Renal Endocannabinoid Dysregulation in Obesity-Induced Chronic Kidney Disease in Humans
Abstract
1. Introduction
2. Results
2.1. Patient Information
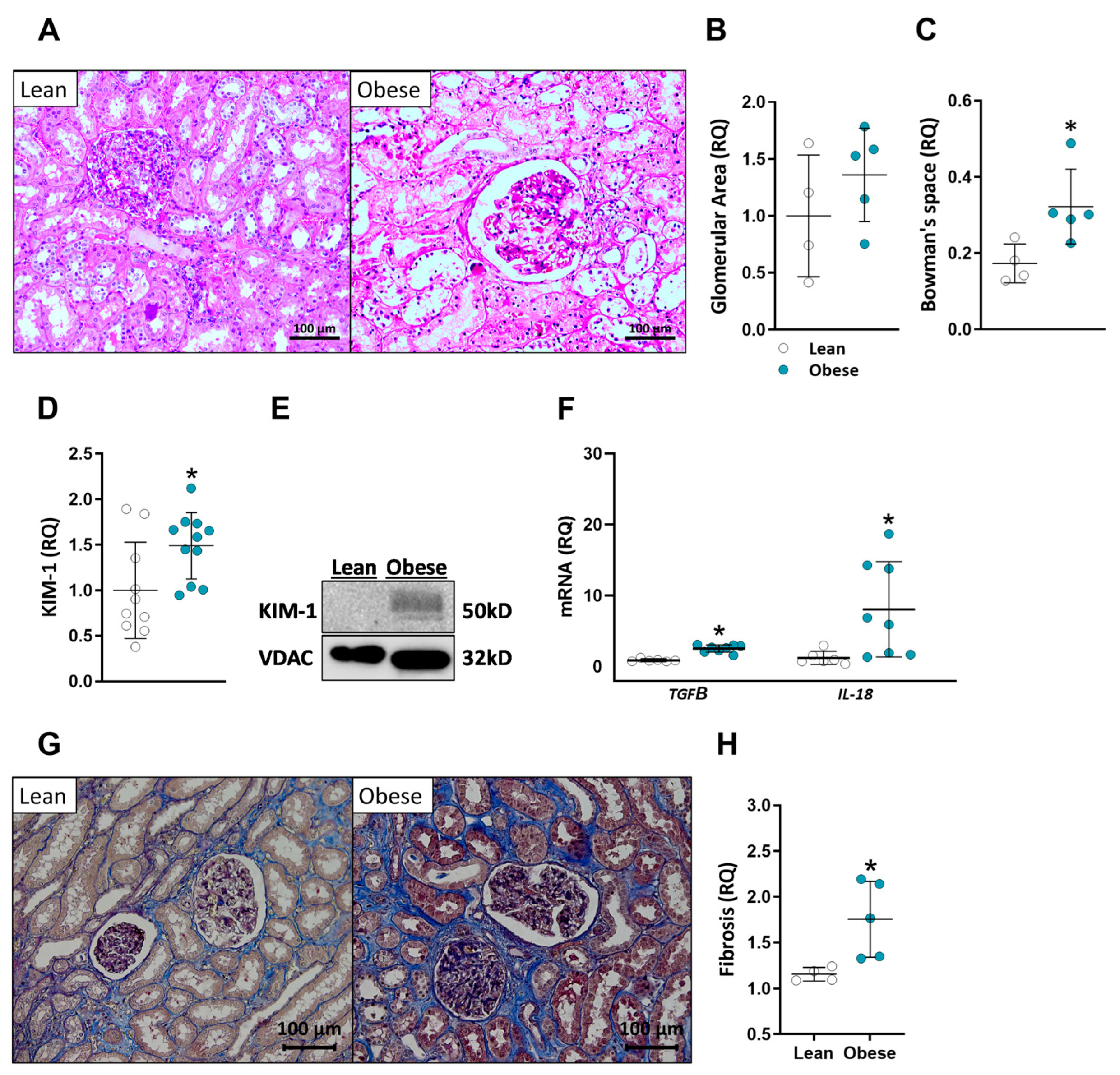
2.2. Patients with High BMI Exhibit Abnormal Kidney Morphology and Elevated Kidney Injury Markers
2.3. AEA Levels Are Increased in the Kidneys of Obese Patients
2.4. Abnormal Expression of CB1R and ECS-Related Enzymes in Obese Patients
2.5. Multiple-Parameter Correlations
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Study Protocol
4.3. Biochemistry Measurements
4.4. Endocannabinoid Extraction and Measurement by LC-MS/MS
4.5. Real-Time PCR
4.6. Western Blotting
4.7. Histopathology
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Correlation | p-Value |
|---|---|
| Glucose/AEA | 0.0093 |
| ALT/kidney 2-AG | 0.0042 |
| ALT/kidney PEA | 0.014 |
| ALT/kidney AA | 0.0014 |
| ALT/kidney OEA | 0.0178 |
| AST/kidney PEA | 0.0477 |
| AST/kidney AA | 0.0294 |
| ALP/kidney AA | −0.0311 |
| LDL/kidney AEA | −0.0051 |
| AST/serum PEA | 0.0284 |
| ALP/serum AA | −0.0216 |

References
- Hall, J.E.; Henegar, J.R.; Dwyer, T.M.; Liu, J.; da Silva, A.A.; Kuo, J.J.; Tallam, L. Is obesity a major cause of chronic kidney disease? Adv. Ren. Replace. Ther. 2004, 11, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Furth, S.L.; Zoccali, C. World Kidney Day Steering Committee. Obesity and Kidney Disease: Hidden Consequences of the Epidemic. Indian J. Nephrol. 2017, 27, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Eknoyan, G. Obesity and chronic kidney disease. Nefrología 2011, 31, 397–403. [Google Scholar] [CrossRef]
- Levey, A.S.; Schwartz, W.B.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- El Nahas, A.M.; Bello, A.K. Chronic kidney disease: The global challenge. Lancet 2005, 365, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Hung, C.C.; Hwang, S.J.; Wang, S.L.; Hsiao, S.M.; Lin, M.Y.; Kung, L.F.; Hsiao, P.N.; Chen, H.C. Quality of life predicts risks of end-stage renal disease and mortality in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2010, 25, 1621–1626. [Google Scholar] [CrossRef]
- Dalrymple, L.S.; Katz, R.; Kestenbaum, B.; Shlipak, M.G.; Sarnak, M.J.; Stehman-Breen, C.; Seliger, S.; Siscovick, D.; Newman, A.B.; Fried, L. Chronic Kidney Disease and the Risk of End-Stage Renal Disease versus Death. J. Gen. Intern. Med. 2011, 26, 379–385. [Google Scholar] [CrossRef]
- Hall, J.; Juncos, L.; Wang, Z.; Hall, M.; Carmo, J.M.D.; da Silva, A. Obesity, hypertension, and chronic kidney disease. Int. J. Nephrol. Renov. Dis. 2014, 7, 75–88. [Google Scholar] [CrossRef]
- Czaja-Stolc, S.; Potrykus, M.; Stankiewicz, M.; Kaska, Ł.; Małgorzewicz, S. Pro-Inflammatory Profile of Adipokines in Obesity Contributes to Pathogenesis, Nutritional Disorders, and Cardiovascular Risk in Chronic Kidney Disease. Nutrients 2022, 14, 1457. [Google Scholar] [CrossRef]
- Chua, J.T.; Argueta, D.A.; DiPatrizio, N.V.; Kovesdy, C.P.; Vaziri, N.D.; Kalantar-Zadeh, K.; Moradi, H. Endocannabinoid System and the Kidneys: From Renal Physiology to Injury and Disease. Cannabis Cannabinoid Res. 2019, 4, 10–20. [Google Scholar] [CrossRef]
- Battista, N.; Di Tommaso, M.; Bari, M.; Maccarrone, M. The endocannabinoid system: An overview. Front. Behav. Neurosci. 2012, 6, 9. [Google Scholar] [CrossRef]
- Marsicano, G.; Kuner, R. Anatomical Distribution of Receptors, Ligands and Enzymes in the Brain and in the Spinal Cord: Cir-cuitries and Neurochemistry. In Cannabinoids and the Brain; Springer: New York, NY, USA, 2008. [Google Scholar]
- Tsuboi, K.; Uyama, T.; Okamoto, Y.; Ueda, N. Endocannabinoids and related N-acylethanolamines: Biological activities and metabolism. Inflamm. Regen. 2018, 38, 28. [Google Scholar] [CrossRef] [PubMed]
- Re, G.; Barbero, R.; Miolo, A.; Di Marzo, V. Palmitoylethanolamide, endocannabinoids and related cannabimimetic compounds in protection against tissue inflammation and pain: Potential use in companion animals. Veter. J. 2007, 173, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Ligresti, A.; Dimarzo, V. The endocannabinoid signalling system: Biochemical aspects. Pharmacol. Biochem. Behav. 2005, 81, 224–238. [Google Scholar] [CrossRef] [PubMed]
- LoVerme, J.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The search for the palmitoylethanolamide receptor. Life Sci. 2005, 77, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- Matias, I.; Petrosino, S.; Racioppi, A.; Capasso, R.; Izzo, A.A.; Di Marzo, V. Dysregulation of peripheral endocannabinoid levels in hyperglycemia and obesity: Effect of high fat diets. Mol. Cell. Endocrinol. 2008, 286, S66–S78. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, F.; Carta, G.; Bisogno, T.; Murru, E.; Cordeddu, L.; Berge, K.; Tandy, S.; Cohn, J.S.; Griinari, M.; Banni, S.; et al. Effect of dietary krill oil supplementation on the endocannabinoidome of metabolically relevant tissues from high-fat-fed mice. Nutr. Metab. 2011, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Udi, S.; Hinden, L.; Earley, B.; Drori, A.; Reuveni, N.; Hadar, R.; Cinar, R.; Nemirovski, A.; Tam, J. Proximal Tubular Cannabinoid-1 Receptor Regulates Obesity-Induced CKD. J. Am. Soc. Nephrol. 2017, 28, 3518–3532. [Google Scholar] [CrossRef]
- Nam, D.H.; Lee, M.H.; Kim, J.E.; Song, H.K.; Kang, Y.S.; Lee, J.E.; Kim, H.W.; Cha, J.J.; Hyun, Y.Y.; Kim, S.H.; et al. Blockade of Cannabinoid Receptor 1 Improves Insulin Resistance, Lipid Metabolism, and Diabetic Nephropathy in db/db Mice. Endocrinology 2012, 153, 1387–1396. [Google Scholar] [CrossRef]
- Udi, S.; Hinden, L.; Ahmad, M.; Drori, A.; Iyer, M.R.; Cinar, R.; Herman-Edelstein, M.; Tam, J. Dual inhibition of cannabinoid CB1receptor and inducible NOS attenuates obesity-induced chronic kidney disease. Br. J. Pharmacol. 2020, 177, 110–127. [Google Scholar] [CrossRef]
- Hryciw, D.H.; McAinch, A.J. Cannabinoid receptors in the kidney. Curr. Opin. Nephrol. Hypertens. 2016, 25, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, T.; Szanda, G.; Rosenberg, A.Z.; Tam, J.; Earley, B.J.; Godlewski, G.; Cinar, R.; Liu, Z.; Liu, J.; Ju, C.; et al. Overactive cannabinoid 1 receptor in podocytes drives type 2 diabetic nephropathy. Proc. Natl. Acad. Sci. USA 2014, 111, E5420–E5428. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Corbelli, A.; Mastrocola, R.; Gambino, R.; Di Marzo, V.; Pinach, S.; Rastaldi, M.P.; Perin, P.C.; Gruden, G. Cannabinoid Receptor 1 Blockade Ameliorates Albuminuria in Experimental Diabetic Nephropathy. Diabetes 2010, 59, 1046–1054. [Google Scholar] [CrossRef]
- Lim, J.; Lim, S.; Han, H.; Park, S. Cannabinoid receptor 1 mediates palmitic acid-induced apoptosis via endoplasmic reticulum stress in human renal proximal tubular cells. J. Cell. Physiol. 2010, 225, 654–663. [Google Scholar] [CrossRef]
- Hinden, L.; Kogot-Levin, A.; Tam, J.; Leibowitz, G. Pathogenesis of diabesity-induced kidney disease: Role of kidney nutrient sensing. FEBS J. 2021, 289, 901–921. [Google Scholar] [CrossRef] [PubMed]
- A Jenkin, K.; O’Keefe, L.; Simcocks, A.C.; Grinfeld, E.; Mathai, M.L.; McAinch, A.J.; Hryciw, D.H. Chronic administration of AM251 improves albuminuria and renal tubular structure in obese rats. J. Endocrinol. 2015, 225, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Janiak, P.; Poirier, B.; Bidouard, J.-P.; Cadrouvele, C.; Pierre, F.; Gouraud, L.; Barbosa, I.; Dedio, J.; Maffrand, J.-P.; Le Fur, G.; et al. Blockade of cannabinoid CB1 receptors improves renal function, metabolic profile, and increased survival of obese Zucker rats. Kidney Int. 2007, 72, 1345–1357. [Google Scholar] [CrossRef]
- Lecru, L.; Desterke, C.; Grassin-Delyle, S.; Chatziantoniou, C.; Vandermeersch, S.; Devocelle, A.; Vernochet, A.; Ivanovski, N.; Ledent, C.; Ferlicot, S.; et al. Cannabinoid receptor 1 is a major mediator of renal fibrosis. Kidney Int. 2015, 88, 72–84. [Google Scholar] [CrossRef]
- Hinden, L.; Udi, S.; Drori, A.; Gammal, A.; Nemirovski, A.; Hadar, R.; Baraghithy, S.; Permyakova, A.; Geron, M.; Cohen, M.; et al. Modulation of Renal GLUT2 by the Cannabinoid-1 Receptor: Implications for the Treatment of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2018, 29, 434–448. [Google Scholar] [CrossRef]
- Jourdan, T.; Park, J.K.; Varga, Z.V.; Pálóczi, J.; Coffey, N.J.; Rosenberg, A.Z.; Godlewski, G.; Cinar, R.; Mackie, K.; Pacher, P.; et al. Cannabinoid-1 receptor deletion in podocytes mitigates both glomerular and tubular dysfunction in a mouse model of diabetic nephropathy. Diabetes Obes. Metab. 2018, 20, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.; François, H. Cannabinoid Receptor 1 Inhibition in Chronic Kidney Disease: A New Therapeutic Toolbox. Front. Endocrinol. 2021, 12, 720734. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Grimaldi, S.; Franco, I.; Bellini, S.; Gambino, R.; Pinach, S.; Corbelli, A.; Bruno, G.; Rastaldi, M.P.; Aveta, T.; et al. Deficiency of cannabinoid receptor of type 2 worsens renal functional and structural abnormalities in streptozotocin-induced diabetic mice. Kidney Int. 2014, 86, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.; Hellerbrand, C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.-J.; Huber, T.B.; Isermann, B.; Schiffer, M. CKD in diabetes: Diabetic kidney disease versus nondiabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 361–377. [Google Scholar] [CrossRef]
- Pyram, R.; Kansara, A.; Banerji, M.A.; Loney-Hutchinson, L. Chronic kidney disease and diabetes. Maturitas 2012, 71, 94–103. [Google Scholar] [CrossRef]
- Lee, Y.; Park, S.; Lee, S.; Kim, Y.; Kang, M.W.; Cho, S.; Park, S.; Han, K.; Kim, Y.C.; Han, S.S.; et al. Lipid profiles and risk of major adverse cardiovascular events in CKD and diabetes: A nationwide population-based study. PLoS ONE 2020, 15, e0231328. [Google Scholar] [CrossRef]
- Middleton, R.J.; Foley, R.N.; Hegarty, J.; Cheung, C.M.; McElduff, P.; Gibson, J.M.; Kalra, P.A.; O’Donoghue, D.J.; New, J.P. The unrecognized prevalence of chronic kidney disease in diabetes. Nephrol. Dial. Transplant. 2006, 21, 88–92. [Google Scholar] [CrossRef]
- Targher, G.; Chonchol, M.B.; Byrne, C.D. CKD and Nonalcoholic Fatty Liver Disease. Am. J. Kidney Dis. 2014, 64, 638–652. [Google Scholar] [CrossRef]
- Orlić, L.; Mikolasevic, I.; Bagic, Z.; Racki, S.; Stimac, D.; Milic, S. Chronic Kidney Disease and Nonalcoholic Fatty Liver Disease—Is There a Link? Gastroenterol. Res. Pract. 2014, 2014, 847539. [Google Scholar] [CrossRef]
- Thomas, R.; Kanso, A.; Sedor, J.R. Chronic Kidney Disease and Its Complications. Prim. Care Clin. Off. Pract. 2008, 35, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Stephany, B.R.; Alao, B.; Budev, M.; Boumitri, M.; Poggio, E.D. Hyperlipidemia Is Associated with Accelerated Chronic Kidney Disease Progression After Lung Transplantation. Am. J. Transplant. 2007, 7, 2553–2560. [Google Scholar] [CrossRef]
- Câmara, N.O.S.; Iseki, K.; Kramer, H.; Liu, Z.-H.; Sharma, K. Kidney disease and obesity: Epidemiology, mechanisms and treatment. Nat. Rev. Nephrol. 2017, 13, 181–190. [Google Scholar] [CrossRef]
- Cignarelli, M.; Lamacchia, O. Obesity and kidney disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Rea, D.; Heimbach, J.; Grande, J.; Textor, S.; Taler, S.; Prieto, M.; Larson, T.; Cosio, F. Stegall Glomerular volume and renal histology in obese and non-obese living kidney donors. Kidney Int. 2006, 70, 1636–1641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goumenos, D.S.; Kawar, B.; El Nahas, M.; Conti, S.; Wagner, B.; Spyropoulos, C.; Vlachojannis, J.G.; Benigni, A.; Kalfarentzos, F. Early histological changes in the kidney of people with morbid obesity. Nephrol. Dial. Transplant. 2009, 24, 3732–3738. [Google Scholar] [CrossRef]
- Tobar, A.; Ori, Y.; Benchetrit, S.; Milo, G.; Herman-Edelstein, M.; Zingerman, B.; Lev, N.; Gafter, U.; Chagnac, A. Proximal Tubular Hypertrophy and Enlarged Glomerular and Proximal Tubular Urinary Space in Obese Subjects with Proteinuria. PLoS ONE 2013, 8, e75547. [Google Scholar] [CrossRef]
- Wen, Y.; Parikh, C.R. Current concepts and advances in biomarkers of acute kidney injury. Crit. Rev. Clin. Lab. Sci. 2021, 58, 354–368. [Google Scholar] [CrossRef]
- Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A specific and sensitive biomarker of kidney injury. Scand. J. Clin. Lab. Investig. 2008, 68, 78–83. [Google Scholar] [CrossRef]
- Lim, A.I.; Tang, S.C.W.; Lai, K.N.; Leung, J.C.K. Kidney injury molecule-1: More than just an injury marker of tubular epithelial cells? J. Cell. Physiol. 2013, 228, 917–924. [Google Scholar] [CrossRef]
- van Timmeren, M.M.; van den Heuvel, M.C.; Bailly, V.; Bakker, S.J.L.; van Goor, H.; Stegeman, C.A. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J. Pathol. 2007, 212, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Chen, B.; Hong, W.; Liang, Y.; Bai, Y. Transforming growth factor-β1 stimulates hedgehog signaling to promote epithelial-mesenchymal transition after kidney injury. FEBS J. 2016, 283, 3771–3790. [Google Scholar] [CrossRef] [PubMed]
- Parikh, C.R.; Mishra, J.; Thiessen-Philbrook, H.; Dursun, B.; Ma, Q.; Kelly, C.; Dent, C.; Devarajan, P.; Edelstein, C. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006, 70, 199–203. [Google Scholar] [CrossRef]
- Zdziechowska, M.; Gluba-Brzózka, A.; Poliwczak, A.R.; Franczyk, B.; Kidawa, M.; Zielinska, M.; Rysz, J. Serum NGAL, KIM-1, IL-18, L-FABP: New biomarkers in the diagnostics of acute kidney injury (AKI) following invasive cardiology procedures. Int. Urol. Nephrol. 2020, 52, 2135–2143. [Google Scholar] [CrossRef]
- Lane, B.R. Molecular markers of kidney injury. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Panizo, S.; Martínez-Arias, L.; Alonso-Montes, C.; Cannata, P.; Martín-Carro, B.; Fernández-Martín, J.L.; Naves-Díaz, M.; Carrillo-López, N.; Cannata-Andía, J.B. Fibrosis in Chronic Kidney Disease: Pathogenesis and Consequences. Int. J. Mol. Sci. 2021, 22, 408. [Google Scholar] [CrossRef]
- Drori, A.; Permyakova, A.; Hadar, R.; Udi, S.; Nemirovski, A.; Tam, J. Cannabinoid-1 receptor regulates mitochondrial dynamics and function in renal proximal tubular cells. Diabetes Obes. Metab. 2019, 21, 146–159. [Google Scholar] [CrossRef]
- Tam, J.; Hinden, L.; Drori, A.; Udi, S.; Azar, S.; Baraghithy, S. The therapeutic potential of targeting the peripheral endocannabinoid/CB 1 receptor system. Eur. J. Intern. Med. 2018, 49, 23–29. [Google Scholar] [CrossRef]
- Di Marzo, V.; De Petrocellis, L. Why do cannabinoid receptors have more than one endogenous ligand? Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3216–3228. [Google Scholar] [CrossRef]
- Engeli, S.; Böhnke, J.; Feldpausch, M.; Gorzelniak, K.; Janke, J.; Bátkai, S.; Pacher, P.; Harvey-White, J.; Luft, F.C.; Sharma, A.M.; et al. Activation of the Peripheral Endocannabinoid System in Human Obesity. Diabetes 2005, 54, 2838–2843. [Google Scholar] [CrossRef]
- Matias, I.; Gatta-Cherifi, B.; Cota, D. Obesity and the Endocannabinoid System: Circulating Endocannabinoids and Obesity. Curr. Obes. Rep. 2012, 1, 229–235. [Google Scholar] [CrossRef]
- Côté, M.; Matias, I.; Lemieux, I.; Petrosino, S.; Alméras, N.; Després, J.-P.; Di Marzo, V. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int. J. Obes. 2007, 31, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Azar, S.; Sherf-Dagan, S.; Nemirovski, A.; Webb, M.; Raziel, A.; Keidar, A.; Goitein, D.; Sakran, N.; Shibolet, O.; Tam, J.; et al. Circulating Endocannabinoids Are Reduced Following Bariatric Surgery and Associated with Improved Metabolic Homeostasis in Humans. Obes. Surg. 2019, 29, 268–276. [Google Scholar] [CrossRef]
- Castonguay-Paradis, S.; Lacroix, S.; Rochefort, G.; Parent, L.; Perron, J.; Martin, C.; Lamarche, B.; Raymond, F.; Flamand, N.; Di Marzo, V.; et al. Dietary fatty acid intake and gut microbiota determine circulating endocannabinoidome signaling beyond the effect of body fat. Sci. Rep. 2020, 10, 15975. [Google Scholar] [CrossRef]
- Hillard, C.J. Circulating Endocannabinoids: From Whence Do They Come and Where are They Going? Neuropsychopharmacology 2018, 43, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.S.; Colangeli, R.; Dong, A.; George, A.G.; Addo-Osafo, K.; Kingsley, P.J.; Morena, M.; Wolff, M.D.; Dudok, B.; He, K.; et al. In vivo endocannabinoid dynamics at the timescale of physiological and pathological neural activity. Neuron 2021, 109, 2398–2403.e4. [Google Scholar] [CrossRef]
- Gorzalka, B.B.; Dang, S.S. Minireview: Endocannabinoids and Gonadal Hormones: Bidirectional Interactions in Physiology and Behavior. Endocrinology 2012, 153, 1016–1024. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Marczylo, T.H.; Lam, P.M.; Rana, S.; Franchi, A.M.; Konje, J.C.; Perez-Martinez, S. Anandamide Levels Fluctuate in the Bovine Oviduct during the Oestrous Cycle. PLoS ONE 2013, 8, e72521. [Google Scholar] [CrossRef]
- Ritter, J.K.; Li, G.; Xia, M.; Boini, K. Anandamide and its metabolites: What are their roles in the kidney? Front. Biosci. 2016, 8, 264–277. [Google Scholar] [CrossRef]
- Tam, J. The emerging role of the endocannabinoid system in the pathogenesis and treatment of kidney diseases. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 267–276. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Pan, H.; Rajesh, M.; Bátkai, S.; Patel, V.; Harvey-White, J.; Mukhopadhyay, B.; Haskó, G.; Gao, B.; Mackie, K.; et al. CB1 cannabinoid receptors promote oxidative/nitrosative stress, inflammation and cell death in a murine nephropathy model. Br. J. Pharmacol. 2010, 160, 657–668. [Google Scholar] [CrossRef]
- Hinden, L.; Ahmad, M.; Hamad, S.; Nemirovski, A.; Szanda, G.; Glasmacher, S.; Kogot-Levin, A.; Abramovitch, R.; Thorens, B.; Gertsch, J.; et al. Opposite physiological and pathological mTORC1-mediated roles of the CB1 receptor in regulating renal tubular function. Nat. Commun. 2022, 13, 1783. [Google Scholar] [CrossRef] [PubMed]
- Vanni, E.; Bugianesi, E.; Kotronen, A.; De Minicis, S.; Yki-Järvinen, H.; Svegliati-Baroni, G. From the metabolic syndrome to NAFLD or vice versa? Dig. Liver Dis. 2010, 42, 320–330. [Google Scholar] [CrossRef]
- Bonora, E.; Targher, G. Increased risk of cardiovascular disease and chronic kidney disease in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Marcuccilli, M.; Chonchol, M. NAFLD and Chronic Kidney Disease. Int. J. Mol. Sci. 2016, 17, 562. [Google Scholar] [CrossRef]
- Iacobini, C.; Pugliese, G.; Blasetti Fantauzzi, C.; Federici, M.; Menini, S. Metabolically healthy versus metabolically unhealthy obesity. Metabolism 2019, 92, 51–60. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Low BMI (n = 10) | High BMI (n = 11) | p Value |
|---|---|---|---|
| Age (years) | 57.3 (40–64) | 54.91 (38–71) | 0.5214 |
| BMI (kg/m2) | 23.18 (19.02–25.99) | 33.69 (30.02–42.50) | <0.0001 |
| Fasting serum glucose (mg/dL) # | 157 (75-362) | 122.86 (89–199) | 0.7396 |
| Creatinine (mg/dL) * | 1.41 (0.56–4.73) | 0.78 (0.64–1.00) | 0.7546 |
| BUN (mg/dL) * | 14.07 (9.07–31.09) | 15.37 (10.67–24.22) | 0.2414 |
| Glucose (mmol/L) * | 5.87 (3.99–6.67) | 6.57 (3.34–9.97) | 0.4908 |
| Lactate (mmol/L) * | 2.55 (1.13–4.53) | 3.86 (2.3–6.5) | 0.1079 |
| ALT (U/L) * | 18 (9–29) | 18.87 (10–29) | 0.323 |
| AST (U/L) * | 17.11 (9.2–24.1) | 13.56 (9.2–18.2) | 0.5987 |
| ALP (U/L) * | 90.97 (61.1–136.2) | 74.33 (54.9–119.3) | 0.1812 |
| TG (mg/dL) * | 182.55 (119.58–852.44) | 214.33 (100.81–609.23) | 0.4908 |
| Cholesterol (mg/dL) * | 164.91 (112.26–221.72) | 151.21 (111.4–218.61) | 0.5728 |
| HDL (mg/dL) * | 0.98 (0.63–1.13) | 0.91 (0.61–1.36) | 0.8258 |
| LDL (mg/dL) * | 70.33 (18.10–106.95) | 69.51 (1.63–139.25) | 0.8765 |
| Analyte | Molecular Ion [M + H]+ [M − H]− for AA [m/z] | Fragment (m/z) | DP (Volts) | CE (Volts) | CXP (Volts) |
|---|---|---|---|---|---|
| 2-AG | 379.2 | 287.1 (quantifier) | 70 | 19 | 14 |
| 91 (qualifier) | 70 | 67 | 10 | ||
| AEA | 348.2 | 287.1 (quantifier) | 26 | 13 | 16 |
| 62 (qualifier) | 26 | 13 | 8 | ||
| PEA | 300.3 | 283.2 (quantifier) | 130 | 19 | 24 |
| 62 (qualifier) | 130 | 17 | 8 | ||
| AA | 305.3 | 91 (quantifier) | 1 | 49 | 10 |
| 287.1 (qualifier) | 1 | 13 | 22 | ||
| OEA | 326.3 | 61.9 (quantifier) | 146 | 21 | 24 |
| 309.1 (qualifier) | 146 | 21 | 42 | ||
| d4-AEA | 352.3 | 287.1 (quantifier) | 66 | 15 | 20 |
| 66 (qualifier) | 66 | 21 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Permyakova, A.; Rothner, A.; Knapp, S.; Nemirovski, A.; Ben-Zvi, D.; Tam, J. Renal Endocannabinoid Dysregulation in Obesity-Induced Chronic Kidney Disease in Humans. Int. J. Mol. Sci. 2023, 24, 13636. https://doi.org/10.3390/ijms241713636
Permyakova A, Rothner A, Knapp S, Nemirovski A, Ben-Zvi D, Tam J. Renal Endocannabinoid Dysregulation in Obesity-Induced Chronic Kidney Disease in Humans. International Journal of Molecular Sciences. 2023; 24(17):13636. https://doi.org/10.3390/ijms241713636
Chicago/Turabian StylePermyakova, Anna, Ariel Rothner, Sarah Knapp, Alina Nemirovski, Danny Ben-Zvi, and Joseph Tam. 2023. "Renal Endocannabinoid Dysregulation in Obesity-Induced Chronic Kidney Disease in Humans" International Journal of Molecular Sciences 24, no. 17: 13636. https://doi.org/10.3390/ijms241713636
APA StylePermyakova, A., Rothner, A., Knapp, S., Nemirovski, A., Ben-Zvi, D., & Tam, J. (2023). Renal Endocannabinoid Dysregulation in Obesity-Induced Chronic Kidney Disease in Humans. International Journal of Molecular Sciences, 24(17), 13636. https://doi.org/10.3390/ijms241713636






