APPA Increases Lifespan and Stress Resistance via Lipid Metabolism and Insulin/IGF-1 Signal Pathway in Caenorhabditis elegans
Abstract
:1. Introduction
2. Results
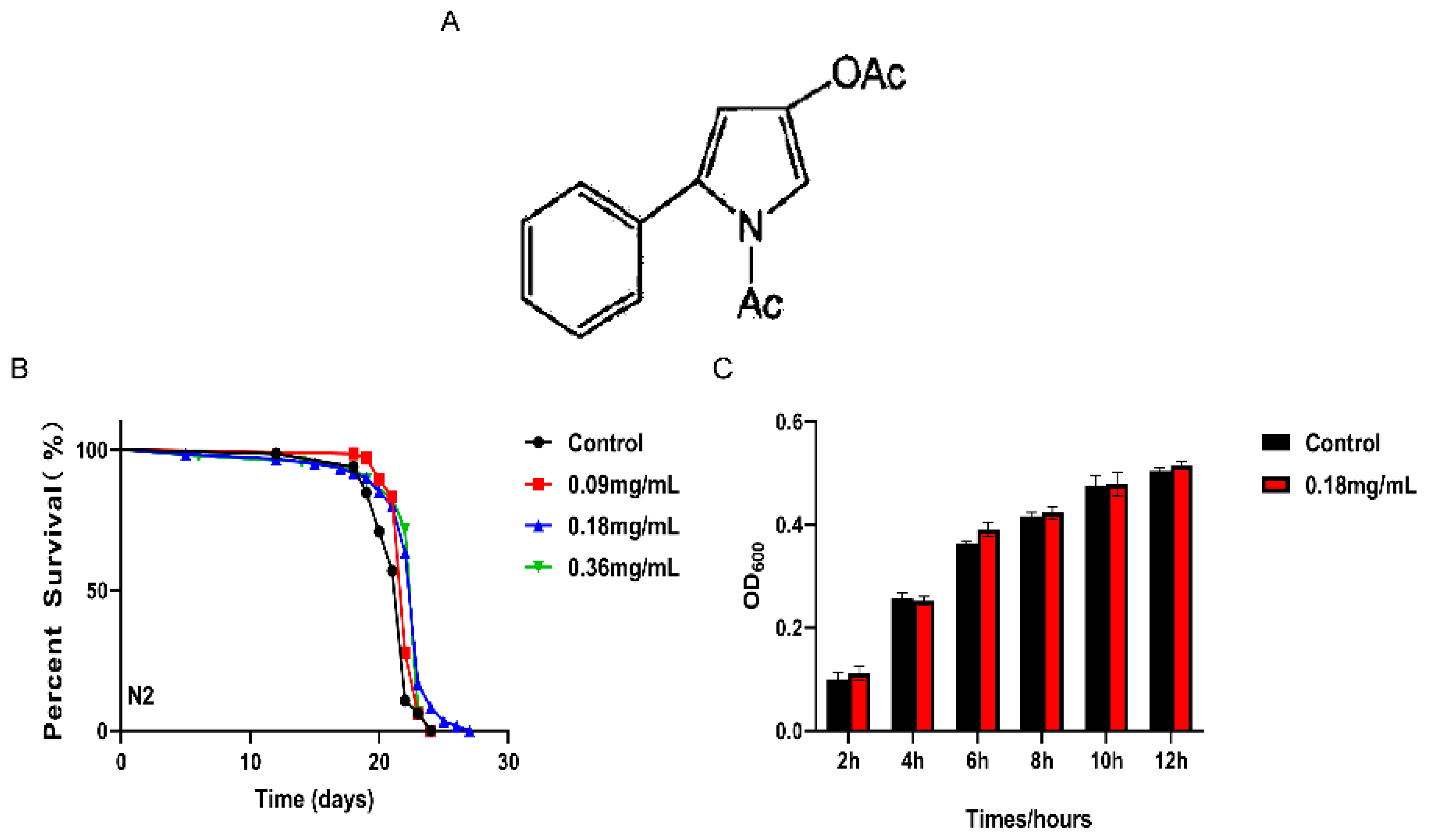
2.1. APPA Extended the Lifespan of C. elegans
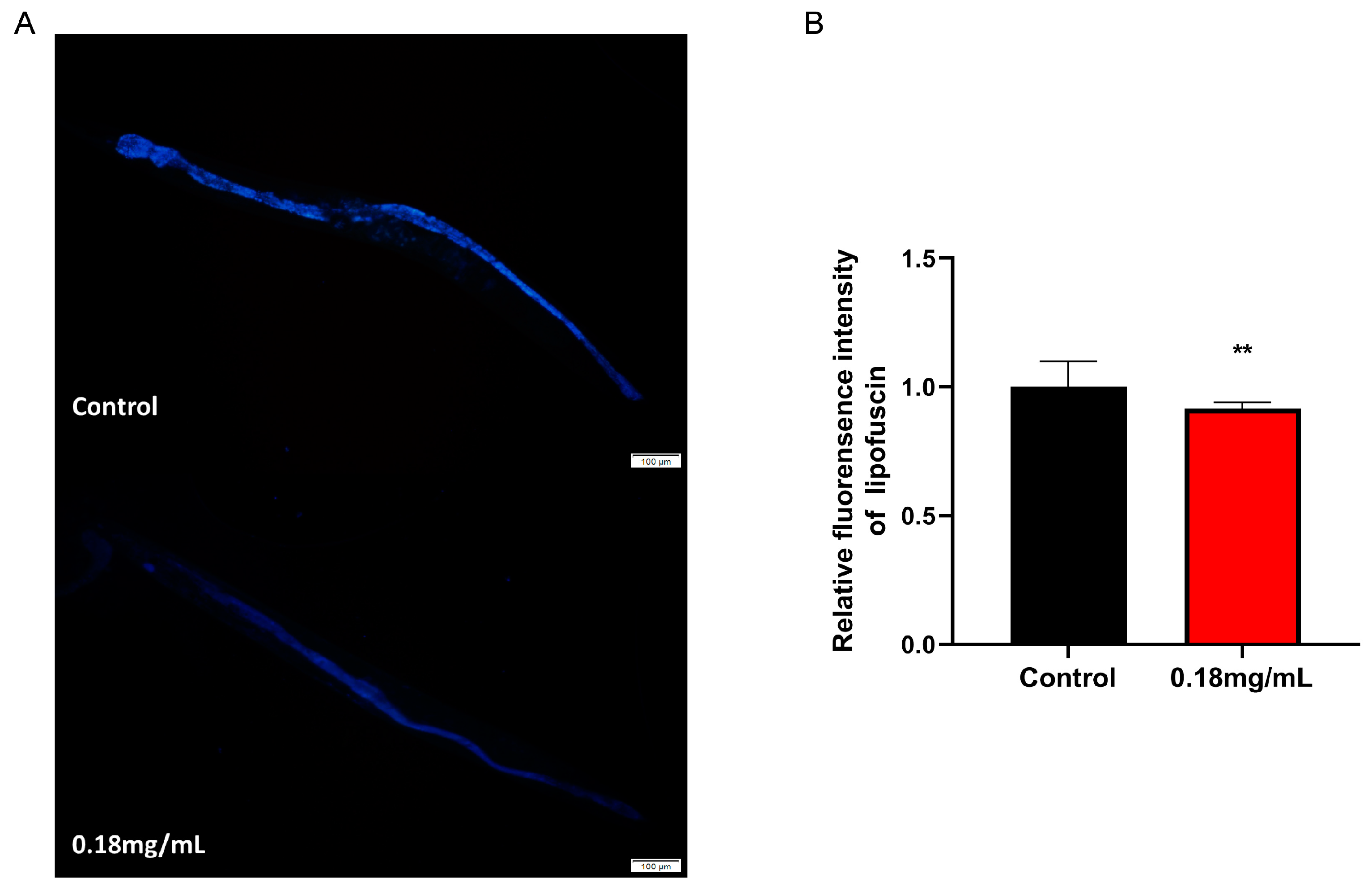
2.2. APPA Reduces Lipofuscin Accumulation in C. elegans
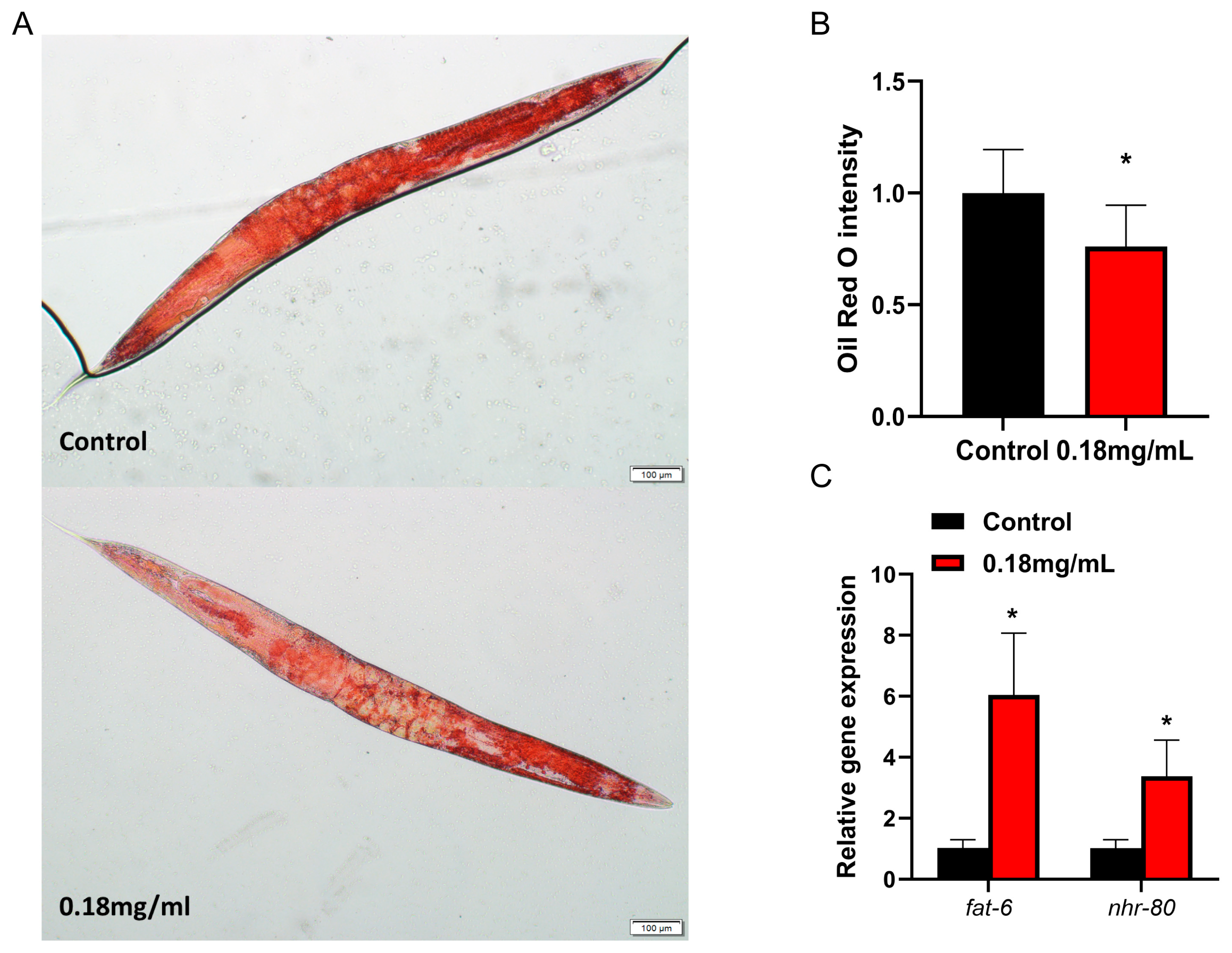
2.3. APPA Reduces Fat Accumulation in Nematodes
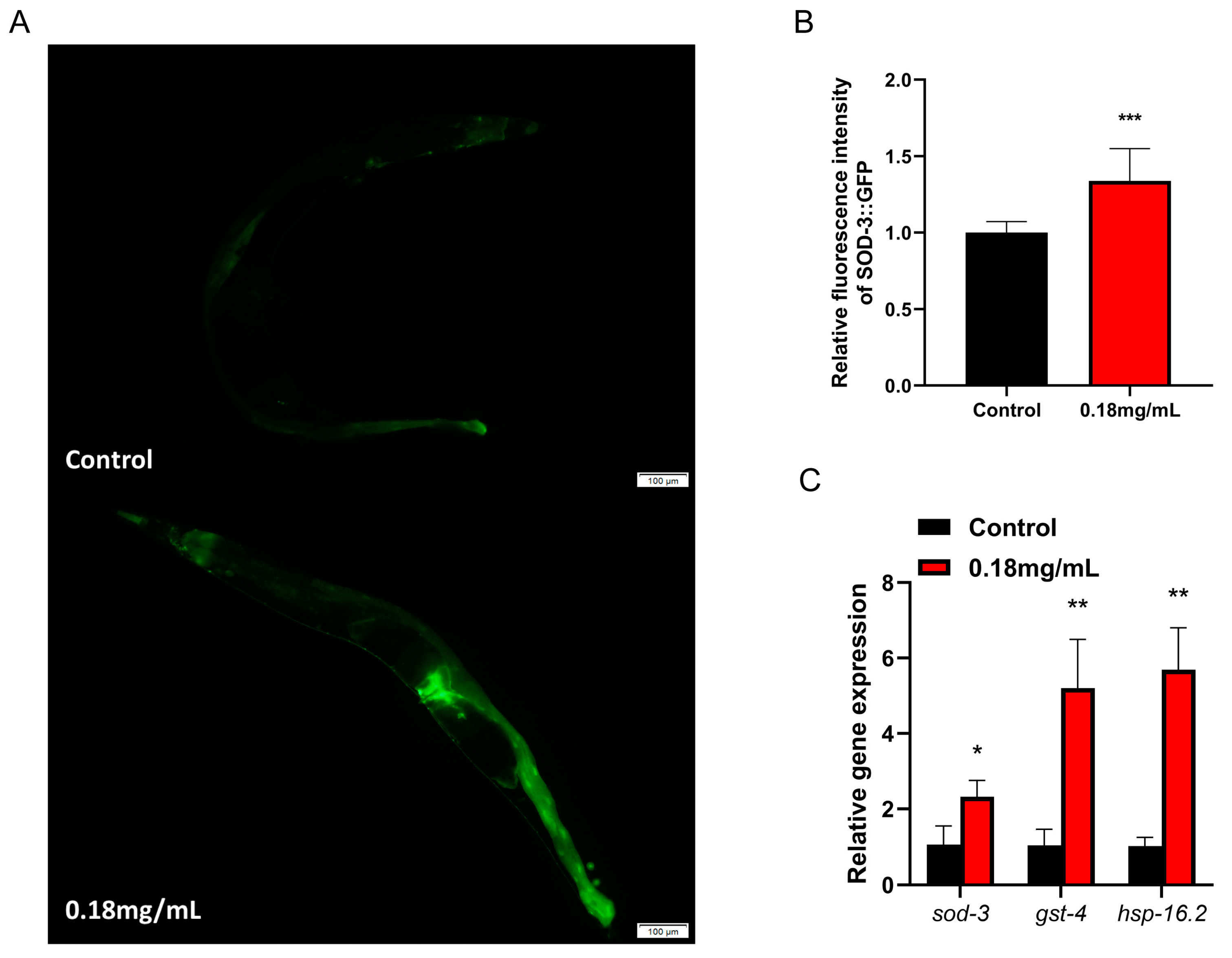
2.4. APPA Promotes the Expression and Activity of Antioxidant Defense Enzymes, Which Increase Survival and Reduces ROS Levels in Stress-Induced C. elegans
2.5. APPA Does Not Extend the Lifespan of C. elegans through Dietary Restriction
2.6. APPA Extended C. elegans Lifespan through the Insulin Signal Pathway
2.7. SKN-1 Was Required for APPA-Mediated Lifespan Extension
2.8. HSF-1 Was Required for APPA-Mediated Lifespan Extension
2.9. APPA Enhances the Expression of Stress Resistance Gene in C. elegans
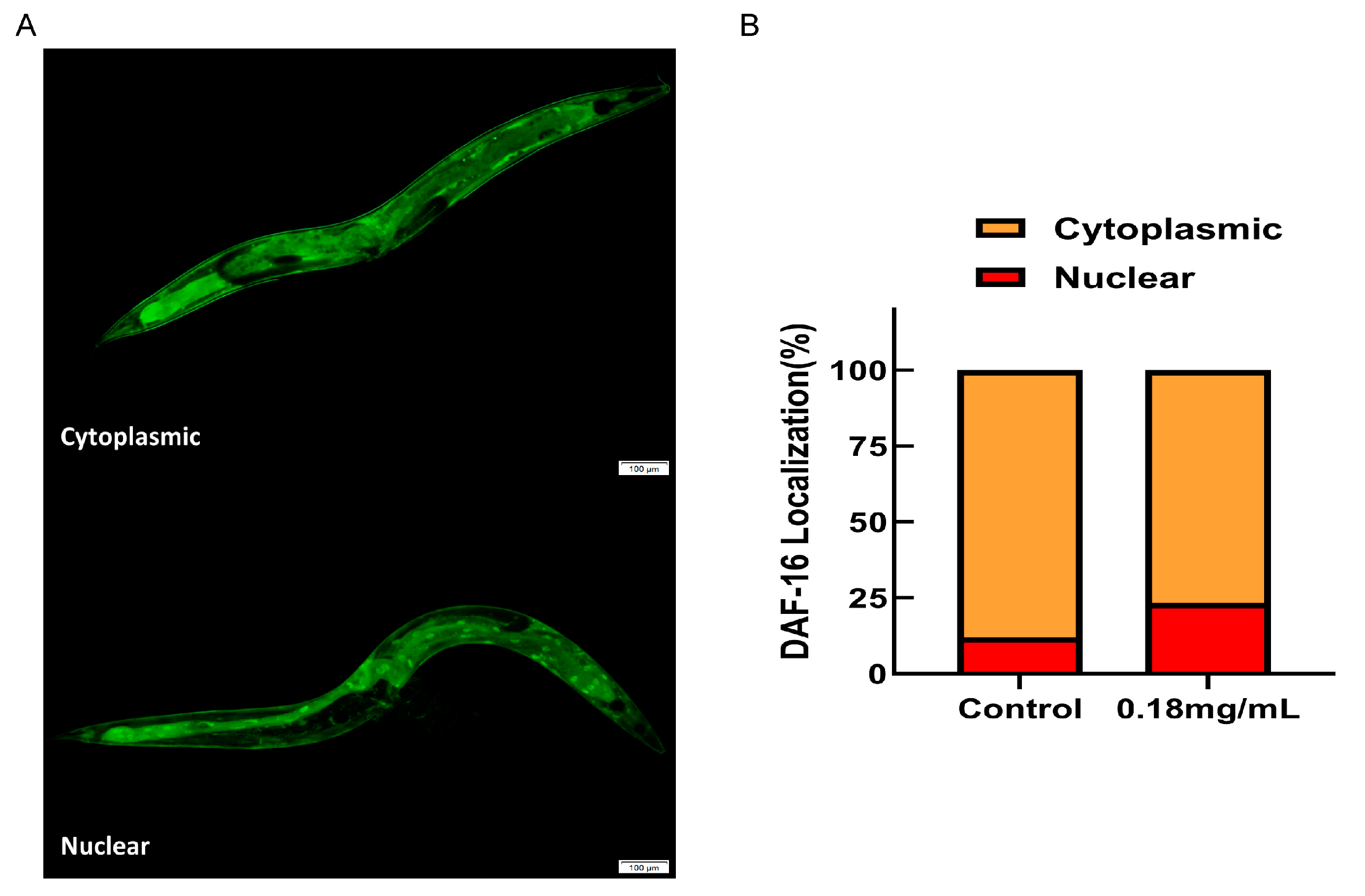
2.10. The Longevity Extension of APPA Depends on DAF-16/FOXO
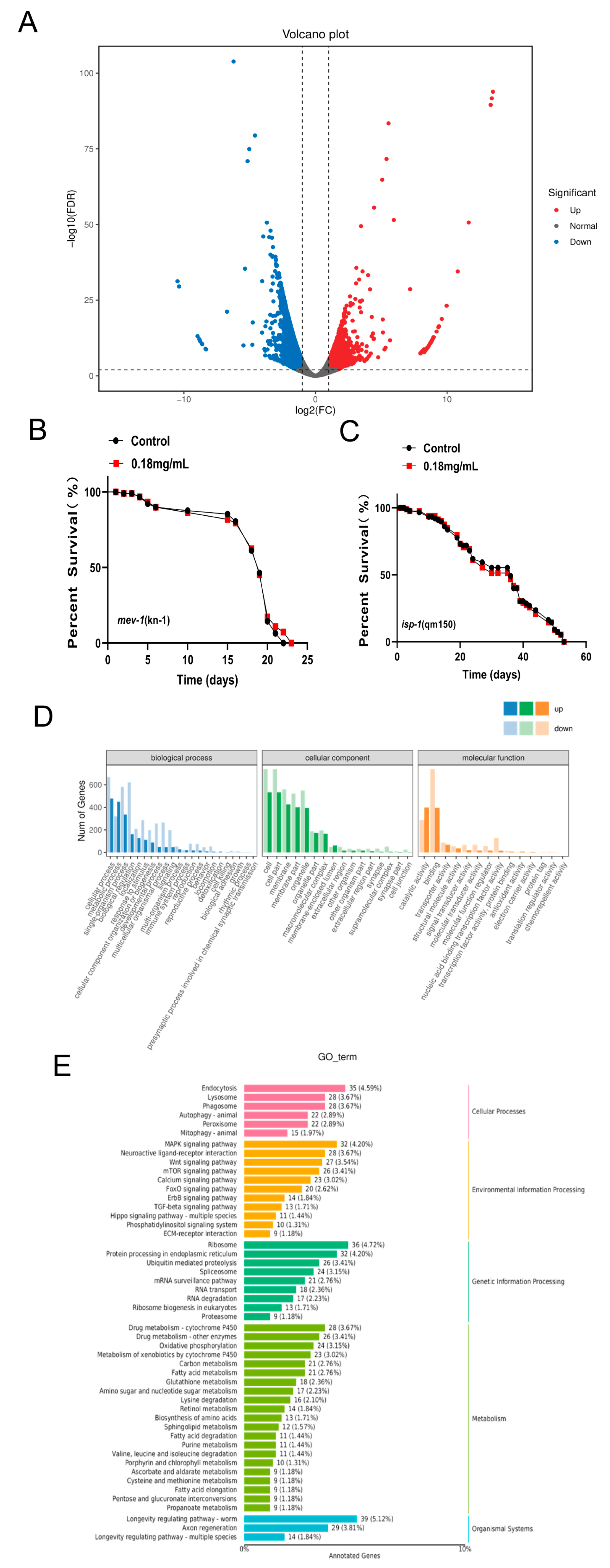
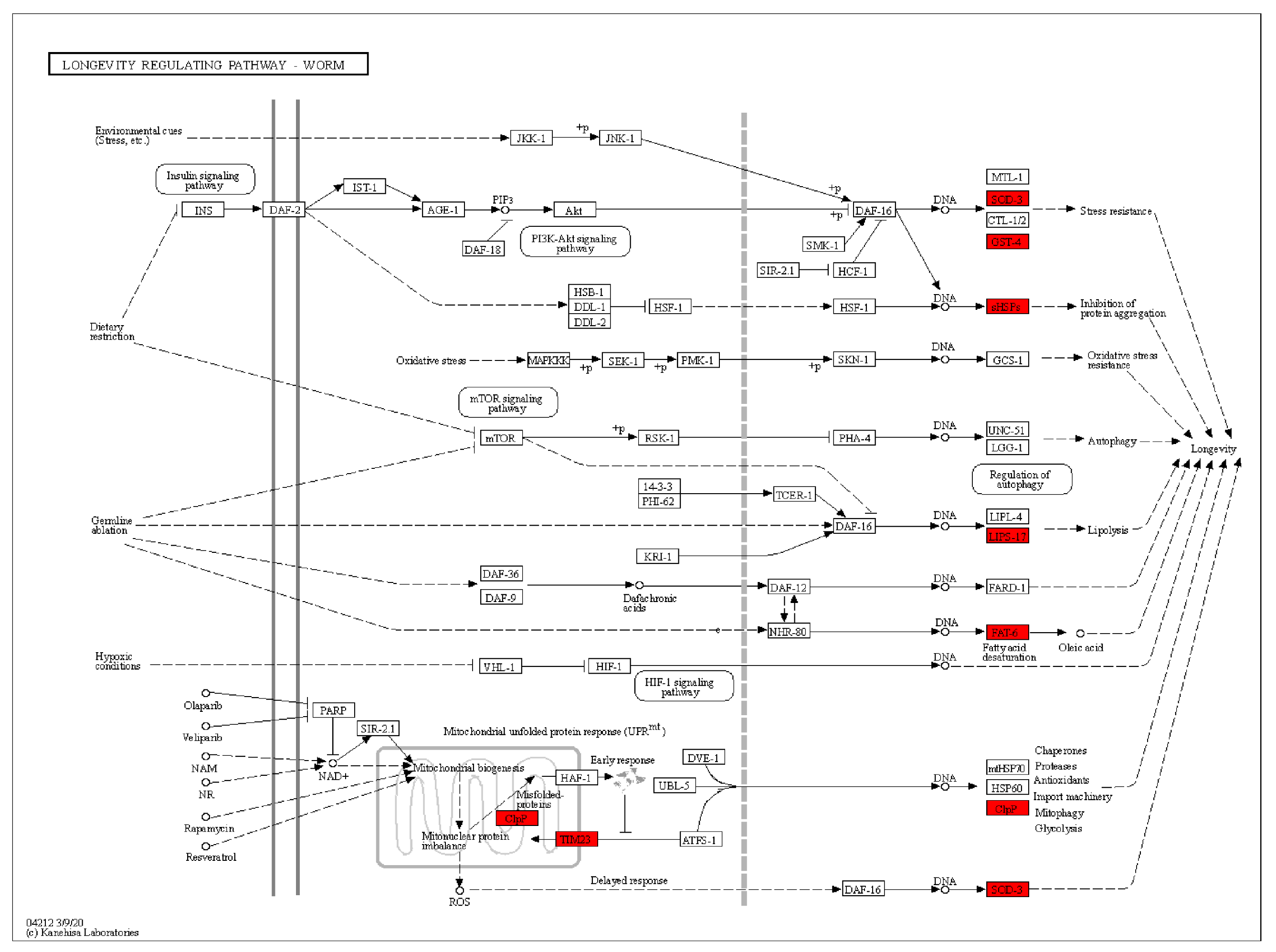
2.11. Effect of APPA on the Whole Genome Transcription Profile of C. elegans
2.12. Lifespan Extension by APPA Depends on the Mitochondrial Pathway
3. Discussion
3.1. Effects of APPA on Longevity and Physiological Indices of C. elegans
3.2. APPA Enhances the Stress Capacity of Nematodes
3.3. APPA Extended C. elegans Lifespan through the Insulin Signal Pathway
4. Materials and Methods
4.1. Strains
4.2. Lifespan Assay
4.3. OP50 Growth Assay
4.4. Lipofuscin Assay
4.5. Body Bend Assay
4.6. Pharyngeal Pumping Assay
4.7. Fertility Assay
4.8. Body Length and Body Width Assay
4.9. Oil Red O Staining
4.10. Quantification of Reactive Oxygen Species (ROS)
4.11. Heat Stress Assay
4.12. Oxidative Stress Assay
4.13. Fluorescence Measurement
4.14. DAF-16 Nuclear Localization Assays
4.15. Enzymatic Activity Assays
4.16. Quantitative Real-Time PCR
4.17. RNA Sequencing
4.18. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Govindhan, T.; Amirthalingam, M.; Duraisamy, K.; Muthusamy, V.; Periyakali, S.B.; Paramasivam, P.; Shinkichi, T.; Palanisamy, S. Geroprotective Effect of Levilactobacillus brevis and Weizmannia coagulans in Caenorhabditis elegans. Probiotics Antimicrob. Proteins 2023. [Google Scholar] [CrossRef]
- Man, X.; Bocen, C.; Kun, N.; Ziyu, L.; Fan, Y.; Yiqiang, X. Alpiniae oxyphylla fructus extract promotes longevity and stress resistance of C. elegans via DAF-16 and SKN-1. Front. Pharmacol. 2022, 13, 1034515. [Google Scholar]
- Wang, Y.; Shi, J.; Liu, K.; Wang, Y.; Xu, Y.; Liu, Y. Metabolomics and gene expression levels reveal the positive effects of teaseed oil on lifespan and aging process in Caenorhabditis elegans. Food Sci. Hum. Wellness 2023, 12, 1391–1401. [Google Scholar] [CrossRef]
- Duangjan, C.; Rangsinth, P.; Gu, X.; Zhang, S.; Wink, M.; Tencomnao, T. Glochidion zeylanicum leaf extracts exhibit lifespan extending and oxidative stress resistance properties in Caenorhabditis elegans via DAF-16/FoxO and SKN-1/Nrf-2 signaling pathways. Phytomedicine 2019, 64, 153061. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, X.; Lin, Y.; Sun, J.; Chen, S.; Wang, W.; Li, J. Insights into the Oxidative Stress Alleviation Potential of Enzymatically Prepared Dendrobium officinale Polysaccharides. Molecules 2023, 28, 3071. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Zhou, X.; Wang, T.; Zhan, H.; Wu, F.; Li, H.; Bian, P.; Xie, Z. Investigation into the communication between unheated and heat-stressed Caenorhabditis elegans via volatile stress signals. Sci. Rep. 2023, 13, 3225. [Google Scholar] [CrossRef] [PubMed]
- Ixchel, O.; Xareni, V.; Regina, B.; Silvestre, A. Vanillic acid improves stress resistance and substantially extends lifespan in Caenorhabditis elegans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2023, 78, 1100–1107. [Google Scholar]
- The C. elegans Sequencing Consortium. Genome Sequence of the Nematode C. elegans: A Platform for Investigating Biology. Science 1998, 282, 2012–2018. [Google Scholar] [CrossRef]
- Lant, B.; Storey, K.B. An Overview of Stress Response and Hypometabolic Strategies in Caenorhabditis elegans: Conserved and Contrasting Signals with the Mammalian System. Int. J. Biol. Sci. 2010, 6, 9–50. [Google Scholar] [CrossRef]
- Debabrata, D.; Swathi, A. Conserved insulin signaling in the regulation of oocyte growth, development, and maturation. Mol. Reprod. Dev. 2017, 84, 444–459. [Google Scholar]
- Agnieszka, L.; Milena, D.; Elzbieta, P.; Alicja, J.; Jozef, D. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. CMLS 2016, 73, 3221–3247. [Google Scholar]
- Fuse, Y.; Kobayashi, M. Conservation of the Keap1-Nrf2 System: An Evolutionary Journey through Stressful Space and Time. Molecules 2017, 22, 436. [Google Scholar] [CrossRef]
- White, J.G.; Southgate, E.; Thomson, J.N.; Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1986, 314, 1–340. [Google Scholar]
- Leung, M.C.K.; Williams, P.L.; Alexandre, B.; Catherine, A.; Helmcke, K.J.; Michael, A.; Meyer, J.N. Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology. Toxicol. Sci. Off. J. Soc. Toxicol. 2008, 106, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, J.; Chen, Y.; Chen, C.; Zhang, K.; Huang, X. The olfactory signal transduction for attractive odorants in Caenorhabditis elegans. Biotechnol. Adv. 2014, 32, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Lin, L.; Zhang, X.Y.; Wang, X.Y.; Ding, J. Covalent modification of soy protein hydrolysates by EGCG: Improves the emulsifying and antioxidant properties. Food Res. Int. 2023, 164, 112317. [Google Scholar] [CrossRef]
- Goudeau, J.; Bellemin, S.; Toselli-Mollereau, E.; Shamalnasab, M.; Chen, Y.Q.; Aguilaniu, H. Fatty Acid Desaturation Links Germ Cell Loss to Longevity Through NHR-80/HNF4 in C. elegans. PLoS Biol. 2011, 9, e1000599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.A.; Wu, B.X.; Liang, S.Y.; Min, D.Y.; Jiang, H.R. Insight of Silkworm Pupa Oil Regulating Oxidative Stress and Lipid Metabolism in Caenorhabditis elegans. Foods 2022, 11, 4084. [Google Scholar] [CrossRef] [PubMed]
- Guarente, L.; Kenyon, C. Genetic pathways that regulate ageing in model organisms. Nature 2000, 408, 255–262. [Google Scholar] [CrossRef]
- Lapierre, L.R.; Hansen, M. Lessons from C. elegans: Signaling pathways for longevity. Trends Endocrinol. Metab. 2012, 23, 637–644. [Google Scholar] [CrossRef]
- Shen, P.Y.; Yue, Y.R.; Park, Y. A living model for obesity and aging research: Caenorhabditis elegans. Crit. Rev. Food Sci. Nutr. 2018, 58, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Qianmin, L.; Bingbing, S.; Yingxiong, Z.; Huan, Y.; Ziyu, L.; Zhuo, W.; KitLeong, C.; Riming, H.; Saiyi, Z. Effect of Sodium Hyaluronate on Antioxidant and Anti-Ageing Activities in Caenorhabditis elegans. Foods 2023, 12, 1400. [Google Scholar]
- Yaoru, Y.; Jing, C.; Lu, A.; Tianci, H.; Wenbo, W.; Ziqi, C.; Lu, W.; Xuesong, X.; Zhizhuang, Z.; Xueqi, F.; et al. Knockdown of phosphatases of regenerating liver-1 prolongs the lifespan of Caenorhabditis elegans via activating DAF-16/FOXO. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2023, 37, e22844. [Google Scholar]
- Shanmughapriya, S.; Rajan, S.; Hoffman, N.E.; Zhang, X.; Guo, S.; Kolesar, J.E.; Hines, K.J.; Ragheb, J.; Jog, N.R.; Caricchio, R.; et al. Ca2+ signals regulate mitochondrial metabolism by stimulating CREB-mediated expression of the mitochondrial Ca2+ uniporter gene MCU. Sci. Signal. 2015, 8, ra23. [Google Scholar] [CrossRef] [PubMed]
- Miyabayashi, T.; Palfreyman, M.T.; Sluder, A.E.; Slack, F.; Sengupta, P. Expression and Function of Members of a Divergent Nuclear Receptor Family in Caenorhabditis elegans. Dev. Biol. 1999, 215, 314–331. [Google Scholar] [CrossRef]
- Zuoyi, S.; Jianxin, Q.; Hailiang, Y.; Menghao, G.; Mengting, S.; Shuyan, N.; Yunjing, L.; Qiang, L.; Yuying, X. Recombinant human metallothionein-III alleviates oxidative damage induced by copper and cadmium in Caenorhabditis elegans. J. Appl. Toxicol. JAT 2023, 43, 1242–1252. [Google Scholar]
- Labuschagne, C.F.; Broek, N.J.F.v.d.; Postma, P.; Berger, R.; Brenkman, A.B. A protocol for quantifying lipid peroxidation in cellular systems by F2-isoprostane analysis. PLoS ONE 2017, 8, e80935. [Google Scholar] [CrossRef]
- Yiman, H.; Zhaofa, X.; Qian, P.; Long, M. Casein kinase 1 gamma regulates oxidative stress response via interacting with the NADPH dual oxidase complex. PLoS Genet. 2023, 19, e1010740. [Google Scholar]
- Yu, C.; BingHao, H.; GuiLin, X.; YaTing, S.; Jie, Y.; Chen, X. Transient inhibition of mitochondrial function by chrysin and apigenin prolong longevity via mitohormesis in C. elegans. Free Radic. Biol. Med. 2023, 203, 24–33. [Google Scholar]
- Alugoju, P.; Babu, J.D.; Latha, P. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. IJCB 2015, 30, 11–26. [Google Scholar]
- Yanase, S.; Yasuda, K.; Ishii, N. Adaptive responses to oxidative damage in three mutants of Caenorhabditis elegans (age-1, mev-1 and daf-16) that affect life span. Mech. Ageing Dev. 2002, 123, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Hyung, A.J.; Keith, B.T. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003, 17, 1882–1893. [Google Scholar]
- Javier, G.-R.F.; Carmen, M.-F.; David, B.; Dmytro, K.; Xènia, S.; Ernest, N.; Mike, B.; Sebastian, H.; Antonio, M.-V.; Alberto, V.; et al. Genetic and cellular sensitivity of Caenorhabditis elegans to the chemotherapeutic agent cisplatin. Dis. Models Mech. 2018, 11, dmm033506. [Google Scholar]
- Hyung, A.J.; Kelly, V.; Michael, L.; Hideki, I.; Naoki, H.; Kunihiro, M.; Keith, B.T. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc. Natl. Acad. Sci. USA 2005, 102, 16275–16280. [Google Scholar]
- Hoeven, R.v.d.; McCallum, K.C.; Cruz, M.R.; Garsin, D.A. Ce-Duox1/BLI-3 generated reactive oxygen species trigger protective SKN-1 activity via p38 MAPK signaling during infection in C. elegans. PLoS Pathog. 2011, 7, e1002453. [Google Scholar]
- Jeong-Min, C.; Sarala, M.; Hyang-Rim, L.; Hyun-Min, P.; Mi-Kyoung, K. Role of the Nrf2-antioxidant system in cytotoxicity mediated by anticancer cisplatin: Implication to cancer cell resistance. Cancer Lett. 2008, 260, 96–108. [Google Scholar]
- Ren, D.; Villeneuve, N.F.; Jiang, T.; Wu, T.; Lau, A.; Toppin, H.A.; Zhang, D.D. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc. Natl. Acad. Sci. USA 2011, 108, 1433–1438. [Google Scholar] [CrossRef]
- Satomi, M.; Sonu, K.; Eduardo, C.; Thomas, V.Z. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar]
- Oberst, A.; Bender, C.; Green, D.R. Living with death: The evolution of the mitochondrial pathway of apoptosis in animals. Cell Death Differ. 2008, 15, 1139–1146. [Google Scholar] [CrossRef]
- Rendan, Y.; Yamei, L.; Yanli, W.; Jingjing, Z.; Qijing, F.; Jianlin, T.; Weizhen, L.; Xiaoju, Z.; Bin, L. NHR-80 senses the mitochondrial UPR to rewire citrate metabolism for lipid accumulation in Caenorhabditis elegans. Cell Rep. 2022, 38, 110206. [Google Scholar]
- Jin, P.C.Z.; Xiaqing, Z.; Lesly, T.; Morrigan, M.; Morteza, A.; Siming, M.; Daniel, P.; Matt, K.; Alaattin, K. Genetic perturbation of mitochondrial function reveals functional role for specific mitonuclear genes, metabolites, and pathways that regulate lifespan. GeroScience 2023. [Google Scholar] [CrossRef]
- Michael, V.R.J.; Siegfried, H. Reactive Oxygen Species and Aging in Caenorhabditis elegans: Causal or Casual Relationship? Antioxid. Redox Signal. 2010, 13, 1911–1953. [Google Scholar]
- Hwang, A.B.; Jeong, D.-E.; Lee, S.-J. Mitochondria and organismal longevity. Curr. Genom. 2012, 13, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.; Dillin, A. The trifecta of aging in Caenorhabditis elegans. Exp. Gerontol. 2006, 41, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.D.; Kennedy, B.K.; Matt, K. Genome-wide identification of conserved longevity genes in yeast and worms. Mech. Ageing Dev. 2007, 128, 106–111. [Google Scholar] [CrossRef]
- Kennedy, B.K. The genetics of ageing: Insight from genome-wide approaches in invertebrate model organisms. J. Intern. Med. 2008, 263, 142–152. [Google Scholar] [CrossRef] [PubMed]
- NaeCherng, Y.; ChiaYu, C.; YaXin, Z.; Inn, L. The Attenuation of Insulin/IGF-1 Signaling Pathway Plays a Crucial Role in the Myo-Inositol-Alleviated Aging in Caenorhabditis elegans. Int. J. Mol. Sci. 2023, 24, 6194. [Google Scholar]
- Yage, L.; Yu, J.; Rong, H.; Xuan, W.; Xiujuan, H.; Yonggang, L.; Peng, T. Polygonati Rhizoma Polysaccharide Prolongs Lifespan and Healthspan in Caenorhabditis elegans. Molecules 2023, 28, 2235. [Google Scholar]
- Prapaporn, J.; Sirin, S.; Warisra, W.; Kawita, C.; Pichnaree, K.; Tanatcha, S.; Prachayaporn, P.; Salinthip, T.; Prasert, S.; Krai, M. 2-Butoxytetrahydrofuran and Palmitic Acid from Holothuria scabra Enhance C. elegans Lifespan and Healthspan via DAF-16/FOXO and SKN-1/NRF2 Signaling Pathways. Pharmaceuticals 2022, 15, 1374. [Google Scholar]
- Esmaeil, D.; Yiqiang, Z.; Bahar, S.; Sivaramakrishna, Y.; Amirmansoor, H.; Maryam, D.; Mohammad, G.; Xiaoqin, T.; Scott, R.; Rueyling, L.; et al. Hydralazine induces stress resistance and extends C. elegans lifespan by activating the NRF2/SKN-1 signalling pathway. Nat. Commun. 2017, 8, 2223. [Google Scholar]
- Yue, W.; Jingjuan, Y.; Chengmei, X.; Qiuqi, L.; Yage, M.; Shenglan, Z.; Jiachen, Z.; Fei, S.; Qianqian, W.; Fengqin, F.; et al. Sea cucumber (Acaudina leucoprocta) peptides extended the lifespan and enhanced antioxidant capacity via DAF-16/DAF-2/SOD-3/OLD-1/PEPT-1 in Caenorhabditis elegans. Front. Nutr. 2022, 9, 1065145. [Google Scholar]
- Takahiro, O.; Yukihiro, K.; Dai, H.; Keith, B.T.; Masaki, M. Natural thioallyl compounds increase oxidative stress resistance and lifespan in Caenorhabditis elegans by modulating SKN-1/Nrf. Sci. Rep. 2016, 6, 21611. [Google Scholar]
- Jong, W.W.d.; Caspers, G.-J.; Leunissen, J.A.M. Genealogy of the α-crystallin—Small heat-shock protein superfamily. Int. J. Biol. Macromol. 1998, 22, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Valdez, M.M.; Clark, J.I.; Wu, G.J.S.; Muchowski, P.J. Functional similarities between the small heat shock proteins Mycobacterium tuberculosis HSP 16.3 and human alphaB-crystallin. Eur. J. Biochem. FEBS 2002, 269, 1806–1813. [Google Scholar] [CrossRef]
- Link, C.D.; Taft, A.; Kapulkin, V.; Duke, K.; Kim, S.; Fei, Q.; Wood, D.E.; Sahagan, B.G. Gene expression analysis in a transgenic Caenorhabditis elegans Alzheimer’s disease model. Neurobiol. Aging 2003, 24, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Söti, C.; Péter, C. Protein stress and stress proteins: Implications in aging and disease. J. Biosci. 2007, 32, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.A.; Lithgow, G.J. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell 2003, 2, 131–139. [Google Scholar] [CrossRef]
- Rea, S.L.; Wu, D.; Cypser, J.R.; Vaupel, J.W.; Johnson, T.E. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat. Genet. 2005, 37, 894–898. [Google Scholar] [CrossRef]
- Dhondt, I.; Petyuk, V.A.; Cai, H.; Vandemeulebroucke, L.; Vierstraete, A.; Smith, R.D.; Depuydt, G.; Braeckman, B.P. FOXO/DAF-16 Activation Slows Down Turnover of the Majority of Proteins in C. elegans. Cell Rep. 2016, 16, 3028–3040. [Google Scholar] [CrossRef]
- Franziska, P.; Andreia, T.-C.; Daniela, C.M.; Victoria, L.; Juliana, F.-F.; Marie, G.; Giovanna, B.; Wendy, R.; Patrícia, M.; Paul, K.T.L. GST-4-Dependent Suppression of Neurodegeneration in C. elegans Models of Parkinson’s and Machado-Joseph Disease by Rapeseed Pomace Extract Supplementation. Front. Neurosci. 2019, 13, 1091. [Google Scholar]
- Kai, H.; Tanja, H.; Jan, M.; Hannelore, D.; Uwe, W. Feeding a ROS-generator to Caenorhabditis elegans leads to increased expression of small heat shock protein HSP-16.2 and hormesis. Genes Nutr. 2009, 4, 59–67. [Google Scholar]
- Lin, T.; Ya, Z.Z.; Lv, H.; Zhong, J.; Lian, L.S.; Sheng, W.G.; Rong, L.H. Flavonol glycoside complanatoside A requires FOXO/DAF-16, NRF2/SKN-1, and HSF-1 to improve stress resistances and extend the life span of Caenorhabditis elegans. Front. Pharmacol. 2022, 13, 931886. [Google Scholar]
- Lee, D.; An, S.W.A.; Jung, Y.; Yamaoka, Y.; Ryu, Y.; Goh, G.Y.S.; Beigi, A.; Yang, J.-S.; Jung, G.Y.; Ma, D.K.; et al. MDT-15/MED15 permits longevity at low temperature via enhancing lipidostasis and proteostasis. PLoS Biol. 2019, 17, e3000415. [Google Scholar] [CrossRef] [PubMed]
- Kumsta, C.; Chang, J.T.; Schmalz, J.; Hansen, M. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat. Commun. 2017, 8, 14337. [Google Scholar] [CrossRef]
- Jia, W.; Peng, Q.; Su, L.; Yu, X.; Ma, C.W.; Liang, M.; Yin, X.; Zou, Y.; Huang, Z. Novel Bioactive Peptides from Meretrix meretrix Protect Caenorhabditis elegans against Free Radical-Induced Oxidative Stress through the Stress Response Factor DAF-16/FOXO. Mar. Drugs 2018, 16, 444. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Lin, D.; Cao, J.; Wang, L. APPA Increases Lifespan and Stress Resistance via Lipid Metabolism and Insulin/IGF-1 Signal Pathway in Caenorhabditis elegans. Int. J. Mol. Sci. 2023, 24, 13682. https://doi.org/10.3390/ijms241813682
Wang S, Lin D, Cao J, Wang L. APPA Increases Lifespan and Stress Resistance via Lipid Metabolism and Insulin/IGF-1 Signal Pathway in Caenorhabditis elegans. International Journal of Molecular Sciences. 2023; 24(18):13682. https://doi.org/10.3390/ijms241813682
Chicago/Turabian StyleWang, Shiyao, Dongfa Lin, Jiaofei Cao, and Liping Wang. 2023. "APPA Increases Lifespan and Stress Resistance via Lipid Metabolism and Insulin/IGF-1 Signal Pathway in Caenorhabditis elegans" International Journal of Molecular Sciences 24, no. 18: 13682. https://doi.org/10.3390/ijms241813682




