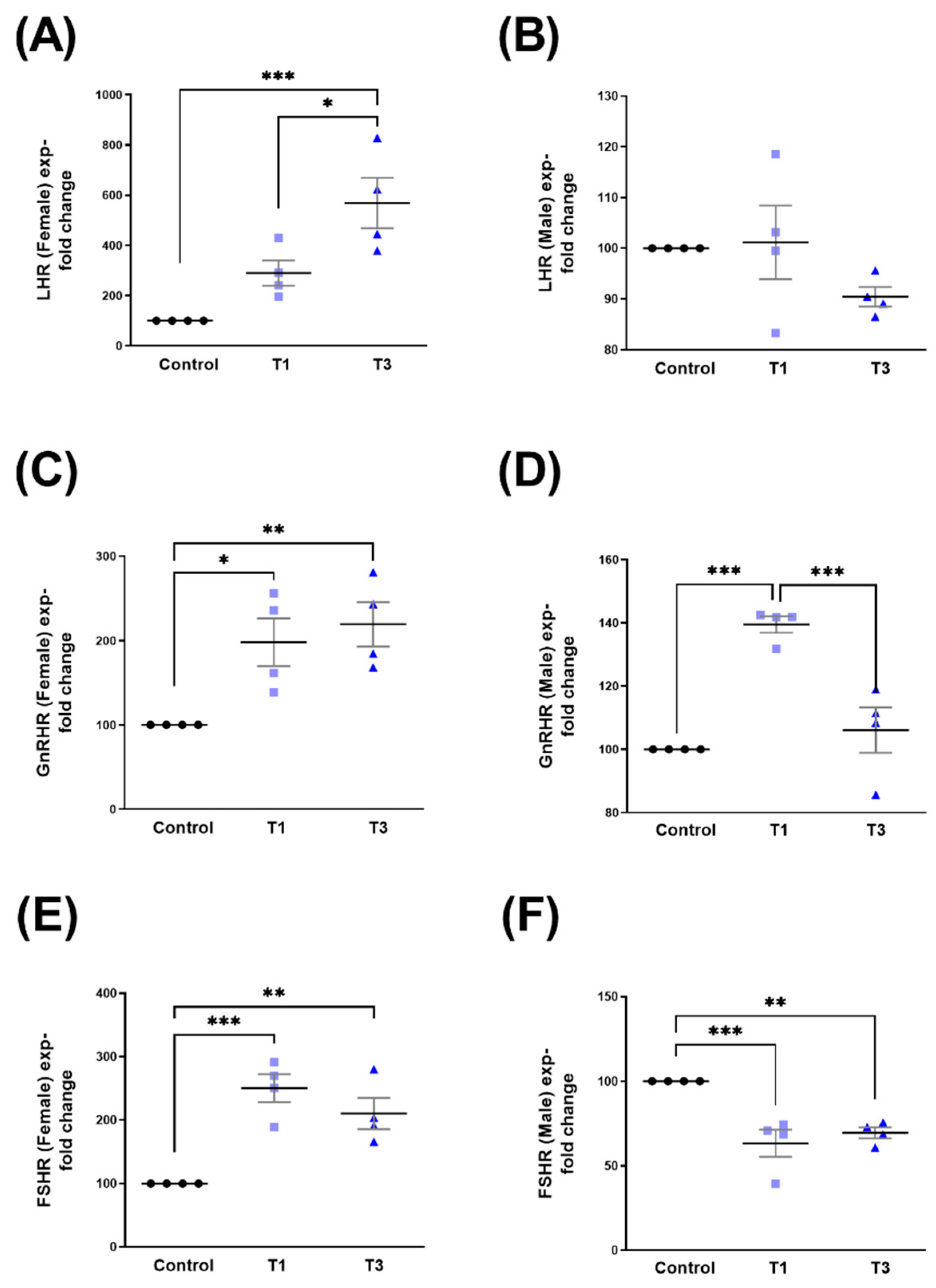
2.3. Luteinizing Hormone Receptor (LHR), Gonadotropin-Releasing Hormone Receptor (GnRHR), and Follicle-Stimulating Hormone Receptor (FSHR)
2.4. Thyrotropin-Releasing Hormone Receptor (TRHR) and KiSS1-Derived Peptide Receptor (KISS1R/GPR54)
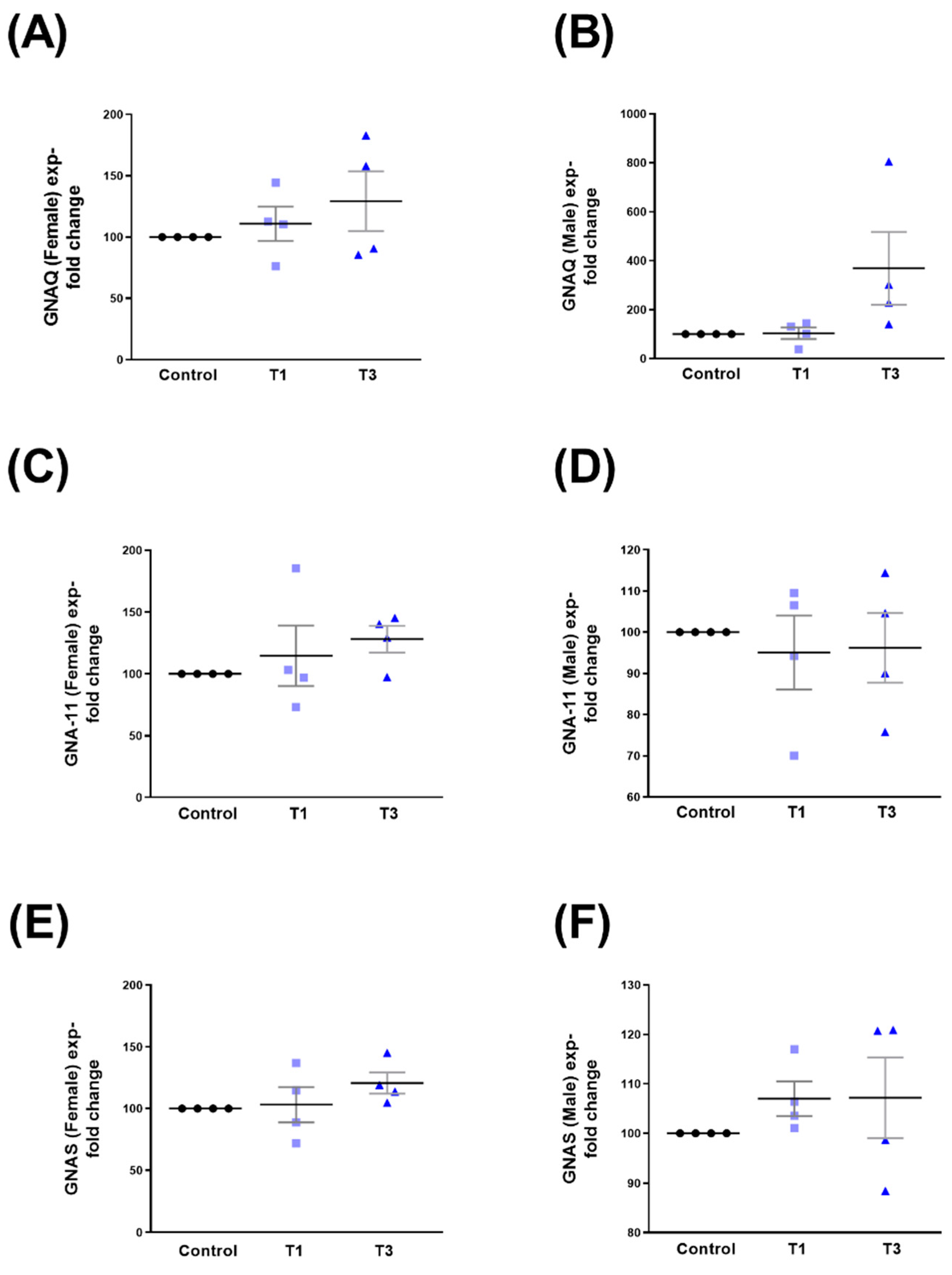
2.5. G Protein Subunit Alpha Q (GNAQ), G Protein Subunit Alpha 11 (GNA-11), and G Protein Alpha Stimulating (GNAS)
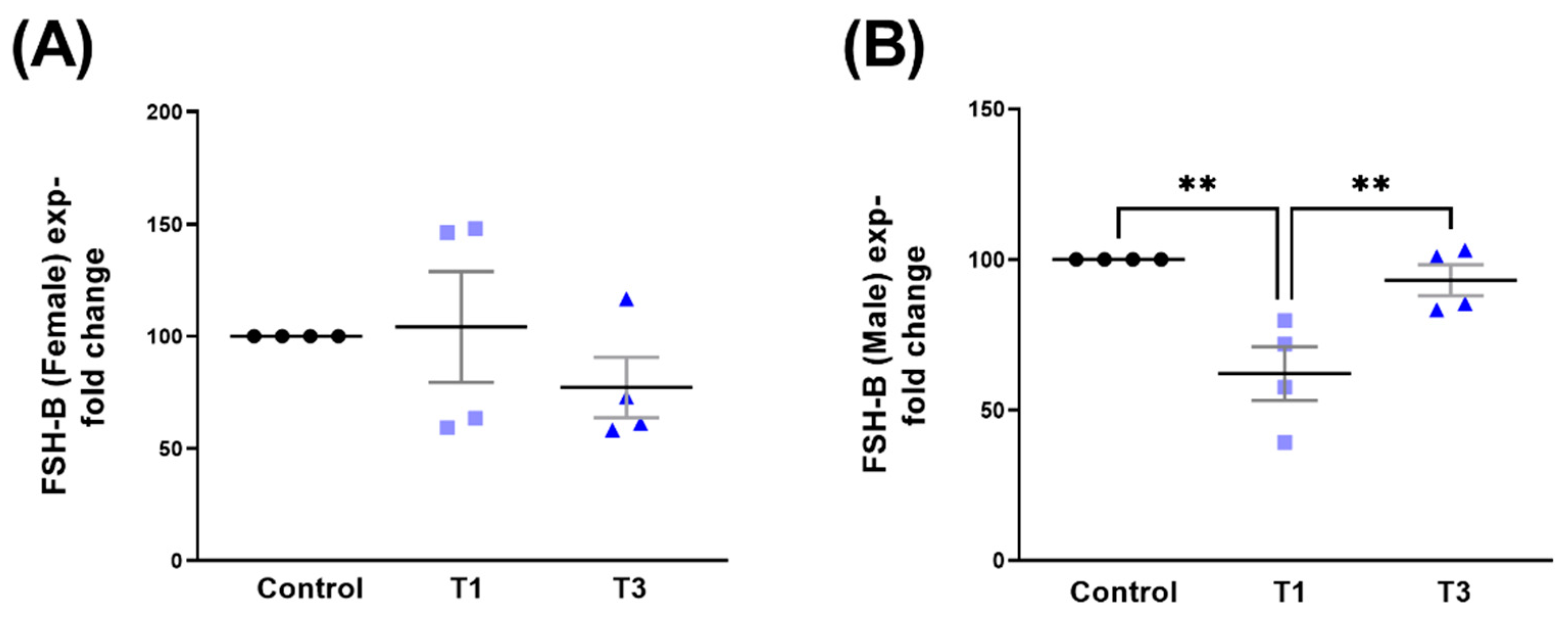
2.6. Follicle-Stimulating Hormone Subunit Beta (FSH-B)
3. Discussion
4. Materials and Methods
4.1. Ethical Consent
4.2. Cell Isolation, Cell Cultures, and ECM Assembly
4.3. Protein Extraction and Quantification
4.4. Western Blot
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- Escandon, P.; Vasini, B.; Whelchel, A.E.; Nicholas, S.E.; Matlock, H.G.; Ma, J.X.; Karamichos, D. The role of peroxisome proliferator-activated receptors in healthy and diseased eyes. Exp. Eye Res. 2021, 208, 108617. [Google Scholar] [CrossRef] [PubMed]
- Sugioka, K.; Fukuda, K.; Nishida, T.; Kusaka, S. The fibrinolytic system in the cornea: A key regulator of corneal wound healing and biological defense. Exp. Eye Res. 2021, 204, 108459. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Bu, Y.; Lau, D.A.; Lin, Z.; Sun, T.; Lu, W.W.; Lu, S.; Ruan, C.; Chan, C.J. Advances in 3D bioprinting technology for functional corneal reconstruction and regeneration. Front. Bioeng. Biotechnol. 2022, 10, 1065460. [Google Scholar] [CrossRef]
- Kelly, D.S.; Sabharwal, S.; Ramsey, D.J.; Morkin, M.I. The effects of female sex hormones on the human cornea across a woman’s life cycle. BMC Ophthalmol. 2023, 23, 358. [Google Scholar] [CrossRef]
- Zhou, C.; Robert, M.C.; Kapoulea, V.; Lei, F.; Stagner, A.M.; Jakobiec, F.A.; Dohlman, C.H.; Paschalis, E.I. Sustained Subconjunctival Delivery of Infliximab Protects the Cornea and Retina Following Alkali Burn to the Eye. Investig. Ophthalmol. Vis. Sci. 2017, 58, 96–105. [Google Scholar] [CrossRef]
- Abdelkader, H.; Patel, D.V.; McGhee, C.; Alany, R.G. New therapeutic approaches in the treatment of diabetic keratopathy: A review. Clin. Exp. Ophthalmol. 2011, 39, 259–270. [Google Scholar] [CrossRef]
- Sharma, N.; Goel, M.; Velpandian, T.; Titiyal, J.S.; Tandon, R.; Vajpayee, R.B. Evaluation of umbilical cord serum therapy in acute ocular chemical burns. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1087–1092. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef]
- Yeung, V.; Boychev, N.; Farhat, W.; Ntentakis, D.P.; Hutcheon, A.E.K.; Ross, A.E.; Ciolino, J.B. Extracellular Vesicles in Corneal Fibrosis/Scarring. Int. J. Mol. Sci. 2022, 23, 5921. [Google Scholar] [CrossRef]
- Buzzi, M.; Versura, P.; Grigolo, B.; Cavallo, C.; Terzi, A.; Pellegrini, M.; Giannaccare, G.; Randi, V.; Campos, E.C. Comparison of growth factor and interleukin content of adult peripheral blood and cord blood serum eye drops for cornea and ocular surface diseases. Transfus. Apher. Sci. 2018, 57, 549–555. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.B.; Karamichos, D.; Hutcheon, A.E.K.; Guo, X.; Zieske, J.D. Corneal Epithelial-Stromal Fibroblast Constructs to Study Cell-Cell Communication in Vitro. Bioengineering 2019, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Yeung, V.; Sriram, S.; Tran, J.A.; Guo, X.; Hutcheon, A.E.K.; Zieske, J.D.; Karamichos, D.; Ciolino, J.B. FAK Inhibition Attenuates Corneal Fibroblast Differentiation In Vitro. Biomolecules 2021, 11, 1682. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.S.; Marino, G.K.; Santhiago, M.R.; Wilson, S.E. The Corneal Basement Membranes and Stromal Fibrosis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4044–4053. [Google Scholar] [CrossRef]
- Karamichos, D.; Hutcheon, A.E.; Zieske, J.D. Reversal of fibrosis by TGF-beta3 in a 3D in vitro model. Exp. Eye Res. 2014, 124, 31–36. [Google Scholar] [CrossRef]
- Kamil, S.; Mohan, R.R. Corneal stromal wound healing: Major regulators and therapeutic targets. Ocul. Surf. 2021, 19, 290–306. [Google Scholar] [CrossRef]
- Liang, W.; Ma, J.X.; Van, L.; Vasini, B.; Karamichos, D. Prolactin-Induced Protein facilitates corneal wound healing. Exp. Eye Res. 2022, 225, 109300. [Google Scholar] [CrossRef]
- Soleimani, M.; Ebrahimi, Z.; Ebrahimi, K.S.; Farhadian, N.; Shahlaei, M.; Cheraqpour, K.; Ghasemi, H.; Moradi, S.; Chang, A.Y.; Sharifi, S.; et al. Application of biomaterials and nanotechnology in corneal tissue engineering. J. Int. Med. Res. 2023, 51, 3000605231190473. [Google Scholar] [CrossRef]
- Musa, M.; Zeppieri, M.; Enaholo, E.S.; Chukwuyem, E.; Salati, C. An Overview of Corneal Transplantation in the Past Decade. Clin. Pract. 2023, 13, 264–279. [Google Scholar] [CrossRef]
- Moramarco, A.; Gardini, L.; Iannetta, D.; Versura, P.; Fontana, L. Post Penetrating Keratoplasty Ectasia: Incidence, Risk Factors, Clinical Features, and Treatment Options. J. Clin. Med. 2022, 11, 2678. [Google Scholar] [CrossRef]
- Kirkness, C.M.; Ficker, L.A.; Steele, A.D.; Rice, N.S. The success of penetrating keratoplasty for keratoconus. Eye 1990, 4 Pt 5, 673–688. [Google Scholar] [CrossRef]
- Chandler, J.W.; Kaufman, H.E. Graft reactions after keratoplasty for keratoconus. Am. J. Ophthalmol. 1974, 77, 543–547. [Google Scholar] [CrossRef]
- Zemba, M.; Stamate, A.C. Glaucoma after penetrating keratoplasty. Rom. J. Ophthalmol. 2017, 61, 159–165. [Google Scholar] [CrossRef]
- Malbran, E.S.; Fernandez-Meijide, R.E. Bilateral versus unilateral penetrating graft in keratoconus. Ophthalmology 1982, 89, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.D.; Johar, K., Sr.; Nagpal, K.; Vasavada, A.R. Sex hormone receptors in the human eye. Surv. Ophthalmol. 2005, 50, 274–284. [Google Scholar] [CrossRef]
- McKay, T.B.; Priyadarsini, S.; Karamichos, D. Sex Hormones, Growth Hormone, and the Cornea. Cells 2022, 11, 224. [Google Scholar] [CrossRef]
- Karamichos, D.; Barrientez, B.; Nicholas, S.; Ma, S.; Van, L.; Bak-Nielsen, S.; Hjortdal, J. Gonadotropins in Keratoconus: The Unexpected Suspects. Cells 2019, 8, 1494. [Google Scholar] [CrossRef] [PubMed]
- Escandon, P.; Nicholas, S.E.; Cunningham, R.L.; Murphy, D.A.; Riaz, K.M.; Karamichos, D. The Role of Estriol and Estrone in Keratoconic Stromal Sex Hormone Receptors. Int. J. Mol. Sci. 2022, 23, 916. [Google Scholar] [CrossRef]
- Suzuki, T.; Kinoshita, Y.; Tachibana, M.; Matsushima, Y.; Kobayashi, Y.; Adachi, W.; Sotozono, C.; Kinoshita, S. Expression of sex steroid hormone receptors in human cornea. Curr. Eye Res. 2001, 22, 28–33. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.C.; Wilson, S.E. Fibrocytes, Wound Healing, and Corneal Fibrosis. Investig. Ophthalmol. Vis. Sci. 2020, 61, 28. [Google Scholar] [CrossRef]
- Ziller, N.; Kotolloshi, R.; Esmaeili, M.; Liebisch, M.; Mrowka, R.; Baniahmad, A.; Liehr, T.; Wolf, G.; Loeffler, I. Sex Differences in Diabetes- and TGF-beta1-Induced Renal Damage. Cells 2020, 9, 2236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, C.; Shen, S.; Xia, Y.; Yi, L.; Gao, Q.; Wang, Y. Dehydroepiandrosterone induces ovarian and uterine hyperfibrosis in female rats. Hum. Reprod. 2013, 28, 3074–3085. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tan, Y.; Wang, X.; Zhu, M. Paeoniflorin attenuates DHEA-induced polycystic ovary syndrome via inactivation of TGF-beta1/Smads signaling pathway in vivo. Aging (Albany NY) 2021, 13, 7084–7095. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E. Corneal myofibroblasts and fibrosis. Exp. Eye Res. 2020, 201, 108272. [Google Scholar] [CrossRef]
- Wilson, S.E. Fibrosis Is a Basement Membrane-Related Disease in the Cornea: Injury and Defective Regeneration of Basement Membranes May Underlie Fibrosis in Other Organs. Cells 2022, 11, 309. [Google Scholar] [CrossRef]
- Mallone, F.; Costi, R.; Marenco, M.; Plateroti, R.; Minni, A.; Attanasio, G.; Artico, M.; Lambiase, A. Understanding Drivers of Ocular Fibrosis: Current and Future Therapeutic Perspectives. Int. J. Mol. Sci. 2021, 22, 1748. [Google Scholar] [CrossRef]
- Friedlander, M. Fibrosis and diseases of the eye. J. Clin. Investig. 2007, 117, 576–586. [Google Scholar] [CrossRef]
- Tandon, A.; Tovey, J.C.; Sharma, A.; Gupta, R.; Mohan, R.R. Role of transforming growth factor Beta in corneal function, biology and pathology. Curr. Mol. Med. 2010, 10, 565–578. [Google Scholar] [CrossRef]
- Wilson, S.E. TGF beta -1, -2 and -3 in the modulation of fibrosis in the cornea and other organs. Exp. Eye Res. 2021, 207, 108594. [Google Scholar] [CrossRef]
- Nuwormegbe, S.; Park, N.Y.; Kim, S.W. Lobeglitazone attenuates fibrosis in corneal fibroblasts by interrupting TGF-beta-mediated Smad signaling. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 149–162. [Google Scholar] [CrossRef]
- Li, S.; Gu, X.; Yi, S. The Regulatory Effects of Transforming Growth Factor-beta on Nerve Regeneration. Cell Transplant. 2017, 26, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Hariyanto, N.I.; Yo, E.C.; Wanandi, S.I. Regulation and Signaling of TGF-beta Autoinduction. Int. J. Mol. Cell Med. 2021, 10, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Funderburgh, J.L.; Khandaker, I.; Geary, M.L.; Yang, T.; Basu, R.; Funderburgh, M.L.; Du, Y.; Yam, G.H. The anti-scarring effect of corneal stromal stem cell therapy is mediated by transforming growth factor beta3. Eye Vis. 2020, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, A.A.; Santhanam, A.; Wu, J.; Singh, V.; Wilson, S.E. The corneal fibrosis response to epithelial-stromal injury. Exp. Eye Res. 2016, 142, 110–118. [Google Scholar] [CrossRef]
- Benito, M.J.; Calder, V.; Corrales, R.M.; Garcia-Vazquez, C.; Narayanan, S.; Herreras, J.M.; Stern, M.E.; Calonge, M.; Enriquez-de-Salamanca, A. Effect of TGF-beta on ocular surface epithelial cells. Exp. Eye Res. 2013, 107, 88–100. [Google Scholar] [CrossRef]
- Karamichos, D.; Rich, C.B.; Zareian, R.; Hutcheon, A.E.; Ruberti, J.W.; Trinkaus-Randall, V.; Zieske, J.D. TGF-beta3 stimulates stromal matrix assembly by human corneal keratocyte-like cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6612–6619. [Google Scholar] [CrossRef]
- Mak, K.M.; Png, C.Y.; Lee, D.J. Type V Collagen in Health, Disease, and Fibrosis. Anat. Rec. (Hoboken) 2016, 299, 613–629. [Google Scholar] [CrossRef]
- Karamichos, D.; Hutcheon, A.E.; Zieske, J.D. Transforming growth factor-beta3 regulates assembly of a non-fibrotic matrix in a 3D corneal model. J. Tissue Eng. Regen. Med. 2011, 5, e228–e238. [Google Scholar] [CrossRef]
- Tripathi, R.; Giuliano, E.A.; Gafen, H.B.; Gupta, S.; Martin, L.M.; Sinha, P.R.; Rodier, J.T.; Fink, M.K.; Hesemann, N.P.; Chaurasia, S.S.; et al. Is sex a biological variable in corneal wound healing? Exp. Eye Res. 2019, 187, 107705. [Google Scholar] [CrossRef]
- Ahn, J.H.; Choi, Y.H.; Paik, H.J.; Kim, M.K.; Wee, W.R.; Kim, D.H. Sex differences in the effect of aging on dry eye disease. Clin. Interv. Aging 2017, 12, 1331–1338. [Google Scholar] [CrossRef]
- Aoki, C.A.; Borchers, A.T.; Li, M.; Flavell, R.A.; Bowlus, C.L.; Ansari, A.A.; Gershwin, M.E. Transforming growth factor beta (TGF-beta) and autoimmunity. Autoimmun. Rev. 2005, 4, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Toma, I.; McCaffrey, T.A. Transforming growth factor-beta and atherosclerosis: Interwoven atherogenic and atheroprotective aspects. Cell Tissue Res. 2012, 347, 155–175. [Google Scholar] [CrossRef]
- Crowe, M.J.; Doetschman, T.; Greenhalgh, D.G. Delayed wound healing in immunodeficient TGF-beta 1 knockout mice. J. Investig. Dermatol. 2000, 115, 3–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, Y.; Raman, A.; Daniel, E.; Metcalf, J.; Reif, G.; Pierucci-Alves, F.; Wallace, D.P. Overexpression of TGF-beta1 induces renal fibrosis and accelerates the decline in kidney function in polycystic kidney disease. Am. J. Physiol. Renal Physiol. 2020, 319, F1135–F1148. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yi, L.; Du, M.; Gong, G.; Zhu, Y. Overexpression of TGF-beta enhances the migration and invasive ability of ectopic endometrial cells via ERK/MAPK signaling pathway. Exp. Ther. Med. 2019, 17, 4457–4464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.Y.; Chen, Y.; Wang, J.; Wang, Q.; Lu, H. TGF-beta Signaling and Resistance to Cancer Therapy. Front. Cell Dev. Biol. 2021, 9, 786728. [Google Scholar] [CrossRef]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-beta and the TGF-beta Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef]
- Zhang, Y.; Alexander, P.B.; Wang, X.F. TGF-beta Family Signaling in the Control of Cell Proliferation and Survival. Cold Spring Harb. Perspect. Biol. 2017, 9, a022145. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-beta-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef]
- Tzavlaki, K.; Moustakas, A. TGF-beta Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef]
- Hata, A.; Chen, Y.G. TGF-beta Signaling from Receptors to Smads. Cold Spring Harb. Perspect. Biol. 2016, 8, a022061. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, F.; Qin, L.; Liao, Z.; Song, J.; Liang, H.; Chen, X.; Zhang, Z.; Zhang, B. Characterization of TGFbeta-associated molecular features and drug responses in gastrointestinal adenocarcinoma. BMC Gastroenterol. 2021, 21, 284. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.M.; Cian, A.A.; Weng, T.H.; Liang, C.M.; Pao, S.I.; Chen, Y.J. Beneficial Effects of Hypercapnic Acidosis on the Inhibition of Transforming Growth Factor beta-1-induced Corneal Fibrosis in Vitro. Curr. Eye Res. 2021, 46, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Zahir-Jouzdani, F.; Soleimani, M.; Mahbod, M.; Mottaghitalab, F.; Vakhshite, F.; Arefian, E.; Shahhoseini, S.; Dinarvand, R.; Atyabi, F. Corneal chemical burn treatment through a delivery system consisting of TGF-beta1 siRNA: In vitro and in vivo. Drug Deliv. Transl. Res. 2018, 8, 1127–1138. [Google Scholar] [CrossRef]
- Moretti, L.; Stalfort, J.; Barker, T.H.; Abebayehu, D. The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J. Biol. Chem. 2022, 298, 101530. [Google Scholar] [CrossRef]
- Guo, X.; Hutcheon, A.E.K.; Zieske, J.D. Molecular insights on the effect of TGF-beta1/-beta3 in human corneal fibroblasts. Exp. Eye Res. 2016, 146, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Wickham, L.A.; Gao, J.; Toda, I.; Rocha, E.M.; Ono, M.; Sullivan, D.A. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol. Scand. 2000, 78, 146–153. [Google Scholar] [CrossRef]
- Kanda, N.; Watanabe, S. Regulatory roles of sex hormones in cutaneous biology and immunology. J. Dermatol. Sci. 2005, 38, 1–7. [Google Scholar] [CrossRef]
- Hammes, S.R.; Levin, E.R. Impact of estrogens in males and androgens in females. J. Clin. Investig. 2019, 129, 1818–1826. [Google Scholar] [CrossRef]
- Handelsman, D.J.; Hirschberg, A.L.; Bermon, S. Circulating Testosterone as the Hormonal Basis of Sex Differences in Athletic Performance. Endocr. Rev. 2018, 39, 803–829. [Google Scholar] [CrossRef]
- Roper, L.K.; Briguglio, J.S.; Evans, C.S.; Jackson, M.B.; Chapman, E.R. Sex-specific regulation of follicle-stimulating hormone secretion by synaptotagmin 9. Nat. Commun. 2015, 6, 8645. [Google Scholar] [CrossRef] [PubMed]
- Vrtacnik, P.; Ostanek, B.; Mencej-Bedrac, S.; Marc, J. The many faces of estrogen signaling. Biochem. Med. 2014, 24, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.A.; Cooke, P.S. Estrogen in the male: A historical perspective. Biol. Reprod. 2018, 99, 27–44. [Google Scholar] [CrossRef]
- Nuzzi, R.; Caselgrandi, P. Sex Hormones and Their Effects on Ocular Disorders and Pathophysiology: Current Aspects and Our Experience. Int. J. Mol. Sci. 2022, 23, 3269. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, S.K.; Singh, S.; Bajwa, S.J. Ocular tissue responses to sex hormones. Indian. J. Endocrinol. Metab. 2012, 16, 488–489. [Google Scholar] [CrossRef]
- Bujor, I.A.; Iancu, R.C.; Istrate, S.L.; Ungureanu, E.; Iancu, G. Corneal Biomechanical Changes in Third Trimester of Pregnancy. Medicina 2021, 57, 600. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.G.; Carr, B.R. The Normal Menstrual Cycle and the Control of Ovulation. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Patel, S.; Homaei, A.; Raju, A.B.; Meher, B.R. Estrogen: The necessary evil for human health, and ways to tame it. Biomed. Pharmacother. 2018, 102, 403–411. [Google Scholar] [CrossRef]
- Rao, A.; Douglas, S.C.; Hall, J.M. Endocrine Disrupting Chemicals, Hormone Receptors, and Acne Vulgaris: A Connecting Hypothesis. Cells 2021, 10, 1439. [Google Scholar] [CrossRef]
- Shah, T.A.; Rogers, M.B. Unanswered Questions Regarding Sex and BMP/TGF-beta Signaling. J. Dev. Biol. 2018, 6, 14. [Google Scholar] [CrossRef]
- Perry, K.J.; Johnson, V.R.; Malloch, E.L.; Fukui, L.; Wever, J.; Thomas, A.G.; Hamilton, P.W.; Henry, J.J. The G-protein-coupled receptor, GPR84, is important for eye development in Xenopus laevis. Dev. Dyn. 2010, 239, 3024–3037. [Google Scholar] [CrossRef]
- Martemyanov, K.A. G protein signaling in the retina and beyond: The Cogan lecture. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8201–8207. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yu, F.S. ERK1/2 mediate wounding- and G-protein-coupled receptor ligands-induced EGFR activation via regulating ADAM17 and HB-EGF shedding. Investig. Ophthalmol. Vis. Sci. 2009, 50, 132–139. [Google Scholar] [CrossRef]
- Matysik-Wozniak, A.; Wnorowski, A.; Turski, W.A.; Jozwiak, K.; Junemann, A.; Rejdak, R. The presence and distribution of G protein-coupled receptor 35 (GPR35) in the human cornea—Evidences from in silico gene expression analysis and immunodetection. Exp. Eye Res. 2019, 179, 188–192. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Chen, P.; Meng, J.; Zhang, H. G-protein-coupled estrogen receptor protects retinal ganglion cells via inhibiting endoplasmic reticulum stress under hyperoxia. J. Cell Physiol. 2021, 236, 3780–3788. [Google Scholar] [CrossRef]
- Hu, J.; Li, T.; Du, X.; Wu, Q.; Le, Y.Z. G protein-coupled receptor 91 signaling in diabetic retinopathy and hypoxic retinal diseases. Vision. Res. 2017, 139, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Matteson, P.G.; Desai, J.; Korstanje, R.; Lazar, G.; Borsuk, T.E.; Rollins, J.; Kadambi, S.; Joseph, J.; Rahman, T.; Wink, J.; et al. The orphan G protein-coupled receptor, Gpr161, encodes the vacuolated lens locus and controls neurulation and lens development. Proc. Natl. Acad. Sci. USA 2008, 105, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dohlman, H.G. Regulation of G protein and mitogen-activated protein kinase signaling by ubiquitination: Insights from model organisms. Circ. Res. 2006, 99, 1305–1314. [Google Scholar] [CrossRef]
- Syrovatkina, V.; Alegre, K.O.; Dey, R.; Huang, X.Y. Regulation, Signaling, and Physiological Functions of G-Proteins. J. Mol. Biol. 2016, 428, 3850–3868. [Google Scholar] [CrossRef]
- Roberts, D.J.; Waelbroeck, M. G protein activation by G protein coupled receptors: Ternary complex formation or catalyzed reaction? Biochem. Pharmacol. 2004, 68, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Vetter, I.R.; Wittinghofer, A. The guanine nucleotide-binding switch in three dimensions. Science 2001, 294, 1299–1304. [Google Scholar] [CrossRef]
- Karamichos, D.; Guo, X.Q.; Hutcheon, A.E.; Zieske, J.D. Human corneal fibrosis: An in vitro model. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, S.; McKay, T.B.; Sarker-Nag, A.; Karamichos, D. Keratoconus in vitro and the key players of the TGF-beta pathway. Mol. Vis. 2015, 21, 577–588. [Google Scholar]
- Nicholas, S.E.; Choi, A.J.; Lam, T.N.; Basu, S.K.; Mandal, N.; Karamichos, D. Potentiation of Sphingolipids and TGF-beta in the human corneal stroma reveals intricate signaling pathway crosstalks. Exp. Eye Res. 2023, 231, 109487. [Google Scholar] [CrossRef] [PubMed]
- Escandon, P.; Nicholas, S.E.; Vasini, B.; Cunningham, R.L.; Murphy, D.A.; Riaz, K.M.; Karamichos, D. Selective Modulation of the Keratoconic Stromal Microenvironment by FSH and LH. Am. J. Pathol. 2023. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, A.J.; Hefley, B.S.; Nicholas, S.E.; Cunningham, R.L.; Karamichos, D. Novel Correlation between TGF-β1/-β3 and Hormone Receptors in the Human Corneal Stroma. Int. J. Mol. Sci. 2023, 24, 13635. https://doi.org/10.3390/ijms241713635
Choi AJ, Hefley BS, Nicholas SE, Cunningham RL, Karamichos D. Novel Correlation between TGF-β1/-β3 and Hormone Receptors in the Human Corneal Stroma. International Journal of Molecular Sciences. 2023; 24(17):13635. https://doi.org/10.3390/ijms241713635
Chicago/Turabian StyleChoi, Alexander J., Brenna S. Hefley, Sarah E. Nicholas, Rebecca L. Cunningham, and Dimitrios Karamichos. 2023. "Novel Correlation between TGF-β1/-β3 and Hormone Receptors in the Human Corneal Stroma" International Journal of Molecular Sciences 24, no. 17: 13635. https://doi.org/10.3390/ijms241713635
APA StyleChoi, A. J., Hefley, B. S., Nicholas, S. E., Cunningham, R. L., & Karamichos, D. (2023). Novel Correlation between TGF-β1/-β3 and Hormone Receptors in the Human Corneal Stroma. International Journal of Molecular Sciences, 24(17), 13635. https://doi.org/10.3390/ijms241713635





