Salivary Chemical Barrier Proteins in Oral Squamous Cell Carcinoma—Alterations in the Defense Mechanism of the Oral Cavity
Abstract
1. Introduction
2. Results
2.1. Differential Expression of Chemical Barrier Proteins in Saliva of Patients with OSCC
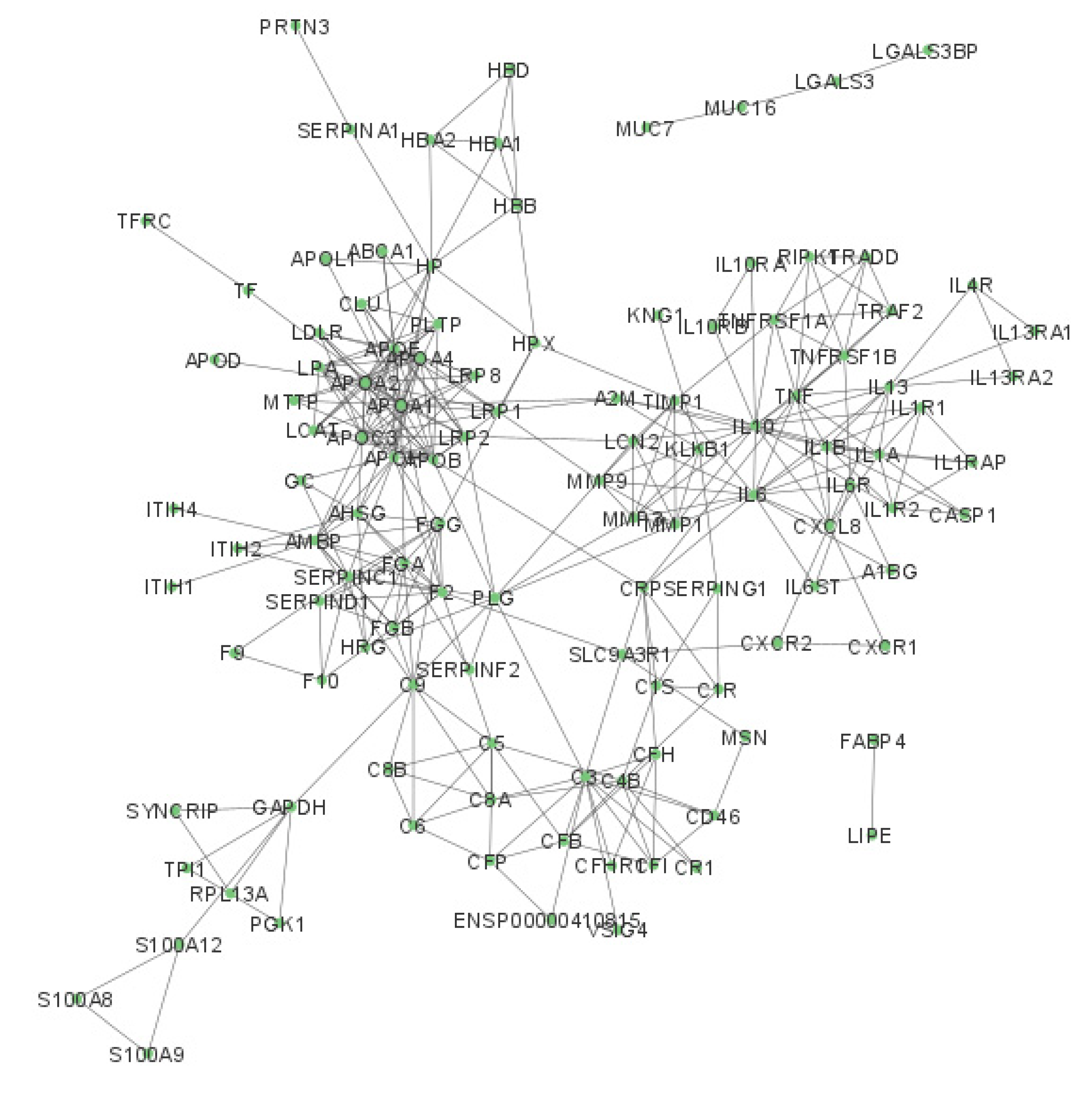
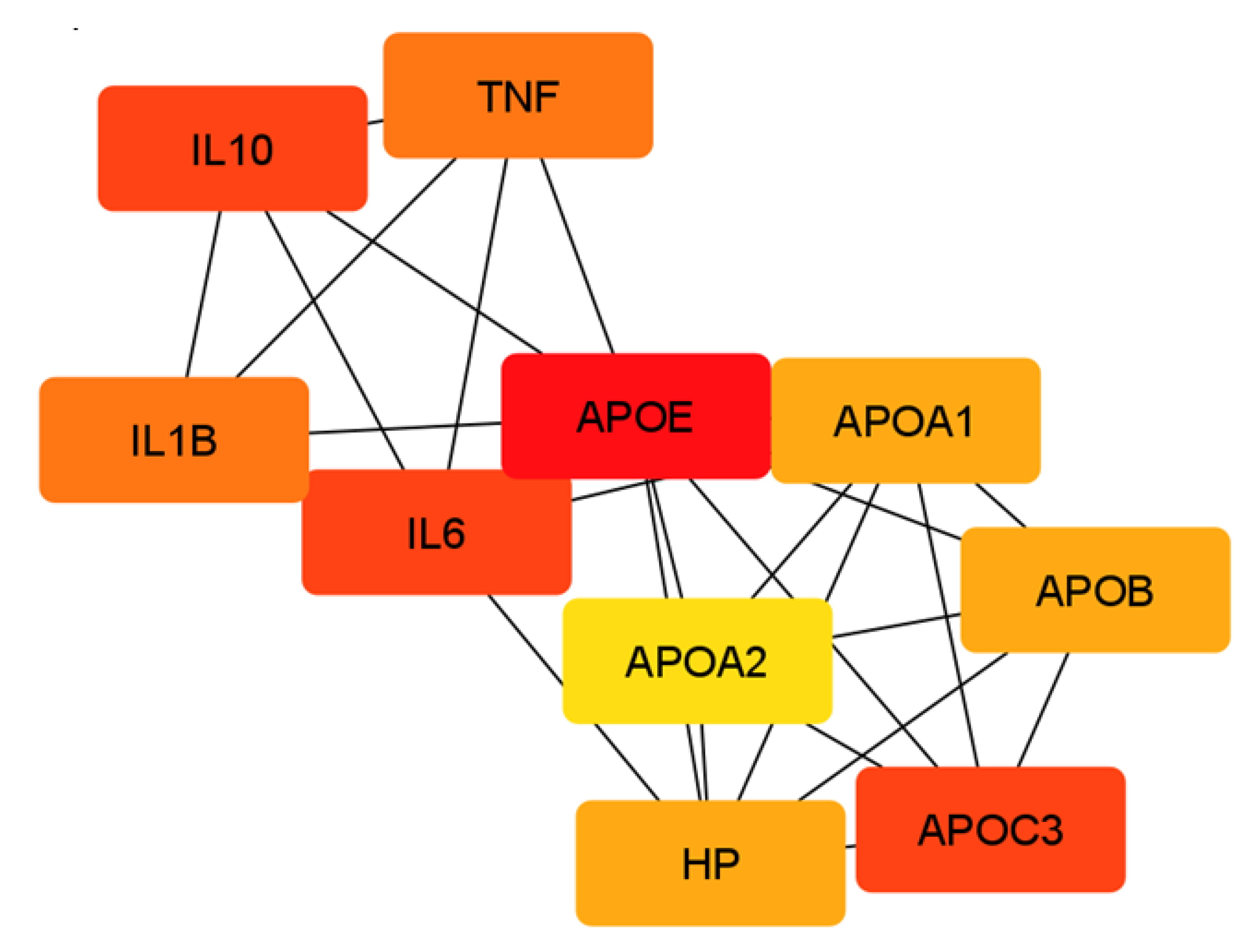
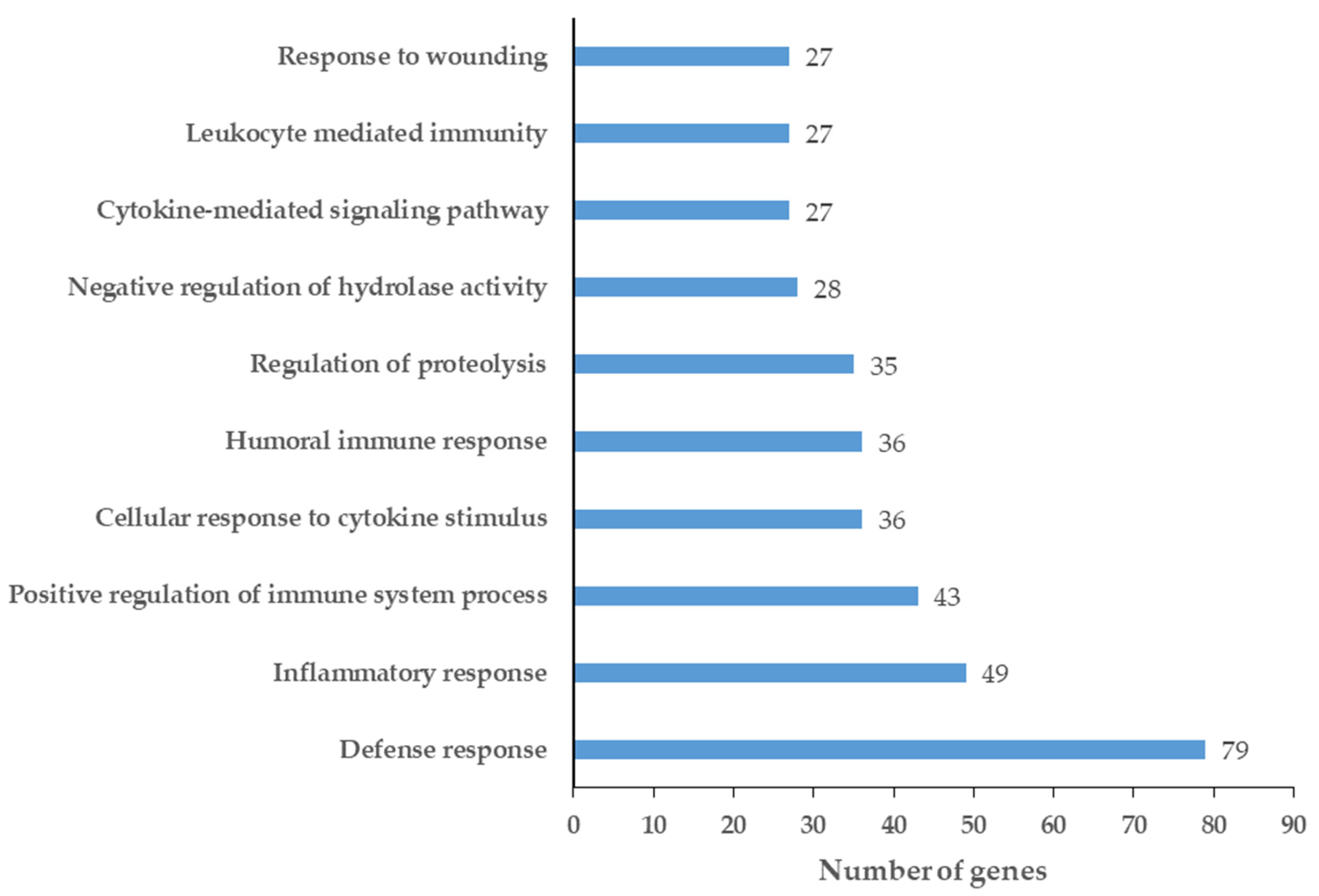
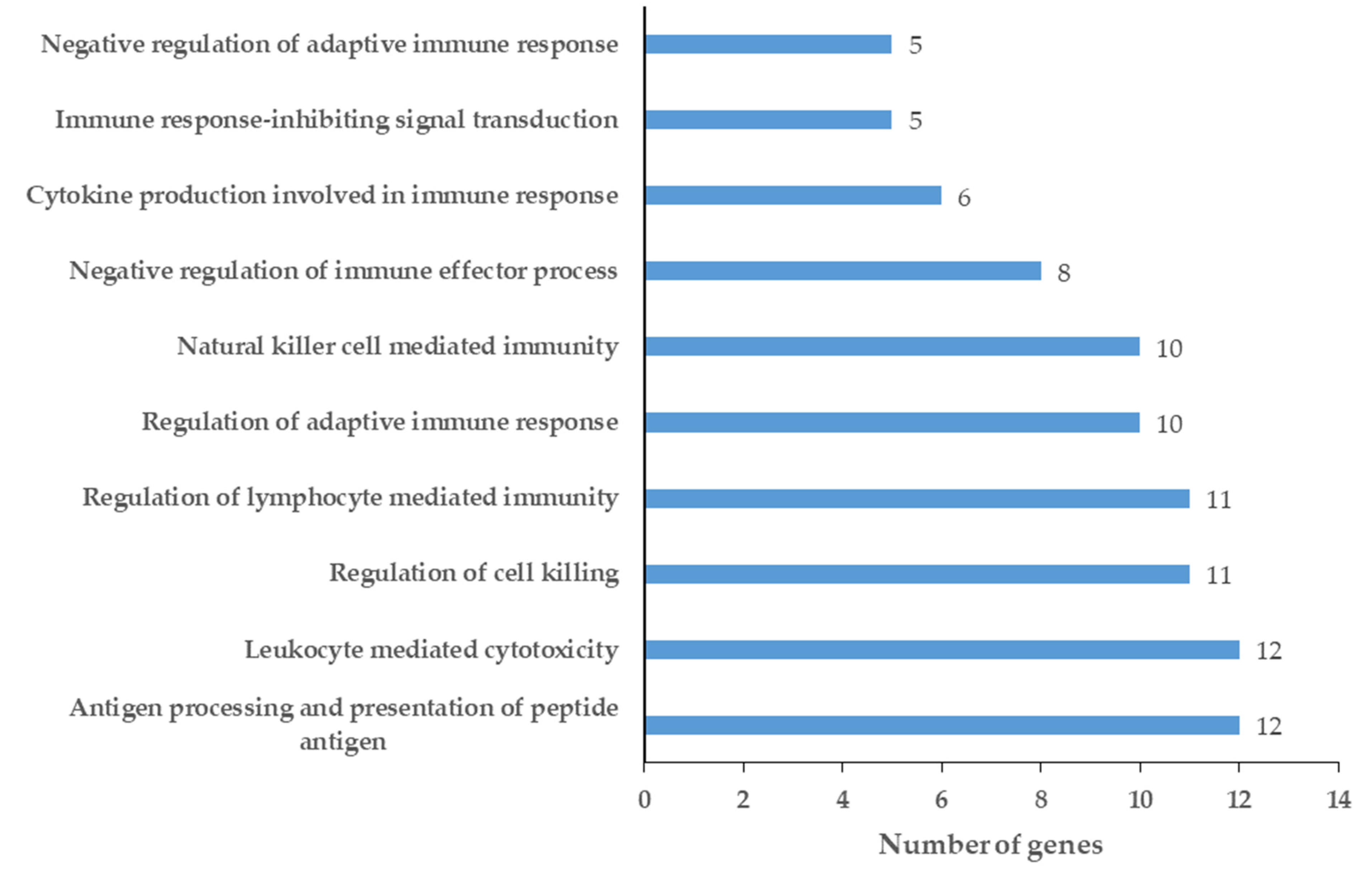
2.2. Network Analysis of Chemical Barrier Proteins Affected by OSCC
3. Discussion
3.1. Amylases and Mucins in the Chemical Barrier of Patients with OSCC
3.2. Proteases and Protease Inhibitors in the Salivary Chemical Barrier of Patients with OSCC
3.3. Contribution of Cytokines in the Salivary Chemical Barrier of Patients with OSCC
3.4. Members of S100 Protein Family Are Affected by OSCC
3.5. OSCC Can Enhance the Complement System in the Salivary Chemical Barrier
3.6. Elevated Levels of Apolipoproteins in the Saliva of Patients with OSCC
3.7. Salivary Chemical Barrier Proteins with Contradictory Expression Profile in OSCC
3.8. Alterations in the Defense Mechanism of the Oral Cavity
3.9. Limitations of the Study
4. Materials and Methods
4.1. Examination of Chemical Barrier Proteins in OSCC Datasets
4.2. Network Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Massano, J.; Regateiro, F.S.; Januário, G.; Ferreira, A. Oral squamous cell carcinoma: Review of prognostic and predictive factors. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, D.; Sreenivasan, P.; Öhman, J.; Wallström, M.; Braz-Silva, P.H.; Giglio, D.; Kjeller, G.; Hasséus, B. Potentially Malignant Oral Disorders and Cancer Transformation. Anticancer Res. 2018, 38, 3223–3229. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Diz, P.; Meleti, M.; Diniz-Freitas, M.; Vescovi, P.; Warnakulasuriya, S.; Johnson, N.W.; Kerr, A.R. Oral and pharyngeal cancer in Europe: Incidence, mortality and trends as presented to the Global Oral Cancer Forum. Transl. Res. Oral Oncol. 2017, 2, 2057178. [Google Scholar] [CrossRef]
- Zhang, H.; Dziegielewski, P.T.; Biron, V.L.; Szudek, J.; Al-Qahatani, K.H.; O’Connell, D.A.; Harris, J.R.; Seikaly, H. Survival outcomes of patients with advanced oral cavity squamous cell carcinoma treated with multimodal therapy: A multi-institutional analysis. J. Otolaryngol. Head Neck Surg. 2013, 42, 30. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Torras, C.; Gay-Escoda, C. Techniques for early diagnosis of oral squamous cell carcinoma: Systematic review. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e305–e315. [Google Scholar] [CrossRef]
- Chang, J.T.; Wang, H.-M.; Chang, K.-W.; Chen, W.-H.; Wen, M.-C.; Hsu, Y.-M.; Yung, B.Y.-M.; Chen, I.-H.; Liao, C.-T.; Hsieh, L.-L.; et al. Identification of differentially expressed genes in oral squamous cell carcinoma (OSCC): Overexpression of NPM, CDK1 and NDRG1 and underexpression of CHES1. Int. J. Cancer 2005, 114, 942–949. [Google Scholar] [CrossRef]
- Gandini, S.; Botteri, E.; Iodice, S.; Boniol, M.; Lowenfels, A.B.; Maisonneuve, P.; Boyle, P. Tobacco smoking and cancer: A meta-analysis. Int. J. Cancer 2008, 122, 155–164. [Google Scholar] [CrossRef]
- Bagnardi, V.; Blangiardo, M.; La Vecchia, C.; Corrao, G. A meta-analysis of alcohol drinking and cancer risk. Br. J. Cancer 2001, 85, 1700–1705. [Google Scholar] [CrossRef]
- Rosenquist, K. Risk factors in oral and oropharyngeal squamous cell carcinoma: A population-based case-control study in southern Sweden. Swed. Dent. J. Suppl. 2005, 179, 1–66. [Google Scholar]
- Gupta, S.; Gupta, S. Role of human papillomavirus in oral squamous cell carcinoma and oral potentially malignant disorders: A review of the literature. Indian J. Dent. 2015, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hu, Y.; Zhou, X.; Liu, S.; Han, Q.; Cheng, L. Role of Oral Bacteria in the Development of Oral Squamous Cell Carcinoma. Cancers 2020, 12, 2797. [Google Scholar] [CrossRef] [PubMed]
- Robayo, D.A.G.; Erira, H.A.T.; Jaimes, F.O.G.; Torres, A.M.; Galindo, A.I.C. Oropharyngeal Squamous Cell Carcinoma: Human Papilloma Virus Coinfection with Streptococcus anginosus. Braz. Dent. J. 2019, 30, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Henskens, Y.M.; van der Velden, U.; Veerman, E.C.; Nieuw Amerongen, A.V. Protein, albumin and cystatin concentrations in saliva of healthy subjects and of patients with gingivitis or periodontitis. J. Periodontal Res. 1993, 28, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kalló, G.; Kumar, A.; Tőzsér, J.; Csősz, É. Chemical Barrier Proteins in Human Body Fluids. Biomedicines 2022, 10, 1472. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Epand, R.M.; Vogel, H.J. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta 1999, 1462, 11–28. [Google Scholar] [CrossRef]
- Wiesner, J.; Vilcinskas, A. Antimicrobial peptides: The ancient arm of the human immune system. Virulence 2010, 1, 440–464. [Google Scholar] [CrossRef]
- Csősz, É.; Kalló, G.; Márkus, B.; Deák, E.; Csutak, A.; Tőzsér, J. Quantitative body fluid proteomics in medicine—A focus on minimal invasiveness. J. Proteom. 2017, 153, 30–43. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chen, H.W.; Wu, C.F.; Chu, L.J.; Chiang, W.F.; Wu, C.C.; Yu, J.S.; Tsai, C.H.; Liang, K.H.; Chang, Y.S.; et al. Development of a Multiplexed Liquid Chromatography Multiple-Reaction-Monitoring Mass Spectrometry (LC-MRM/MS) Method for Evaluation of Salivary Proteins as Oral Cancer Biomarkers. Mol. Cell. Proteom. 2017, 16, 799–811. [Google Scholar] [CrossRef]
- Kawahara, R.; Bollinger, J.G.; Rivera, C.; Ribeiro, A.C.P.; Brandão, T.B.; Leme, A.F.P.; Maccoss, M.J. A targeted proteomic strategy for the measurement of oral cancer candidate biomarkers in human saliva. Proteomics 2016, 16, 159–173. [Google Scholar] [CrossRef]
- Wu, C.C.; Chu, H.W.; Hsu, C.W.; Chang, K.P.; Liu, H.P. Saliva proteome profiling reveals potential salivary biomarkers for detection of oral cavity squamous cell carcinoma. Proteomics 2015, 15, 3394–3404. [Google Scholar] [CrossRef]
- Sivadasan, P.; Gupta, M.K.; Sathe, G.; Sudheendra, H.V.; Sunny, S.P.; Renu, D.; Hari, P.S.; Gowda, H.; Suresh, A.; Kuriakose, M.A.; et al. Salivary proteins from dysplastic leukoplakia and oral squamous cell carcinoma and their potential for early detection. J. Proteom. 2020, 212, 103574. [Google Scholar] [CrossRef]
- Csosz, E.; Lábiscsák, P.; Kalló, G.; Márkus, B.; Emri, M.; Szabó, A.; Tar, I.; Tozsér, J.; Kiss, C.; Márton, I. Proteomics investigation of OSCC-specific salivary biomarkers in a Hungarian population highlights the importance of identification of population-tailored biomarkers. PLoS ONE 2017, 12, e0177282. [Google Scholar] [CrossRef]
- Csősz, É.; Márkus, B.; Darula, Z.; Medzihradszky, K.F.; Nemes, J.; Szabó, E.; Tőzsér, J.; Kiss, C.; Márton, I. Salivary proteome profiling of oral squamous cell carcinoma in a Hungarian population. FEBS Open Bio. 2018, 8, 556–569. [Google Scholar] [CrossRef]
- Márton, I.J.; Horváth, J.; Lábiscsák, P.; Márkus, B.; Dezső, B.; Szabó, A.; Tar, I.; Piffkó, J.; Jakus, P.; Barabás, J.; et al. Salivary IL-6 mRNA is a Robust Biomarker in Oral Squamous Cell Carcinoma. J. Clin. Med. 2019, 8, 1958. [Google Scholar] [CrossRef]
- Scholtz, B.; Vo Minh, D.; Kiss, C.; Tar, I.; Kumar, A.; Tőzsér, J.; Csősz, É.; Márton, I. Examination of Oral Squamous Cell Carcinoma and Precancerous Lesions Using Proximity Extension Assay and Salivary RNA Quantification. Biomedicines 2020, 8, 610. [Google Scholar] [CrossRef]
- Vizcaíno, J.A.; Deutsch, E.W.; Wang, R.; Csordas, A.; Reisinger, F.; Ríos, D.; Dianes, J.A.; Sun, Z.; Farrah, T.; Bandeira, N.; et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 2014, 32, 223–226. [Google Scholar] [CrossRef]
- PubMed. Available online: www.pubmed.ncbi.nlm.nih.gov (accessed on 22 May 2023).
- Kumar, A.; Doan, V.M.; Kunkli, B.; Csősz, É. Construction of Unified Human Antimicrobial and Immunomodulatory Peptide Database and Examination of Antimicrobial and Immunomodulatory Peptides in Alzheimer’s Disease Using Network Analysis of Proteomics Datasets. Front. Genet. 2021, 12, 633050. [Google Scholar] [CrossRef] [PubMed]
- Ceciliani, F.; Lecchi, C. The Immune Functions of α 1 Acid Glycoprotein. Curr. Protein Pept. Sci. 2019, 20, 505–524. [Google Scholar] [CrossRef] [PubMed]
- Dimberg, J.; Ström, K.; Löfgren, S.; Zar, N.; Hugander, A.; Matussek, A. Expression of the serine protease inhibitor serpinA3 in human colorectal adenocarcinomas. Oncol. Lett. 2011, 2, 413. [Google Scholar] [CrossRef] [PubMed]
- Janciauskiene, S.; Wrenger, S.; Immenschuh, S.; Olejnicka, B.; Greulich, T.; Welte, T.; Chorostowska-Wynimko, J. The multifaceted effects of Alpha1-Antitrypsin on neutrophil functions. Front. Pharmacol. 2018, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Cederfur, C.; Salomonsson, E.; Nilsson, J.; Halim, A.; Öberg, C.T.; Larson, G.; Nilsson, U.J.; Leffler, H. Different affinity of galectins for human serum glycoproteins: Galectin-3 binds many protease inhibitors and acute phase proteins. Glycobiology 2008, 18, 384–394. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, S.; Saleem, S.; Reed, G.L. Alpha2-Antiplasmin: The Devil You Don’t Know in Cerebrovascular and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 363. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sama, A.E. Anti-inflammatory role of fetuin-A in injury and infection. Curr. Mol. Med. 2012, 12, 625–633. [Google Scholar] [CrossRef]
- Vandooren, J.; Itoh, Y. Alpha-2-Macroglobulin in Inflammation, Immunity and Infections. Front. Immunol. 2021, 12, 5411. [Google Scholar] [CrossRef]
- Mulligan, M.S.; Lentsch, A.B.; Huber-Lang, M.; Guo, R.F.; Sarma, V.; Wright, C.D.; Ulich, T.R.; Ward, P.A. Anti-inflammatory effects of mutant forms of secretory leukocyte protease inhibitor. Am. J. Pathol. 2000, 156, 1033–1039. [Google Scholar] [CrossRef]
- Roemisch, J.; Gray, E.; Hoffmann, J.N.; Wiedermann, C.J.; Kalina, U. Antithrombin: A new look at the actions of a serine protease inhibitor. Blood Coagul. Fibrinolysis 2002, 13, 657–670. [Google Scholar] [CrossRef]
- Tada, N.; Sakamoto, T.; Kagami, A.; Mochizuki, K.; Kurosaka, K. Antimicrobial activity of lipoprotein particles containing apolipoprotein Al. Mol. Cell. Biochem. 1993, 119, 171–178. [Google Scholar] [CrossRef]
- Recalde, D.; Ostos, M.A.; Badell, E.; Garcia-Otin, A.L.; Pidoux, J.; Castro, G.; Zakin, M.M.; Scott-Algara, D. Human apolipoprotein A-IV reduces secretion of proinflammatory cytokines and atherosclerotic effects of a chronic infection mimicked by lipopolysaccharide. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 756–761. [Google Scholar] [CrossRef]
- Gaglione, R.; Cesaro, A.; Dell’Olmo, E.; Della Ventura, B.; Casillo, A.; Di Girolamo, R.; Velotta, R.; Notomista, E.; Veldhuizen, E.J.A.; Corsaro, M.M.; et al. Effects of human antimicrobial cryptides identified in apolipoprotein B depend on specific features of bacterial strains. Sci. Rep. 2019, 9, 6728. [Google Scholar] [CrossRef]
- Zewinger, S.; Reiser, J.; Jankowski, V.; Alansary, D.; Hahm, E.; Triem, S.; Klug, M.; Schunk, S.J.; Schmit, D.; Kramann, R.; et al. Apolipoprotein C3 induces inflammation and organ damage by alternative inflammasome activation. Nat. Immunol. 2019, 21, 30–41. [Google Scholar] [CrossRef]
- Do Carmo, S.; Jacomy, H.; Talbot, P.J.; Rassart, E. Neuroprotective effect of apolipoprotein D against human coronavirus OC43-induced encephalitis in mice. J. Neurosci. 2008, 28, 10330–10338. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, L.M.; Wu, J. Cross-talk between apolipoprotein E and cytokines. Mediat. Inflamm. 2011, 2011, 949072. [Google Scholar] [CrossRef]
- Fang, J.; Yao, X.; Hou, M.; Duan, M.; Xing, L.; Huang, J.; Wang, Y.; Zhu, B.; Chen, Q.; Wang, H. ApoL1 induces kidney inflammation through RIG-I/NF-κB activation. Biochem. Biophys. Res. Commun. 2020, 527, 466–473. [Google Scholar] [CrossRef]
- Serrano, M.; Morán, L.; Martinez-Flores, J.A.; Mancebo, E.; Pleguezuelo, D.; Cabrera-Marante, O.; Delgado, J.; Serrano, A. Immune Complexes of Beta-2-Glycoprotein I and IgA Antiphospholipid Antibodies Identify Patients With Elevated Risk of Thrombosis and Early Mortality After Heart Transplantation. Front. Immunol. 2019, 10, 2891. [Google Scholar] [CrossRef]
- Liu, Y.; Bartlett, J.A.; Di, M.E.; Bomberger, J.M.; Chan, Y.R.; Gakhar, L.; Mallampalli, R.K.; McCray, P.B.; Di, Y.P. SPLUNC1/BPIFA1 Contributes to Pulmonary Host Defense against Klebsiella pneumoniae Respiratory Infection. Am. J. Pathol. 2013, 182, 1519–1531. [Google Scholar] [CrossRef]
- Kono, Y. Apparent antibacterial activity of catalase: Role of lipid hydroperoxide contamination. J. Biochem. 1995, 117, 42–46. [Google Scholar] [CrossRef]
- Stafford, S.L.; Bokil, N.J.; Achard, M.E.S.; Kapetanovic, R.; Schembri, M.A.; Mcewan, A.G.; Sweet, M.J. Metal ions in macrophage antimicrobial pathways: Emerging roles for zinc and copper. Biosci. Rep. 2013, 33, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Ledee, D.R.; Gordon, G.M.; Itakura, T.; Patel, N.; Martin, A.; Fini, M.E. Interaction of clusterin and matrix metalloproteinase-9 and its implication for epithelial homeostasis and inflammation. Am. J. Pathol. 2012, 180, 2028–2039. [Google Scholar] [CrossRef] [PubMed]
- Gardill, B.R.; Vogl, M.R.; Lin, H.Y.; Hammond, G.L.; Muller, Y.A. Corticosteroid-Binding Globulin: Structure-Function Implications from Species Differences. PLoS ONE 2012, 7, 52759. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Torrent, M.; de la Torre, B.G.; Nogués, V.M.; Andreu, D.; Boix, E. Bactericidal and membrane disruption activities of the eosinophil cationic protein are largely retained in an N-terminal fragment. Biochem. J. 2009, 421, 425–434. [Google Scholar] [CrossRef]
- Gong, Y.; Yu, Z.; Gao, Y.; Deng, L.; Wang, M.; Chen, Y.; Li, J.; Cheng, B. FABP4 inhibitors suppress inflammation and oxidative stress in murine and cell models of acute lung injury. Biochem. Biophys. Res. Commun. 2018, 496, 1115–1121. [Google Scholar] [CrossRef]
- Ko, Y.P.; Flick, M.J. Fibrinogen Is at the Interface of Host Defense and Pathogen Virulence in Staphylococcus aureus Infection. Semin. Thromb. Hemost. 2016, 42, 408–421. [Google Scholar] [CrossRef]
- Loimaranta, V.; Hepojoki, J.; Laaksoaho, O.; Pulliainen, A.T. Galectin-3-binding protein: A multitask glycoprotein with innate immunity functions in viral and bacterial infections. J. Leukoc. Biol. 2018, 104, 777–786. [Google Scholar] [CrossRef]
- Ullrich, A.; Sures, I.; D’Egidio, M.; Jallal, B.; Powell, T.J.; Herbst, R.; Dreps, A.; Azam, M.; Rubinstein, M.; Natoli, C.; et al. The secreted tumor-associated antigen 90K is a potent immune stimulator. J. Biol. Chem. 1994, 269, 18401–18407. [Google Scholar] [CrossRef]
- Bucki, R.; Janmey, P.A. Interaction of the gelsolin-derived antibacterial PBP 10 peptide with lipid bilayers and cell membranes. Antimicrob. Agents Chemother. 2006, 50, 2932–2940. [Google Scholar] [CrossRef]
- Hughes, M.M.; McGettrick, A.F.; O’Neill, L.A.J. Glutathione and Glutathione Transferase Omega 1 as Key Posttranslational Regulators in Macrophages. Microbiol. Spectr. 2017, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, X.; Pham, T.H.; Feuerbacher, L.A.; Lubos, M.L.; Huang, M.; Olsen, R.; Mushegian, A.; Slawson, C.; Hardwidge, P.R. NleB, a bacterial effector with glycosyltransferase activity, targets GAPDH function to inhibit NF-κB activation. Cell Host Microbe 2013, 13, 87–99. [Google Scholar] [CrossRef] [PubMed]
- MacKellar, M.; Vigerust, D.J. Role of Haptoglobin in Health and Disease: A Focus on Diabetes. Clin. Diabetes 2016, 34, 148. [Google Scholar] [CrossRef] [PubMed]
- Drain, J.; Bishop, J.R.; Hajduk, S.L. Haptoglobin-related protein mediates trypanosome lytic factor binding to trypanosomes. J. Biol. Chem. 2001, 276, 30254–30260. [Google Scholar] [CrossRef] [PubMed]
- Parish, C.A.; Jiang, H.; Tokiwa, Y.; Berova, N.; Nakanishi, K.; McCabe, D.; Zuckerman, W.; Xia, M.M.; Gabay, J.E. Broad-spectrum antimicrobial activity of hemoglobin. Bioorg. Med. Chem. 2001, 9, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Li, X.; Chen, N.; Mu, L.; Wu, H.; Yang, Y.; Han, K.; Huang, Y.; Wang, B.; Jian, J.; et al. Hemopexin as an acute phase protein regulates the inflammatory response against bacterial infection of Nile tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2021, 187, 166–178. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Vicente, C.P.; Westrick, R.J.; Eitzman, D.T.; Tollefsen, D.M. Heparin cofactor II inhibits arterial thrombosis after endothelial injury. J. Clin. Investig. 2002, 109, 213. [Google Scholar] [CrossRef] [PubMed]
- Rydengård, V.; Olsson, A.K.; Mörgelin, M.; Schmidtchen, A. Histidine-rich glycoprotein exerts antibacterial activity. FEBS J. 2007, 274, 377–389. [Google Scholar] [CrossRef]
- Hoeksema, M.; Van Eijk, M.; Haagsman, H.P.; Hartshorn, K.L. Histones as mediators of host defense, inflammation and thrombosis. Future Microbiol. 2016, 11, 441–453. [Google Scholar] [CrossRef]
- Zhuo, L.; Kimata, K. Structure and function of inter-alpha-trypsin inhibitor heavy chains. Connect. Tissue Res. 2008, 49, 311–320. [Google Scholar] [CrossRef]
- Rapala-Kozik, M.; Karkowska, J.; Jacher, A.; Golda, A.; Barbasz, A.; Guevara-Lora, I.; Kozik, A. Kininogen adsorption to the cell surface of Candida spp. Int. Immunopharmacol. 2008, 8, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Ben Nasr, A.; Herwald, H.; Muller-Esterl, W.; Bjorck, L. Human kininogens interact with M protein, a bacterial surface protein and virulence determinant. Biochem. J. 1995, 305, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Ra, H.J.; Parks, W.C. Control of Matrix Metalloproteinase Catalytic Activity. Matrix Biol. 2007, 26, 587. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-S.; Greenlee, K.J.; Pitchumani, R.; Lee, S.-H.; Song, L.; Shan, M.; Chang, S.H.; Park, P.W.; Dong, C.; Werb, Z.; et al. Dual protective mechanisms of matrix metalloproteinases 2 and 9 in immune defense against Streptococcus pneumoniae. J. Immunol. 2011, 186, 6427–6436. [Google Scholar] [CrossRef] [PubMed]
- Serrador, J.M.; Alonso-Lebrero, J.L.; Del Pozo, M.A.; Furthmayr, H.; Schwartz-Albiez, R.; Calvo, J.; Lozano, F.; Sánchez-Madrid, F. Moesin interacts with the cytoplasmic region of intercellular adhesion molecule-3 and is redistributed to the uropod of T lymphocytes during cell polarization. J. Cell Biol. 1997, 138, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Linden, S.K.; Sutton, P.; Karlsson, N.G.; Korolik, V.; McGuckin, M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008, 1, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, H.; Schwarting, A.; Kriegsmann, J.; Petrow, P.; Gaumann, A.; Müller, K.M.; Galle, P.R.; Mayet, W. Proteinase-3 as the major autoantigen of c-ANCA is strongly expressed in lung tissue of patients with Wegener’s granulomatosis. Arthritis Res. 2002, 4, 220–225. [Google Scholar] [CrossRef]
- Flo, T.H.; Smith, K.D.; Sato, S.; Rodriguez, D.J.; Holmes, M.A.; Strong, R.K.; Akira, S.; Aderem, A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004, 432, 917–921. [Google Scholar] [CrossRef]
- Kang, D.; Liu, G.; Lundström, A.; Gelius, E.; Steiner, H. A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc. Natl. Acad. Sci. USA 1998, 95, 10078–10082. [Google Scholar] [CrossRef]
- Steele, F.R.; Chader, G.J.; Johnson, L.V.; Tombran-Tink, J. Pigment epithelium-derived factor: Neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc. Natl. Acad. Sci. USA 1993, 90, 1526–1530. [Google Scholar] [CrossRef]
- Law, R.H.P.; Zhang, Q.; McGowan, S.; Buckle, A.M.; Silverman, G.A.; Wong, W.; Rosado, C.J.; Langendorf, C.G.; Pike, R.N.; Bird, P.I.; et al. An overview of the serpin superfamily. Genome Biol. 2006, 7, 216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, X.; Kugadas, A.; Smith-Page, K.; Lamb, J.; Lin, T.; Ru, Y.; Morley, S.C.; Fichorova, R.; Mittal, S.K.; Chauhan, S.K.; et al. Neutrophil L-Plastin Controls Ocular Paucibacteriality and Susceptibility to Keratitis. Front. Immunol. 2020, 11, 547. [Google Scholar] [CrossRef] [PubMed]
- Tamir, A.; Gangadharan, A.; Balwani, S.; Tanaka, T.; Patel, U.; Hassan, A.; Benke, S.; Agas, A.; D’Agostino, J.; Shin, D.; et al. The serine protease prostasin (PRSS8) is a potential biomarker for early detection of ovarian cancer. J. Ovarian Res. 2016, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Vetr, H.; Gebhard, W. Structure of the human alpha 1-microglobulin-bikunin gene. Biol. Chem. Hoppe. Seyler. 1990, 371, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, T.; Miao, H.; Liang, B. The Calcium Binding Protein S100A11 and Its Roles in Diseases. Front. cell Dev. Biol. 2021, 9, 693262. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Braunstein, Z.; Toomey, A.C.; Zhong, J.; Rao, X. S100 proteins as an important regulator of macrophage inflammation. Front. Immunol. 2018, 8, 1908. [Google Scholar] [CrossRef] [PubMed]
- Donato, R. Intracellular and extracellular roles of S100 proteins. Microsc. Res. Tech. 2003, 60, 540–551. [Google Scholar] [CrossRef]
- Bruhn, K.W.; Spellberg, B. Transferrin-Mediated Iron Sequestration as a Novel Therapy for Bacterial and Fungal Infections. Curr. Opin. Microbiol. 2015, 27, 57. [Google Scholar] [CrossRef]
- Gatt, M.E.; Urieli-Shoval, S.; Preciado-Patt, L.; Fridkin, M.; Calco, S.; Azar, Y.; Matzner, Y. Effect of serum amyloid A on selected in vitro functions of isolated human neutrophils. J. Lab. Clin. Med. 1998, 132, 414–420. [Google Scholar] [CrossRef]
- Gomis-Rüth, F.X.; Maskos, K.; Betz, M.; Bergner, A.; Huber, R.; Suzuki, K.; Yoshida, N.; Nagase, H.; Brew, K.; Bourenkov, G.P.; et al. Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature 1997, 389, 77–81. [Google Scholar] [CrossRef]
- Jirasakuldech, B.; Schussler, G.C.; Yap, M.G.; Drew, H.; Josephson, A.; Michl, J. A characteristic serpin cleavage product of thyroxine-binding globulin appears in sepsis sera. J. Clin. Endocrinol. Metab. 2000, 85, 3996–3999. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Park, J.S.; Karabulut, H.; Yasmin, F.; Jun, C.D. Transgelin-2: A Double-Edged Sword in Immunity and Cancer Metastasis. Front. Cell Dev. Biol. 2021, 9, 825. [Google Scholar] [CrossRef] [PubMed]
- Kew, R.R. The Vitamin D binding protein and inflammatory injury: A mediator or sentinel of tissue damage? Front. Endocrinol. 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement system part I-molecular mechanisms of activation and regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, Inflammation and Pain. Int. Anesthesiol. Clin. 2007, 45, 27. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.; Nag, M.; Banerjee, R.; Mukherjee, D.; Garai, S.; Sarkar, T.; Dey, A.; Sheikh, H.I.; Pathak, S.K.; Edinur, H.A.; et al. Amylases: Biofilm Inducer or Biofilm Inhibitor? Front. Cell. Infect. Microbiol. 2021, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.J.; Ko, H.J.; Hwang, C.C.; Hong, Y.R. The Double-Edged Sword of Beta2-Microglobulin in Antibacterial Properties and Amyloid Fibril-Mediated Cytotoxicity. Int. J. Mol. Sci. 2021, 22, 6330. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yi, Q.; Uchanska-Ziegler, B.; Ziegler, A. β2-microglobulin as a potential initiator of inflammatory responses. Trends Immunol. 2003, 24, 228–229. [Google Scholar] [CrossRef]
- Koo, I.C.; Ohol, Y.M.; Wu, P.; Morisaki, J.H.; Cox, J.S.; Brown, E.J. Role for lysosomal enzyme β-hexosaminidase in the control of mycobacteria infection. Proc. Natl. Acad. Sci. USA 2008, 105, 710. [Google Scholar] [CrossRef]
- Prokopovic, V.; Popovic, M.; Andjelkovic, U.; Marsavelski, A.; Raskovic, B.; Gavrovic-Jankulovic, M.; Polovic, N. Isolation, biochemical characterization and anti-bacterial activity of BPIFA2 protein. Arch. Oral Biol. 2014, 59, 302–309. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, M.; Hong, Y.; Bu, X.; Luan, G.; Wang, Y.; Li, Y.; Lou, H.; Wang, C.; Zhang, L. Reduced Expression of Antimicrobial Protein Secretory Leukoprotease Inhibitor and Clusterin in Chronic Rhinosinusitis with Nasal Polyps. J. Immunol. Res. 2021, 2021, 1057186. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.J.; Quinnell, R.J.; Raiko, A.; Lagog, M.; Siba, P.; Morroll, S.; Falcone, F.H. Chitotriosidase deficiency is not associated with human hookworm infection in a Papua New Guinean population. Infect. Genet. Evol. 2007, 7, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Zavasnik-Bergant, T. Cystatin protease inhibitors and immune functions. Front. Biosci. 2008, 13, 4625–4637. [Google Scholar] [CrossRef] [PubMed]
- Haroon, N.; Inman, R.D. Endoplasmic reticulum aminopeptidases: Biology and pathogenic potential. Nat. Rev. Rheumatol. 2010, 6, 461–467. [Google Scholar] [CrossRef] [PubMed]
- McKown, R.L.; Coleman Frazier, E.V.; Zadrozny, K.K.; Deleault, A.M.; Raab, R.W.; Ryan, D.S.; Sia, R.K.; Lee, J.K.; Laurie, G.W. A cleavage-potentiated fragment of tear lacritin is bactericidal. J. Biol. Chem. 2014, 289, 22172–22182. [Google Scholar] [CrossRef] [PubMed]
- Suojalehto, H.; Kinaret, P.; Kilpeläinen, M.; Toskala, E.; Ahonen, N.; Wolff, H.; Alenius, H.; Puustinen, A. Level of Fatty Acid Binding Protein 5 (FABP5) Is Increased in Sputum of Allergic Asthmatics and Links to Airway Remodeling and Inflammation. PLoS ONE 2015, 10, e0127003. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Fan, Y.; Xue, B.; Luo, L.; Shen, J.; Zhang, S.; Jiang, Y.; Yin, Z. Human glutathione S-transferase P1-1 interacts with TRAF2 and regulates TRAF2–ASK1 signals. Oncogene 2006, 25, 5787–5800. [Google Scholar] [CrossRef] [PubMed]
- Diamandis, E.P.; Okui, A.; Mitsui, S.; Luo, L.-Y.; Soosaipillai, A.; Grass, L.; Nakamura, T.; Howarth, D.J.C.; Yamaguchi, N. Human Kallikrein 11: A New Biomarker of Prostate and Ovarian Carcinoma. Cancer Res. 2002, 62, 295–300. [Google Scholar]
- Jeong, J.K.; Diano, S. Prolyl carboxypeptidase and its inhibitors in metabolism. Trends Endocrinol. Metab. 2013, 24, 61. [Google Scholar] [CrossRef][Green Version]
- Chavanas, S.; Bodemer, C.; Rochat, A.; Hamel-Teillac, D.; Ali, M.; Irvine, A.D.; Bonafé, J.L.; Wilkinson, J.; Taïeb, A.; Barrandon, Y.; et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat. Genet. 2000, 25, 141–142. [Google Scholar] [CrossRef]
- Turato, C.; Pontisso, P. SERPINB3 (serpin peptidase inhibitor, clade B (ovalbumin), member 3). Atlas Genet. Cytogenet. Oncol. Haematol. 2015, 19, 202. [Google Scholar] [CrossRef] [PubMed]
- Carion, T.W.; Ebrahim, A.S.; Alluri, S.; Ebrahim, T.; Parker, T.; Burns, J.; Sosne, G.; Berger, E.A. Antimicrobial Effects of Thymosin Beta-4 and Ciprofloxacin Adjunctive Therapy in Pseudomonas aeruginosa Induced Keratitis. Int. J. Mol. Sci. 2020, 21, 6840. [Google Scholar] [CrossRef] [PubMed]
- Yasuoka, S.; Ohnishi, T.; Kawano, S.; Tsuchihashi, S.; Ogawara, M.; Masuda, K.I.; Yamaoka, K.; Takahashi, M.; Sano, T. Purification, characterization, and localization of a novel trypsin-like protease found in the human airway. Am. J. Respir. Cell Mol. Biol. 1997, 16, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Oddo, M.; Calandra, T.; Bucala, R.; Meylan, P.R.A. Macrophage migration inhibitory factor reduces the growth of virulent Mycobacterium tuberculosis in human macrophages. Infect. Immun. 2005, 73, 3783–3786. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.I.; Waheed, A.; Yadav, S.; Singh, T.P.; Ahmad, F. Prolactin inducible protein in cancer, fertility and immunoregulation: Structure, function and its clinical implications. Cell. Mol. Life Sci. 2009, 66, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef]
- Theofilou, D.V.I.; Ghita, D.I.; Elnaggar, D.M.; Chaisuparat, D.R.; Dyalram, D.D.; Ord, P.R.A.; Lubek, D.J.E.; Younis, D.R.H. Stromal inflammatory subtypes of oral squamous cell carcinoma correlate with patient clinical characteristics, demographics and gene expression. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, e125. [Google Scholar] [CrossRef]
- Goertzen, C.; Mahdi, H.; Laliberte, C.; Meirson, T.; Eymael, D.; Gil-Henn, H.; Magalhaes, M. Oral inflammation promotes oral squamous cell carcinoma invasion. Oncotarget 2018, 9, 29047–29063. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ye, P.; Zhang, W.; Zhu, H.; Yu, H. Prognostic role of an inflammation scoring system in radical resection of oral squamous cell carcinoma. BMC Oral Health 2022, 22, 226. [Google Scholar] [CrossRef] [PubMed]
- Navale, A.M.; Deshpande, A.; Mistry, B.; Chauhan, P.; Bhagat, C. Salivary protein biomarkers for diagnosis of oral squamous cell carcinoma. Curr. Cancer Drug Targets 2023, 23. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, Q.; Chen, J.; Yi, P.; Xu, X.; Fan, Y.; Cui, B.; Yu, Y.; Li, X.; Du, Y.; et al. Salivary protease spectrum biomarkers of oral cancer. Int. J. Oral Sci. 2019, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Agbowuro, A.A.; Huston, W.M.; Gamble, A.B.; Tyndall, J.D.A. Proteases and protease inhibitors in infectious diseases. Med. Res. Rev. 2018, 38, 1295–1331. [Google Scholar] [CrossRef] [PubMed]
- Piyarathne, N.S.; Weerasekera, M.M.; Fonseka, P.F.D.; Karunatilleke, A.H.T.S.; Liyanage, R.L.P.R.; Jayasinghe, R.D.; De Silva, K.; Yasawardene, S.; Gupta, E.; Jayasinghe, J.A.P.; et al. Salivary Interleukin Levels in Oral Squamous Cell Carcinoma and Oral Epithelial Dysplasia: Findings from a Sri Lankan Study. Cancers 2023, 15, 1510. [Google Scholar] [CrossRef] [PubMed]
- Zackular, J.P.; Chazin, W.J.; Skaar, E.P. Nutritional Immunity: S100 Proteins at the Host-Pathogen Interface. J. Biol. Chem. 2015, 290, 18991. [Google Scholar] [CrossRef]
- Ajona, D.; Pajares, M.J.; Chiara, M.D.; Rodrigo, J.P.; Jantus-Lewintre, E.; Camps, C.; Suarez, C.; Bagán, J.V.; Montuenga, L.M.; Pio, R. Complement activation product C4d in oral and oropharyngeal squamous cell carcinoma. Oral Dis. 2015, 21, 899–904. [Google Scholar] [CrossRef]
- Ain, D.; Shaikh, T.; Manimala, S.; Ghebrehiwet, B. The role of complement in the tumor microenvironment. Fac. Rev. 2021, 10, 899–904. [Google Scholar] [CrossRef]
- Dominiczak, M.H.; Caslake, M.J. Apolipoproteins: Metabolic role and clinical biochemistry applications. Ann. Clin. Biochem. 2011, 48, 498–515. [Google Scholar] [CrossRef] [PubMed]
- Dell’Olmo, E.; Gaglione, R.; Sabbah, M.; Schibeci, M.; Cesaro, A.; Di Girolamo, R.; Porta, R.; Arciello, A. Host defense peptides identified in human apolipoprotein B as novel food biopreservatives and active coating components. Food Microbiol. 2021, 99, 103804. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The Oral Microbiota: Community Composition, Influencing Factors, Pathogenesis, and Interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, M.; Scarano, E.; Passali, G.C.; Messana, I.; Cabras, T.; Iavarone, F.; Cintio, G.D.; Fiorita, A.; Corso, E.D.; Paludetti, G. Salivary biomarkers and proteomics:future diagnostic and clinical utilities. Acta Otorhinolaryngol. Ital. 2017, 37, 94. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, Y.; Wang, H.; Wang, Z.; Hu, S.; Cao, C.; Xiao, H. In-Depth Metaproteomics Analysis of Oral Microbiome for Lung Cancer. Research 2022, 2022, 9781578. [Google Scholar] [CrossRef] [PubMed]
- Grassl, N.; Kulak, N.A.; Pichler, G.; Geyer, P.E.; Jung, J.; Schubert, S.; Sinitcyn, P.; Cox, J.; Mann, M. Ultra-deep and quantitative saliva proteome reveals dynamics of the oral microbiome. Genome Med. 2016, 8, 44. [Google Scholar] [CrossRef]
- Rabe, A.; Gesell Salazar, M.; Michalik, S.; Fuchs, S.; Welk, A.; Kocher, T.; Völker, U. Metaproteomics analysis of microbial diversity of human saliva and tongue dorsum in young healthy individuals. J. Oral Microbiol. 2019, 11, 1654786. [Google Scholar] [CrossRef]
- Bostanci, N.; Grant, M.; Bao, K.; Silbereisen, A.; Hetrodt, F.; Manoil, D.; Belibasakis, G.N. Metaproteome and metabolome of oral microbial communities. Periodontol 2021, 85, 46–81. [Google Scholar] [CrossRef]
- Garley, M.; Dziemiańczyk-Pakieła, D.; Ratajczak-Wrona, W.; Pryczynicz, A.; Nowak, K.; Łazarczyk, B.; Jabłońska, E. NETs biomarkers in saliva and serum OSCC patients: One hypothesis, two conclusions. Adv. Med. Sci. 2022, 67, 45–54. [Google Scholar] [CrossRef]
- Nijakowski, K.; Gruszczyński, D.; Kopała, D.; Surdacka, A. Salivary Metabolomics for Oral Squamous Cell Carcinoma Diagnosis: A Systematic Review. Metabolites 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Jou, Y.J.; Lin, C.D.; Lai, C.H.; Chen, C.H.; Kao, J.Y.; Chen, S.Y.; Tsai, M.H.; Huang, S.H.; Lin, C.W. Proteomic identification of salivary transferrin as a biomarker for early detection of oral cancer. Anal. Chim. Acta 2010, 681, 41–48. [Google Scholar] [CrossRef] [PubMed]
- G, D.; Nandan, S.R.K.; Kulkarni, P.G. Salivary Tumour Necrosis Factor-α as a Biomarker in Oral Leukoplakia and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2019, 20, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Yi, C.; Chung, H.R.; Wang, D.J.; Chang, W.C.; Lee, S.Y.; Lin, C.T.; Yang, Y.C.; Yang, W.C.V. Potential biomarkers in saliva for oral squamous cell carcinoma. Oral Oncol. 2010, 46, 226–231. [Google Scholar] [CrossRef]
- Heawchaiyaphum, C.; Pientong, C.; Phusingha, P.; Vatanasapt, P.; Promthet, S.; Daduang, J.; Teeramatwanich, W.; Kongyingyoes, B.; Chuerduangphui, J.; Ekalaksananan, T. Peroxiredoxin-2 and zinc-alpha-2-glycoprotein as potentially combined novel salivary biomarkers for early detection of oral squamous cell carcinoma using proteomic approaches. J. Proteom. 2018, 173, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Arellano, M.; Boontheung, P.; Wang, J.; Zhou, H.; Jiang, J.; Elashoff, D.; Wei, R.; Loo, J.A.; Wong, D.T. Salivary proteomics for oral cancer biomarker discovery. Clin. Cancer Res. 2008, 14, 6246–6252. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kotimoole, C.N.; Ghoshal, S.; Bakshi, J.; Chatterjee, A.; Prasad, T.S.K.; Pal, A. Identification of potential salivary biomarker panels for oral squamous cell carcinoma. Sci. Rep. 2021, 11, 3365. [Google Scholar] [CrossRef] [PubMed]
- Gleber-Netto, F.O.; Yakob, M.; Li, F.; Feng, Z.; Dai, J.; Kao, H.K.; Chang, Y.L.; Chang, K.P.; Wong, D.T.W. Salivary Biomarkers for Detection of Oral Squamous Cell Carcinoma in a Taiwanese Population. Clin. Cancer Res. 2016, 22, 3340–3347. [Google Scholar] [CrossRef]
- Hsu, C.W.; Chang, K.P.; Huang, Y.; Liu, H.P.; Hsueh, P.C.; Gu, P.W.; Yen, W.C.; Wu, C.C. Proteomic Profiling of Paired Interstitial Fluids Reveals Dysregulated Pathways and Salivary NID1 as a Biomarker of Oral Cavity Squamous Cell Carcinoma. Mol. Cell. Proteom. 2019, 18, 1939–1949. [Google Scholar] [CrossRef]
- Lin, Y.H.; Eguez, R.V.; Torralba, M.G.; Singh, H.; Golusinski, P.; Golusinski, W.; Masternak, M.; Nelson, K.E.; Freire, M.; Yu, Y. Self-Assembled STrap for Global Proteomics and Salivary Biomarker Discovery. J. Proteome Res. 2019, 18, 1907–1915. [Google Scholar] [CrossRef]
- Winck, F.V.; Ribeiro, A.C.P.; Domingues, R.R.; Ling, L.Y.; Riaño-Pachón, D.M.; Rivera, C.; Brandão, T.B.; Gouvea, A.F.; Santos-Silva, A.R.; Coletta, R.D.; et al. Insights into immune responses in oral cancer through proteomic analysis of saliva and salivary extracellular vesicles. Sci. Rep. 2015, 5, 16305. [Google Scholar] [CrossRef] [PubMed]
- Dowling, P.; Wormald, R.; Meleady, P.; Henry, M.; Curran, A.; Clynes, M. Analysis of the saliva proteome from patients with head and neck squamous cell carcinoma reveals differences in abundance levels of proteins associated with tumour progression and metastasis. J. Proteom. 2008, 71, 168–175. [Google Scholar] [CrossRef]
- Riccardi, G.; Bellizzi, M.G.; Fatuzzo, I.; Zoccali, F.; Cavalcanti, L.; Greco, A.; Vincentiis, M.d.; Ralli, M.; Fiore, M.; Petrella, C.; et al. Salivary Biomarkers in Oral Squamous Cell Carcinoma: A Proteomic Overview. Proteomes 2022, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Jou, Y.J.; Hua, C.H.; Lin, C.D.; Lai, C.H.; Huang, S.H.; Tsai, M.H.; Kao, J.Y.; Lin, C.W. S100A8 as potential salivary biomarker of oral squamous cell carcinoma using nanoLC-MS/MS. Clin. Chim. Acta 2014, 436, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Manzano-Moreno, F.J.; Costela-Ruiz, V.J.; García-Recio, E.; Olmedo-Gaya, M.V.; Ruiz, C.; Reyes-Botella, C. Role of Salivary MicroRNA and Cytokines in the Diagnosis and Prognosis of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2021, 22, 12215. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.W.; Yu, J.S.; Peng, P.H.; Liu, S.C.; Chang, Y.S.; Chang, K.P.; Wu, C.C. Secretome profiling of primary cells reveals that THBS2 is a salivary biomarker of oral cavity squamous cell carcinoma. J. Proteome Res. 2014, 13, 4796–4807. [Google Scholar] [CrossRef] [PubMed]
- Roi, A.; Roi, C.I.; Negruțiu, M.L.; Riviș, M.; Sinescu, C.; Rusu, L.C. The Challenges of OSCC Diagnosis: Salivary Cytokines as Potential Biomarkers. J. Clin. Med. 2020, 9, 2866. [Google Scholar] [CrossRef] [PubMed]
- Jessie, K.; Jayapalan, J.J.; Ong, K.-C.; Abdul Rahim, Z.H.; Zain, R.M.; Wong, K.-T.; Hashim, O.H. Aberrant proteins in the saliva of patients with oral squamous cell carcinoma. Electrophoresis 2013, 34, 2495–2502. [Google Scholar] [CrossRef]
- Hsiao, Y.C.; Lin, S.Y.; Chien, K.Y.; Chen, S.F.; Wu, C.C.; Chang, Y.T.; Chi, L.M.; Chu, L.J.; Chiang, W.F.; Chien, C.Y.; et al. An immuno-MALDI mass spectrometry assay for the oral cancer biomarker, matrix metalloproteinase-1, in dried saliva spot samples. Anal. Chim. Acta 2020, 1100, 118–130. [Google Scholar] [CrossRef]
- Elashoff, D.; Zhou, H.; Reiss, J.; Wang, J.; Xiao, H.; Henson, B.; Hu, S.; Arellano, M.; Sinha, U.; Le, A.; et al. Prevalidation of salivary biomarkers for oral cancer detection. Cancer Epidemiol. Biomark. Prev. 2012, 21, 664–672. [Google Scholar] [CrossRef]
- Faria, P.C.B.; Carneiro, A.P.; Binato, R.; Nascimento, R.; Santos, P.S.; Fagundes, D.; da Silva, S.J.; Loyola, A.M.; Abdelhay, E.; Goulart, L.R. Upregulation of tropomyosin alpha-4 chain in patients’ saliva with oral squamous cell carcinoma as demonstrated by Phage display. Sci. Rep. 2019, 9, 18399. [Google Scholar] [CrossRef] [PubMed]
- Jou, Y.J.; Lin, C.D.; Lai, C.H.; Tang, C.H.; Huang, S.H.; Tsai, M.H.; Chen, S.Y.; Kao, J.Y.; Lin, C.W. Salivary zinc finger protein 510 peptide as a novel biomarker for detection of oral squamous cell carcinoma in early stages. Clin. Chim. Acta 2011, 412, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Panda, M. Recent trends of saliva omics biomarkers for the diagnosis and treatment of oral cancer. J. Oral Biosci. 2019, 61, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, O.; Kastratovic, D.A.; Dimitrijevic, M.V.; Konstantinovic, V.S.; Jelovac, D.B.; Antic, J.; Nesic, V.S.; Markovic, S.Z.; Martinovic, Z.R.; Akin, D.; et al. Oral squamous cell carcinoma detection by salivary biomarkers in a Serbian population. Oral Oncol. 2011, 47, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Sun, Z.; Yang, J.; Xu, J.; Shi, W.; Wu, Y.; Fan, Y.; Li, H. Discovery and preclinical validation of proteomic biomarkers in saliva for early detection of oral squamous cell carcinomas. Oral Dis. 2019, 25, 97–107. [Google Scholar] [CrossRef]

| Uniprot Entry | Protein Name | Function in the Chemical Barrier | Reference |
|---|---|---|---|
| P02763 | Alpha-1-acid glycoprotein 1 | Immunomodulatory effect | [33] |
| P01011 | Alpha-1-antichymotrypsin | Protease inhibitor | [34] |
| P01009 | Alpha-1-antitrypsin | Protease inhibitor | [35] |
| P04217 | Alpha-1B-glycoprotein | Immunomodulatory effect | [36] |
| P08697 | Alpha-2-antiplasmin | Protease inhibitor | [37] |
| P02765 | Alpha-2-HS-glycoprotein | Immunomodulatory effect | [38] |
| P01023 | Alpha-2-macroglobulin | Protease inhibitor | [39] |
| P03973 | Antileukoproteinase | Protease inhibitor/Immunomodulatory effect | [40] |
| P01008 | Antithrombin-III | Protease inhibitor | [41] |
| P02647 | Apolipoprotein A-I | Antimicrobial activity | [42] |
| P02652 | Apolipoprotein A-II | Immunomodulatory effect | [42] |
| P06727 | Apolipoprotein A-IV | Immunomodulatory effect | [43] |
| P04114 | Apolipoprotein B-100 | Antimicrobial activity | [44] |
| P02656 | Apolipoprotein C-III | Immunomodulatory effect | [45] |
| P05090 | Apolipoprotein D | Immunomodulatory effect | [46] |
| P02649 | Apolipoprotein E | Immunomodulatory effect | [47] |
| O14791 | Apolipoprotein L1 | Immunomodulatory effect | [48] |
| P02749 | Beta-2-glycoprotein 1 | Immunomodulatory effect | [49] |
| Q9NP55 | BPI fold-containing family A member 1 | Antimicrobial activity | [50] |
| P04040 | Catalase | Antibacterial activity | [51] |
| P00450 | Ceruloplasmin | Antimicrobial/Cu sequestration | [52] |
| P10909 | Clusterin | Immunomodulatory effect | [53] |
| P08185 | Corticosteroid-binding globulin | Protease inhibitor | [54] |
| P02741 | C-reactive protein | Antimicrobial activity/Acute-phase protein | [55] |
| P12724 | Eosinophil cationic protein | Antimicrobial activity | [56] |
| P15090 | Fatty acid-binding protein 4 | Immunomodulatory effect | [57] |
| P02671 | Fibrinogen alpha chain | Antimicrobial activity | [58] |
| P02675 | Fibrinogen beta chain | Antimicrobial activity | [58] |
| P02679 | Fibrinogen gamma chain | Antimicrobial activity | [58] |
| Q08380 | Galectin-3-binding protein | Immunomodulatory effect/Antimicrobial activity | [59,60] |
| P06396 | Gelsolin | Processed from has antimicrobial activity | [61] |
| P78417 | Glutathione S-transferase omega-1 | Immunomodulatory effect | [62] |
| P04406 | Glyceraldehyde-3-phosphate dehydrogenase | Immunomodulatory effect | [63] |
| P00738 | Haptoglobin | Immunomodulatory effect/iron sequestering | [64] |
| P00739 | Haptoglobin-related protein | Antiparasitic activity | [65] |
| P69905 | Hemoglobin subunit alpha | Processed forms (hemocidins) have antimicrobial activity | [66] |
| P68871 | Hemoglobin subunit beta | Processed forms (hemocidins) have antimicrobial activity | [66] |
| P02042 | Hemoglobin subunit delta | Processed forms (hemocidins) have antimicrobial activity | [66] |
| P02790 | Hemopexin | Immunomodulatory effect/ Antimicrobial activity | [67] |
| P05546 | Heparin cofactor 2 | Protease inhibitor | [68] |
| P04196 | Histidine-rich glycoprotein | Antimicrobial activity | [69] |
| Q96QV6 | Histone H2A type 1-A | Antimicrobial activity | [70] |
| P19827 | Inter-alpha-trypsin inhibitor heavy chain H1 | Protease inhibitor | [71] |
| P19823 | Inter-alpha-trypsin inhibitor heavy chain H2 | Protease inhibitor | [71] |
| Q14624 | Inter-alpha-trypsin inhibitor heavy chain H4 | Protease inhibitor | [71] |
| P01042 | Kininogen-1 | Antimicrobial activity | [72,73] |
| P03956 | Matrix metalloproteinase-1 | Protease activity | [74] |
| P14780 | Matrix metalloproteinase-9 | Protease activity/Protective role against bacterial infections | [75] |
| P26038 | Moesin | Immunomodulatory effect | [76] |
| Q8WXI7 | Mucin-16 | Antimicrobial activity | [77] |
| Q8TAX7 | Mucin-7 | Antimicrobial activity | [77] |
| P24158 | Myeloblastin | Protease activity | [78] |
| P80188 | Neutrophil gelatinase-associated lipocalin | Immunomodulatory effect/iron sequestration | [79] |
| O75594 | Peptidoglycan recognition protein 1 | Antimicrobial activity | [80] |
| P36955 | Pigment epithelium-derived factor | Protease inhibitor | [81] |
| P05155 | Plasma protease C1 inhibitor | Protease inhibitor | [82] |
| P13796 | Plastin-2 | Immunomodulatory effect | [83] |
| Q16651 | Prostasin | Protease activity | [84] |
| P02760 | Protein AMBP | Protease inhibitor | [85] |
| P31949 | Protein S100-A11 | Immunomodulatory effect | [86] |
| P80511 | Protein S100-A12 | Immunomodulatory effect | [87] |
| P29034 | Protein S100-A2 | Immunomodulatory effect | [88] |
| P31151 | Protein S100-A7 | Immunomodulatory effect | [88] |
| Q86SG5 | Protein S100-A7A | Immunomodulatory effect | [88] |
| P05109 | Protein S100-A8 | Immunomodulatory effect | [88] |
| P06702 | Protein S100-A9 | Immunomodulatory effect | [88] |
| P25815 | Protein S100-P | Immunomodulatory effect | [88] |
| O95969 | Secretoglobin family 1D member 2 | Immunomodulatory effect | [88] |
| P02787 | Serotransferrin | Antimicrobial/Iron sequestration | [89] |
| P48594 | Serpin B4 | Protease inhibitor | [82] |
| E9PGN7 | Serpin family G member 1 | Protease inhibitor | [82] |
| P35542 | Serum amyloid A-4 protein | Immunomodulatory effect | [90] |
| P02743 | Serum amyloid P-component | Antiviral activity | [90] |
| P08254 | Stromelysin-1 | Protease activity | [91] |
| P05543 | Thyroxine-binding globulin | Protease inhibitor | [92] |
| P37802 | Transgelin-2 | Immunomodulatory effect | [93] |
| P02774 | Vitamin D-binding protein | Immunomodulatory effect | [94] |
| Uniprot Entry | Protein Name | Function in the Chemical Barrier | Reference |
|---|---|---|---|
| P00736 | Complement C1r subcomponent | Opsonization of bacteria/Immunomodulatory effect | [95] |
| B4E1Z4 | Complement C2 | Opsonization of bacteria/Immunomodulatory effect | [95] |
| P01024 | Complement C3 | Opsonization of bacteria/Immunomodulatory effect | [95] |
| P0C0L5 | Complement C4-B | Opsonization of bacteria/Immunomodulatory effect | [95] |
| P01031 | Complement C5 | Opsonization of bacteria/Immunomodulatory effect | [95] |
| P13671 | Complement component C6 | Opsonization of bacteria/Immunomodulatory effect | [95] |
| P02748 | Complement component C9 | Opsonization of bacteria/Immunomodulatory effect | [95] |
| P00751 | Complement factor B | Opsonization of bacteria/Immunomodulatory effect | [95] |
| P08603 | Complement factor H | Opsonization of bacteria/Immunomodulatory effect | [95] |
| P05156 | Complement factor I | Opsonization of bacteria/Immunomodulatory effect | [95] |
| Uniprot Entry | Protein Name | Function in the Chemical Barrier | Reference |
|---|---|---|---|
| P01583 | Interleukin-1 alpha | Immunomodulatory effect | [96] |
| P01584 | Interleukin-1 beta | Immunomodulatory effect | [96] |
| P22301 | Interleukin-10 | Immunomodulatory effect | [96] |
| P35225 | Interleukin-13 | Immunomodulatory effect | [96] |
| P05231 | Interleukin-6 | Immunomodulatory effect | [96] |
| P10145 | Interleukin-8 | Immunomodulatory effect | [96] |
| P01375 | Tumor necrosis factor | Immunomodulatory effect | [96] |
| Uniprot Entry | Protein Name | Function in the Chemical Barrier | Reference |
|---|---|---|---|
| P0DUB6 | Alpha-amylase 1A | Modulation of biofilm formation | [97] |
| P0DTE7 | Alpha-amylase 1B | Modulation of biofilm formation | [97] |
| P0DTE8 | Alpha-amylase 1C | Modulation of biofilm formation | [97] |
| P17213 | Bactericidal permeability-increasing protein | Antimicrobial activity | [20] |
| P61769 | Beta-2-microglobulin | Immunomodulatory effect/antimicrobial activity | [98,99] |
| P06865 | Beta-hexosaminidase subunit alpha | Antimicrobial activity | [100] |
| Q96DR5 | BPI fold-containing family A member 2 | Antimicrobial activity | [101] |
| Q8N4F0 | BPI fold-containing family B member 2 | Antimicrobial activity | [102] |
| Q13231 | Chitotriosidase-1 | Antifungal activity | [103] |
| P01040 | Cystatin-A | Protease inhibitor | [104] |
| P04080 | Cystatin-B | Protease inhibitor | [104] |
| P01034 | Cystatin-C | Protease inhibitor | [104] |
| P01036 | Cystatin-S | Protease inhibitor | [104] |
| P09228 | Cystatin-SA | Protease inhibitor | [104] |
| P01037 | Cystatin-SN | Protease inhibitor | [104] |
| Q9NZ08 | Endoplasmic reticulum aminopeptidase 1 | Protease activity | [105] |
| Q9GZZ8 | Extracellular glycoprotein lacritin | Antimicrobial activity | [106] |
| Q01469 | Fatty acid-binding protein 5 | Immunomodulatory effect | [107] |
| P09211 | Glutathione S-transferase P | Immunomodulatory effect | [108] |
| Q9UBX7 | Kallikrein-11 | Protease activity | [109] |
| P42785 | Lysosomal Pro-X carboxypeptidase | Protease activity | [110] |
| P61626 | Lysozyme C | Antimicrobial activity | [20] |
| P59665 | Neutrophil defensin 1 | Antimicrobial activity | [20] |
| P26447 | Protein S100-A4 | Immunomodulatory effect | [87] |
| Q9NQ38 | Serine protease inhibitor Kazal-type 5 | Protease inhibitor | [111] |
| Q4VAX6 | Serpin peptidase inhibitor, clade B (Ovalbumin), member 10 | Protease inhibitor | [112] |
| P62328 | Thymosin beta-4 | Antimicrobial activity | [113] |
| O60235 | Transmembrane protease serine 11D | Protease activity | [114] |
| Uniprot Entry | Protein Name | Function | Reference |
|---|---|---|---|
| P14174 | Macrophage migration inhibitory factor | Antimicrobial activity | [115] |
| Q9HC84 | Mucin-5B | Antimicrobial activity | [77] |
| P29508 | Serpin B3 | Protease inhibitor | [82] |
| P36952 | Serpin B5 | Protease inhibitor | [82] |
| P25311 | Zinc-alpha-2-glycoprotein | Immunomodulatory effect | [116] |
| Dataset Identifier | Source Database | Reference | Dataset Identifier | Source Database | Reference |
|---|---|---|---|---|---|
| 29632809 | PubMed | [27] | 21035601 | PubMed | [143] |
| 31350970 | PubMed | [144] | 20138569 | PubMed | [145] |
| 29199150 | PubMed | [146] | 18829504 | PubMed | [147] |
| 28545132 | PubMed | [26] | PXD020263 | ProteomeXchange | [148] |
| 28235782 | PubMed | [22] | PXD015722 | ProteomeXchange | [25] |
| 26847061 | PubMed | [149] | PXD008654 | ProteomeXchange | [150] |
| 26552850 | PubMed | [23] | PXD012436 | ProteomeXchange | [151] |
| 26538482 | PubMed | [152] | 18617144 | PubMed | [153] |
| 26205615 | PubMed | [24] | 36412636 | PubMed | [154] |
| 24863804 | PubMed | [155] | 34830096 | PubMed | [156] |
| 24708169 | PubMed | [157] | 32899735 | PubMed | [158] |
| 23784731 | PubMed | [159] | 31987131 | PubMed | [160] |
| 22301830 | PubMed | [161] | 31804537 | PubMed | [162] |
| 21497587 | PubMed | [163] | 31109866 | PubMed | [164] |
| 21109482 | PubMed | [165] | 30169911 | PubMed | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalló, G.; Bertalan, P.M.; Márton, I.; Kiss, C.; Csősz, É. Salivary Chemical Barrier Proteins in Oral Squamous Cell Carcinoma—Alterations in the Defense Mechanism of the Oral Cavity. Int. J. Mol. Sci. 2023, 24, 13657. https://doi.org/10.3390/ijms241713657
Kalló G, Bertalan PM, Márton I, Kiss C, Csősz É. Salivary Chemical Barrier Proteins in Oral Squamous Cell Carcinoma—Alterations in the Defense Mechanism of the Oral Cavity. International Journal of Molecular Sciences. 2023; 24(17):13657. https://doi.org/10.3390/ijms241713657
Chicago/Turabian StyleKalló, Gergő, Petra Magdolna Bertalan, Ildikó Márton, Csongor Kiss, and Éva Csősz. 2023. "Salivary Chemical Barrier Proteins in Oral Squamous Cell Carcinoma—Alterations in the Defense Mechanism of the Oral Cavity" International Journal of Molecular Sciences 24, no. 17: 13657. https://doi.org/10.3390/ijms241713657
APA StyleKalló, G., Bertalan, P. M., Márton, I., Kiss, C., & Csősz, É. (2023). Salivary Chemical Barrier Proteins in Oral Squamous Cell Carcinoma—Alterations in the Defense Mechanism of the Oral Cavity. International Journal of Molecular Sciences, 24(17), 13657. https://doi.org/10.3390/ijms241713657