Exploring the Anti-Hypoxaemia Effect of Hydromethylthionine: A Prospective Study of Phase 3 Clinical Trial Participants
Abstract
:1. Introduction
2. Results
2.1. Population Characteristics at Baseline
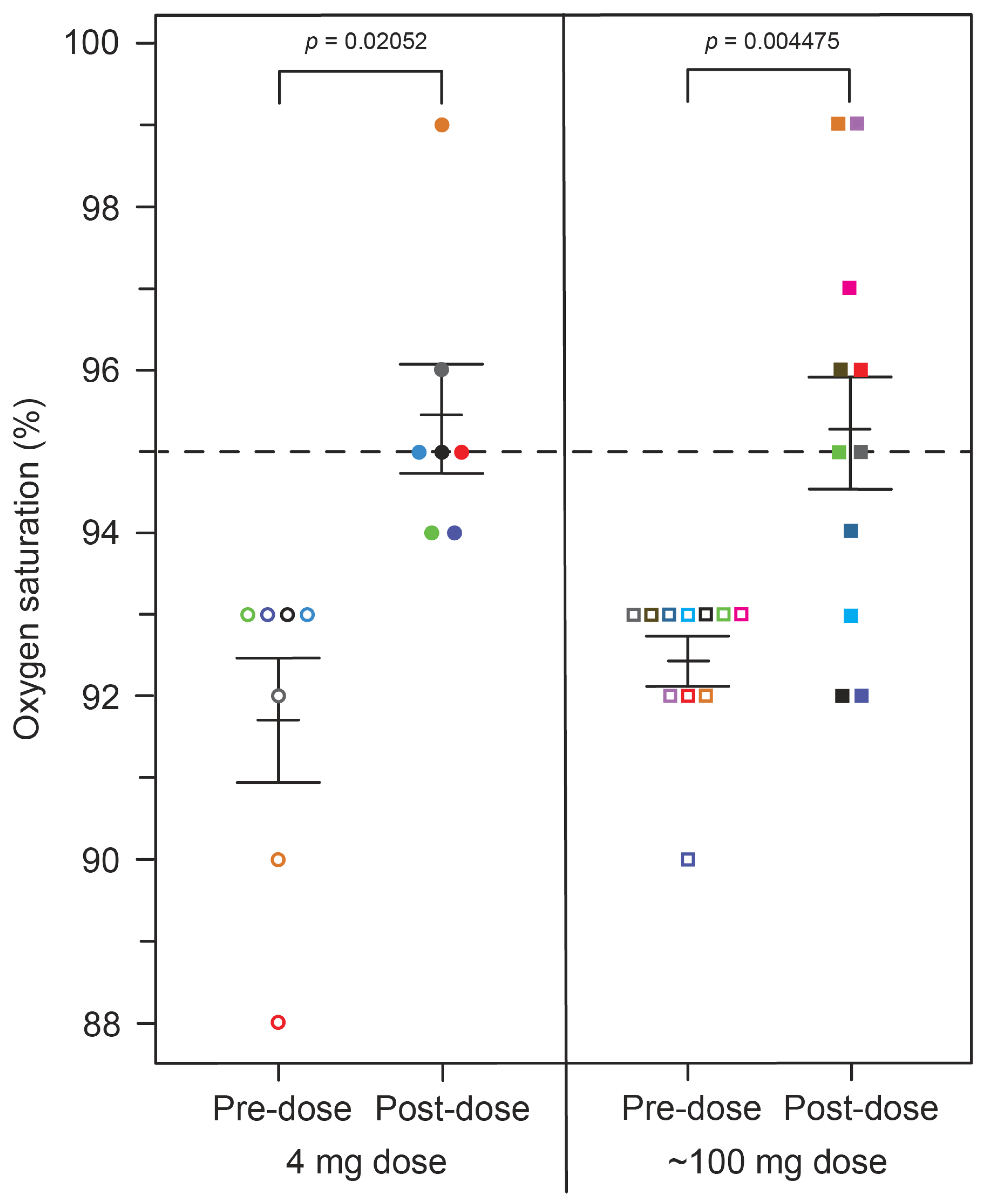
2.2. Prospective Clinical Study of Peripheral Oxygen Saturation and Methaemoglobinaemia in AD Patients Treated with HMTM
2.3. Computational Chemistry Model of HMT-Heme Effect
3. Discussion
3.1. Interpretation
3.2. Take-Home Points
4. Materials and Methods
4.1. Patients
4.2. Estimation of Effect of HMT Treatment on Oxygen–Haemoglobin Dissociation Curve
4.3. Statistics
4.4. Computational Modelling
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sarkar, M.; Niranjan, N.; Banyal, P.K. Mechanisms of hypoxemia. Lung India 2017, 34, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Skold, A.; Cosco, D.L.; Klein, R. Methemoglobinemia. South. Med. J. 2011, 104, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Harrington, C.R.; Storey, J.M.D.; Clunas, S.; Harrington, K.A.; Horsley, D.; Ishaq, A.; Kemp, S.J.; Larch, C.P.; Marshall, C.; Nicoll, S.L.; et al. Cellular models of aggregation-dependent template-directed proteolysis to characterize tau aggregation inhibitors for treatment of Alzheimer disease. J. Biol. Chem. 2015, 290, 10862–10875. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, T.C.; McCaffrey, J.; Storey, J.M.D.; Cheung, J.K.S.; Melis, V.; Horsley, D.; Harrington, C.R.; Wischik, C.M. Complex disposition of methylthioninium redox forms determines efficacy in tau aggregation inhibitor therapy for Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2015, 352, 110–118. [Google Scholar] [CrossRef]
- Linehan, J.H.; Vyprachticky, D.; Brantmeier, B.M.; Gan, Z.; Audi, S.H.; Bongard, R.D.; Gauthier, K.M.; Merker, M.P.; Lindemer, B.J.; Hoffmann, R.; et al. Pulmonary endothelial thiazine uptake: Separation of cell surface reduction from intracellular reoxidation. Am. J. Physiol. Cell. Mol. Physiol. 1997, 272, L673–L680. [Google Scholar] [CrossRef]
- Schelter, B.O.; Shiells, H.; Baddeley, T.C.; Rubino, C.M.; Ganesan, H.; Hammel, J.; Vuksanovic, V.; Staff, R.T.; Murray, A.D.; Bracoud, L.; et al. Concentration-dependent activity of hydromethylthionine on cognitive decline and brain atrophy in mild to moderate Alzheimer’s disease. J. Alzheimer’s Dis. 2019, 72, 931–946. [Google Scholar] [CrossRef]
- Gauthier, S.; Feldman, H.H.; Schneider, L.S.; Wilcock, G.K.; Frisoni, G.B.; Hardlund, J.H.; Moebius, H.J.; Bentham, P.; Kook, K.A.; Wischik, D.J.; et al. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: A randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet 2016, 388, 2873–2884. [Google Scholar] [CrossRef]
- Wilcock, G.K.; Gauthier, S.; Frisoni, G.B.; Jia, J.; Hardlund, J.H.; Moebius, H.J.; Bentham, P.; Kook, K.A.; Schelter, B.O.; Wischik, D.J.; et al. Potential of low dose leuco-methylthioninium bis(hydromethanesulphonate) (LMTM) monotherapy for treatment of mild Alzheimer’s disease: Cohort analysis as modified primary outcome in a Phase III clinical trial. J. Alzheimer’s Dis. 2018, 61, 435–457. [Google Scholar] [CrossRef]
- Al-Hilaly, Y.K.; Pollack, S.J.; Rickard, J.E.; Simpson, M.; Raulin, A.-C.; Baddeley, T.; Schellenberger, P.; Storey, J.M.; Harrington, C.R.; Wischik, C.M.; et al. Cysteine-independent inhibition of Alzheimer’s disease-like paired helical filament assembly by leuco-methylthioninium (LMT). J. Mol. Biol. 2018, 430, 4119–4131. [Google Scholar] [CrossRef]
- Curry, S. Methemoglobinemia. Ann. Emerg. Med. 1982, 11, 214–221. [Google Scholar] [CrossRef]
- Blank, O.; Davioud-Charvet, E.; Elhabiri, M. Interactions of the antimalarial drug methylene blue with methemoglobin and heme targets in Plasmodium falciparum: A physico-biochemical study. Antioxid. Redox Signal. 2012, 17, 544–554. [Google Scholar] [CrossRef]
- Yubisui, T.; Takeshita, M.; Yoneyama, Y. Reduction of methemoglobin through flavin at the physiological concentration by NADPH-flavin reductase of human erythrocytes. J. Biochem. 1980, 87, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Alamdari, D.H.; Moghaddam, A.B.; Amini, S.; Keramati, M.R.; Zarmehri, A.M.; Alamdari, A.H.; Damsaz, M.; Banpour, H.; Yarahmadi, A.; Koliakos, G. Application of methylene blue -vitamin C –N-acetyl cysteine for treatment of critically ill COVID-19 patients, report of a phase-I clinical trial. Eur. J. Pharmacol. 2020, 885, 173494. [Google Scholar] [CrossRef] [PubMed]
- Gendrot, M.; Andreani, J.; Duflot, I.; Boxberger, M.; Le Bideau, M.; Mosnier, J.; Jardot, P.; Fonta, I.; Rolland, C.; Bogreau, H.; et al. Methylene blue inhibits replication of SARS-CoV-2 in vitro. Int. J. Antimicrob. Agents 2020, 56, 106202. [Google Scholar] [CrossRef]
- Cagno, V.; Medaglia, C.; Cerny, A.; Cerny, T.; Zwygart, A.C.-A.; Cerny, E.; Tapparel, C. Methylene blue has a potent antiviral activity against SARS-CoV-2 and H1N1 influenza virus in the absence of UV-activation in vitro. Sci. Rep. 2021, 11, 14295. [Google Scholar] [CrossRef] [PubMed]
- Naymagon, L.; Berwick, S.; Kessler, A.; Lancman, G.; Gidwani, U.; Troy, K. The emergence of methemoglobinemia amidst the COVID-19 pandemic. Am. J. Hematol. 2020, 95, E196–E197. [Google Scholar] [CrossRef]
- Hamidi-Alamdari, D.; Hafizi-Lotfabadi, S.; Bagheri-Moghaddam, A.; Safari, H.; Mozdourian, M.; Javidarabshahi, Z.; Peivandi-Yazdi, A.; Ali-Zeraati, A.; Sedaghat, A.; Poursadegh, F.; et al. Methylene blue for treatment of hospitalized COVID-19 patients: A randomized, controlled, open-label clinical trial, phase 2. Rev. Investig. Clin. 2021, 73, 190–198. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.C.; Cobb, C.E. Reduction and uptake of methylene blue by human erythrocytes. Am. J. Physiol. Cell Physiol. 2004, 286, 1390–1398. [Google Scholar] [CrossRef]
- Schirmer, R.H.; Adler, H.; Pickhardt, M.; Mandelkow, E. “Lest we forget you—Methylene blue...”. Neurobiol. Aging 2011, 32, 2325.e7–2325.e16. [Google Scholar] [CrossRef]
- Jørgensen, C.K. Absorption Spectra and Chemical Bonding in Complexes; Pergamon Press: London, UK; New York, NY, USA; Paris, France, 1962; 352p. [Google Scholar] [CrossRef]
- Jørgensen, C.K. Oxidation Numbers and Oxidation States; Springer: Berlin/Heidelberg, Germany, 1969. [Google Scholar] [CrossRef]
- Perutz, M.F. Proteins and Nucleic Acids-Structure and Function; Elsevier Publishing Co.: Amsterdam, The Netherlands; New York, NY, USA, 1962. [Google Scholar]
- Lima, F.A.; Penfold, T.J.; van der Veen, R.M.; Reinhard, M.; Abela, R.; Tavernelli, I.; Rothlisberger, U.; Benfatto, M.; Milne, C.J.; Chergui, M. Probing the electronic and geometric structure of ferric and ferrous myoglobins in physiological solutions by Fe K-edge absorption spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 1617–1631. [Google Scholar] [CrossRef]
- Bohr, C.; Hasselbalch, K.; Krogh, A. Ueber einen in biologischer Beziehung wichtigen Einfluss, den die Kohlensäurespannung des Blutes auf dessen Sauerstoffbindung übt. Skand. Arch. Physiol. 1904, 16, 402–412. [Google Scholar] [CrossRef]
- Darling, R.C.; Roughton, F.J.W. The effect of methemoglobin on the equilibrium between oxygen and hemoglobin. Am. J. Physiol. Content 1942, 137, 56–68. [Google Scholar] [CrossRef]
- Davies, A.; Moores, C. The Respiratory System: Basic Science and Clinical Conditions, 2nd ed.; Churchill Livingstone: London, UK, 2010. [Google Scholar]
- Bodansky, O. Methemoglobinemia and methemoglobin-producing compounds. Pharmacol. Rev. 1951, 3, 144–196. [Google Scholar] [PubMed]
- Bojadzic, D.; Alcazar, O.; Buchwald, P. Methylene blue inhibits the SARS-CoV-2 spike–ACE2 protein-protein interaction–a mechanism that can contribute to its antiviral activity against COVID-19. Front. Pharmacol. 2021, 11, 600372. [Google Scholar] [CrossRef]
- Mohr, H.; Bachmann, B.; Klein-Struckmeier, A.; Lambrecht, B. Virus inactivation of blood products by phenothiazine dyes and light. Photochem. Photobiol. 1997, 65, 441–445. [Google Scholar] [CrossRef]
- Müller-Breitkreutz, K.; Mohr, H. Hepatitis C and human immunodeficiency virus RNA degradation by methylene blue/light treatment of human plasma. J. Med. Virol. 1998, 56, 239–245. [Google Scholar] [CrossRef]
- Gourlain, H.; Buneaux, F.; Borron, S.W.; Gouget, B.; Levillain, P. Interference of methylene blue with CO-Oximetry of hemoglobin derivatives. Clin. Chem. 1997, 43, 1078–1080. [Google Scholar] [CrossRef]
- Barker, S.J.; Curry, J.; Redford, D.; Morgan, S. Measurement of carboxyhemoglobin and methemoglobin by pulse oximetry: A human volunteer study. Anesthesiology 2006, 105, 892–897. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE). 2020.09; Chemical Computing Group ULC, 1010 Sherbrooke St. West, Suite #910, Montreal, QC, Canada. 2020. Available online: https://eur03.safelinks.protection.outlook.com/?url=https%3A%2F%2Fwww.chemcomp.com%2F&data=05%7C01%7Cc.harrington%40abdn.ac.uk%7C104c77a7b2114334d26a08dba487e1c1%7C8c2b19ad5f9c49d490773ec3cfc52b3f%7C0%7C0%7C638284675731396605%7CUnknown%7CTWFpbGZsb3d8eyJWIjoiMC4wLjAwMDAiLCJQIjoiV2luMzIiLCJBTiI6Ik1haWwiLCJXVCI6Mn0%3D%7C3000%7C%7C%7C&sdata=8VVMeXeGwO6XEKZLL15Q%2B%2Fehf55nohbYUnPliYXLIE4%3D&reserved=0 (accessed on 2 April 2023).
- Park, S.-Y.; Yokoyama, T.; Shibayama, N.; Shiro, Y.; Tame, J.R.H. 1.25 Å resolution crystal structures of human haemoglobin in the oxy, deoxy and carbonmonoxy forms. J. Mol. Biol. 2006, 360, 690–701. [Google Scholar] [CrossRef]





| Characteristic | HMTM Total (n = 18) |
|---|---|
| Age (year) | |
| Mean (SD) | 76.1 (7.8) |
| Median (range) | 77.5 (70–80) |
| Sex, n (%) | |
| Male | 7 (39%) |
| Female | 11 (61%) |
| Race, n (%) | |
| Black or African American | 2 (11%) |
| White | 16 (89%) |
| Subject | Clinical Respiratory/Ventilation Type | Aggravating Clinical Conditions a |
|---|---|---|
| 1 | Sleep apnoea | Hypothyroid/diabetic |
| 2 | Insomnia | Often a sign of obstructive sleep apnoea or other mild hypoxia conditions such as paroxysmal nocturnal dyspnoea |
| 3 | Hypertension/left bundle branch block (LBBB); left ventricular hypertrophy (LVH) | |
| 4 | Asbestosis | |
| 5 | Oedema | Often a sign of right heart failure or congestive cardiac failure/may also be simple sedentary dependent oedema |
| 6 | Hypertension/LBBB/LVH | |
| 7 | Acute myocardial infarction/hypertension | |
| 8 | Hypertension | |
| 9 | Hypertension/coronary artery disease with angioplasty and stent insertion | |
| 10 b | ||
| 11 b | ||
| 12 | Asthma (childhood) | |
| 13 | Sleep apnoea with uvulectomy | |
| 14 | Bronchitis/seasonal allergies/anaemia | |
| 15 | Acute myocardial infarction with stent insertion | |
| 16 b | ||
| 17 | Transient ischaemic attack/hypertension | |
| 18 | Asthma (childhood)/intermittent angioedema/occasional insomnia/pneumonia | Hypertension/hypothyroid/syncope/tachycardia/sepsis |
| 4 mg | 100 mg | |||
|---|---|---|---|---|
| Pre-Dose | Post-Dose | Pre-Dose | Post-Dose | |
| Oxygen saturation, mean % (SE) | 91.71 (0.75) | 95.43 (0.65) | 92.45 (0.28) | 95.27 (0.74) |
| metHb, mean % (SE) | 0.70 (0.195) | 0.87 (0.198) | 0.90 (0.220) | 0.65 (0.154) |
| Visit | Oxygen Saturation | p-Value |
|---|---|---|
| Baseline (1 h), mean % (SE) | 92.167 (0.336) | |
| Baseline (4 h), mean % (SE) | 95.333 (0.505) | 0.000139 |
| Week 2, mean % (SE) | 95.400 (0.798) | 0.0034 |
| Week 6, mean % (SE) | 95.571 (0.618) | 0.0005 |
| Visit | HMTM Dose (mg/day) | |||
|---|---|---|---|---|
| 8 | 150 | 200 | 250 | |
| A Patients with SpO2 < 94% at baseline | ||||
| Baseline, mean % (SE) | 0.709 (0.017) | 0.721 (0.029) | 0.702 (0.025) | 0.696 (0.028) |
| 4 h, mean % (SE) | 0.733 (0.018) | 0.781 (0.030) | 0.760 (0.023) | 0.758 (0.028) |
| Week 2, mean % (SE) | 0.732 (0.018) | 0.835 (0.032) | 0.849 (0.027) | 0.845 (0.033) |
| Week 6, mean % (SE) | 0.769 (0.019) | 0.888 (0.034) | 0.873 (0.030) | 0.858 (0.033) |
| Visit | HMTM dose (mg/day) | |||
| 8 | 150 | 200 | 250 | |
| B All patients | ||||
| Baseline, mean % ± SE (N) | 0.709 ± 0.017 (754) | 0.721 ± 0.029 (268) | 0.702 ± 0.025 (398) | 0.696 ± 0.028 (265) |
| 4 h, mean % ± SE (N) | 0.732 ± 0.017 (754) | 0.7811 ± 0.029 (267) | 0.760 ± 0.023 (398) | 0.758 ± 0.028 (263) |
| p-value | 0.09732 | 0.02212 | 0.01967 | 0.005868 |
| Week 2, mean % ± SE (N) | 0.733 ± 0.018 (721) | 0.831 ± 0.032 (248) | 0.849 ± 0.027 (355) | 0.837 ± 0.034 (239) |
| p-value | 0.1326 | <0.0001 | <0.0001 | <0.0001 |
| Week 6, mean % ± SE (N) | 0.770 ± 0.019 (721) | 0.889 ± 0.034 (248) | 0.873 ± 0.030 (355) | 0.851 ± 0.033 (239) |
| p-value | 0.0006916 | <0.0001 | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arastoo, M.; Mazanetz, M.P.; Miller, S.; Shiells, H.; Hull, C.; Robinson, K.; Storey, J.M.D.; Harrington, C.R.; Wischik, C.M. Exploring the Anti-Hypoxaemia Effect of Hydromethylthionine: A Prospective Study of Phase 3 Clinical Trial Participants. Int. J. Mol. Sci. 2023, 24, 13747. https://doi.org/10.3390/ijms241813747
Arastoo M, Mazanetz MP, Miller S, Shiells H, Hull C, Robinson K, Storey JMD, Harrington CR, Wischik CM. Exploring the Anti-Hypoxaemia Effect of Hydromethylthionine: A Prospective Study of Phase 3 Clinical Trial Participants. International Journal of Molecular Sciences. 2023; 24(18):13747. https://doi.org/10.3390/ijms241813747
Chicago/Turabian StyleArastoo, Mohammad, Michael P. Mazanetz, Sonya Miller, Helen Shiells, Claire Hull, Keith Robinson, John M. D. Storey, Charles R. Harrington, and Claude M. Wischik. 2023. "Exploring the Anti-Hypoxaemia Effect of Hydromethylthionine: A Prospective Study of Phase 3 Clinical Trial Participants" International Journal of Molecular Sciences 24, no. 18: 13747. https://doi.org/10.3390/ijms241813747
APA StyleArastoo, M., Mazanetz, M. P., Miller, S., Shiells, H., Hull, C., Robinson, K., Storey, J. M. D., Harrington, C. R., & Wischik, C. M. (2023). Exploring the Anti-Hypoxaemia Effect of Hydromethylthionine: A Prospective Study of Phase 3 Clinical Trial Participants. International Journal of Molecular Sciences, 24(18), 13747. https://doi.org/10.3390/ijms241813747









