Novel Conformation-Dependent Tau Antibodies Are Modulated by Adjacent Phosphorylation Sites
Abstract
:1. Introduction
2. Results
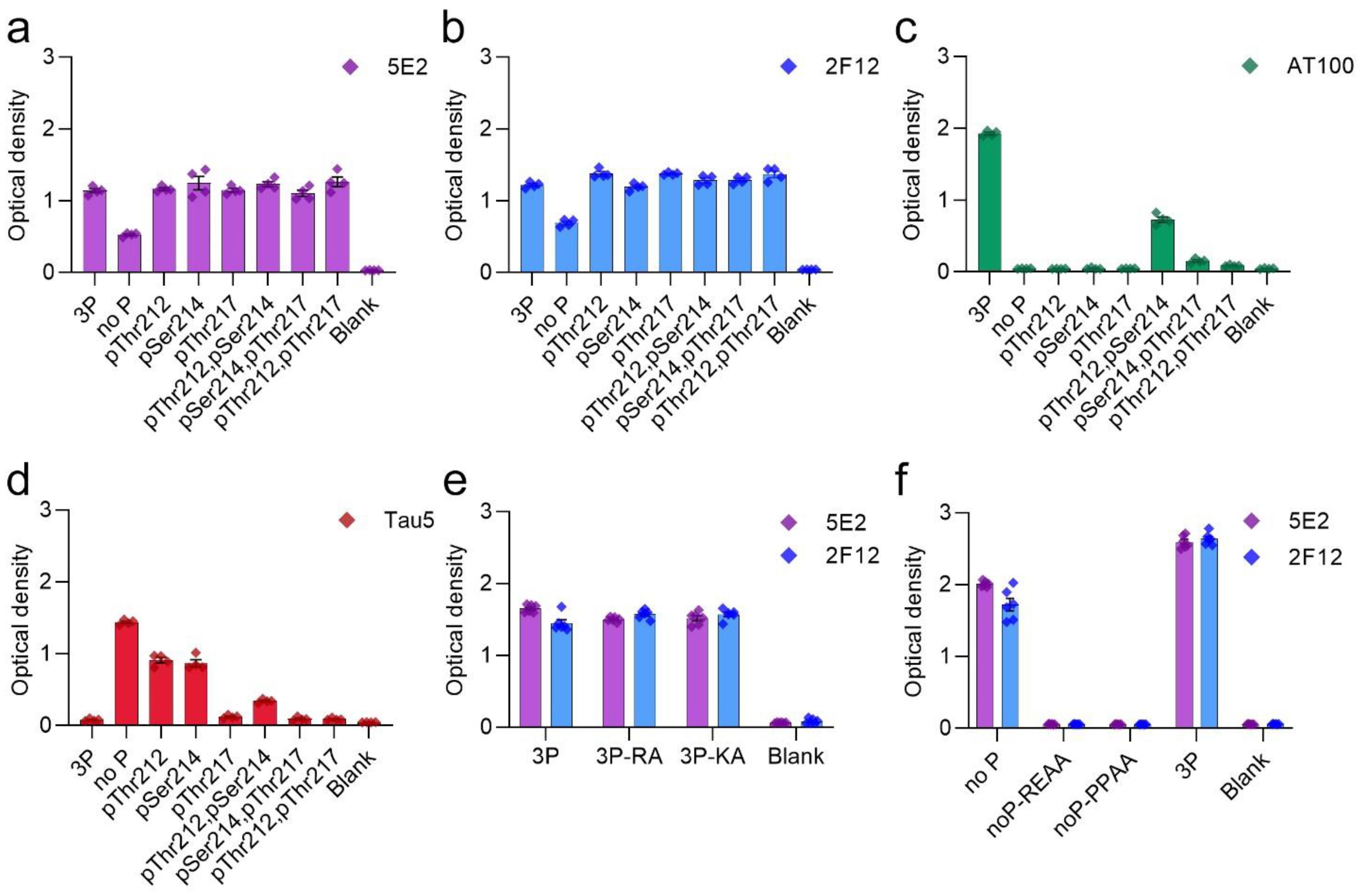
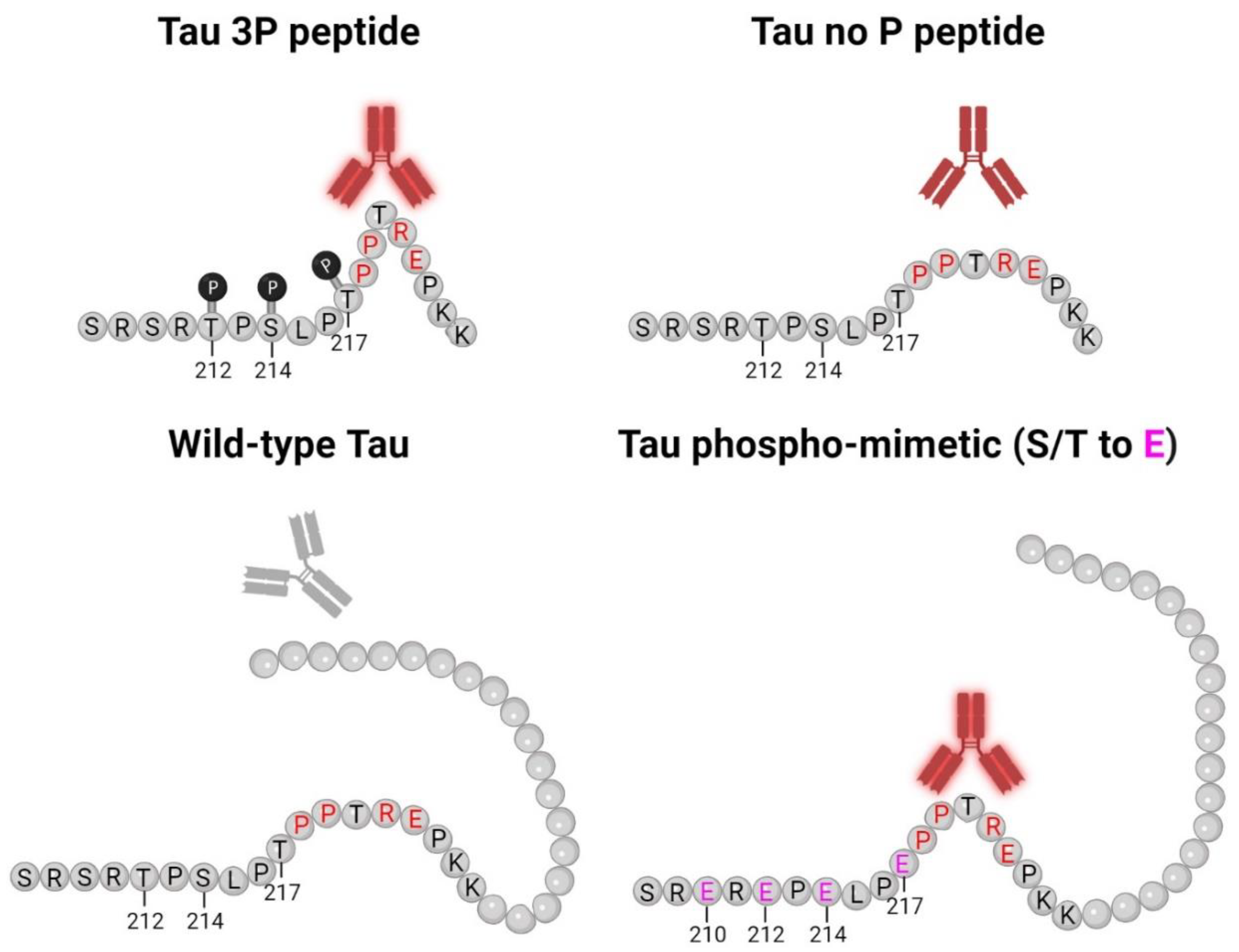
2.1. Epitope Characterization of Novel Tau Antibodies 5E2 and 2F12 by ELISA
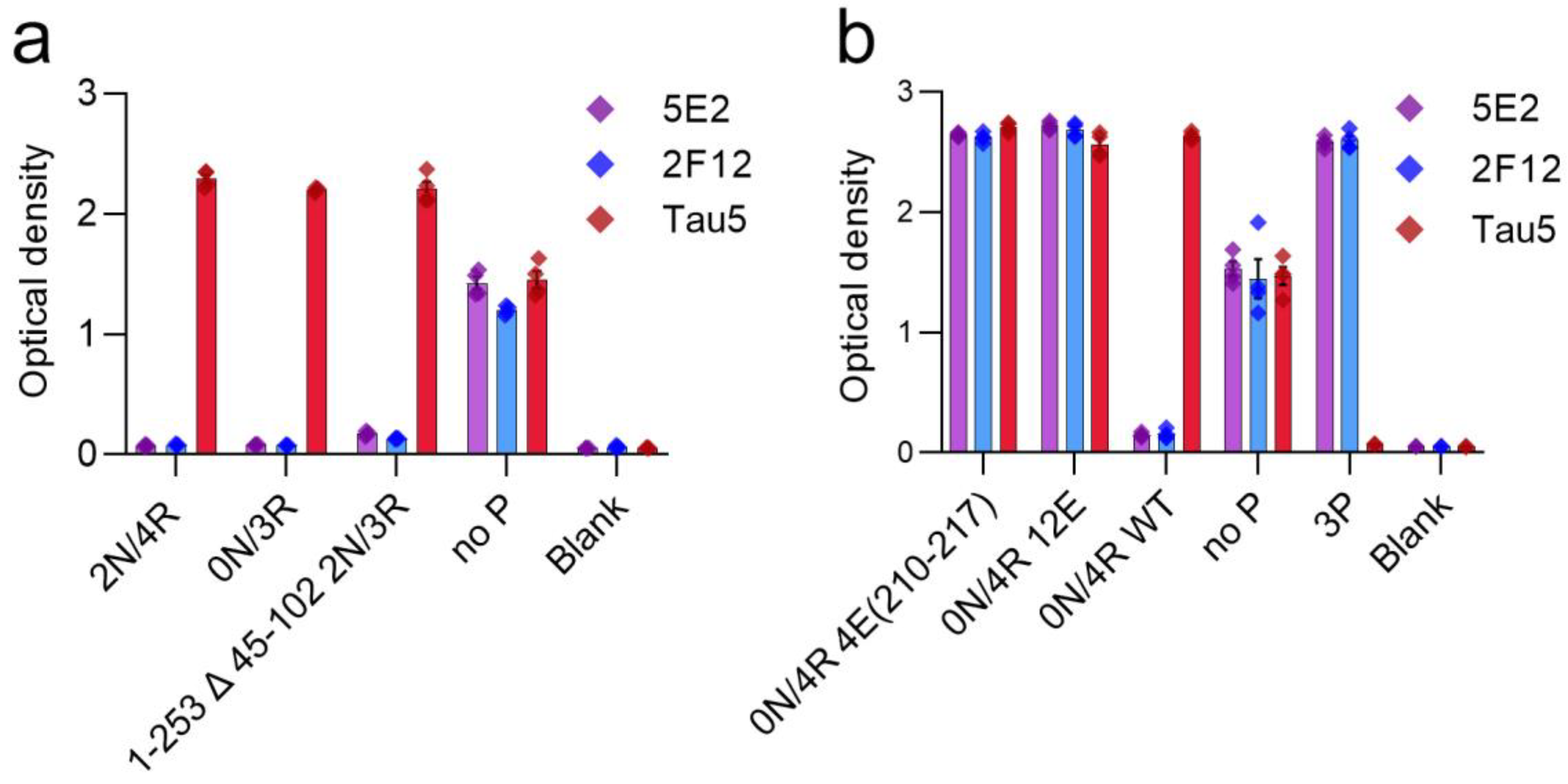
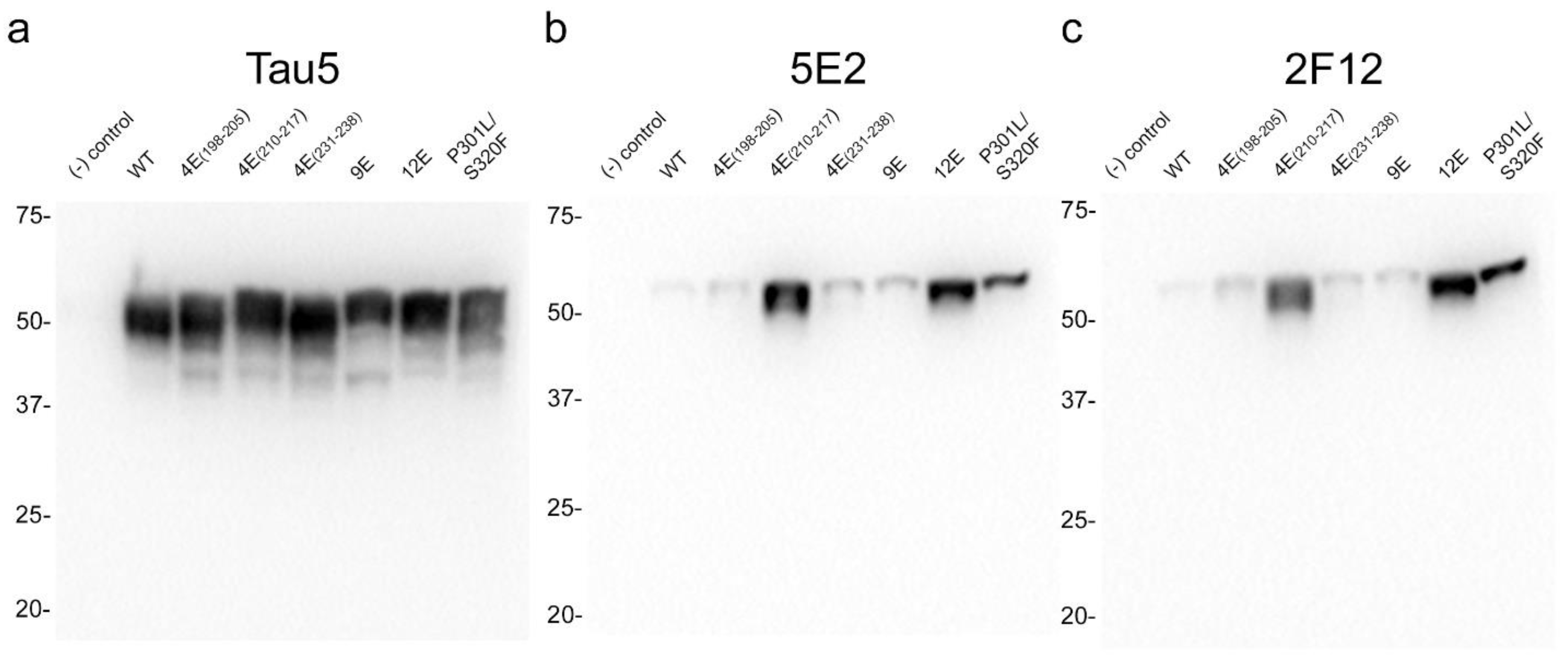
2.2. 5E2 and 2F12 Immunoreactivity Is Dependent on the Phosphorylation State Adjacent to the Core Epitope and Enhanced by Tau Pathogenic Mutants in HEK293T Cells
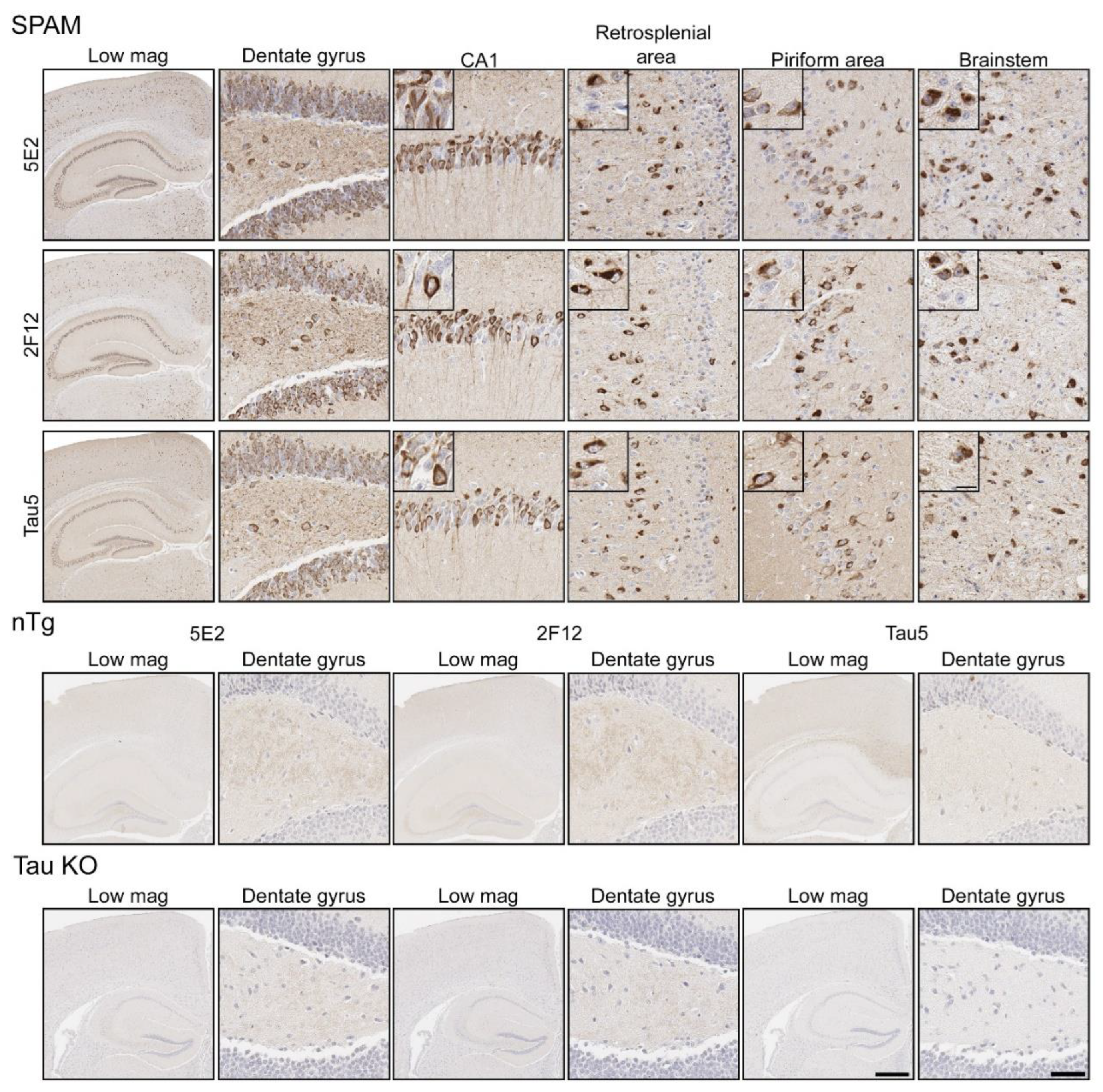
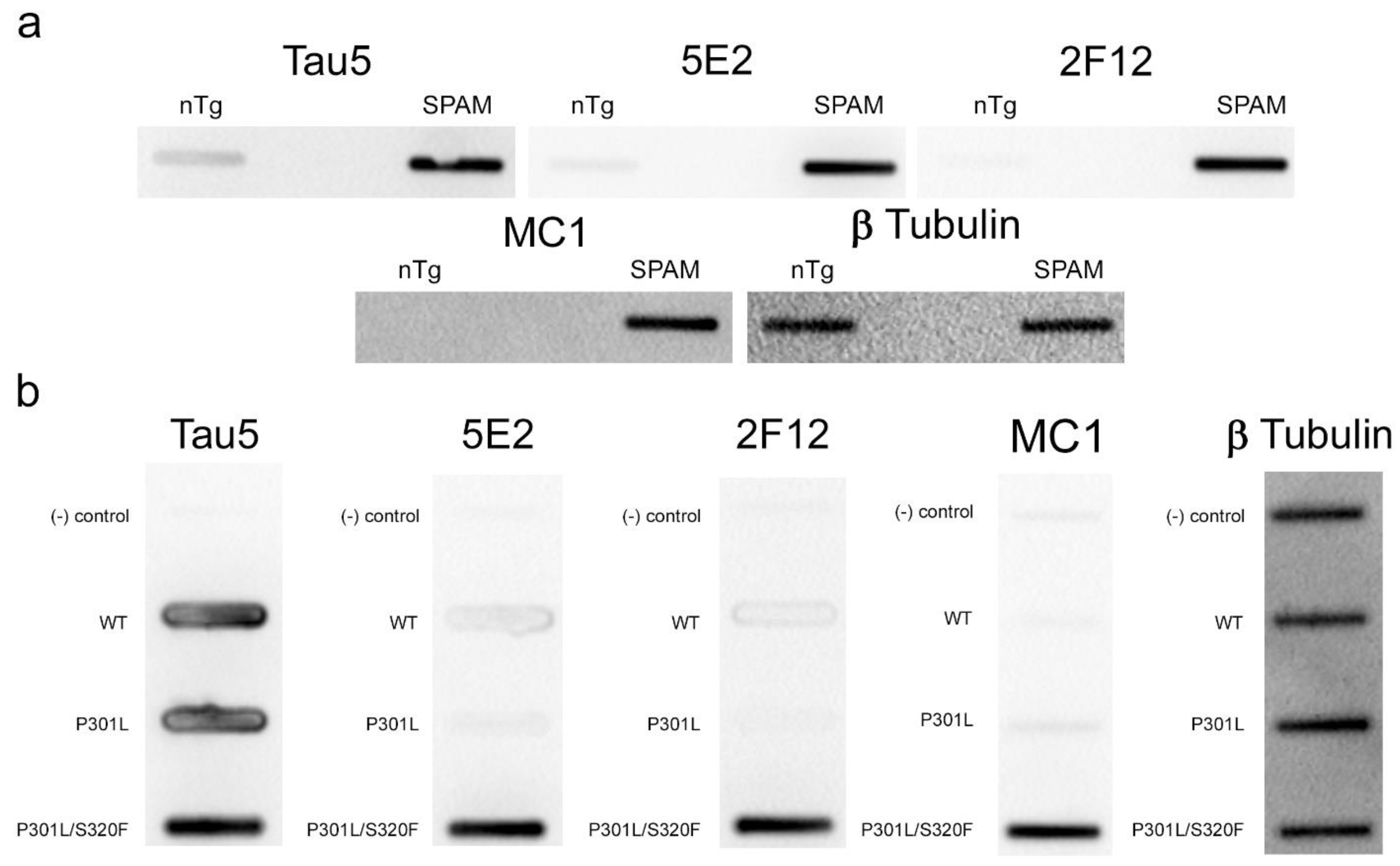
2.3. Immunohistochemical and Biochemical Characterization of 5E2 and 2F12 Antibodies Using Mice
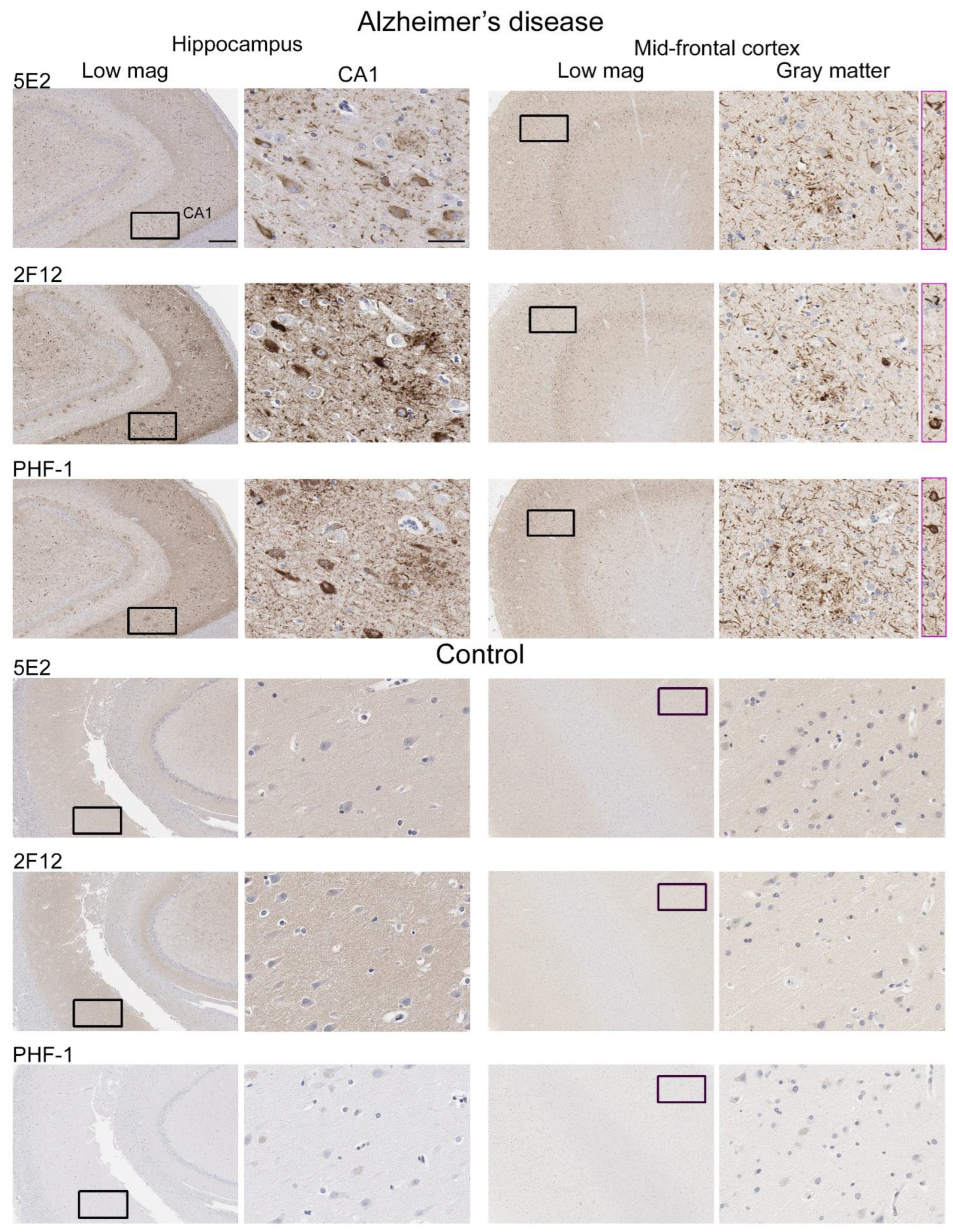
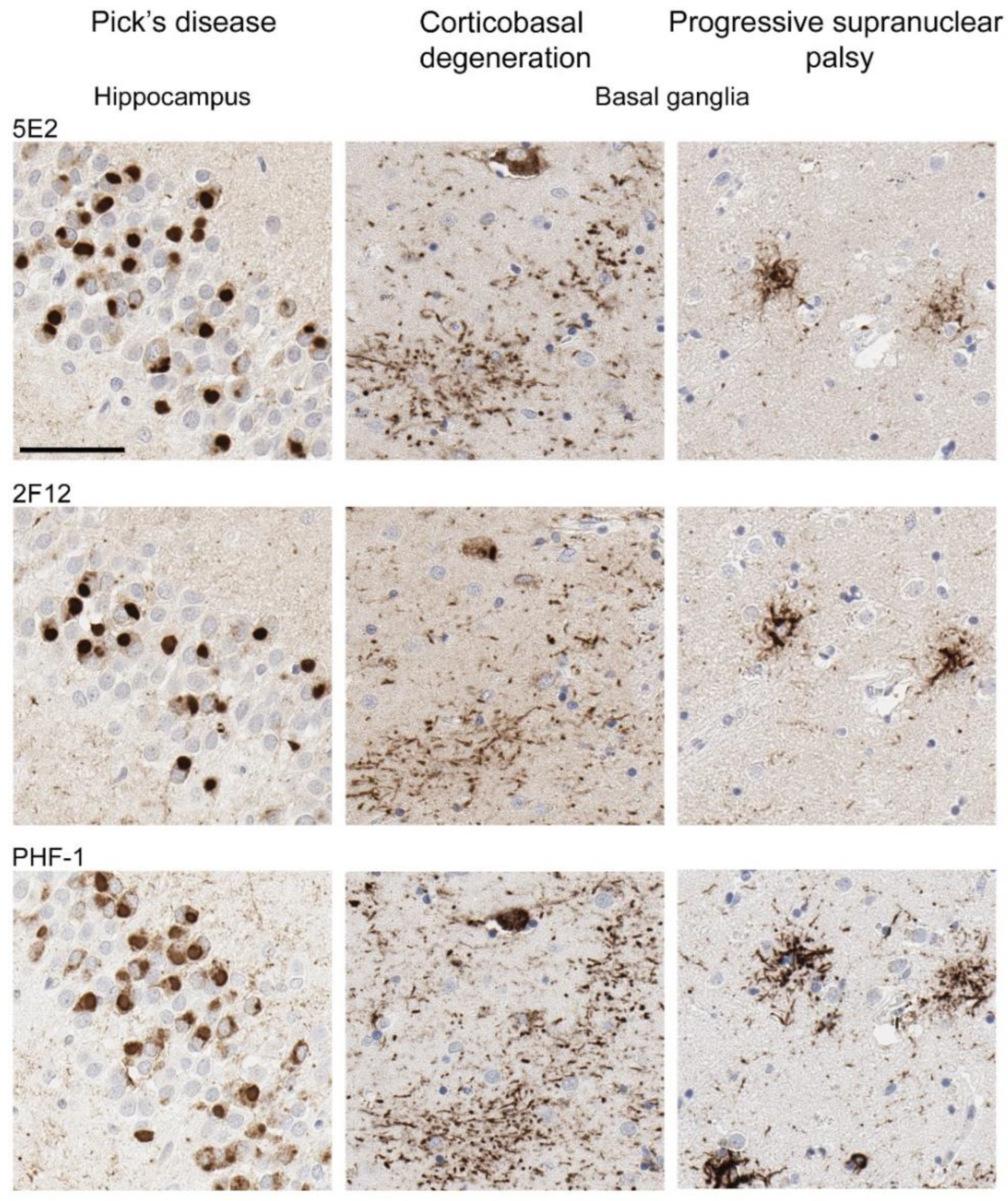
2.4. 5E2 and 2F12 Labelling of Tau Neuropathology in Alzheimer’s Disease and Primary Tauopathies
3. Discussion
4. Materials and Methods
4.1. Antibodies and Peptides
4.2. Generation of New Tau Monoclonal Antibodies 5E2 and 2F12
4.3. ELISA
4.4. Generation of Phospho-Mimetic Constructs
4.5. Recombinant Tau Protein Expression and Purification
4.6. HEK293T Cells and Calcium Phosphate Transfection
4.7. Histological and Biochemical Analysis of Mouse Tissue
4.8. Western Blotting Analysis
4.9. Immunohistochemistry
4.10. Semiquantification of Tau Neuropathology Immunostaining with PHF-1, 5E2, and 2F12 in Human Brain Tissue
4.11. 5E2 and 2F12 Pre-Absorption with 3P Peptide
4.12. Non-Denaturation Immunoblotting
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goedert, M.; Wischik, C.M.; Crowther, R.A.; Walker, J.E.; Klug, A. Cloning and Sequencing of the CDNA Encoding a Core Protein of the Paired Helical Filament of Alzheimer Disease: Identification as the Microtubule-Associated Protein Tau. Proc. Natl Acad. Sci. USA 1988, 85, 4051–4055. [Google Scholar] [CrossRef]
- Weingarten, M.; Lockwood, A.; Hwo, S.; Kirschner, M. A Protein Factor Essential for Microtubule Assembly. Proc. Natl. Acad. Sci. USA 1975, 72, 1858–1862. [Google Scholar] [CrossRef]
- Cleveland, D.W.; Hwo, S.Y.; Kirschner, M.W. Physical and Chemical Properties of Purified Tau Factor and the Role of Tau in Microtubule Assembly. J. Mol. Biol. 1977, 116, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Jeganathan, S.; Von Bergen, M.; Mandelkow, E.-M.; Mandelkow, E. The Natively Unfolded Character of Tau and Its Aggregation to Alzheimer-like Paired Helical Filaments. Biochemistry 2008, 47, 10526–10539. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Spillantini, M.G.; Jakes, R.; Rutherford, D.; Crowther, R.A. Multiple Isoforms of Human Microtubule-Associated Protein Tau: Sequences and Localization in Neurofibrillary Tangles of Alzheimer’s Disease. Neuron 1989, 3, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Spillantini, M.G.; Potier, M.C.; Ulrich, J.; Crowther, R.A. Cloning and Sequencing of the cDNA Encoding an Isoform of Microtubule-Associated Protein Tau Containing Four Tandem Repeats: Differential Expression of Tau Protein MRNAs in Human Brain. EMBO J. 1989, 8, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, P.; Szuchet, S.; Papasozomenos, S.C.; Zinkowski, R.P.; Binder, L.I. Functional Implications for the Microtubule-Associated Protein Tau: Localization in Oligodendrocytes. Proc. Natl. Acad. Sci. USA 1995, 92, 10369–10373. [Google Scholar] [CrossRef]
- Shin, R.-W.; Iwaki, T.; Kitamoto, T.; Tateishi, J. Hydrated Autoclave Pretreatment Enhances TAU Immunoreactivity in Formalin-Fixed Normal and Alzheimer’s Disease Brain Tissue. Lab. Investig. 1991, 64, 693–702. [Google Scholar] [PubMed]
- Buée, L.; Bussière, T.; Buée-Scherrer, V.; Delacourte, A.; Hof, P.R. Tau Protein Isoforms, Phosphorylation and Role in Neurodegenerative Disorders. Brain Res. Rev. 2000, 33, 95–130. [Google Scholar] [CrossRef]
- Lee, V.M.Y.; Goedert, M.; Trojanowski, J.Q. Neurodegenerative Tauopathies. Annu. Rev. Neurosci. 2001, 24, 1121–1159. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The Diagnosis of Dementia Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s Dement. 2012, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W.; Kouri, N.; Murray, M.E.; Josephs, K.A. Neuropathology of Frontotemporal Lobar Degeneration-Tau (FTLD-Tau). J. Mol. Neurosci. 2011, 45, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, P.; Scharf, E.L.; Paolini, M.A.; Graff-Radford, J.; Alden, E.C.; Machulda, M.M.; Jones, D.T.; Fields, J.A.; Murray, M.E.; Graff-Radford, N.R.; et al. Pick’s Disease: Clinicopathologic Characterization of 21 Cases. J. Neurol. 2020, 267, 2697–2704. [Google Scholar] [CrossRef] [PubMed]
- Hoglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Muller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical Diagnosis of Progressive Supranuclear Palsy: The Movement Disorder Society Criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Litvan, I.; Lang, A.E.; Bak, T.H.; Bhatia, K.P.; Borroni, B.; Boxer, A.L.; Dickson, D.W.; Grossman, M.; Hallett, M.; et al. Criteria for the Diagnosis of Corticobasal Degeneration. Neurology 2013, 80, 496–503. [Google Scholar] [CrossRef]
- Hasegawa, M. Biochemistry and Molecular Biology of Tauopathies. Neuropathology 2006, 26, 484–490. [Google Scholar] [CrossRef]
- Wang, Y.; Mandelkow, E. Tau in Physiology and Pathology. Nat. Rev. Neurosci. 2015, 17, 5–21. [Google Scholar] [CrossRef]
- Wesseling, H.; Mair, W.; Kumar, M.; Schlaffner, C.N.; Tang, S.; Beerepoot, P.; Fatou, B.; Guise, A.J.; Cheng, L.; Takeda, S.; et al. Tau PTM Profiles Identify Patient Heterogeneity and Stages of Alzheimer’s Disease. Cell 2020, 183, 1699–1713. [Google Scholar] [CrossRef]
- Barthélemy, N.R.; Saef, B.; Li, Y.; Gordon, B.A.; He, Y.; Horie, K.; Stomrud, E.; Salvadó, G.; Janelidze, S.; Sato, C.; et al. CSF Tau Phosphorylation Occupancies at T217 and T205 Represent Improved Biomarkers of Amyloid and Tau Pathology in Alzheimer’s Disease. Nat. Aging 2023, 3, 391–401. [Google Scholar] [CrossRef]
- Barthélemy, N.R.; Toth, B.; Manser, P.T.; Sanabria-Bohórquez, S.; Teng, E.; Keeley, M.; Bateman, R.J.; Weimer, R.M.; Wildsmith, K.R. Site-Specific Cerebrospinal Fluid Tau Hyperphosphorylation in Response to Alzheimer’s Disease Brain Pathology: Not All Tau Phospho-Sites Are Hyperphosphorylated. J. Alzheimer’s Dis. 2022, 85, 415–429. [Google Scholar] [CrossRef]
- Janelidze, S.; Bali, D.; Ashton, N.J.; Barthélemy, N.R.; Vanbrabant, J.; Stoops, E.; Vanmechelen, E.; He, Y.; Dolado, A.O.; Triana-Baltzer, G.; et al. Head-to-Head Comparison of 10 Plasma Phospho-Tau Assays in Prodromal Alzheimer’s Disease. Brain 2023, 146, 1592–1601. [Google Scholar] [CrossRef] [PubMed]
- Zheng-Fischhöfer, Q.; Biernat, J.; Mandelkow, E.E.M.; Illenberger, S.; Godemann, R.; Mandelkow, E.E.M. Sequential Phosphorylation of Tau by Glycogen Synthase Kinase-3β and Protein Kinase A at Thr212 and Ser214 Generates the Alzheimer-Specific Epitope of Antibody AT100 and Requires a Paired-Helical-Filament-like Conformation. Eur. J. Biochem. 1998, 252, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Porzig, R.; Singer, D.; Hoffmann, R. Epitope Mapping of MAbs AT8 and Tau5 Directed against Hyperphosphorylated Regions of the Human Tau Protein. Biochem. Biophys. Res. Commun. 2007, 358, 644–649. [Google Scholar] [CrossRef]
- Strang, K.H.; Croft, C.L.; Sorrentino, Z.A.; Chakrabarty, P.; Golde, T.E.; Giasson, B.I. Distinct Differences in Prion-like Seeding and Aggregation between Tau Protein Variants Provide Mechanistic Insights into Tauopathies. J. Biol. Chem. 2018, 293, 2408–2421. [Google Scholar] [CrossRef]
- Xia, Y.; Prokop, S.; Bell, B.M.; Gorion, K.M.M.; Croft, C.L.; Nasif, L.; Xu, G.; Riffe, C.J.; Manaois, A.N.; Strang, K.H.; et al. Pathogenic Tau Recruits Wild-Type Tau into Brain Inclusions and Induces Gut Degeneration in Transgenic SPAM Mice. Commun. Biol. 2022, 5, 446–464. [Google Scholar] [CrossRef] [PubMed]
- Dawson, H.N.; Ferreira, A.; Eyster, M.V.; Ghoshal, N.; Binder, L.I.; Vitek, M.P. Inhibition of Neuronal Maturation in Primary Hippocampal Neurons from Tau Deficient Mice. J. Cell Sci. 2001, 114, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Otvos, L., Jr.; Feiner, L.; Lang, E.; Szendrei, G.I.; Goedert, M.; Lee, V.M.-Y. Monoclonal Antibody PHF-1 Recognizes Tau Protein Phosphorylated at Serine Residue 396 and 404. J. Neurosci. Res. 1994, 39, 669–673. [Google Scholar] [CrossRef]
- Paterno, G.; Bell, B.M.; Gorion, K.M.M.; Prokop, S.; Giasson, B.I. Reassessment of Neuronal Tau Distribution in Adult Human Brain and Implications for Tau Pathobiology. Acta Neuropathol. Commun. 2022, 10, 94. [Google Scholar] [CrossRef]
- Greenberg, S.G.; Davies, P. A Preparation of Alzheimer Paired Helical Filaments That Displays Distinct Tau Proteins by Polyacrylamide Gel Electrophoresis. Proc. Natl. Acad. Sci. USA 1990, 87, 5827–5831. [Google Scholar] [CrossRef]
- Kosik, K.S.; Joachim, C.L.; Selkoe, D.J. Microtubule-Associated Protein Tau (T) Is a Major Antigenic Component of Paired Helical Filaments in Alzheimer Disease. Proc. Nati. Acad. Sci. USA 1986, 83, 4044–4048. [Google Scholar] [CrossRef]
- Rubenstein, R.; Kascsak, R.J.; Merz, P.A.; Wisniewski, H.M.; Carp, R.I.; Iqbal, K. Paired Helical Filaments Associated with Alzheimer Disease Are Readily Soluble Structures. Brain Res. 1986, 372, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Lindwall, G.; Cole, R. Phosphorylation Affects the Ability of Tau Protein to Promote Microtubule Assembly. J. Biol. Chem. 1984, 259, 5301–5305. [Google Scholar] [CrossRef] [PubMed]
- Del, C.; Alonso, A.; Grundke-Iqbal, I.; Iqbal, K. Alzheimer’s Disease Hyperphosphorylated Tau Sequesters Normal Tau into Tangles of Filaments and Disassembles Microtubules. Nat. Med. 1996, 2, 783–787. [Google Scholar] [CrossRef]
- Brion, J.; Couck, A.M.; Passareiro, E.; Durand-Flament, J. Neurofibrillary Tangles of Alzheimer’s Disease: An Immunohistochemical Study. J. Submicrosc. Cytol. 1985, 17, 89–96. [Google Scholar] [PubMed]
- Dickson, D.W.; Rademakers, R.; Hutton, M.L. Progressive Supranuclear Palsy: Pathology and Genetics. Brain Pathol. 2007, 17, 74–82. [Google Scholar] [CrossRef]
- Grundke-Iqbal, I.; Iqbal, K.; Tung, Y.-C.; Quinlan, M.; Wisniewski, H.M.; Binder, L.I. Abnormal Phosphorylation of the Microtubule-Associated Protein T (Tau) in Alzheimer Cytoskeletal Pathology. Proc. Natl. Acad. Sci. USA 1986, 83, 4913–4917. [Google Scholar] [CrossRef]
- Ksiezak-reding, H.; Morgan, K.; Mattiace, L.A.; Davies, P.; Liu, W.; Yen, S.; Weidenheim, K.; Dickson, D.W. Ultrastructure and Biochemical Composition of Paired Helical Filaments in Corticobasal Degeneration. Am. J. Pathol. 1994, 145, 1496–1508. [Google Scholar]
- Matsuo, E.S.; Shin, R.-W.; Billingsley, M.L.; Van deVoorde, A.; O’Connor, M.; Trojanowski, J.Q.; Lee, V.M.-Y. Biopsy-Derived Adult Human Brain Tau Is Phosphorylated at Many of the Same Sites as Alzheimer’s Disease Paired Helical Filament Tau. Neuron 1994, 13, 989–1002. [Google Scholar] [CrossRef]
- Moloney, C.M.; Lowe, V.J.; Murray, M.E. Visualization of Neurofibrillary Tangle Maturity in Alzheimer’s Disease: A Clinicopathologic Perspective for Biomarker Research. Alzheimer’s Dement. 2021, 17, 1554–1574. [Google Scholar] [CrossRef]
- Gustke, N.; Trinczek, B.; Biernat, J.; Mandelkow, E.M.; Mandelkow, E. Domains of Tau Protein and Interactions with Microtubules. Biochemistry 1994, 33, 9511–9522. [Google Scholar] [CrossRef]
- Xia, Y.; Prokop, S.; Giasson, B.I. “Don’t Phos Over Tau”: Recent Developments in Clinical Biomarkers and Therapies Targeting Tau Phosphorylation in Alzheimer’s Disease and Other Tauopathies. Mol. Neurodegener. 2021, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Mandelkow, E.M.; Biernat, J.; Drewes, G.; Gustke, N.; Trinczek, B.; Mandelkow, E. Tau Domains, Phosphorylation, and Interactions with Microtubules. Neurobiol. Aging 1995, 16, 355–362. [Google Scholar] [CrossRef]
- McKibben, K.M.; Rhoades, E. Independent Tubulin Binding and Polymerization by the Proline-Rich Region of Tau Is Regulated by Tau’s N-Terminal Domain. J. Biol. Chem. 2019, 294, 19381–19394. [Google Scholar] [CrossRef]
- Mukrasch, M.D.; Von Bergen, M.; Biernat, J.; Fischer, D.; Griesinger, C.; Mandelkow, E.; Zweckstetter, M. The “Jaws” of the Tau-Microtubule Interaction. J. Biol. Chem. 2007, 282, 12230–12239. [Google Scholar] [CrossRef] [PubMed]
- Barthélemy, N.R.; Mallipeddi, N.; Moiseyev, P.; Sato, C.; Bateman, R.J. Tau Phosphorylation Rates Measured by Mass Spectrometry Differ in the Intracellular Brain vs. Extracellular Cerebrospinal Fluid Compartments and Are Differentially Affected by Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 121. [Google Scholar] [CrossRef]
- Engelborghs, S.; De Vreese, K.; Van de Casteele, T.; Vanderstichele, H.; Van Everbroeck, B.; Cras, P.; Martin, J.J.; Vanmechelen, E.; De Deyn, P.P. Diagnostic Performance of a CSF-Biomarker Panel in Autopsy-Confirmed Dementia. Neurobiol. Aging 2008, 29, 1143–1159. [Google Scholar] [CrossRef]
- Hanes, J.; Kovac, A.; Kvartsberg, H.; Kontsekova, E.; Fialova, L.; Katina, S.; Kovacech, B.; Stevens, E.; Hort, J.; Vyhnalek, M.; et al. Evaluation of a Novel Immunoassay to Detect P-Tau Thr217 in the CSF to Distinguish Alzheimer Disease from Other Dementias. Neurology 2020, 95, E3026–E3035. [Google Scholar] [CrossRef]
- Olsson, A.; Vanderstichele, H.; Andreasen, N.; De Meyer, G.; Wallin, A.; Holmberg, B.; Rosengren, L.; Vanmechelen, E.; Blennow, K. Simultaneous Measurement of β-Amyloid(1-42), Total Tau, and Phosphorylated Tau (Thr181) in Cerebrospinal Fluid by the XMAP Technology. Clin. Chem. 2005, 51, 336–345. [Google Scholar] [CrossRef]
- Vanmechelen, E.; Vanderstichele, H.; Davidsson, P.; Van Kerschaver, E.; Van Der Perre, B.; Sjögren, M.; Andreasen, N.; Blennow, K. Quantification of Tau Phosphorylated at Threonine 181 in Human Cerebrospinal Fluid: A Sandwich ELISA with a Synthetic Phosphopeptide for Standardization. Neurosci. Lett. 2000, 285, 49–52. [Google Scholar] [CrossRef]
- Palmqvist, S.; Janelidze, S.; Quiroz, Y.T.; Zetterberg, H.; Lopera, F.; Stomrud, E.; Su, Y.; Chen, Y.; Serrano, G.E.; Leuzy, A.; et al. Discriminative Accuracy of Plasma Phospho-Tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA 2020, 324, 772–781. [Google Scholar] [CrossRef]
- Cleveland, D.W.; Hwo, S.Y.; Kirschner, M.W. Purification of Tau, a Microtubule-Associated Protein That Induces Assembly of Microtubules from Purified Tubulin. J. Mol. Biol. 1977, 116, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Von Bergen, M.; Barghorn, S.; Jeganathan, S.; Mandelkow, E.M.; Mandelkow, E. Spectroscopic Approaches to the Conformation of Tau Protein in Solution and in Paired Helical Filaments. Neurodegener. Dis. 2006, 3, 197–206. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Neuropathological Stageing of Alzheimer-Related Changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, N.; García-Sierra, F.; Fu, Y.; Beckett, L.A.; Mufson, E.J.; Kuret, J.; Berry, R.W.; Binder, L.I. Tau-66: Evidence for a Novel Tau Conformation in Alzheimer’s Disease. J. Neurochem. 2001, 77, 1372–1385. [Google Scholar] [CrossRef]
- Gibbons, G.S.; Kim, S.-J.J.; Wu, Q.; Riddle, D.M.; Leight, S.N.; Changolkar, L.; Xu, H.; Meymand, E.S.; O’reilly, M.; Zhang, B.; et al. Conformation-Selective Tau Monoclonal Antibodies Inhibit Tau Pathology in Primary Neurons and a Mouse Model of Alzheimer’s Disease. Mol. Neurodegener. 2020, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, G.S.; Banks, R.A.; Kim, B.; Changolkar, L.; Riddle, D.M.; Leight, S.N.; Irwin, D.J.; Trojanowski, J.Q.; Lee, V.M.Y. Detection of Alzheimer Disease (AD)-Specific Tau Pathology in AD and NonAD Tauopathies by Immunohistochemistry With Novel Conformation-Selective Tau Antibodies. J. Neuropathol. Exp. Neurol. 2018, 77, 216–228. [Google Scholar] [CrossRef]
- Petry, F.R.; Pelletier, J.; Bretteville, A.; Morin, F.; Calon, F.; Hébert, S.S.; Whittington, R.A.; Planel, E. Specificity of Anti-Tau Antibodies When Analyzing Mice Models of Alzheimer’s Disease: Problems and Solutions. PLoS ONE 2014, 9, e94251. [Google Scholar] [CrossRef]
- Xia, Y.; Prokop, S.; Gorion, K.M.M.; Kim, J.D.; Sorrentino, Z.A.; Bell, B.M.; Manaois, A.N.; Chakrabarty, P.; Davies, P.; Giasson, B.I. Tau Ser208 Phosphorylation Promotes Aggregation and Reveals Neuropathologic Diversity in Alzheimer’s Disease and Other Tauopathies. Acta Neuropathol. Commun. 2020, 8, 88–105. [Google Scholar] [CrossRef]
- Mercken, M.; Vandermeeren, M.; Lübke, U.; Six, J.; Boons, J.; Van de Voorde, A.A.; Martin, J.-J.J.; Gheuens, J.; Liibke, U.; Six, J.; et al. Monoclonal Antibodies with Selective Specificity for Alzheimer Tau Are Directed against Phosphatase-Sensitive Epitopes. Acta Neuropathol. 1992, 84, 265–272. [Google Scholar] [CrossRef]
- Arakhamia, T.; Lee, C.E.; Carlomagno, Y.; Duong, D.M.; Kundinger, S.R.; Wang, K.; Williams, D.; Deture, M.; Dickson, D.W.; Cook, C.N.; et al. Posttranslational Modifications Mediate the Structural Diversity of Tauopathy Strains. Cell 2020, 180, 633–644. [Google Scholar] [CrossRef]
- Goedert, M.; Jakes, R.; Vanmechelen, E. Monoclonal Antibody AT8 Recognises Tau Protein Phosphorylated at Both Serine 202 and Threonine 205. Neurosci. Lett. 1995, 189, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Morishima-Kawashima, M.; Takio, K.; Suzuki, M.; Titani, K.; Ihara, Y. Protein Sequence and Mass Spectrometric Analyses of Tau in the Alzheimer’s Disease Brain. J. Biol. Chem. 1992, 267, 17047–17054. [Google Scholar] [CrossRef] [PubMed]
- Puig, B.; Rey, M.J.; Ferrer, I. Individual and Regional Variations of Phospho-Tau Species in Progressive Supranuclear Palsy. Acta Neuropathol. 2005, 110, 261–268. [Google Scholar] [CrossRef]
- Strang, K.H.; Goodwin, M.S.; Riffe, C.; Moore, B.D.; Chakrabarty, P.; Levites, Y.; Golde, T.E.; Giasson, B.I. Generation and Characterization of New Monoclonal Antibodies Targeting the PHF1 and AT8 Epitopes on Human Tau. Acta Neuropathol. Commun. 2017, 5, 58–69. [Google Scholar] [CrossRef]
- Strang, K.H.; Sorrentino, Z.A.; Riffe, C.J.; Gorion, K.M.M.; Vijayaraghavan, N.; Golde, T.E.; Giasson, B.I. Phosphorylation of Serine 305 in Tau Inhibits Aggregation. Neurosci. Lett. 2019, 692, 187–192. [Google Scholar] [CrossRef]
- Taniguchi-Watanabe, S.; Arai, T.; Kametani, F.; Nonaka, T.; Masuda-Suzukake, M.; Tarutani, A.; Murayama, S.; Saito, Y.; Arima, K.; Yoshida, M.; et al. Biochemical Classification of Tauopathies by Immunoblot, Protein Sequence and Mass Spectrometric Analyses of Sarkosyl-Insoluble and Trypsin-Resistant Tau. Acta Neuropathol. 2016, 131, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Jeganathan, S.; Von Bergen, M.; Brutlach, H.; Steinhoff, H.J.; Mandelkow, E. Global Hairpin Folding of Tau in Solution. Biochemistry 2006, 45, 2283–2293. [Google Scholar] [CrossRef] [PubMed]
- Jicha, G.A.; Bowser, R.; Kazam, I.G.; Davies, P. Alz-50 and MC-1, a New Monoclonal Antibody Raised to Paired Helical Filaments, Recognize Conformational Epitopes on Recombinant Tau Gregory. J. Neurosci. Res. 1997, 48, 128–132. [Google Scholar] [CrossRef]
- Jeganathan, S.; Hascher, A.; Chinnathambi, S.; Biernat, J.; Mandelkow, E.E.M.; Mandelkow, E.E.M. Proline-Directed Pseudo-Phosphorylation at AT8 and PHF1 Epitopes Induces a Compaction of the Paperclip Folding of Tau and Generates a Pathological (MC-1) Conformation. J. Biol. Chem. 2008, 283, 32066–32076. [Google Scholar] [CrossRef]
- Weaver, C.L.; Espinoza, M.; Kress, Y.; Davies, P. Conformational Change as One of the Earliest Alterations of Tau in Alzheimer’s Disease. Neurobiol. Aging 2000, 21, 719–727. [Google Scholar] [CrossRef]
- Jicha, G.A.; Lane, E.; Vincent, I.; Otvos, L.; Hoffmann, R.; Davies, P. A Conformation- and Phosphorylation-Dependent Antibody Recognizing the Paired Helical Filaments of Alzheimer’s Disease. J. Neurochem. 1997, 69, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Vincent, I.; Rosado, M.; Davies, P. Mitotic Mechanisms in Alzheimer’s Disease? J. Cell Biol. 1996, 132, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.; Szendrei, G.I.; Lee, V.M.Y.; Otvos, L. Spectroscopic Evidence That Monoclonal Antibodies Recognize the Dominant Conformation of Medium-Sized Synthetic Peptides. J. Immunol. Methods 1994, 170, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Mukrasch, M.D.; Bibow, S.; Korukottu, J.; Jeganathan, S.; Biernat, J.; Griesinger, C.; Mandelkow, E.; Zweckstetter, M. Structural Polymorphism of 441-Residue Tau at Single Residue Resolution. PLoS Biol. 2009, 7, e34. [Google Scholar] [CrossRef]
- Rutherford, N.J.; Brooks, M.; Giasson, B.I. Novel Antibodies to Phosphorylated α-Synuclein Serine 129 and NFL Serine 473 Demonstrate the Close Molecular Homology of These Epitopes. Acta Neuropathol. Commun. 2016, 4, 80. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Jakes, R. Expression of Separate Isoforms of Human Tau Protein: Correlation with the Tau Pattern in Brain and Effects on Tubulin Polymerization. EMBO J. 1990, 9, 4225–4230. [Google Scholar] [CrossRef]
- Giasson, B.I.; Forman, M.S.; Higuchi, M.; Golbe, L.I.; Graves, C.L.; Kotzbauer, P.T.; Trojanowski, J.Q.; Lee, V.M.Y. Initiation and Synergistic Fibrillization of Tau and Alpha-Synuclein. Science 2003, 300, 636–640. [Google Scholar] [CrossRef]
- Xia, Y.; Sorrentino, Z.A.; Kim, J.D.; Strang, K.H.; Riffe, C.J.; Giasson, B.I. Impaired Tau–Microtubule Interactions Are Prevalent among Pathogenic Tau Variants Arising from Missense Mutations. J. Biol. Chem. 2019, 294, 18488–18503. [Google Scholar] [CrossRef]
| Peptide Name | Peptide Sequence | Additional Note |
|---|---|---|
| Tau 208–225 (3P) | CSRSR(pThr)P(pSer)LP(pThr)PPTREPKK | extra Cys at the N-terminus |
| Tau 208–225 (no P) | CSRSRTPSLPTPPTREPKK | extra Cys at the N-terminus |
| Tau 208–225 (pT212) | CSRSR(pThr)PSLPTPPTREPKK | extra Cys at the N-terminus |
| Tau 208–225 (pS214) | CSRSRTP(pSer)LPTPPTREPKK | extra Cys at the N-terminus |
| Tau 208–225 (pT217) | CSRSRTPSLP(pThr)PPTREPKK | extra Cys at the N-terminus |
| Tau 208–225 (pT212, pS214) | CSRSR(pThr)P(pSer)LPTPPTREPKK | extra Cys at the N-terminus |
| Tau 208–225 (pS214, pT217) | CSRSRTP(pSer)LP(pThr)PPTREPKK | extra Cys at the N-terminus |
| Tau 208–225 (pT212, pT217) | CSRSR(pThr)PSLP(pThr)PPTREPKK | extra Cys at the N-terminus |
| 3P-RA | CSASA(pThr)P(pSer)LP(pThr)PPTREPKK | extra Cys at the N-terminus |
| 3P-KA | CSRSR(pThr)P(pSer)LP(pThr)PPTREPAA | extra Cys at the N-terminus |
| no P-REAA | CSRSRTPSLPTPPTAAPKK | extra Cys at the N-terminus |
| no P-PPAA | CSRSRTPSLPTAATREPKK | extra Cys at the N-terminus |
| Protein Name | Tau Isoform | Mutations |
|---|---|---|
| 4E(198–205) | 0N/4R | S198E, S199E, S202E, T205E |
| 4E(210–217) | 0N/4R | S210E, T212E, S214E, T217E |
| 4E(231–238) | 0N/4R | T231E, S235E, S237E, S238E |
| 12E | 0N/4R | S198E, S199E, S202E, T205E, S210E, T212E, S214E, T217E, T231E, S235E, S237E, S238E |
| 9E | 0N/4R | Y394E, S396E, S400E, T403E, S404E, S409E, S412E, S422E, S435E |
| Case Type | Clinicopathological Diagnosis | Primary Neuropathological Diagnosis | Secondary Neuropathological Diagnosis | Thal | Braak | CERAD | APOE | Sex | Age | Region(s) Investigated |
|---|---|---|---|---|---|---|---|---|---|---|
| PSP-1 | PSP | PSP | AD intermediate | 5 | III | moderate | 4/4 | m | 72 | Striatum |
| PSP-2 | PSP | PSP | PART | 0 | I | none | 3/3 | m | 67 | Striatum |
| PSP-3 | PSP | PSP | AD low | 3 | I | none | 3/3 | f | 77 | Striatum |
| PSP-4 | PSP | PSP | AD low | 2 | II | sparse | 3/3 | m | 79 | Striatum |
| PSP-5 | PSP | PSP | AD low | 3 | 0 | none | 3/3 | m | 69 | Striatum |
| PSP-6 | PSP | PSP | AD intermediate | 3 | III | sparse | 2/3 | f | 74 | Striatum |
| PSP-7 | PSP | PSP | LATE Stage2 | 1 | II | none | 3/3 | m | 77 | Striatum |
| PSP-8 | PSP | PSP | AD low | 3 | I | none | 3/3 | m | 78 | Striatum |
| CBD-1 | CBD | CBD | ARTAG | 0 | 0 | none | 3/3 | f | 71 | Striatum |
| CBD-2 | CBD | CBD | AD low | 1 | II | none | 3/3 | f | 73 | Striatum |
| CBD-3 | CBD | CBD | AD low | 3 | II | none | 2/4 | f | 70 | Striatum |
| CBD-4 | CBD | CBD | AD low | 1 | II | none | 2/3 | m | 69 | Striatum |
| CBD-5 | vascular dementia/AD | CBD | remote infarct in basal ganglia | 0 | II | none | 3/3 | m | 82 | Striatum |
| CBD-6 | FTD/PPA | CBD | AD low | 1 | I | none | 3/3 | m | 66 | Striatum |
| PiD-1 | FTLD | PiD | AD low | 3 | II | sparse | 3/3 | m | 75 | Hippocampus |
| PiD-2 | PiD | PiD | AD intermediate | 5 | III | sparse | 3/3 | m | 82 | Hippocampus |
| AD-1 | AD | AD high | CAA focal, mild | 5 | V | frequent | 3/3 | f | 85 | Hippocampus, Mid-frontal cortex |
| AD-2 | AD | AD high | CAA focal, mild | 5 | V | frequent | 3/3 | f | 83 | Hippocampus |
| AD-3 | dementia | AD high | LATE stage 2, hippocampal sclerosis | 5 | VI | frequent | 3/3 | f | 77 | Mid-frontal cortex |
| AD-4 | AD | AD high | CAA widespread, mild to moderate | 5 | VI | frequent | 3/3 | m | 78 | Mid-frontal cortex |
| AD-5 | AD | AD high | LBD amygdala predominant | 5 | VI | frequent | 3/3 | m | 70 | Mid-frontal cortex |
| AD-6 | AD | AD high | CAA focal, mild to moderate | 5 | VI | frequent | 3/3 | f | 79 | Mid-frontal cortex |
| AD-7 | AD | AD high | CAA widespread, mild to moderate | 5 | V | frequent | 3/3 | f | 83 | Mid-frontal cortex |
| AD-8 | LBD | AD high | CAA focal, moderate | 5 | V | frequent | 3/4 | m | 74 | Hippocampus, Mid-frontal cortex |
| AD-9 | AD | AD high | CAA, moderate, widespread | 5 | V | frequent | 3/4 | f | 86 | Mid-frontal cortex |
| AD-10 | AD | AD high | CAA widespread, severe | 5 | V | frequent | 3/4 | f | 72 | Mid-frontal cortex |
| AD-11 | AD | AD high | CAA widespread, mild to moderate | 4 | VI | frequent | 3/4 | f | 82 | Mid-frontal cortex |
| AD-12 | vascular dementia, possibly mixed | AD high | CAA moderate to severe | 4 | VI | frequent | 3/4 | m | 73 | Mid-frontal cortex |
| AD-13 | AD | AD high | CAA focal, moderate | 4 | V | frequent | 3/4 | f | 85 | Mid-frontal cortex |
| AD-14 | AD | AD high | CAA widespread, mild to moderate | 5 | V | frequent | 3/4 | m | 77 | Mid-frontal cortex |
| AD-15 | dementia | AD high | CAA widespread, moderate to severe | 5 | V | frequent | 3/4 | m | 80 | Mid-frontal cortex |
| AD-16 | dementia | AD high | LBD diffuse neocortical | 5 | VI | frequent | 3/4 | f | 84 | Mid-frontal cortex |
| AD-17 | AD | AD high | CAA widespread, moderate | 4 | VI | frequent | 4/4 | f | 86 | Mid-frontal cortex |
| AD-18 | AD | AD high | CAA widespread, moderate | 4 | VI | frequent | 4/4 | m | 75 | Mid-frontal cortex |
| AD-19 | AD | AD high | CAA widespread, moderate | 5 | VI | frequent | 4/4 | m | 70 | Mid-frontal cortex |
| AD-20 | AD | AD high | CAA widespread, moderate | 5 | VI | frequent | 4/4 | m | 72 | Mid-frontal cortex |
| AD-21 | AD | AD high | CAA widespread, mild | 5 | VI | frequent | 4/4 | f | 76 | Mid-frontal cortex |
| AD-22 | AD | AD high | CAA widespread, severe | 5 | V | frequent | 4/4 | f | 84 | Mid-frontal cortex |
| Control-1 | PART | subacute microinfarct corpus callosum | 0 | II | none | 3/3 | f | 90 | Mid-frontal cortex | |
| Control-2 | PART | 0 | II | none | 3/3 | f | 72 | Mid-frontal cortex | ||
| Control-3 | PART | CAA widespread, mild | 0 | I | none | 3/3 | m | 71 | Mid-frontal cortex | |
| Control-4 | cognitive impairments | PART | CAA focal, mild to moderate | 0 | II | none | 3/3 | m | 75 | Mid-frontal cortex |
| Control-5 | PART | 0 | I | none | 2/3 | f | 73 | Mid-frontal cortex | ||
| Control-6 | metastatic cancer | rare Aβ plaques in neocortex | 1 | 0 | none | 3/3 | m | 46 | Hippocampus, Striatum | |
| Control-7 | Unverricht Lundborg disease | 0 | 0 | none | 2/3 | m | 65 | Hippocampus, Striatum | ||
| Control-8 | metastatic melanoma | AD low | 2 | 0 | none | 3/3 | m | 61 | Hippocampus, Striatum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paterno, G.; Torrellas, J.; Bell, B.M.; Gorion, K.-M.M.; Quintin, S.S.; Hery, G.P.; Prokop, S.; Giasson, B.I. Novel Conformation-Dependent Tau Antibodies Are Modulated by Adjacent Phosphorylation Sites. Int. J. Mol. Sci. 2023, 24, 13676. https://doi.org/10.3390/ijms241813676
Paterno G, Torrellas J, Bell BM, Gorion K-MM, Quintin SS, Hery GP, Prokop S, Giasson BI. Novel Conformation-Dependent Tau Antibodies Are Modulated by Adjacent Phosphorylation Sites. International Journal of Molecular Sciences. 2023; 24(18):13676. https://doi.org/10.3390/ijms241813676
Chicago/Turabian StylePaterno, Giavanna, Jose Torrellas, Brach M. Bell, Kimberly-Marie M. Gorion, Stephan S. Quintin, Gabriela P. Hery, Stefan Prokop, and Benoit I. Giasson. 2023. "Novel Conformation-Dependent Tau Antibodies Are Modulated by Adjacent Phosphorylation Sites" International Journal of Molecular Sciences 24, no. 18: 13676. https://doi.org/10.3390/ijms241813676
APA StylePaterno, G., Torrellas, J., Bell, B. M., Gorion, K.-M. M., Quintin, S. S., Hery, G. P., Prokop, S., & Giasson, B. I. (2023). Novel Conformation-Dependent Tau Antibodies Are Modulated by Adjacent Phosphorylation Sites. International Journal of Molecular Sciences, 24(18), 13676. https://doi.org/10.3390/ijms241813676






