Actinidia deliciosa Extract as a Promising Supplemental Agent for Hepatic and Renal Complication-Associated Type 2 Diabetes (In Vivo and In Silico-Based Studies)
Abstract
:1. Introduction
2. Results
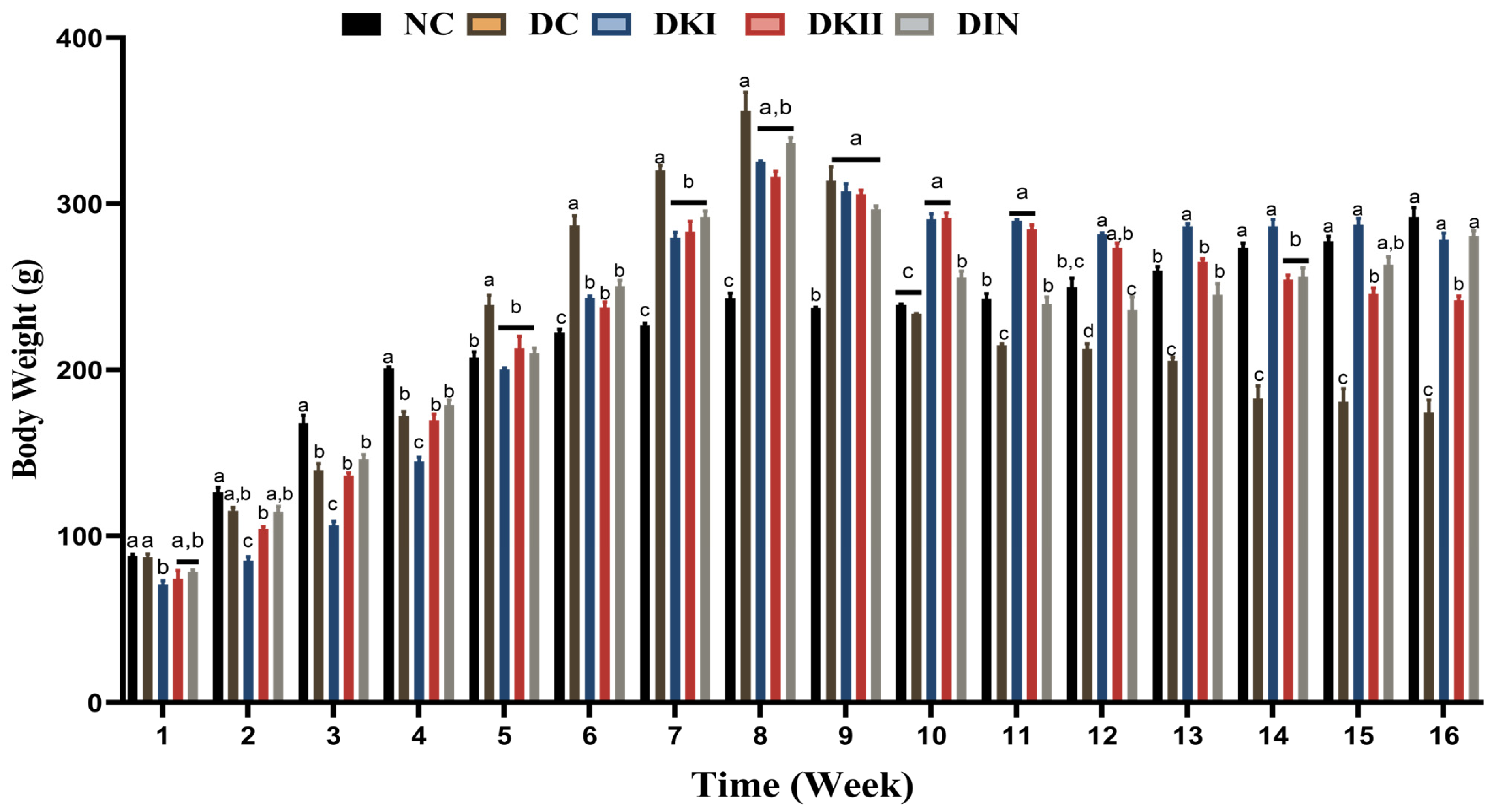
2.1. Body Weight Changes after Administration of Kiwi Extract
2.2. Effect of Kiwi Extract Administration on Serum Glucose and Insulin Levels of T2DM-Induced Rats
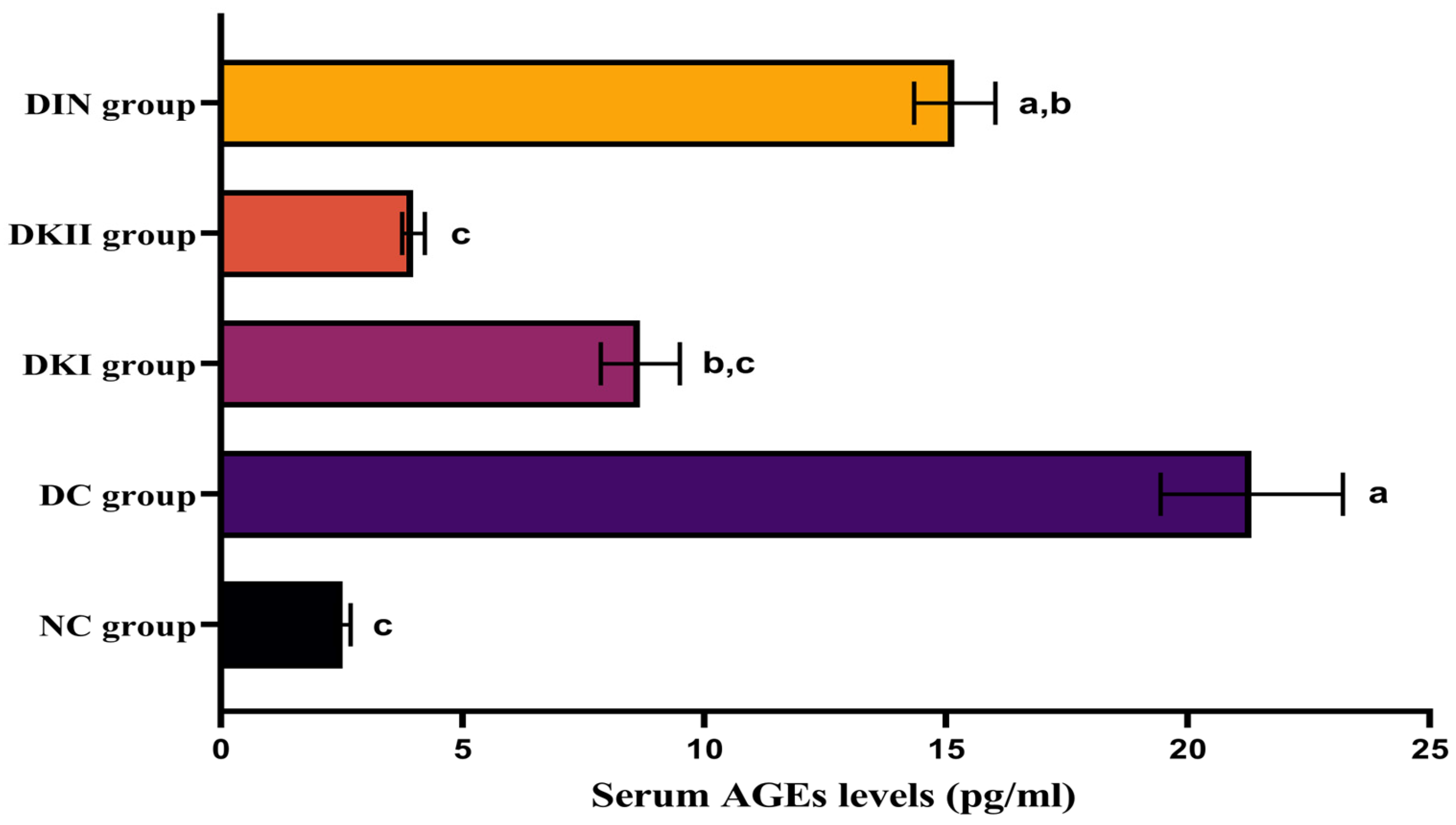
2.3. Effect of Kiwi Extract Administration on Serum AGEs Levels
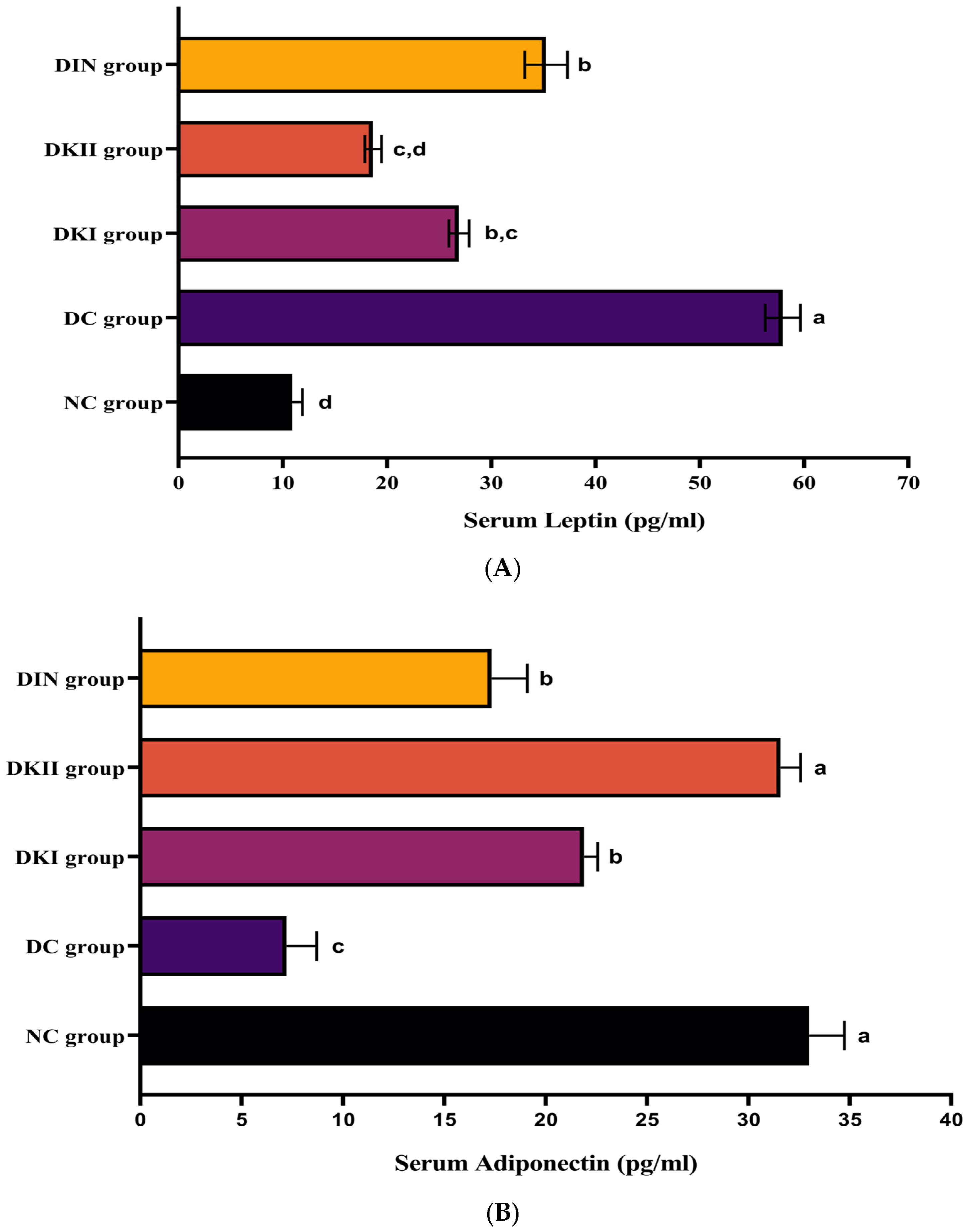
2.4. Effect of Kiwi Extract Administration on Serum Adipokines Levels
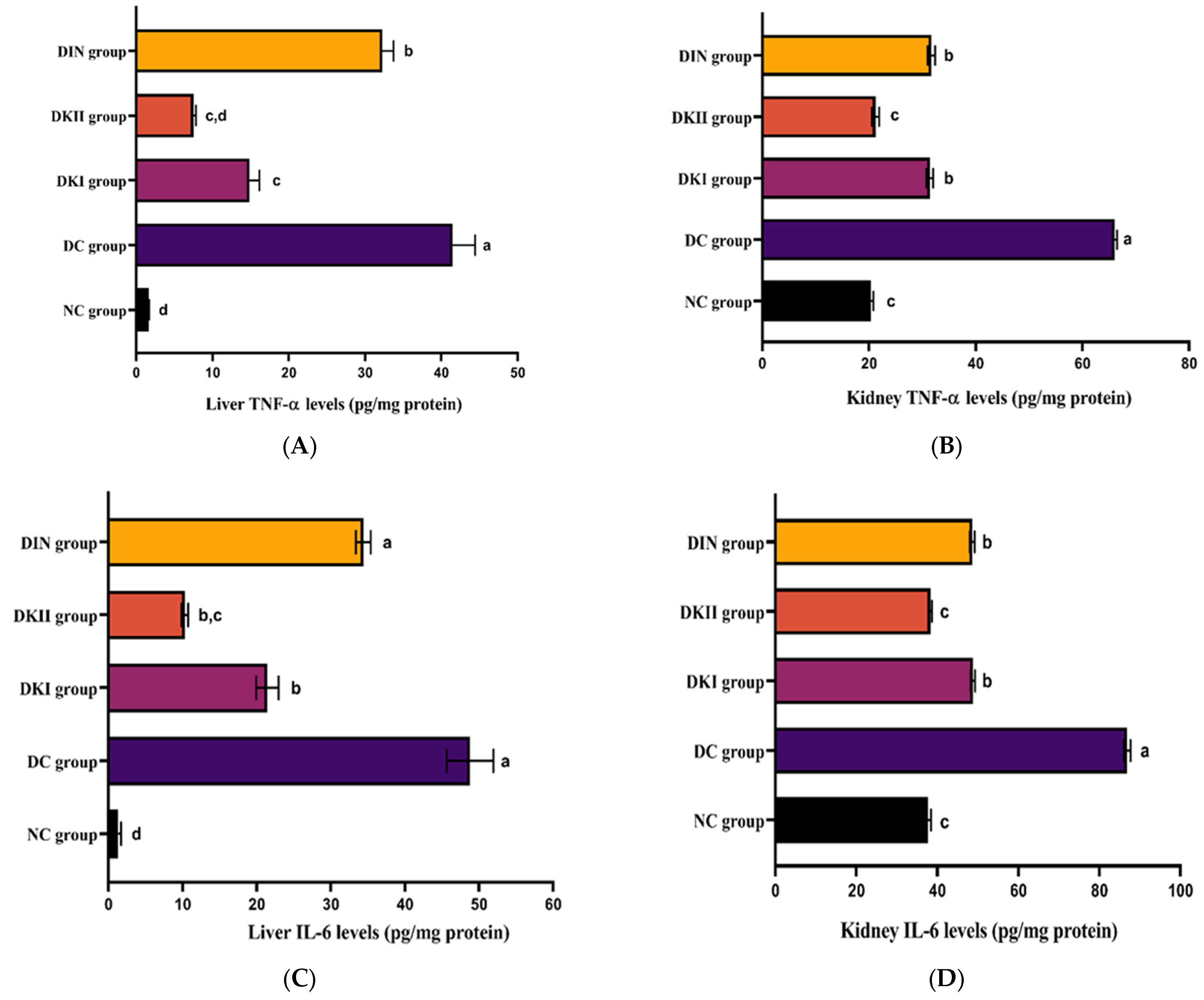
2.5. Kiwi Extract Attenuates Inflammation in the Hepatic and Renal Tissues of T2DM-Induced Rats
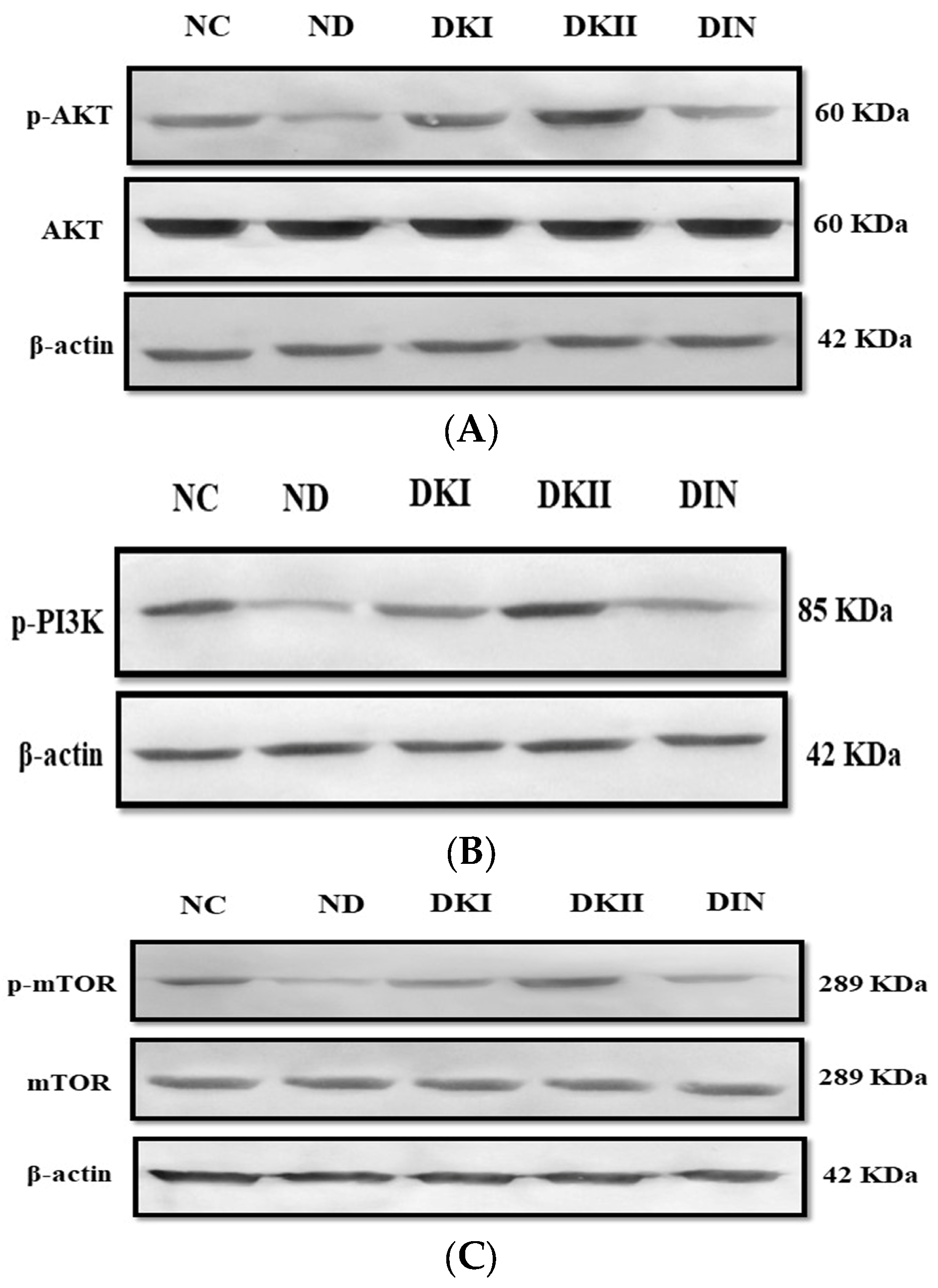
2.6. Kiwi Extract Promotes the Phosphorylation of the AKT Signaling Pathway in Liver of T2DM-Induced Rats
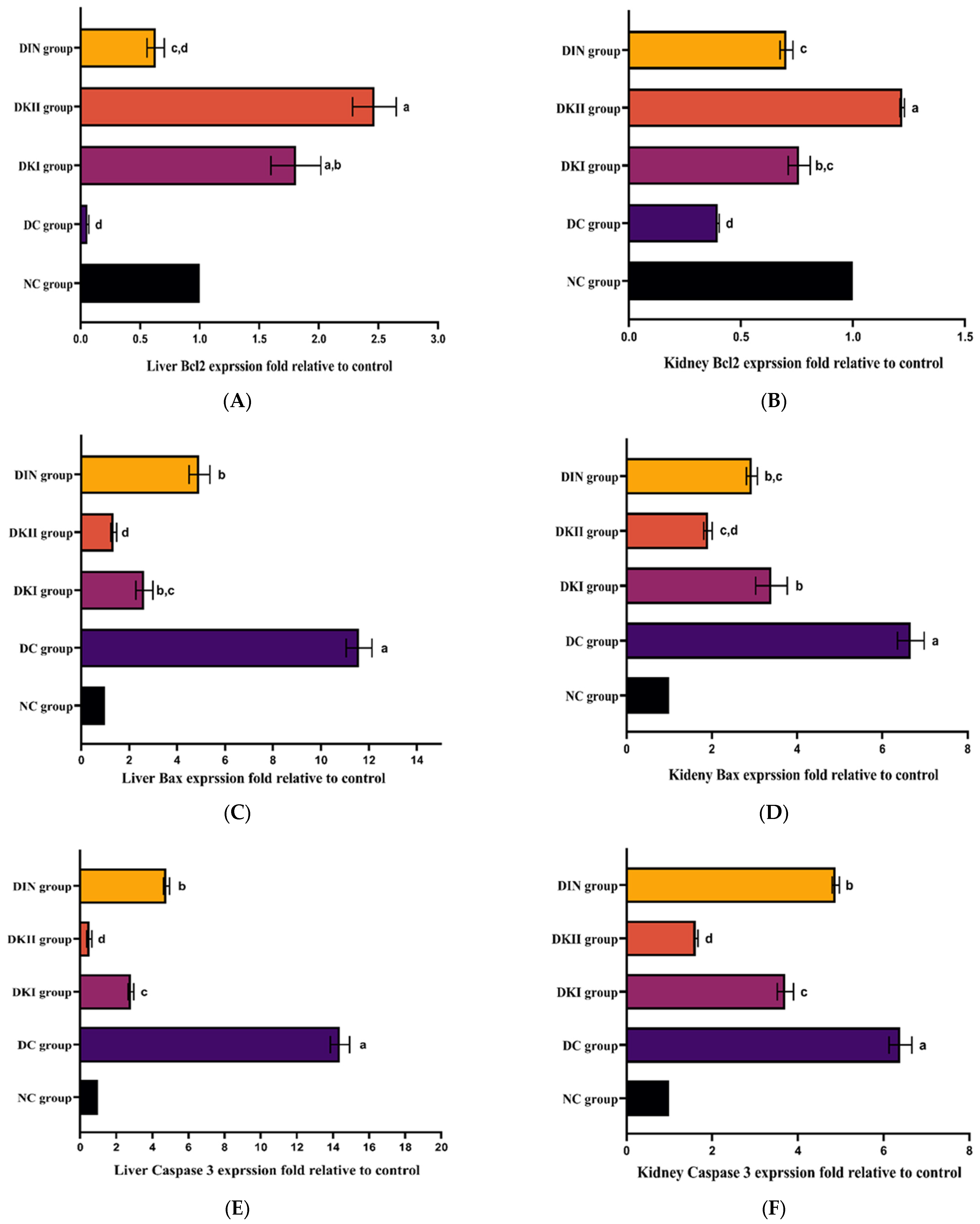
2.7. Effect of Kiwi Extract on an Apoptotic Pathway in the Liver and Kidney of T2DM-Induced Rats
2.8. Evaluation of the Antioxidant Upshot of Kiwi Extract Administration in HFD/STZ-Induced Rats
2.9. Evaluation of Kiwi Extract Upshot on Serum Lipid Profile in HFD/STZ-Induced Rats
2.10. Evaluation of Kiwi Extract Upshot on Serum Renal and Hepatic Functions in HFD/STZ-Induced Rats
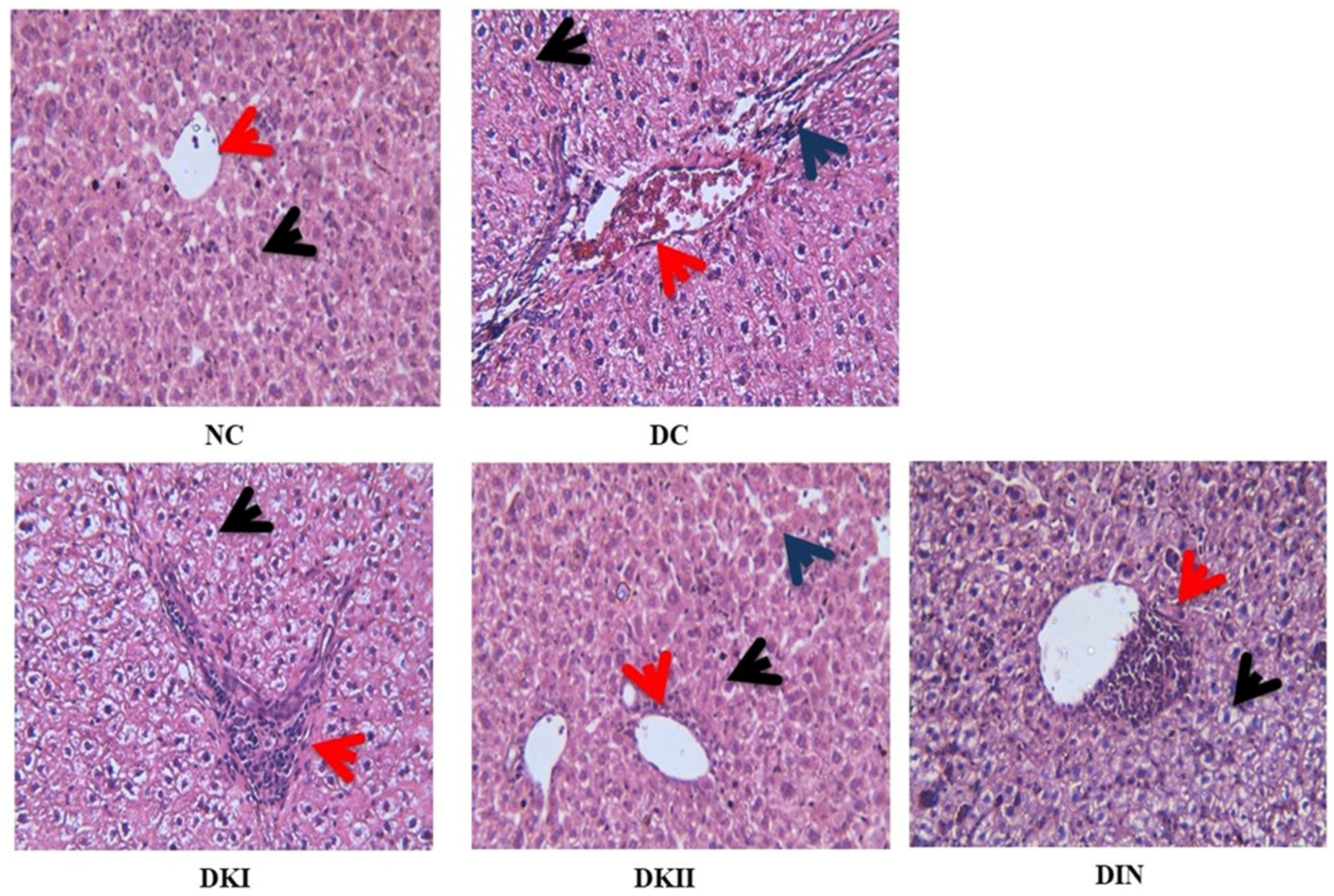
2.11. Histopathological Analysis of the Liver
2.12. Molecular Docking
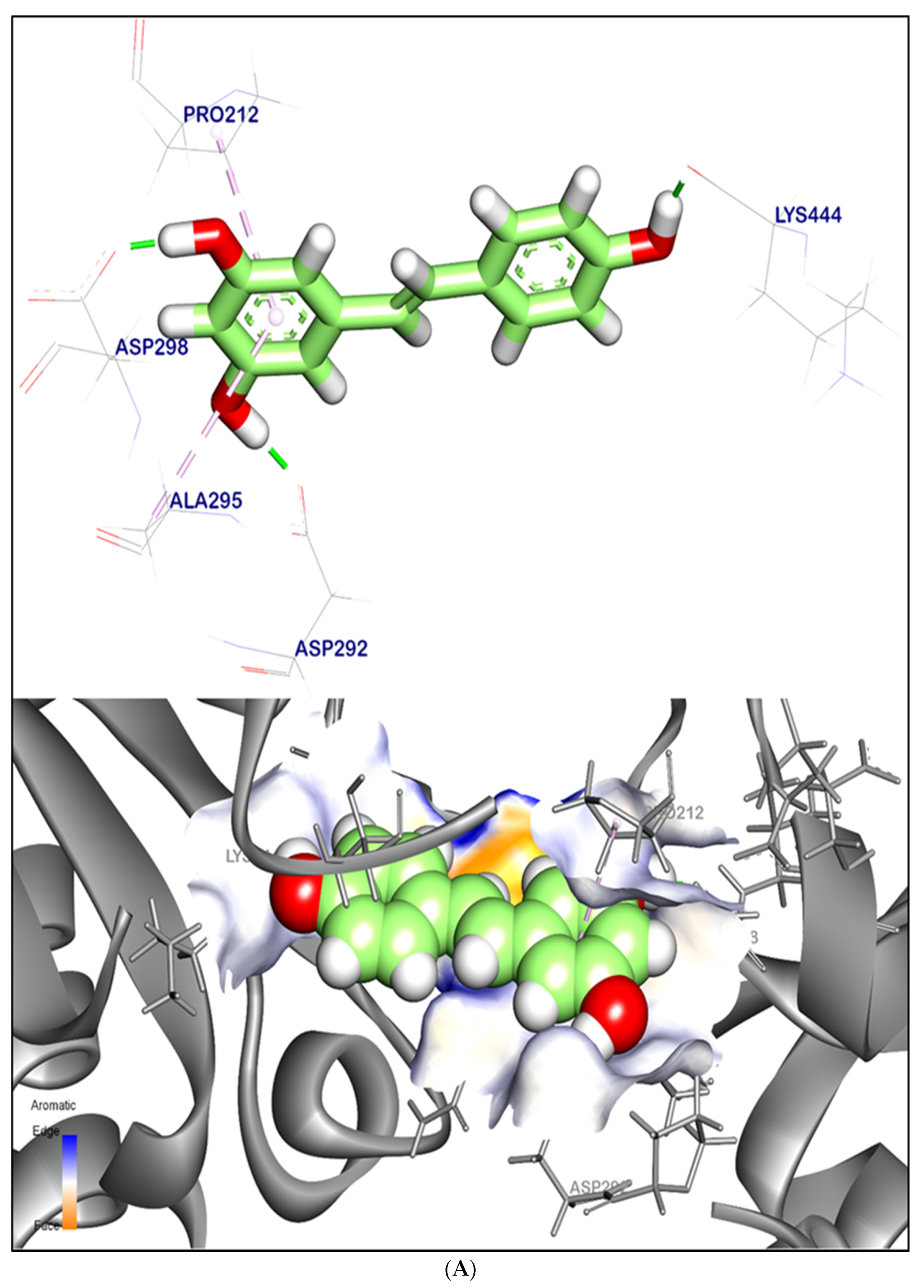
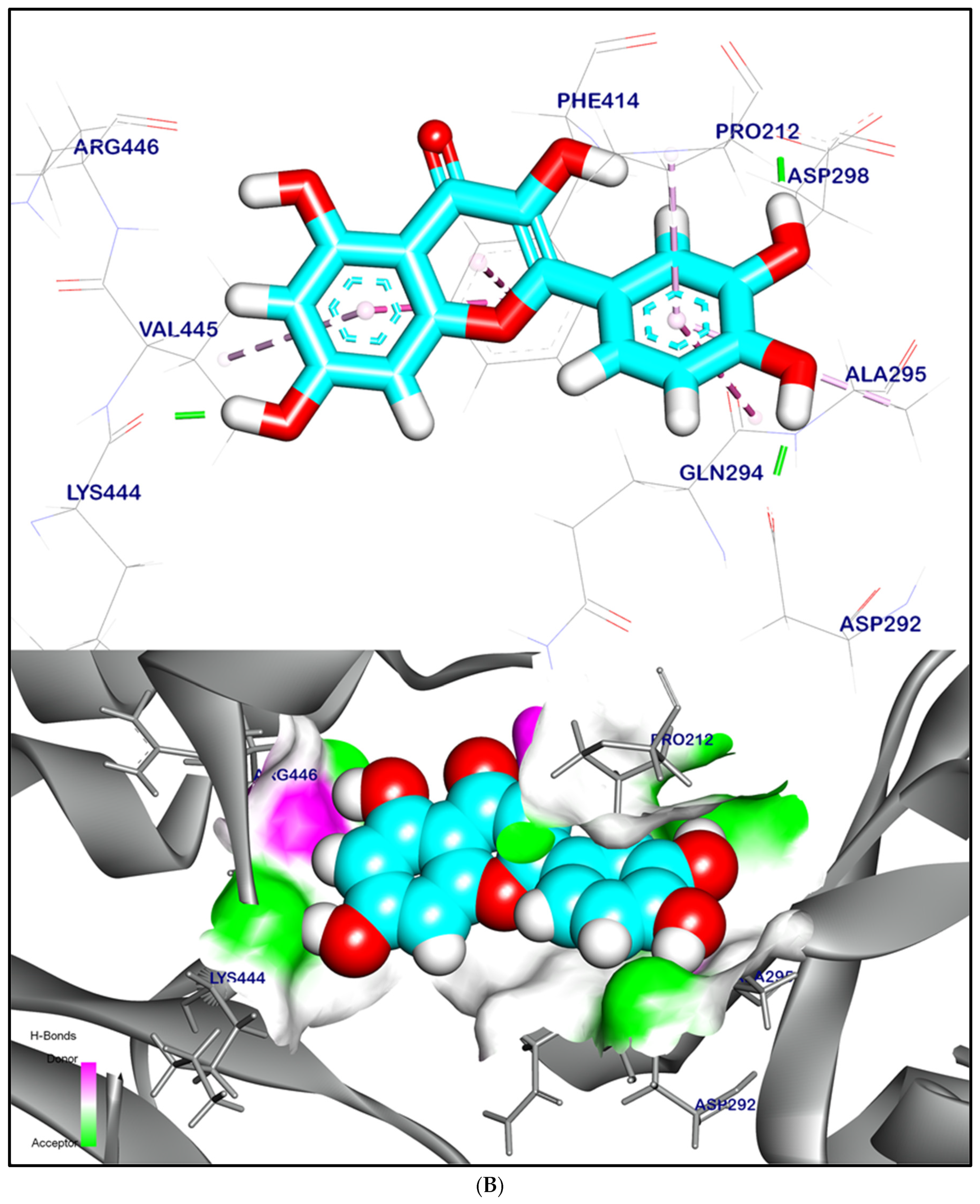
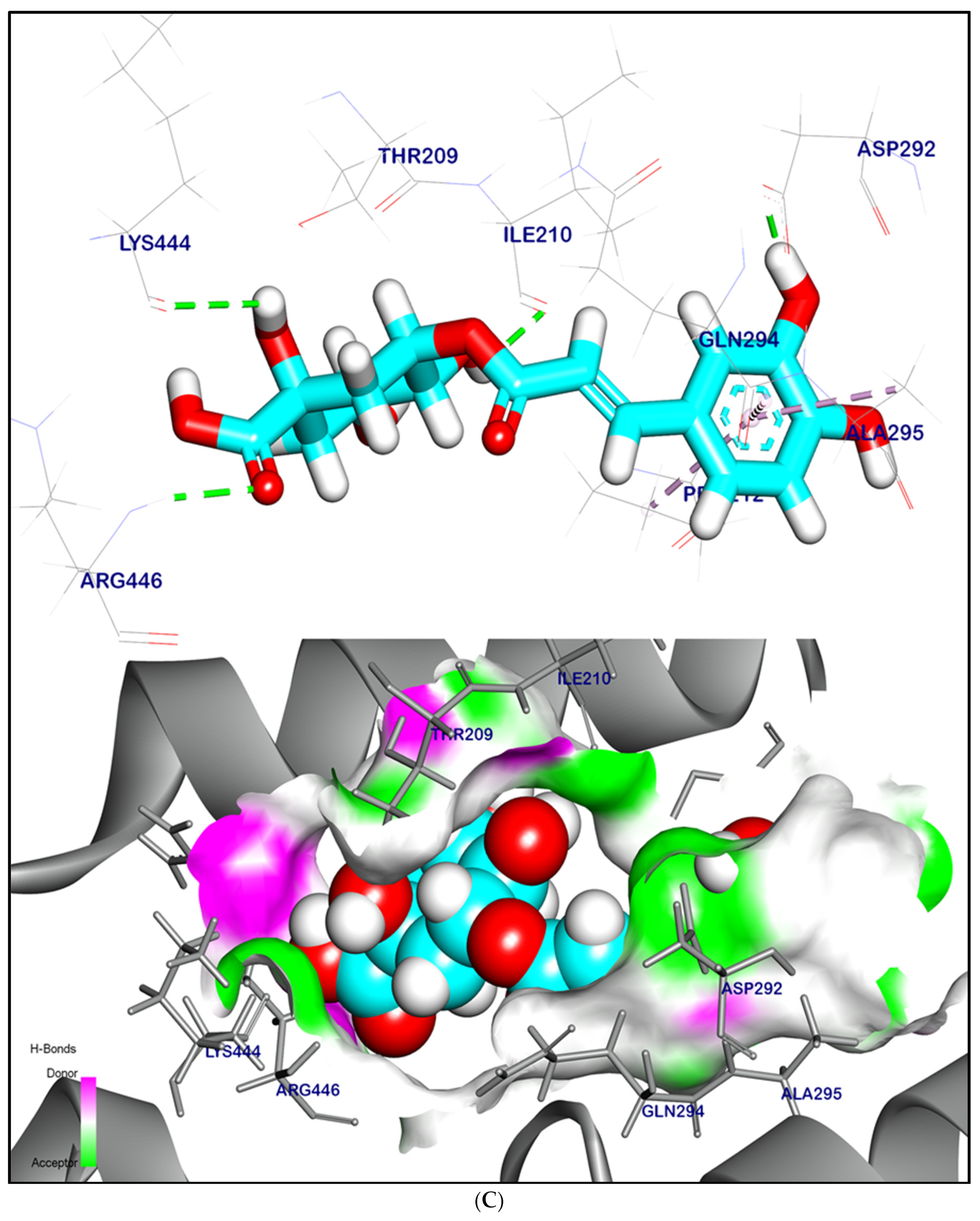
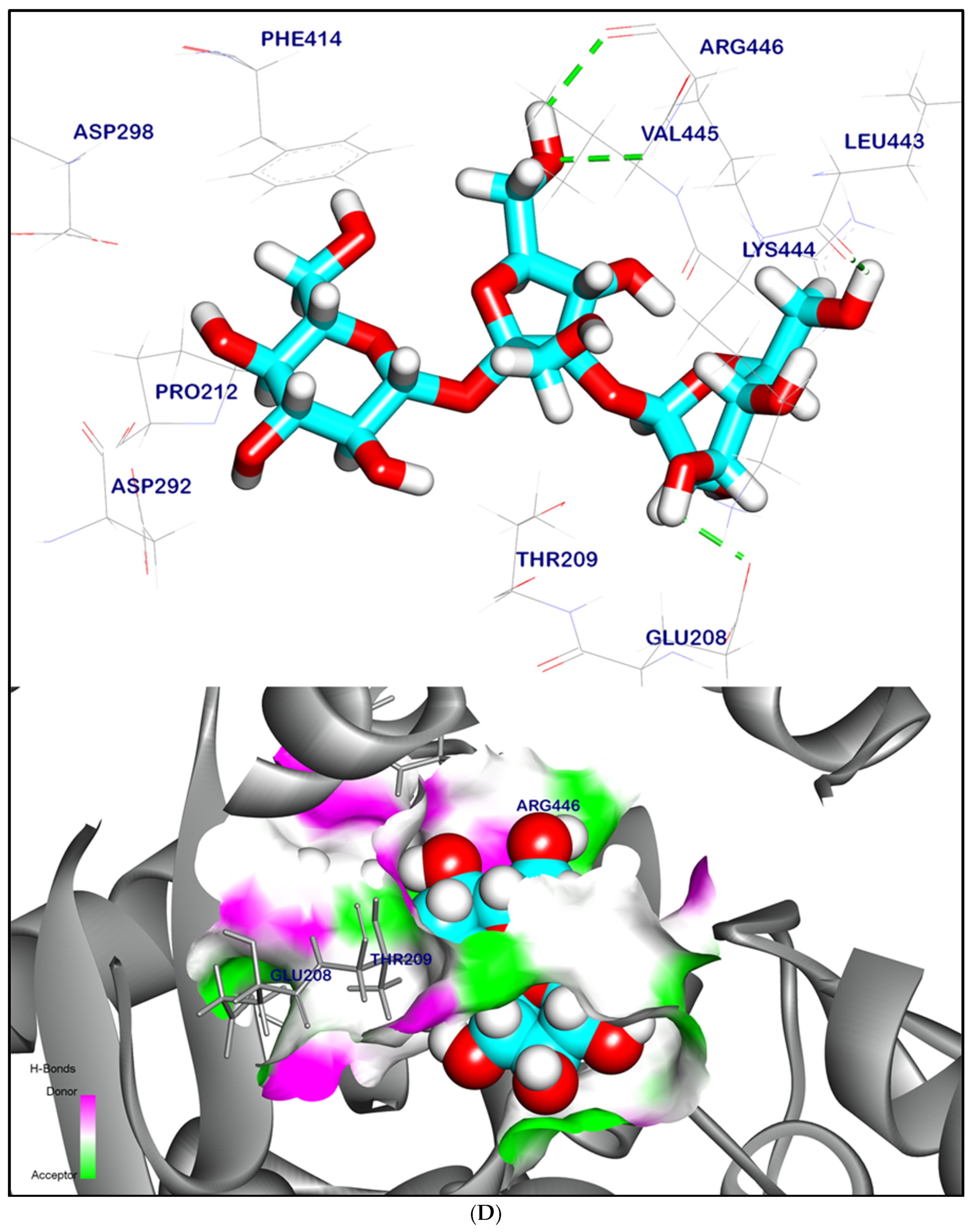
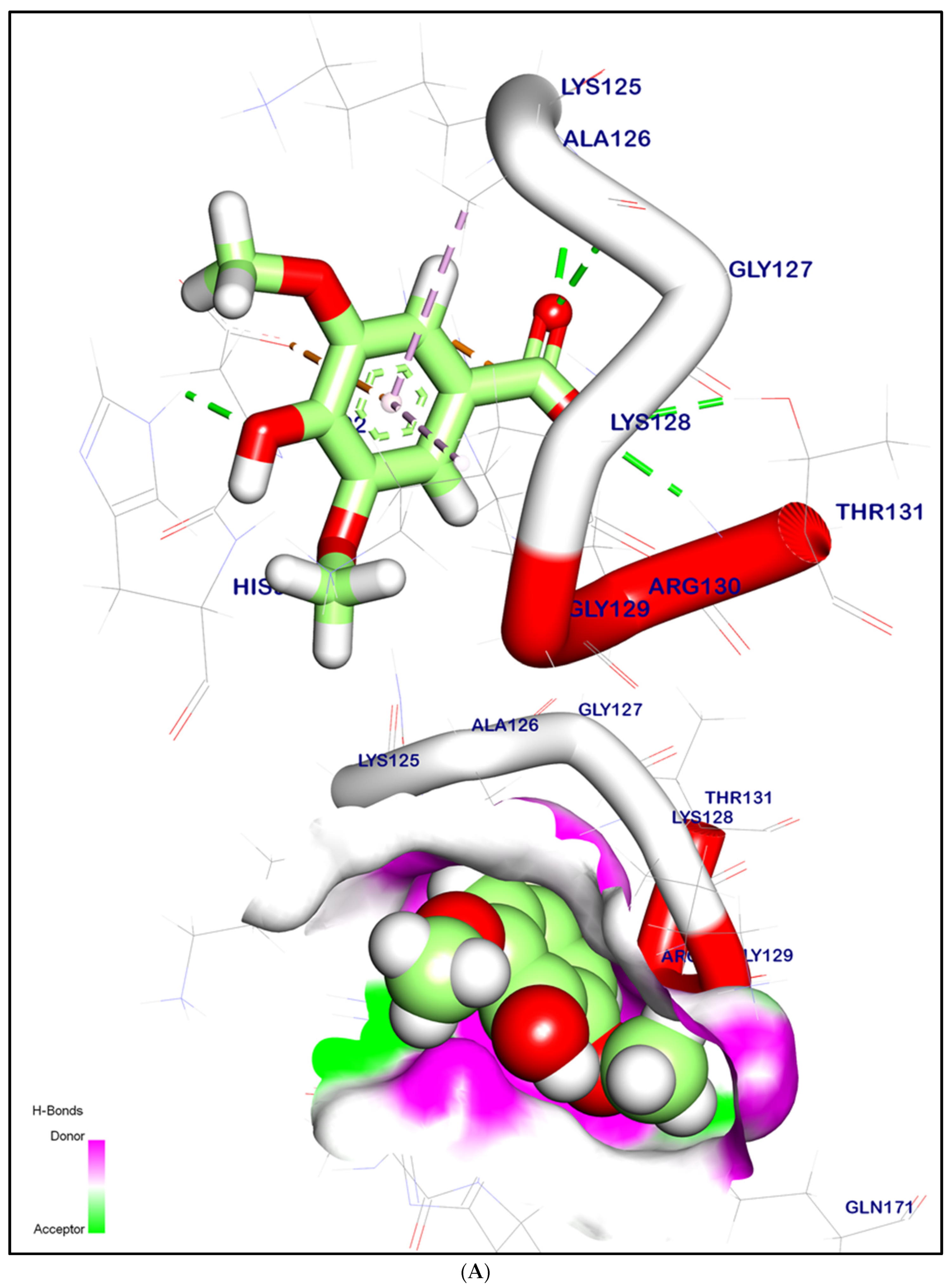
2.12.1. Molecular Docking Illustration Confirms Silent Information Regulator 1 (SIRT-1) and Novel Components of Kiwi Extract Interaction
2.12.2. Molecular Docking Illustration Confirms Phosphatase and Tensin Homolog (PTEN) Inhibition and Novel Components of Kiwi Extract Interaction
2.12.3. Molecular Dynamic (MD) Simulation Studies
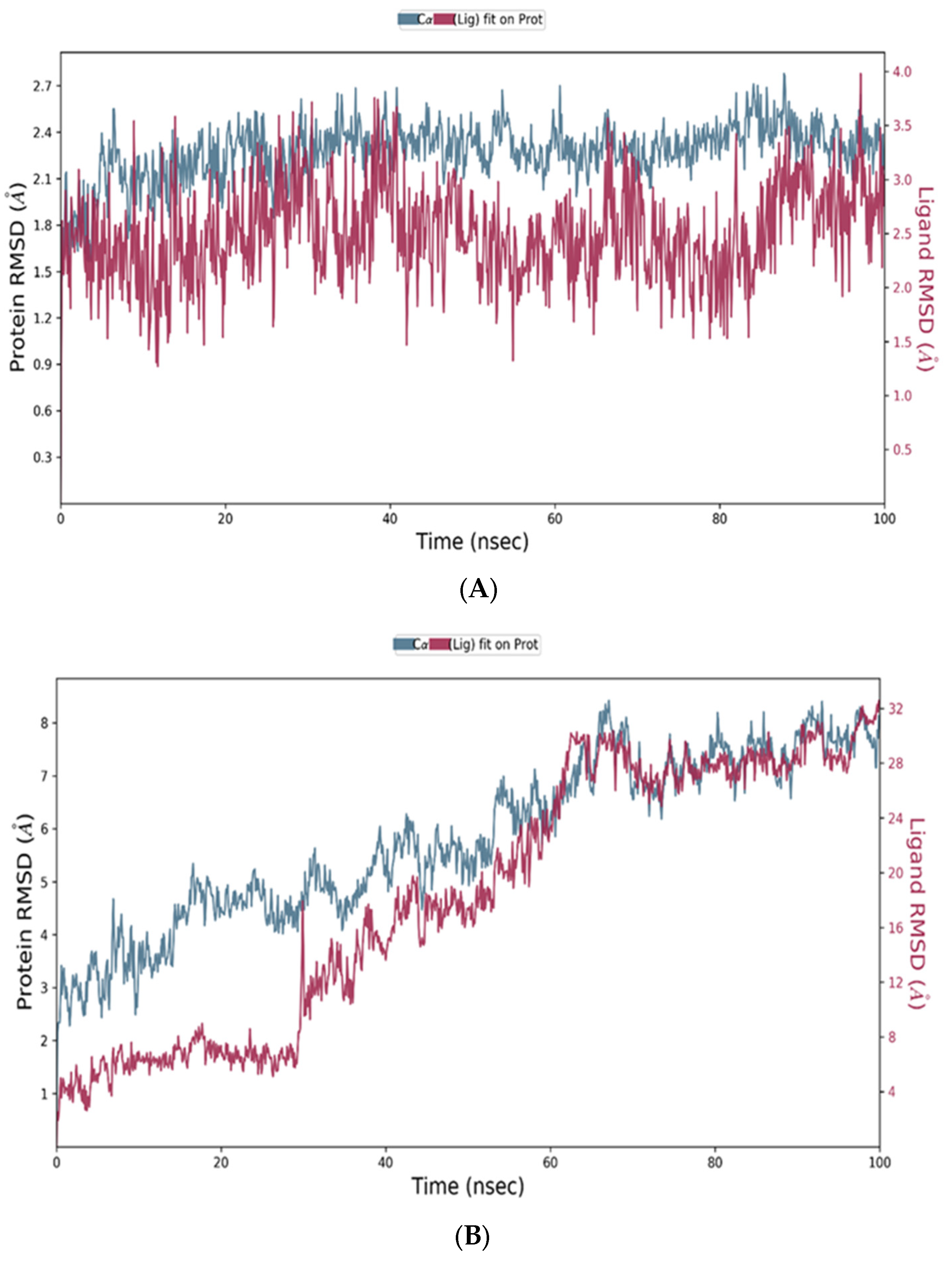
Protein and Ligand RMSD Analysis
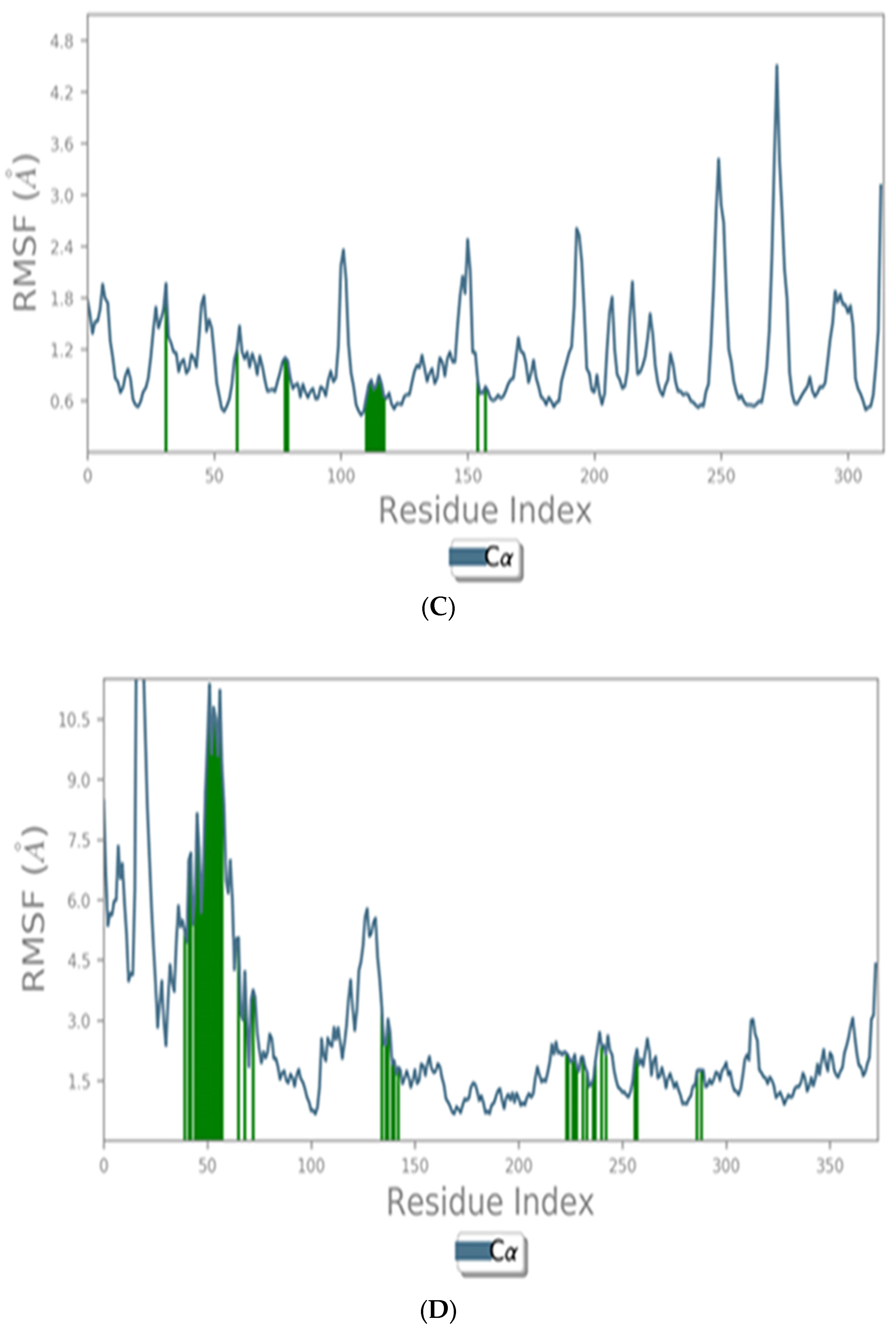
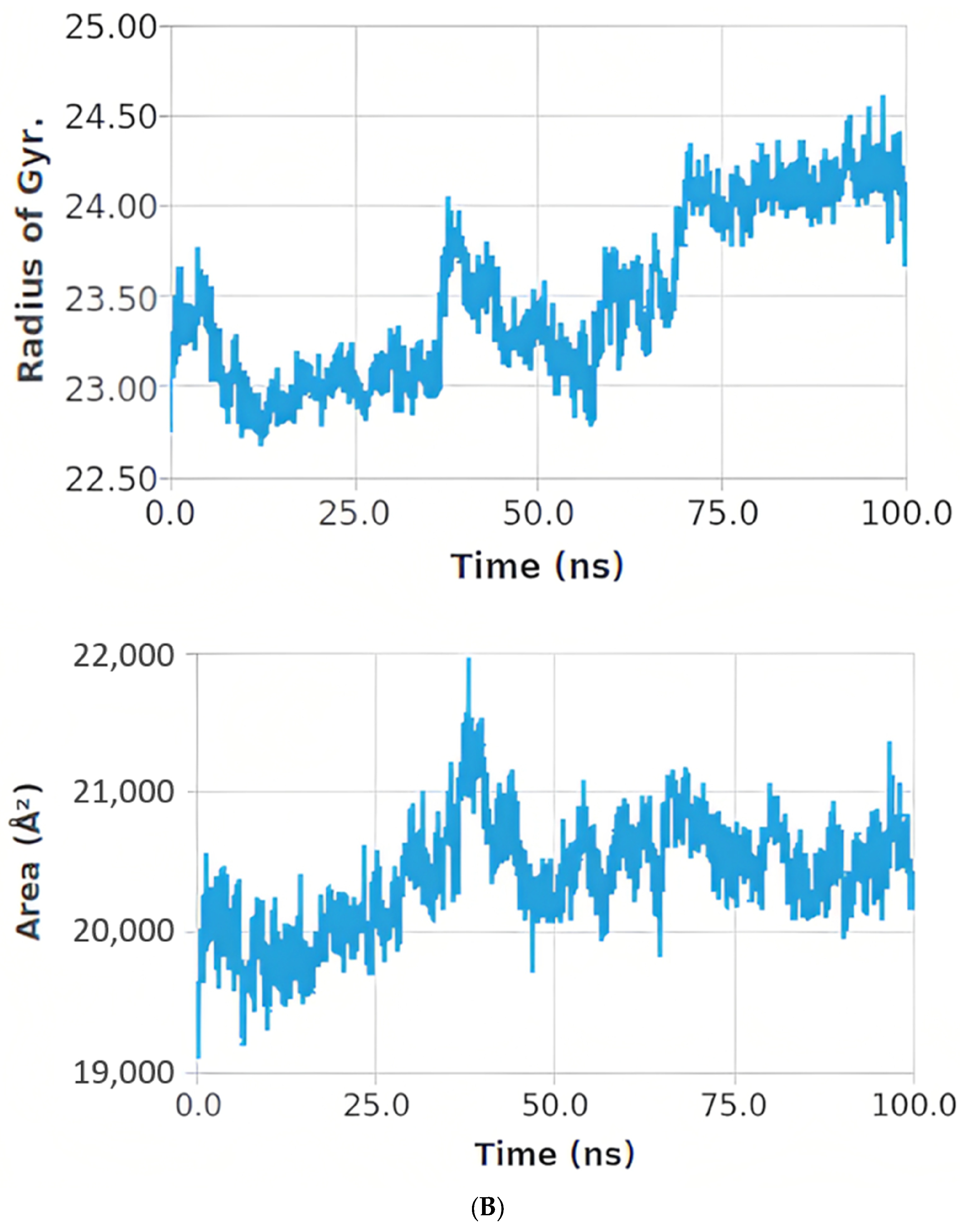
Histogram and Heat Map Analysis
MM-GBSA Calculations
3. Discussion
4. Materials and Methods
4.1. Preparation of Aqueous Extract of Kiwifruit
4.2. Animals and the Experimental Design
4.2.1. Experimental Design
4.2.2. Evaluation of Body Weight Changes
4.2.3. Biological Samples
4.3. Biochemical Marker Estimation in Blood and Tissues
4.3.1. The Diabetic Markers
4.3.2. Assessment of Diabetogenic-Related Parameters in the Serum of Rats
4.3.3. ELISA Iimmunoassay for Quantification of Inflammatory Parameters
4.3.4. Nuclear Protein Isolation and Assays for Renal and Hepatic NF-κB
4.3.5. Immunoblotting
4.3.6. Quantitative Real-Time PCR Analysis
4.3.7. The Antioxidant Markers of the Kidney and Liver Tissues
4.3.8. The Markers of the Lipid Profile
4.3.9. Biochemical Assessment of Serum Biomarkers of the Kidney and Liver Functions
4.4. Histopathology Study
4.5. Method of Molecular Docking
4.5.1. Preparation of Protein
4.5.2. Ligand Preparation
4.5.3. Molecular Dynamic (MD) Simulation Studies
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Dabet, M.D.M.; Shahzad, K.; Elwakiel, A.; Sulaj, A.; Kopf, S.; Bock, F.; Isermann, B. Reversal of the renal hyperglycemic memory in diabetic kidney disease by targeting sustained tubular p21 expression. Nat. Commun. 2022, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hesp, A.C.; Schaub, J.A.; Prasad, P.V.; Vallon, V.; Laverman, G.D.; Bjornstad, P.; Van Raalte, D.H. The role of renal hypoxia in the pathogenesis of diabetic kidney disease: A promising target for newer renoprotective agents including SGLT2 inhibitors. Kidney Int. 2020, 98, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Arabloei Sani, M.; Yaghmaei, P.; Hajebrahimi, Z.; Hayati Roodbari, N. Therapeutic Effect of P-Cymene on Lipid Profile, Liver Enzyme, and Akt/Mtor Pathway in Streptozotocin-Induced Diabetes Mellitus in Wistar Rats. J. Obes. 2022, 2022, 1015669. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef] [PubMed]
- AlAmri, O.D.; Albeltagy, R.S.; Akabawy, A.M.; Mahgoub, S.; AbdelMohsen, D.M.; Moneim, A.E.A.; Amin, H.K. Investigation of antioxidant and anti-inflammatory activities as well as the renal protective potential of green coffee extract in high fat-diet/streptozotocin-induced diabetes in male albino rats. J. Funct. Foods. 2020, 71, 103996. [Google Scholar] [CrossRef]
- Gothai, S.; Ganesan, P.; Park, S.Y.; Fakurazi, S.; Choi, D.K.; Arulselvan, P. Natural phyto-bioactive compounds for the treatment of type 2 diabetes: Inflammation as a target. Nutrients 2016, 8, 461. [Google Scholar] [CrossRef]
- DasNandy, A.; Virge, R.; Hegde, H.V.; Chattopadhyay, D. A review of patent literature on the regulation of glucose metabolism by six phytocompounds in the management of diabetes mellitus and its complications. J. Integr. Med. 2023, 21, 226–235. [Google Scholar] [CrossRef]
- Satpal, D.; Kaur, J.; Bhadariya, V.; Sharma, K. Actinidia deliciosa (Kiwi fruit): A comprehensive review on the nutritional composition, health benefits, traditional utilization, and commercialization. J. Food Process. Preserv. 2021, 45, e15588. [Google Scholar] [CrossRef]
- Hunter, D.C.; Skinner, M.A.; Ferguson, A.R. Kiwifruit and health. In Fruits, Vegetables, and Herbs; Academic Press: Cambridge, MA, USA, 2016; pp. 239–269. [Google Scholar] [CrossRef]
- Duttaroy, A.K. Cardioprotective properties of kiwifruit. Adv. Food Nutr. Res. 2013, 68, 273–282. [Google Scholar] [CrossRef]
- Sarkar, T.; Salauddin, M.; Roy, A.; Sharma, N.; Sharma, A.; Yadav, S.; Simal-Gandara, J. Minor tropical fruits as a potential source of bioactive and functional foods. Crit. Rev. Food Sci. Nutr. 2022, 1–45. [Google Scholar] [CrossRef]
- El Azab, E.F.; Mostafa, H.S. Phytochemical analysis and antioxidant defense of kiwifruit (Actinidia deliciosa) against pancreatic cancer and AAPH-induced RBCs hemolysis. Food Sci. Technol. 2021, 42, e06021. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wang, W.H.; Zhan, J.Y.; Huang, Y.L.; Cheng, W.Y. Beneficial Effects of Golden Kiwifruit Consumption in Overweight and Obese Young Adults. J. Nutr. Sci. Vitaminol. 2020, 66, S356–S360. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Y.; Chen, S.; Gu, J.; Ni, Y. Insoluble and Soluble Dietary Fibers from Kiwifruit (Actinidia deliciosa) Modify Gut Microbiota to Alleviate High-Fat Diet and Streptozotocin-Induced TYPE 2 Diabetes in Rats. Nutrients 2022, 14, 3369. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.N.; Reddy, N.M.; Patil, K.R.; Nakhate, K.T.; Ojha, S.; Patil, C.R.; Agrawal, Y.O. Challenges and issues with streptozotocin-induced diabetes–a clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem. Biol. Interact. 2016, 244, 49–63. [Google Scholar] [CrossRef]
- Sah, S.P.; Singh, B.; Choudhary, S.; Kumar, A. Animal models of insulin resistance: A review. Pharmacol. Rep. 2016, 68, 1165–1177. [Google Scholar] [CrossRef]
- Abdulmalek, S.; Eldala, A.; Awad, D.; Balbaa, M. Ameliorative effect of curcumin and zinc oxide nanoparticles on multiple mechanisms in obese rats with induced type 2 diabetes. Sci. Rep. 2021, 11, 20677. [Google Scholar] [CrossRef] [PubMed]
- Rebecca Roy, J.; Janaki, C.S.; Jayaraman, S.; Periyasamy, V.; Balaji, T.; Vijayamalathi, M.; Prasad, M. Carica Papaya Reduces High Fat Diet and Streptozotocin-Induced Development of Inflammation in Adipocyte via IL-1β/IL-6/TNF-α Mediated Signaling Mechanisms in Type-2 Diabetic Rats. Curr. Issues Mol. Biol. 2023, 45, 852–884. [Google Scholar] [CrossRef]
- Cao, R.; Tian, H.; Zhang, Y.; Liu, G.; Xu, H.; Rao, G.; Fu, X. Signaling pathways and intervention for therapy of type 2 diabetes mellitus. MedComm 2023, 4, e283. [Google Scholar] [CrossRef]
- Baraskar, K.; Thakur, P.; Shrivastava, R.; Shrivastava, V.K. Ameliorative effects of gallic acid on GLUT-4 expression and insulin resistance in high fat diet-induced obesity animal model mice, Mus musculus. J. Diabetes Metab. 2023, 22, 721–733. [Google Scholar] [CrossRef]
- Czech, M.P.; Tencerova, M.; Pedersen, D.J.; Aouadi, M. Insulin signalling mechanisms for triacylglycerol storage. Diabetologia 2013, 56, 949–964. [Google Scholar] [CrossRef]
- Zhou, J.; Shi, Y.; Yang, C.; Lu, S.; Zhao, L.; Liu, X.; Yin, Z. γ-glutamylcysteine alleviates insulin resistance and hepatic steatosis by regulating adenylate cyclase and IGF-1R/IRS1/PI3K/Akt signaling pathways. J. Nutr. Biochem. 2023, 119, 109404. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, A.H.; Casolaro, V.; Bermúdez-Humarán, L.G.; Keyvani, H.; Taghinezhad-S, S. Modulation of the PI3K/Akt/mTOR signaling pathway by probiotics as a fruitful target for orchestrating the immune response. Gut Microbes 2021, 13, 1886844. [Google Scholar] [CrossRef] [PubMed]
- Shokouh, P.; Jeppesen, P.B.; Christiansen, C.B.; Mellbye, F.B.; Hermansen, K.; Gregersen, S. Efficacy of arabica versus robusta coffee in improving weight, insulin resistance, and liver steatosis in a rat model of type-2 diabetes. Nutrients 2019, 11, 2074. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, F.M.; Talaat, Y.; El-Sayed, R.A.; Kang, W.; Ghanem, N.F. Hepatoprotective effect of Actinidia deliciosa against streptozotocin-induced oxidative stress, apoptosis, and inflammations in rats. Oxid. Med. Cell. Longev. 2022, 2022, 1499510. [Google Scholar] [CrossRef] [PubMed]
- Dinić, S.; Arambašić Jovanović, J.; Uskoković, A.; Mihailović, M.; Grdović, N.; Tolić, A.; Vidaković, M. Oxidative stress-mediated beta cell death and dysfunction as a target for diabetes management. Front. Endocrinol. 2022, 13, 1006376. [Google Scholar] [CrossRef] [PubMed]
- Reza, A.A.; Rashid, M.M.; Islam, M.S.; Hossen, M.A.; Ahmed, A.A.; Haque, M.A.; Rahman, M.A. Polyphenolics of purple devil fruits potentiate anti-inflammatory action by regulating the expression of inflammatory cytokines and apoptotic signalling molecules evident in extensive and combined experimental models. J. Funct. Foods 2023, 106, 105610. [Google Scholar] [CrossRef]
- Cho, G.W.; Altamirano, F.; Hill, J.A. Chronic heart failure: Ca2+, catabolism, and catastrophic cell death. Biochim. Biophys. Acta-Mol. Basis Dis. 2016, 1862, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Ramírez, A.; Castillo-Rodríguez, R.A.; Zavala-Vega, S.; Jimenez-Farfan, D.; Anaya-Rubio, I.; Briseño, E.; Trejo-Solís, C. Autophagy as a potential therapy for malignant glioma. Pharmaceuticals 2020, 13, 156. [Google Scholar] [CrossRef]
- Singh, R.; Kishore, L.; Kaur, N. Diabetic peripheral neuropathy: Current perspective and future directions. Pharmacol. Res. 2014, 80, 21–35. [Google Scholar] [CrossRef]
- Laleethambika, N.; Anila, V.; Manojkumar, C.; Muruganandam, I.; Giridharan, B.; Ravimanickam, T.; Balachandar, V. Diabetes and sperm DNA damage: Efficacy of antioxidants. SN Compr. Clin. Med. 2019, 1, 49–59. [Google Scholar] [CrossRef]
- Roy, J.R.; Janaki, C.S.; Jayaraman, S.; Veeraraghavan, V.P.; Periyasamy, V.; Balaji, T.; Swetha, P. Hypoglycemic Potential of Carica papaya in Liver Is Mediated through IRS-2/PI3K/SREBP-1c/GLUT2 Signaling in High-Fat-Diet-Induced Type-2 Diabetic Male Rats. Toxics 2023, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.S.; Khaled, A.M.; Al-Bagawi, A.H.; Fareid, M.A.; Ghany, R.A.; Habotta, O.A.; Moneim, A.E.A. Hepatorenal protective efficacy of flavonoids from Ocimum basilicum extract in diabetic albino rats: A focus on hypoglycemic, antioxidant, anti-inflammatory and anti-apoptotic activities. Biomed. Pharmacother. 2021, 144, 112287. [Google Scholar] [CrossRef] [PubMed]
- Aleid, I.S.; Alfheeaid, H.A.; Aljutaily, T.; Alhomaid, R.M.; Alharbi, H.F.; Althwab, S.A.; Barakat, H. Gastroprotective effects of Spirulina Platensis, golden kiwifruit flesh, and golden kiwifruit peel extracts individually or in combination against indomethacin-induced gastric ulcer in rats. Nutrients 2021, 13, 3499. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Karuppiah, M.; Thiruppathi, M. Antihyperglycaemic potential of rosmarinic acid attenuates glycoprotein moiety in high-fat diet and streptozotocin-induced diabetic rats. All Life 2020, 13, 120–130. [Google Scholar] [CrossRef]
- El Azab, E.F.; Mostafa, H.S. Geraniol ameliorates the progression of high fat-diet/streptozotocin-induced type 2 diabetes mellitus in rats via regulation of caspase-3, Bcl-2, and Bax expression. J. Food Biochem. 2022, 46, e14142. [Google Scholar] [CrossRef] [PubMed]
- Buzanovskii, V.A. Methods for the determination of glucose in blood. Part 2. Rev. J. Chem. 2015, 5, 143–191. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dignam, J.D.; Lebovitz, R.M.; Roeder, R.G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983, 11, 1475–1489. [Google Scholar] [CrossRef]
- Burnette, W.N. “Western blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 1981, 112, 195–203. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta-Gen. Subj. 1979, 582, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Riedel, H.D.; Stremmel, W. Determination of catalase activity at physiological hydrogen peroxide concentrations. Anal. Biochem. 1997, 245, 55–60. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Cribb, A.E.; Leeder, J.S.; Spielberg, S.P. Use of a microplate reader in an assay of glutathione reductase using 5,5′-dithiobis (2-nitrobenzoic acid). Anal. Biochem. 1989, 183, 195–196. [Google Scholar] [CrossRef]
- Türkan, F.; Huyut, Z.; Atalar, M.N. The toxicological impact of some avermectins on human erythrocytes glutathione S-transferase enzyme. J. Biochem. Mol. Toxicol. 2018, 32, e22205. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Yuan, Z.; Zhao, Z.; Gao, X. Mechanism of pyrogallol autoxidation and determination of superoxide dismutase enzyme activity. Bioelectrochem. Bioenerg. 1998, 45, 41–45. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. (Eds.) Theory and Practice of Histological Techniques, 4th ed.; Elsevier Health Sciences: Livingston, NY, USA, 2008. [Google Scholar]
- Schüttelkopf, A.W.; Van Aalten, D.M. PRODRG: A tool for high-throughput crystallography of protein–ligand complexes. Acta Crystallogr. D 2004, 60, 1355–1363. [Google Scholar] [CrossRef]


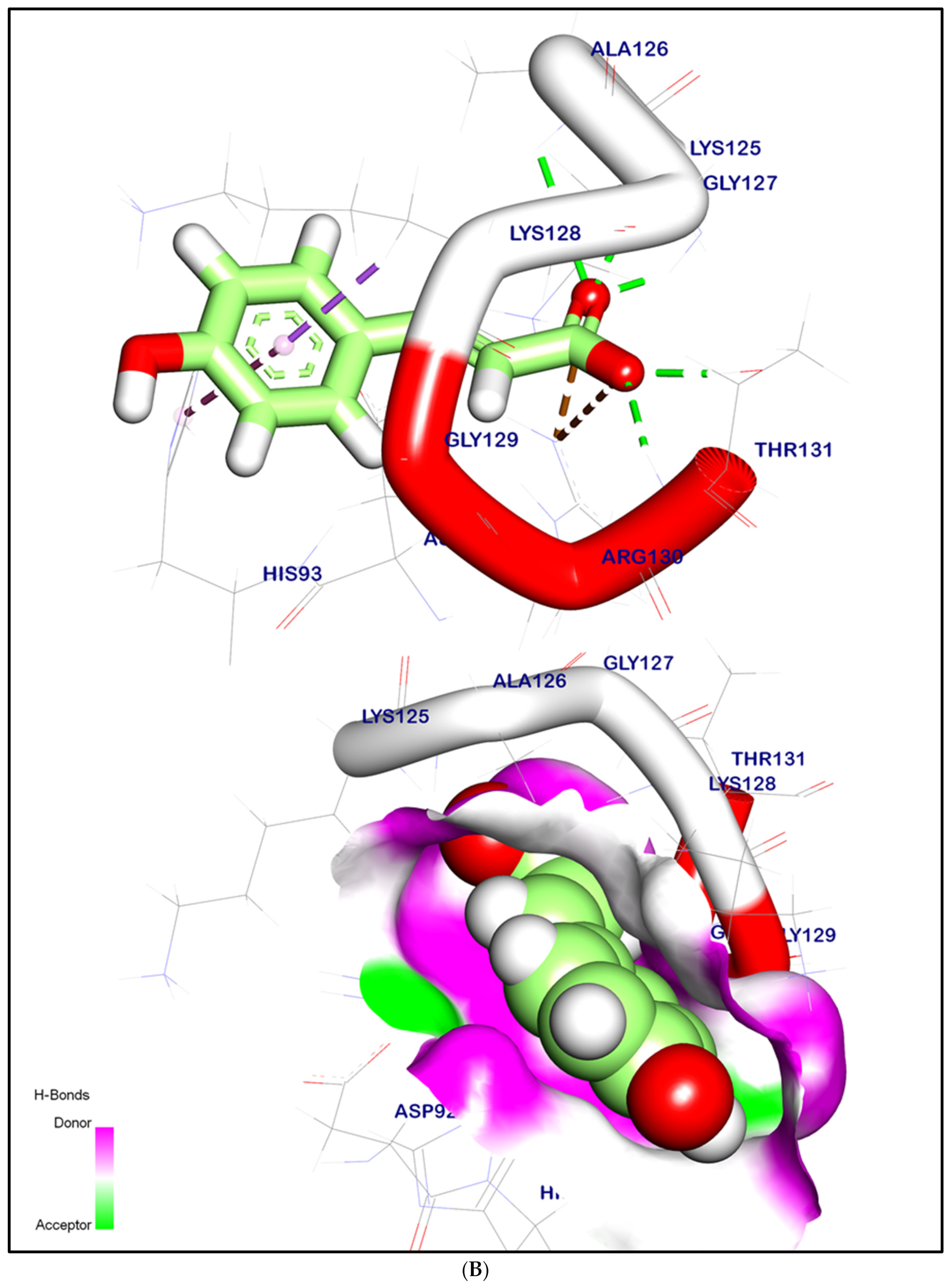
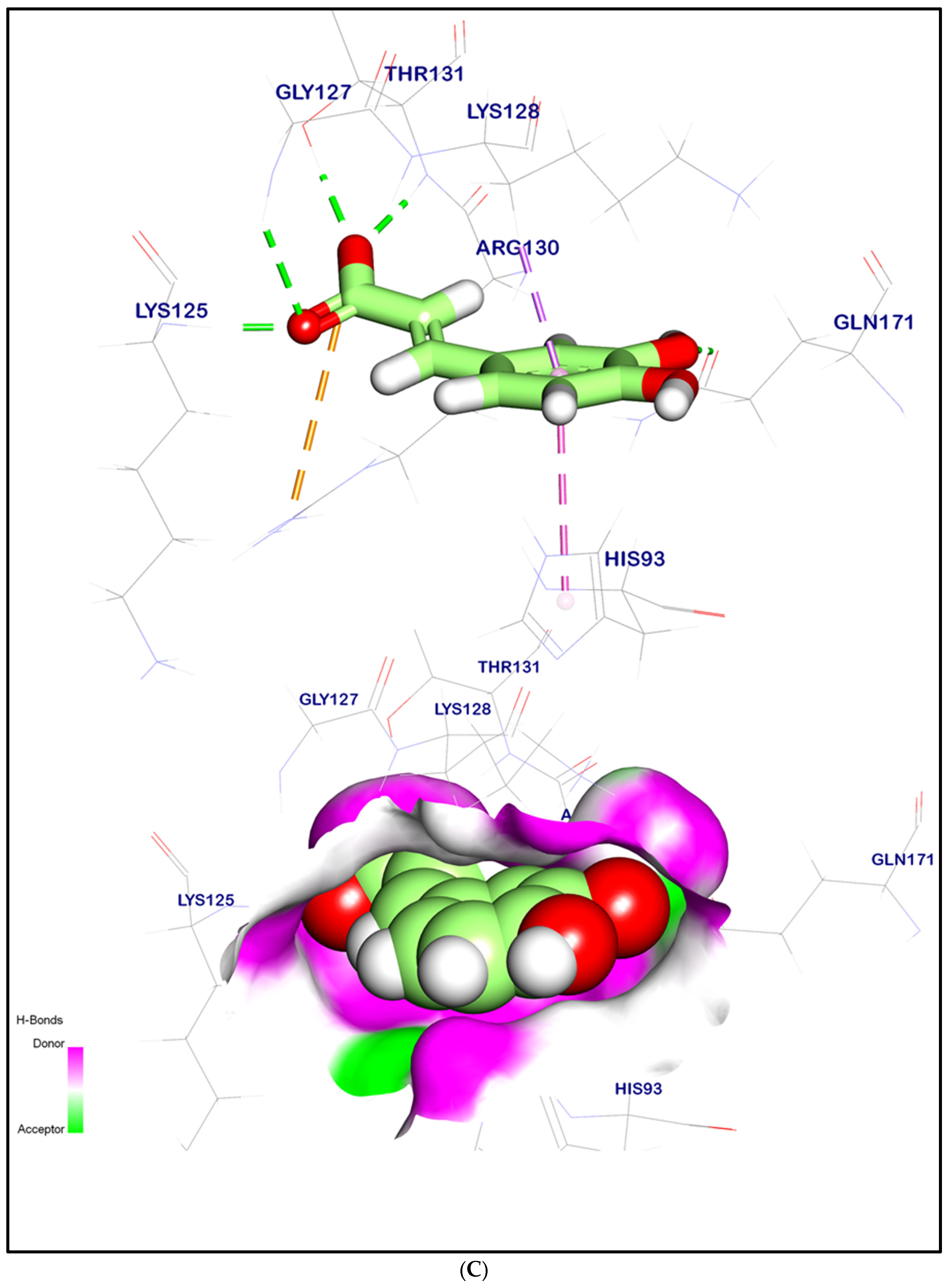
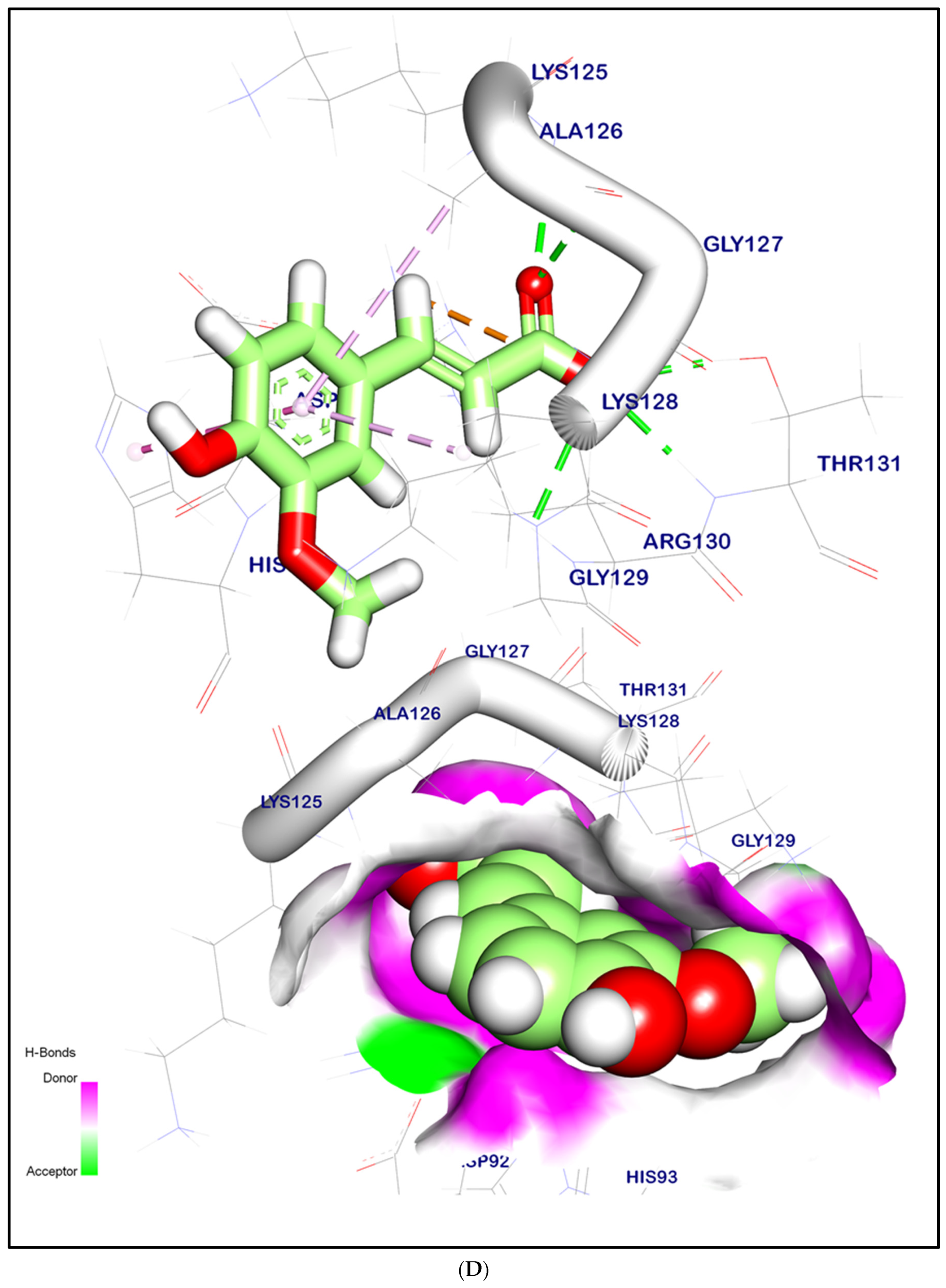
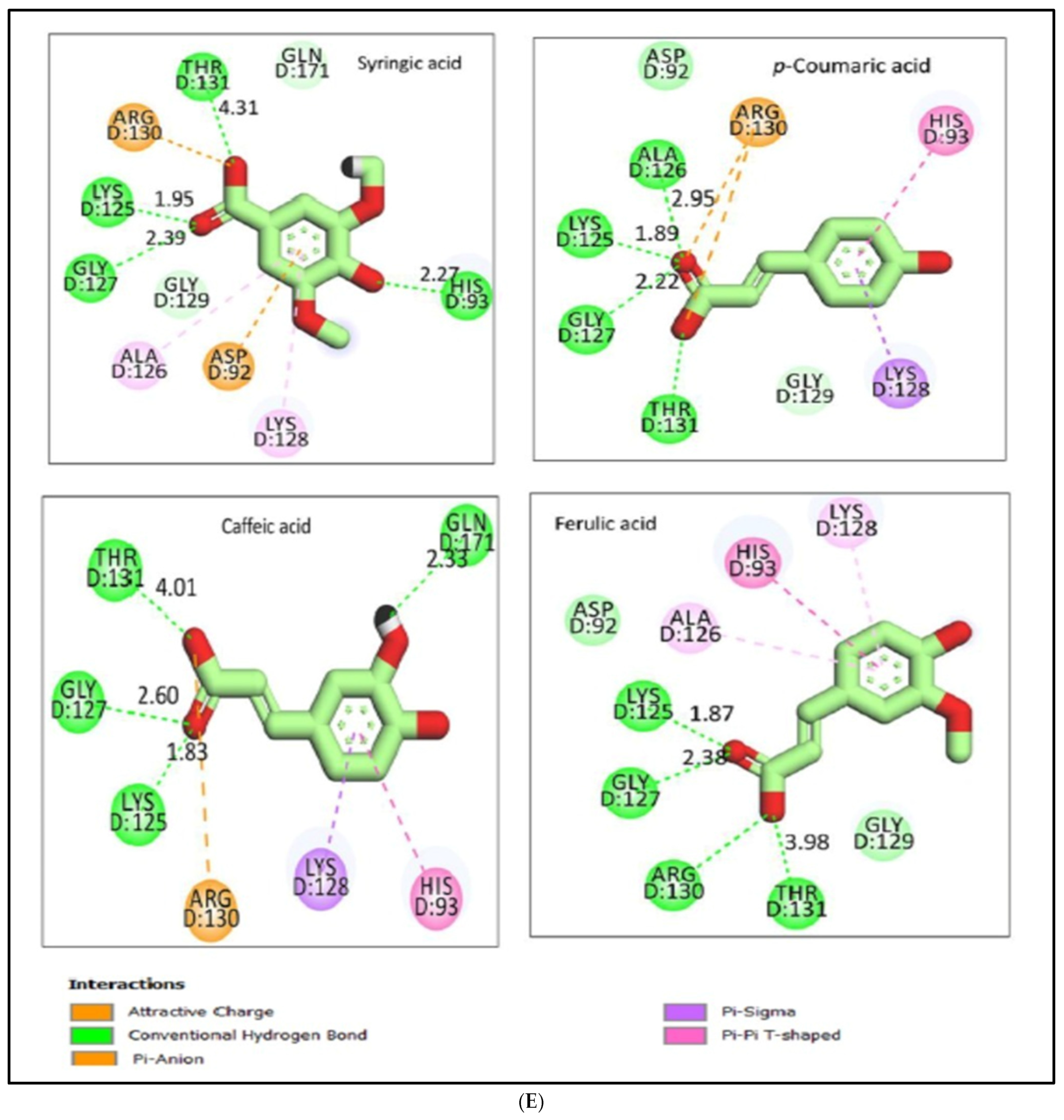





| Animal Groups | Glucose (mg/dL) | Insulin (µIU/mL) | HbA1c (%) | HOMA-IR |
|---|---|---|---|---|
| NC group | 92.74 ± 3.49 d | 5.16 ± 0.26 c | 5.54 ± 0.11 d | 1.18 ± 0.68 d |
| DC group | 371.86 ± 2.42 a | 78.23 ± 2.23 a | 11.69 ± 0.42 a | 71.8 ± 2.74 a |
| DKI group | 115.70 ± 2.32 b | 14.65 ± 2.27 b | 6.65 ± 0.36 b,c | 4.2 ± 0.86 c |
| DKII group | 96.59 ± 3.46 d | 11.13 ± 1.34 b | 5.80 ± 0.76 d,c | 2.7 ± 0.81 c,d |
| DIN group | 132.57 ± 3.38 b | 73.12 ± 3.25 a | 9.54 ± 0.38 b | 23.9 ± 0.91 b |
| Animal Groups | LPO (nmol/mg Protein) | GSH (ng/mg Protein) | ||
|---|---|---|---|---|
| Kidney | Liver | Kidney | Liver | |
| NC group | 51.25 ± 1.01 d | 5.71 ± 0.55 d | 5.11 ± 0.07 a | 50.98 ± 2.73 a |
| DC group | 102.69 ± 1.45 a | 39.13 ± 0.65 a | 1.49 ± 0.06 d | 9.22 ± 0.60 d |
| DKI group | 58.92 ± 2.68 b,c | 8.27 ± 1.75 b,c | 4.23 ± 1.97 b,c | 43.86 ± 1.59 b,c |
| DKII group | 52.35 ± 2.38 c,d | 6.17 ± 0.75 c,d | 5.05 ± 1.07 a,b | 47.32 ± 2.44 a |
| DIN group | 60.39 ± 2.69 b | 8.74 ± 0.61 b | 3.96 ± 0.08 c | 40.48 ± 2.52 c |
| Animal Groups | SOD (Units/mg Protein) | CAT (n mol of H2O2 Released/min/mg Protein) | GPx (µ moles of GSH Oxidized/min/mg Protein) | GST (µ moles of CDNB Utilized/min/mg Protein) | GR (μg GSSG Utilized/min/mg Protein) |
|---|---|---|---|---|---|
| NC group | 8.05 ± 0.17 a | 6.12 ± 0.15 a | 30.32 ± 0.83 a | 121.73 ± 1.52 a | 69.91 ± 0.47 a |
| DC group | 1.84 ± 0.09 d | 2.12 ± 0.03 d | 15.08 ± 0.17 d | 56.60 ± 0.90 d | 19.10 ± 0.47 d |
| DKI group | 6.97 ± 1.28 b,c | 5.67 ± 0.94 b,c | 25.28 ± 1.62 b,c | 114.24 ± 2.91 b,c | 63.75 ± 2.63 b,c |
| DKII group | 7.81 ± 1.25 a,b | 6.00 ± 0.93 a,b | 29.43 ± 2.71 a,b | 118.82 ± 1.92 a,b | 68.31 ± 1.85 a,b |
| DIN group | 6.36 ± 1.01 c | 4.91 ± 1.03 c | 23.57 ± 1.42 c | 108.16 ± 2.73 c | 59.51 ± 1.87 c |
| Animal Groups | SOD (Units/mg Protein) | CAT (n mol of H2O2 Released/min/mg Protein) | GPx (µ moles of GSH Oxidized/min/mg Protein) | GST (µmoles of CDNB Utilized/min/mg Protein) | GR (μg GSSG Utilized/min/mg Protein) |
|---|---|---|---|---|---|
| NC group | 18.35 ± 0.82 a | 179.59 ± 5.82 a | 25.04 ± 1.05 a | 2.02 ± 0.14 a | 3.82 ± 0.09 a |
| DC group | 5.76 ± 0.59 d | 84.42 ± 2.36 c | 11.68 ± 0.53 d | 0.53 ± 0.06 d | 1.88 ± 0.04 c |
| DKI group | 13.89 ± 1.84 b,c | 163.49 ± 2.61 b | 21.81 ± 1.28 b,c | 1.72 ± 1.09 b | 3.41 ± 0.43 b |
| DKII group | 16.23 ± 1.92 a,b | 175.98 ± 3.80 a | 24.96 ± 1.01 a,b | 1.96 ± 0.97 a | 3.80 ± 0.08 a |
| DIN group | 11.75 ± 1.79 c | 160.10 ± 1.98 b | 20.81 ± 1.29 c | 1.51 ± 0.67 c | 3.38 ± 0.29 b |
| Animal Groups | Total Cholesterol (mg/dL) | HDL-Cholesterol (mg/dL) | LDL-Cholesterol (mg/dL) | Triglyceride (mg/dL) | Total Lipid (mg/dL) |
|---|---|---|---|---|---|
| NC group | 100.95 ± 2.88 d | 50.87 ± 1.69 a | 32.25 ± 1.73 d | 63.56 ± 2.34 d | 399.44 ± 3.12 d |
| DC group | 236.20 ± 3.83 a | 30.51 ± 1.90 d | 69.12 ± 2.20 a | 255.08 ± 2.74 a | 898.66 ± 2.28 a |
| DKI group | 116.46 ± 5.64 c | 44.78 ± 1.66 b,c | 44.86 ± 1.29 b,c | 81.59 ± 4.17 c | 436.07 ± 3.23 c |
| DKII group | 103.30 ± 4.35 c,d | 49.13 ± 2.23 a,b | 33.46 ± 1.98 d | 67.53 ± 5.47 c,d | 400.51 ± 3.85 d |
| DIN group | 137.20 ± 2.45 b | 42.59 ± 2.14 c | 46.77 ± 1.75 b | 143.09 ± 3.06 b | 439.02 ± 2.62 b |
| Animal Groups | Creatinine (mg/dL) | Urea (mg/dL) | Uric Acid (mg/dL) | ALT (U/mL) | AST (U/mL) | ALP (IU/L) |
|---|---|---|---|---|---|---|
| NC group | 0.75 ± 0.14 d | 24.30 ± 1.99 d | 1.49 ± 0.06 c | 17.52 ± 1.27 d | 37.29 ± 1.68 c | 44.89 ± 1.86 c |
| DC group | 7.19 ± 0.54 a | 71.20 ± 1.9 a | 8.87 ± 0.70 a | 71.36 ± 1.44 a | 117.87 ± 1.66 a | 123.52 ± 1.65 a |
| DKI group | 1.02 ± 0.35 c | 29.09 ± 2.72 c | 2.36 ± 0.84 b | 21.02 ± 2.29 b,c | 55.91 ± 1.44 a | 53.26 ± 1.93 b |
| DKII group | 0.77 ± 0.43 d | 24.25 ± 2.84 c,d | 1.51 ± 0.13 c | 18.18 ± 1.24 c,d | 38.53 ± 1.04 c | 46.10 ± 1.67 c |
| DIN group | 1.25 ± 0.67 b | 34.16 ± 2.09 b | 2.59 ± 0.39 b | 25.89 ± 1.48 b | 57.63 ± 1.62 b | 55.19 ± 1.64 b |
| Targets | Tested Compounds | RMSD Value (Å) | Docking (Affinity) Score (kcal/mol) | Interactions | |
|---|---|---|---|---|---|
| H.B Pi Interaction | |||||
| SIRT-1 | Resveratrol | 0.75 | −6.90 | 3 | 2 |
| Quercetin | 1.03 | −6.50 | 3 | 6 | |
| Chlorogenic acid | 1.73 | −6.04 | 4 | 3 | |
| Melezitose | 1.69 | −7.65 | 4 | 0 | |
| PTEN | Syringic acid | 1.54 | −6.07 | 4 | 3 |
| p-Coumaric acid | 1.76 | −5.98 | 4 | 2 | |
| Caffeic acid | 1.46 | −6.06 | 4 | 2 | |
| Ferulic acid | 1.53 | −6.15 | 4 | 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Azab, E.F.; Alakilli, S.Y.M.; Saleh, A.M.; Alhassan, H.H.; Alanazi, H.H.; Ghanem, H.B.; Yousif, S.O.; Alrub, H.A.; Anber, N.; Elfaki, E.M.; et al. Actinidia deliciosa Extract as a Promising Supplemental Agent for Hepatic and Renal Complication-Associated Type 2 Diabetes (In Vivo and In Silico-Based Studies). Int. J. Mol. Sci. 2023, 24, 13759. https://doi.org/10.3390/ijms241813759
El Azab EF, Alakilli SYM, Saleh AM, Alhassan HH, Alanazi HH, Ghanem HB, Yousif SO, Alrub HA, Anber N, Elfaki EM, et al. Actinidia deliciosa Extract as a Promising Supplemental Agent for Hepatic and Renal Complication-Associated Type 2 Diabetes (In Vivo and In Silico-Based Studies). International Journal of Molecular Sciences. 2023; 24(18):13759. https://doi.org/10.3390/ijms241813759
Chicago/Turabian StyleEl Azab, Eman Fawzy, Saleha Y. M. Alakilli, Abdulrahman M. Saleh, Hassan H. Alhassan, Hamad H. Alanazi, Heba Bassiony Ghanem, Sara Osman Yousif, Heba Abu Alrub, Nahla Anber, Elyasa Mustafa Elfaki, and et al. 2023. "Actinidia deliciosa Extract as a Promising Supplemental Agent for Hepatic and Renal Complication-Associated Type 2 Diabetes (In Vivo and In Silico-Based Studies)" International Journal of Molecular Sciences 24, no. 18: 13759. https://doi.org/10.3390/ijms241813759







