Anti-Inflammatory Effects of Peripheral Dopamine
Abstract
:1. Introduction
1.1. Dopamine and Immunomodulation
1.2. Anti-Inflammatory Effects of Dopamine in Peripheral Organs, Tissues, and Cells
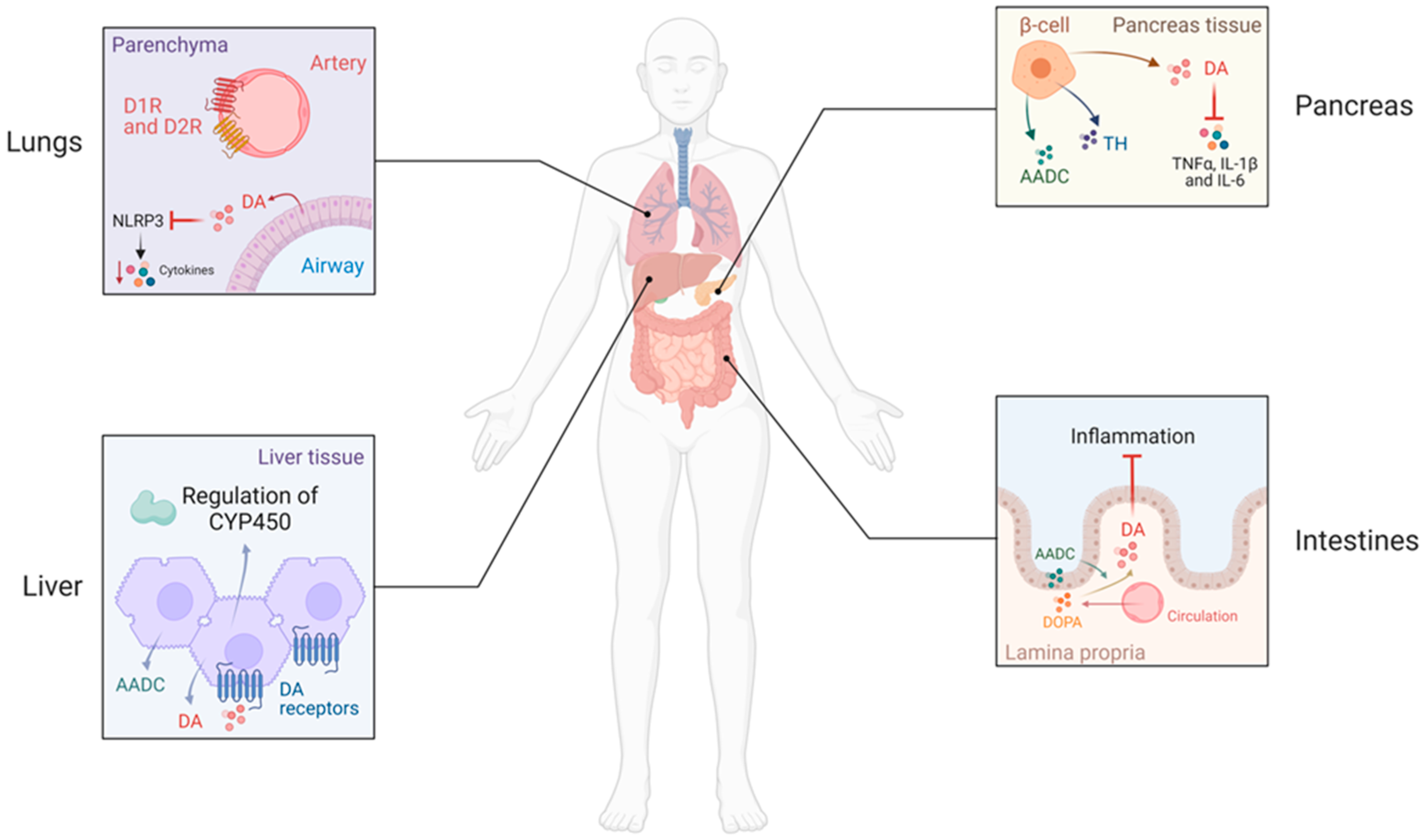
1.2.1. Dopamine in Abdominal Organs
1.2.2. Dopamine in the Liver
1.2.3. Dopamine in the Lung
1.2.4. Dopamine in the Cardiovascular System
1.2.5. Dopamine in Adipose Tissue
1.2.6. Dopamine in Bone and Connective Tissues
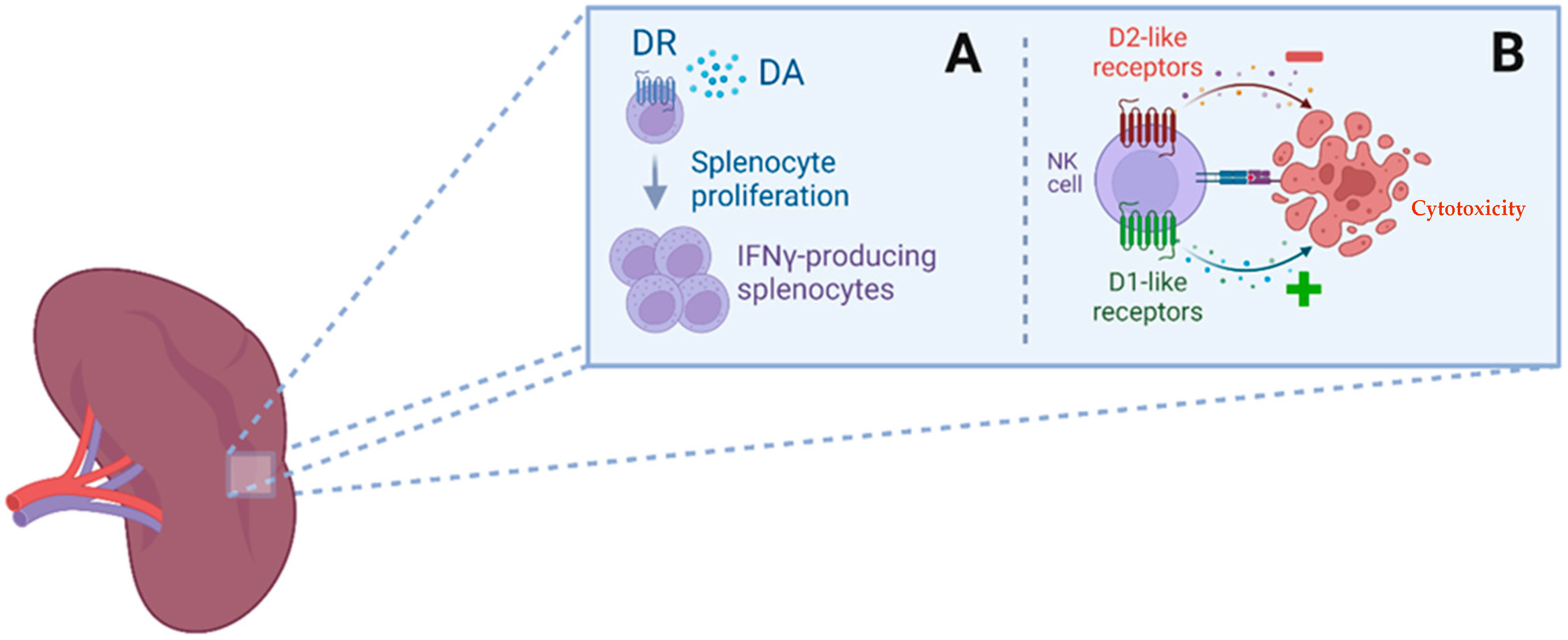
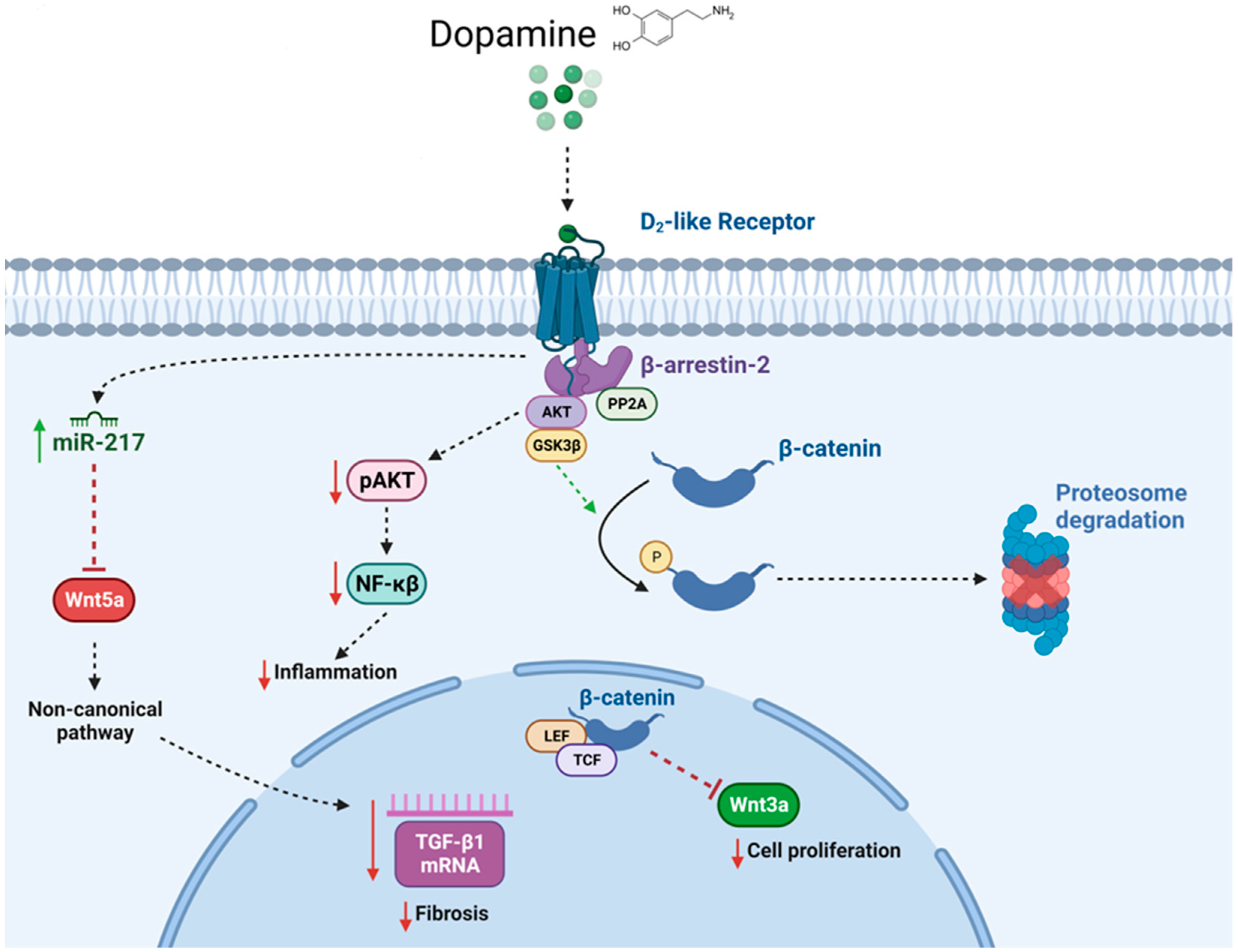
1.2.7. Dopamine and the Kidney
2. Clinical Relevance
2.1. Genetics
2.2. Effects of Antipsychotics on Systemic Inflammation
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Asghar, M.; Tayebati, S.K.; Lokhandwala, M.F.; Hussain, T. Potential Dopamine-1 Receptor Stimulation in Hypertension Management. Curr. Hypertens. Rep. 2011, 13, 294–302. [Google Scholar] [CrossRef]
- Armando, I.; Villar, V.A.M.; Jose, P.A. Dopamine and Renal Function and Blood Pressure Regulation. Compr. Physiol. 2011, 1, 1075–1117. [Google Scholar] [CrossRef]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Dopamine Outside the Brain: The Eye, Cardiovascular System and Endocrine Pancreas. Pharmacol. Ther. 2019, 203, 107392. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ucsfhealth.org/medical-tests/catecholamine-blood-test#:~:text=Normal%20Results&text=The%20normal%20range%20for%20norepinephrine,to%2010048.7%20pmol%2FL (accessed on 7 March 2023).
- Goldstein, D.S.; Swoboda, K.J.; Miles, J.M.; Coppack, S.W.; Aneman, A.; Holmes, C.; Lamensdorf, I.; Eisenhofer, G. Sources and Physiological Significance of Plasma Dopamine Sulfate. J. Clin. Endocrinol. Metab. 1999, 84, 2523–2531. [Google Scholar] [CrossRef]
- Miyajima, K.; Kawamoto, C.; Hara, S.; Mori-Kojima, M.; Ohye, T.; Sumi-Ichinose, C.; Saito, N.; Sasaoka, T.; Metzger, D.; Ichinose, H. Tyrosine Hydroxylase Conditional KO Mice Reveal Peripheral Tissue-Dependent Differences in Dopamine Biosynthetic Pathways. J. Biol. Chem. 2021, 296, 100544. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Mezey, E.; Yamamoto, T.; Aneman, A.; Friberg, P.; Eisenhofer, G. Is There a Third Peripheral Catecholaminergic System? Endogenous Dopamine as an Autocrine/Paracrine Substance Derived from Plasma DOPA and Inactivated by Conjugation. Hypertens. Res. 1995, 18 (Suppl. S1), S93–S99. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Lu, Y. Immunomodulatory Effects of Dopamine in Inflammatory Diseases. Front. Immunol. 2021, 12, 663102. [Google Scholar] [CrossRef] [PubMed]
- McKenna, F.; McLaughlin, P.J.; Lewis, B.J.; Sibbring, G.C.; Cummerson, J.A.; Bowen-Jones, D.; Moots, R.J. Dopamine Receptor Expression on Human T- and B-Lymphocytes, Monocytes, Neutrophils, Eosinophils and NK Cells: A Flow Cytometric Study. J. Neuroimmunol. 2002, 132, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Basu, B.; Chakroborty, D.; Dasgupta, P.S.; Basu, S. The immunoregulatory role of dopamine: An update. Brain Behav. Immun. 2010, 24, 525–528. [Google Scholar] [CrossRef]
- Shao, W.; Zhang, S.; Tang, M.; Zhang, X.; Zhou, Z.; Yin, Y.; Zhou, Q.; Huang, Y.; Liu, Y.; Wawrousek, E.; et al. Suppression of Neuroinflammation by Astrocytic Dopamine D2 Receptors via AB-Crystallin. Nature 2013, 494, 90–94. [Google Scholar] [CrossRef]
- Torres-Rosas, R.; Yehia, G.; Peña, G.; Mishra, P.; del Rocio Thompson-Bonilla, M.; Moreno-Eutimio, M.A.; Arriaga-Pizano, L.A.; Isibasi, A.; Ulloa, L. Dopamine Mediates Vagal Modulation of the Immune System by Electroacupuncture. Nat. Med. 2014, 20, 291–295. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Liu, L.; Wang, X.; Ding, C.; Tian, Z.; Zhou, R. Dopamine Controls Systemic Inflammation through Inhibition of NLRP3 Inflammasome. Cell 2015, 160, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Levite, M. Dopamine and T Cells: Dopamine Receptors and Potent Effects on T Cells, Dopamine Production in T Cells, and Abnormalities in the Dopaminergic System in T Cells in Autoimmune, Neurological and Psychiatric Diseases. Acta Physiol. 2016, 216, 42–89. [Google Scholar] [CrossRef]
- Brito-Melo, G.E.A.; Nicolato, R.; de Oliveira, A.C.P.; Menezes, G.B.; Lélis, F.J.N.; Avelar, R.S.; Sá, J.; Bauer, M.E.; Souza, B.R.; Teixeira, A.L.; et al. Increase in Dopaminergic, but Not Serotoninergic, Receptors in T-Cells as a Marker for Schizophrenia Severity. J. Psychiatr. Res. 2012, 46, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.; Contreras, F.; González, H.; Díaz, P.; Elgueta, D.; Barrientos, M.; Herrada, A.A.; Lladser, Á.; Bernales, S.; Pacheco, R. Stimulation of Dopamine Receptor D5 Expressed on Dendritic Cells Potentiates Th17-Mediated Immunity. J. Immunol. 2012, 188, 3062–3070. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.; Pinoli, M.; Rasini, E.; Martini, S.; Luini, A.; Pulze, L.; Dalla Gasperina, D.; Grossi, P.; Legnaro, M.; Ferrari, M.; et al. Dopaminergic Inhibition of Human Neutrophils Is Exerted through D1-like Receptors and Affected by Bacterial Infection. Immunology 2022, 167, 508–527. [Google Scholar] [CrossRef]
- Mori, T.; Kabashima, K.; Fukamachi, S.; Kuroda, E.; Sakabe, J.; Kobayashi, M.; Nakajima, S.; Nakano, K.; Tanaka, Y.; Matsushita, S.; et al. D1-like Dopamine Receptors Antagonist Inhibits Cutaneous Immune Reactions Mediated by Th2 and Mast Cells. J. Dermatol. Sci. 2013, 71, 37–44. [Google Scholar] [CrossRef]
- Kustrimovic, N.; Rasini, E.; Legnaro, M.; Marino, F.; Cosentino, M. Expression of Dopaminergic Receptors on Human CD4+ T Lymphocytes: Flow Cytometric Analysis ofIe and Memory Subsets and Relevance for the Neuroimmunology of Neurodegenerative Disease. J. Neuroimmune Pharmacol. 2014, 9, 302–312. [Google Scholar] [CrossRef]
- Pacheco, R.; Contreras, F.; Zouali, M. The Dopaminergic System in Autoimmune Diseases. Front. Immunol. 2014, 5, 117. [Google Scholar] [CrossRef]
- González, H.; Contreras, F.; Prado, C.; Elgueta, D.; Franz, D.; Bernales, S.; Pacheco, R. Dopamine Receptor D3 Expressed on CD4+ T Cells Favors Neurodegeneration of Dopaminergic Neurons during Parkinson’s Disease. J. Immunol. 2013, 190, 5048–5056. [Google Scholar] [CrossRef]
- Bach, F.; Grundmann, U.; Bauer, M.; Buchinger, H.; Soltész, S.; Graeter, T.; Larsen, R.; Silomon, M. Modulation of the Inflammatory Response to Cardiopulmonary Bypass by Dopexamine and Epidural Anesthesia. Acta Anaesthesiol. Scand. 2002, 46, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, J.; Klotz, E.; Spies, C.D.; Lorenz, B.; Stuebs, P.; Hein, O.V.; Grundling, M.; Pavlovic, D.; Usichenko, T.; Wendt, M.; et al. Effects of Dopexamine on the Intestinal Microvascular Blood Flow and Leukocyte Activation in a Sepsis Model in Rats. Crit. Care 2006, 10, R117. [Google Scholar] [CrossRef]
- Ghosh, M.C.; Mondal, A.C.; Basu, S.; Banerjee, S.; Majumder, J.; Bhattacharya, D.; Dasgupta, P.S. Dopamine Inhibits Cytokine Release and Expression of Tyrosine Kinases, Lck and Fyn in Activated T Cells. Int. Immunopharmacol. 2003, 3, 1019–1026. [Google Scholar] [CrossRef]
- Nakano, K.; Higashi, T.; Hashimoto, K.; Takagi, R.; Tanaka, Y.; Matsushita, S. Antagonizing Dopamine D1-like Receptor Inhibits Th17 Cell Differentiation: Preventive and Therapeutic Effects on Experimental Autoimmune Encephalomyelitis. Biochem. Biophys. Res. Commun. 2008, 373, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Inoue, T.; Higashi, T.; Takei, S.-I.; Awata, T.; Katayama, S.; Takagi, R.; Okada, H.; Matsushita, S. Dopamine D1-like Receptor Antagonist, SCH23390, Exhibits a Preventive Effect on Diabetes Mellitus That Occurs Naturally in NOD Mice. Biochem. Biophys. Res. Commun. 2009, 383, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Inoue, T.; Hashimoto, K.; Suzuki, H.; Matsushita, S. D1-like receptor antagonist inhibits IL-17 expression and attenuates crescent formation in nephrotoxic serum nephritis. Am. J. Nephrol. 2009, 30, 274–279. [Google Scholar] [CrossRef]
- Haskó, G.; Szabó, C.; Németh, Z.H.; Deitch, E.A. Dopamine Suppresses IL-12 P40 Production by Lipopolysaccharide-Stimulated Macrophages via a Beta-Adrenoceptor-Mediated Mechanism. J. Neuroimmunol. 2002, 122, 34–39. [Google Scholar] [CrossRef]
- Liu, A.; Ding, S. Anti-Inflammatory Effects of Dopamine in Lipopolysaccharide (LPS)-Stimulated RAW264.7 Cells via Inhibiting NLRP3 Inflammasome Activation. Ann. Clin. Lab. Sci. 2019, 49, 353–360. [Google Scholar]
- Sadeghi, H.; Parishani, M.; Akbartabar Touri, M.; Ghavamzadeh, M.; Jafari Barmak, M.; Zarezade, V.; Delaviz, H.; Sadeghi, H. Pramipexole reduces inflammation in the experimental animal models of inflammation. Immunopharmacol. Immunotoxicol. 2017, 39, 80–86. [Google Scholar] [CrossRef]
- Bendele, A.M.; Spaethe, S.M.; Benslay, D.N.; Bryant, H.U. Anti-Inflammatory Activity of Pergolide, a Dopamine Receptor Agonist. J. Pharmacol. Exp. Ther. 1991, 259, 169–175. [Google Scholar]
- Huang, Y.; Chen, C.-C.; Wang, T.-T.; Qiu, Y.-H.; Peng, Y.-P. Dopamine Receptors Modulate T Lymphocytes via Inhibition of CAMP-CREB Signaling Pathway. Neuro Endocrinol. Lett. 2016, 37, 491–500. [Google Scholar]
- Han, X.; Ni, J.; Wu, Z.; Wu, J.; Li, B.; Ye, X.; Dai, J.; Chen, C.; Xue, J.; Wan, R.; et al. Myeloid-Specific Dopamine D2 Receptor Signalling Controls Inflammation in Acute Pancreatitis via Inhibiting M1 Macrophage. Br. J. Pharmacol. 2020, 177, 2991–3008. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ohta, N.; Matsumoto, A.; Horiguchi, Y.; Koide, M.; Fujino, Y. Haloperidol Suppresses NF-KappaB to Inhibit Lipopolysaccharide-Induced Pro-Inflammatory Response in RAW 264 Cells. Med. Sci. Monit. 2016, 22, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Schetz, J.A.; Benjamin, P.S.; Sibley, D.R. Nonconserved residues in the second transmembrane-spanning domain of the D(4) dopamine receptor are molecular determinants of D(4)-selective pharmacology. Mol. Pharmacol. 2000, 57, 144–152. [Google Scholar]
- Anlauf, M.; Schäfer, M.K.-H.; Eiden, L.; Weihe, E. Chemical Coding of the Human Gastrointestinal Nervous System: Cholinergic, VIPergic, and Catecholaminergic Phenotypes. J. Comp. Neurol. 2003, 459, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Pham, T.D.; Tamir, H.; Chen, J.J.; Gershon, M.D. Enteric Dopaminergic Neurons: Definition, Developmental Lineage, and Effects of Extrinsic Denervation. J. Neurosci. 2004, 24, 1330–1339. [Google Scholar] [CrossRef]
- Vieira-Coelho, M.A.; Soares-da-Silva, P. Dopamine Formation, from Its Immediate Precursor 3,4-Dihydroxyphenylalanine, along the Rat Digestive Tract. Fundam. Clin. Pharmacol. 1993, 7, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, C.; Zhou, L.; Wang, T.; Fu, F. Continuous Activation of Dopamine Receptors Alleviates LPS-Induced Liver Injury in Mice via β-arrestin2 Dependent Akt/NF-κB Pathway. Front. Pharmacol. 2022, 13, 853834. [Google Scholar] [CrossRef]
- Herak-Perković, V.; Grabarević, Z.; Banić, M.; Anić, B.; Novosel, V.; Pogacnik, M. Effects of Dopaminergic Drugs on Inflammatory Bowel Disease Induced with 2,4-Dinitrofluorbenzene in BALB/c Mice. J. Vet. Pharmacol. Ther. 2001, 24, 267–273. [Google Scholar] [CrossRef]
- Ugalde, V.; Contreras, F.; Prado, C.; Chovar, O.; Espinoza, A.; Pacheco, R. Dopaminergic Signalling Limits Suppressive Activity and Gut Homing of Regulatory T Cells upon Intestinal Inflammation. Mucosal Immunol. 2021, 14, 652–666. [Google Scholar] [CrossRef]
- Magro, F.; Vieira-Coelho, M.A.; Fraga, S.; Serrão, M.P.; Veloso, F.T.; Ribeiro, T.; Soares-da-Silva, P. Impaired Synthesis or Cellular Storage of Norepinephrine, Dopamine, and 5-Hydroxytryptamine in Human Inflammatory Bowel Disease. Dig. Dis. Sci. 2002, 47, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Tolstanova, G.; Deng, X.; Ahluwalia, A.; Paunovic, B.; Prysiazhniuk, A.; Ostapchenko, L.; Tarnawski, A.; Sandor, Z.; Szabo, S. Role of Dopamine and D2 Dopamine Receptor in the Pathogenesis of Inflammatory Bowel Disease. Dig. Dis. Sci. 2015, 60, 2963–2975. [Google Scholar] [CrossRef] [PubMed]
- Aslanoglou, D.; Bertera, S.; Friggeri, L.; Sánchez-Soto, M.; Lee, J.; Xue, X.; Logan, R.W.; Lane, J.R.; Yechoor, V.K.; McCormick, P.J.; et al. Dual Pancreatic Adrenergic and Dopaminergic Signaling as a Therapeutic Target of Bromocriptine. iScience 2022, 25, 104771. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-W.; Li, J.; Feng, X.-Y.; Chen, H.; Chen, Y.; Liu, J.-H.; Zhang, Y.; Hong, F.; Zhu, J.-X. Pancreatic Acinar Cells Utilize Tyrosine to Synthesize L-Dihydroxyphenylalanine. Exp. Biol. Med. 2021, 246, 2533–2542. [Google Scholar] [CrossRef]
- Rubí, B.; Ljubicic, S.; Pournourmohammadi, S.; Carobbio, S.; Armanet, M.; Bartley, C.; Maechler, P. Dopamine D2-like Receptors Are Expressed in Pancreatic Beta Cells and Mediate Inhibition of Insulin Secretion. J. Biol. Chem. 2005, 280, 36824–36832. [Google Scholar] [CrossRef]
- Farino, Z.J.; Morgenstern, T.J.; Maffei, A.; Quick, M.; De Solis, A.J.; Wiriyasermkul, P.; Freyberg, R.J.; Aslanoglou, D.; Sorisio, D.; Inbar, B.P.; et al. New Roles for Dopamine D2 and D3 Receptors in Pancreatic Beta Cell Insulin Secretion. Mol. Psychiatry 2020, 25, 2070–2085. [Google Scholar] [CrossRef]
- Mezey, E.; Eisenhofer, G.; Harta, G.; Hansson, S.; Gould, L.; Hunyady, B.; Hoffman, B.J. A Novel Nonneuronal Catecholaminergic System: Exocrine Pancreas Synthesizes and Releases Dopamine. Proc. Natl. Acad. Sci. USA 1996, 93, 10377–10382. [Google Scholar] [CrossRef]
- Karanjia, N.D.; Widdison, A.L.; Lutrin, F.J.; Chang, Y.-B.; Reber, H.A. The Antiinflammatory Effect of Dopamine in Alcoholic Hemorrhagic Pancreatitis in Cats: Studies on the Receptors and Mechanisms of Action. Gastroenterology 1991, 101, 1635–1641. [Google Scholar] [CrossRef]
- Han, X.; Li, B.; Ye, X.; Mulatibieke, T.; Wu, J.; Dai, J.; Wu, D.; Ni, J.; Zhang, R.; Xue, J.; et al. Dopamine D2 Receptor Signalling Controls Inflammation in Acute Pancreatitis via a PP2A-Dependent Akt/NF-ΚB Signalling Pathway. Br. J. Pharmacol. 2017, 174, 4751–4770. [Google Scholar] [CrossRef]
- Ye, X.; Han, X.; Li, B.; Dai, J.; Wu, Z.; He, Y.; Wen, L.; Hu, G. Dopamine D2 Receptor Activator Quinpirole Protects against Trypsinogen Activation during Acute Pancreatitis via Upregulating HSP70. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G1000–G1012. [Google Scholar] [CrossRef]
- Mignini, F.; Tomassoni, D.; Traini, E.; Amenta, F. Dopamine, Vesicular Transporters and Dopamine Receptor Expression and Localization in Rat Thymus and Spleen. J. Neuroimmunol. 2009, 206, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Carr, L.; Tucker, A.; Fernandez-Botran, R. In Vivo Administration of L-Dopa or Dopamine Decreases the Number of Splenic IFN Gamma-Producing Cells. J. Neuroimmunol. 2003, 137, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Huang, Y.; Liu, Z.; Cao, B.-B.; Peng, Y.-P.; Qiu, Y.-H. Dopamine Receptors Modulate Cytotoxicity of Natural Killer Cells via CAMP-PKA-CREB Signaling Pathway. PLoS ONE 2013, 8, e65860. [Google Scholar] [CrossRef]
- Feketeova, E.; Li, Z.; Joseph, B.; Shah, R.; Spolarics, Z.; Ulloa, L. Dopaminergic Control of Inflammation and Glycemia in Sepsis and Diabetes. Front. Immunol. 2018, 9, 943. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Wang, T.; Yang, Y.; Fan, Y.; Zhou, L.; Li, M.; Fu, F. Lipopolysaccharide/D-galactosamine-induced acute liver injury could be attenuated by dopamine receptor agonist rotigotine via regulating NF-κB signaling pathway. Int. Immunopharmacol. 2021, 96, 107798. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tang, L.; Yang, Y.; Lin, L.; Dai, J.; Pu, G.; Ai, Q.; Jiang, R.; Zhang, L. Dopamine Alleviated Acute Liver Injury Induced by Lipopolysaccharide/d-Galactosamine in Mice. Int. Immunopharmacol. 2018, 61, 249–255. [Google Scholar] [CrossRef]
- Peng, X.; Yang, Y.; Tang, L.; Wan, J.; Dai, J.; Li, L.; Huang, J.; Shen, Y.; Lin, L.; Gong, X.; et al. Therapeutic benefits of apocynin in mice with lipopolysaccharide/D-galactosamine-induced acute liver injury via suppression of the late stage pro-apoptotic AMPK/JNK pathway. Biomed. Pharmacother. 2020, 125, 110020. [Google Scholar] [CrossRef]
- Yang, C.; He, L.; Wang, C.; Huang, Y.; Wang, A.; Li, X.; Ao, J. Dexmedetomidine alleviated lipopolysaccharide/D-galactosamine-induced acute liver injury in mice. Int. Immunopharmacol. 2019, 72, 367–373. [Google Scholar] [CrossRef]
- Xue, R.; Zhang, H.; Pan, J.; Du, Z.; Zhou, W.; Zhang, Z.; Tian, Z.; Zhou, R.; Bai, L. Peripheral Dopamine Controlled by Gut Microbes Inhibits Invariant Natural Killer T Cell-Mediated Hepatitis. Front. Immunol. 2018, 9, 2398. [Google Scholar] [CrossRef]
- Liu, X.-F.; Long, H.-J.; Miao, X.-Y.; Liu, G.-L.; Yao, H.-L. Fisetin Inhibits Liver Cancer Growth in a Mouse Model: Relation to Dopamine Receptor. Oncol. Rep. 2017, 38, 53–62. [Google Scholar] [CrossRef]
- Harkitis, P.; Daskalopoulos, E.P.; Malliou, F.; Lang, M.A.; Marselos, M.; Fotopoulos, A.; Albucharali, G.; Konstandi, M. Dopamine D2-Receptor Antagonists Down-Regulate CYP1A1/2 and CYP1B1 in the Rat Liver. PLoS ONE 2015, 10, e0128708. [Google Scholar] [CrossRef]
- Aviado, D.M.; Sadavongvivad, C. Pharmacological Significance of Biogenic Amines in the Lungs: Noradrenaline and Dopamine. Br. J. Pharmacol. 1970, 38, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Adir, Y.; Azzam, Z.S.; Lecuona, E.; Leal, S.; Pesce, L.; Dumasius, V.; Bertorello, A.M.; Factor, P.; Young, J.B.; Ridge, K.M.; et al. Augmentation of Endogenous Dopamine Production Increases Lung Liquid Clearance. Am. J. Respir. Crit. Care Med. 2004, 169, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.O.; Koh, P.O.; Kim, J.H.; Kim, J.S.; Kang, S.S.; Cho, G.J.; Kim, K.; Choi, W.S. Localization of Dopamine D1 and D2 Receptor mRNAs in the Rat Systemic and Pulmonary Vasculatures. Mol. Cells 1999, 9, 417–421. [Google Scholar] [PubMed]
- Kobayashi, Y.; Ricci, A.; Amenta, F. Autoradiographic Localization of Dopamine D1-like Receptors in the Rabbit Pulmonary Circulation. Eur. J. Pharmacol. 1994, 253, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Cavallotti, D.; Ricci, A.; Amenta, F. Localisation of Dopamine D2-like Receptors in Pulmonary Artery of the Human and Rabbit but Not of the Rat. Eur. J. Pharmacol. 1994, 261, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Bairam, A.; Frenette, J.; Dauphin, C.; Carroll, J.L.; Khandjian, E.W. Expression of Dopamine D1-Receptor MRNA in the Carotid Body of Adult Rabbits, Cats and Rats. Neurosci. Res. 1998, 31, 147–154. [Google Scholar] [CrossRef]
- Bairam, A.; Néji, H.; De-Grandpré, P.; Carroll, J.L. Autoreceptor Mechanism Regulating Carotid Body Dopamine Release from Adult and 10-Day-Old Rabbits. Respir. Physiol. 2000, 120, 27–34. [Google Scholar] [CrossRef]
- Ciarka, A.; Vincent, J.-L.; van de Borne, P. The Effects of Dopamine on the Respiratory System: Friend or Foe? Pulm. Pharmacol. Ther. 2007, 20, 607–615. [Google Scholar] [CrossRef]
- Peiser, C.; Trevisani, M.; Groneberg, D.A.; Dinh, Q.T.; Lencer, D.; Amadesi, S.; Maggiore, B.; Harrison, S.; Geppetti, P.; Fischer, A. Dopamine Type 2 Receptor Expression and Function in Rodent Sensory Neurons Projecting to the Airways. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, L153–L158. [Google Scholar] [CrossRef]
- Alan, P.N.D.M.J.; Ince, Y.I.G.D.F.; Holt, K.M.R.P.R. Dual D2 Dopamine Receptor and SS2-Adrenoceptor Agonists for the Modulation of Sensory Nerves in COPD. New Drugs Asthma Allergy COPD 2001, 31, 68–71. [Google Scholar]
- Bone, N.B.; Liu, Z.; Pittet, J.-F.; Zmijewski, J.W. Frontline Science: D1 Dopaminergic Receptor Signaling Activates the AMPK-Bioenergetic Pathway in Macrophages and Alveolar Epithelial Cells and Reduces Endotoxin-Induced ALI. J. Leukoc. Biol. 2017, 101, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, N.; Shibata, S.; Matoba, A.; Kudo, T.-A.; Danielsson, J.; Kohjitani, A.; Masaki, E.; Emala, C.W.; Mizuta, K. The Dopamine D1 Receptor Is Expressed and Induces CREB Phosphorylation and MUC5AC Expression in Human Airway Epithelium. Respir. Res. 2018, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Vohra, P.K.; Hoeppner, L.H.; Sagar, G.; Dutta, S.K.; Misra, S.; Hubmayr, R.D.; Mukhopadhyay, D. Dopamine Inhibits Pulmonary Edema through the VEGF-VEGFR2 Axis in a Murine Model of Acute Lung Injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L185–L192. [Google Scholar] [CrossRef]
- Tonnarini, G.; Parlapiano, C.; Cavallotti, D.; Tego, A.; Curione, M.; Giancaspro, G.; Vincentelli, G.M.; Leone, S.; Cavallotti, C. Dopamine Receptor Subtypes in the Human Coronary Vessels of Healthy Subjects. J. Recept. Signal Transduct. Res. 2011, 31, 33–38. [Google Scholar] [CrossRef]
- Cavallotti, C.; Mancone, M.; Bruzzone, P.; Sabbatini, M.; Mignini, F. Dopamine Receptor Subtypes in the Native Human Heart. Heart Vessels 2010, 25, 432–437. [Google Scholar] [CrossRef]
- Liu, J.; Jin, Y.; Wang, B.; Wang, Y.; Zuo, S.; Zhang, J. Dopamine D1 receptor alleviates doxorubicin-induced cardiac injury by inhibiting NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2021, 561, 7–13. [Google Scholar] [CrossRef]
- Li, H.; Shi, S.; Sun, Y.-H.; Zhao, Y.-J.; Li, Q.-F.; Li, H.-Z.; Wang, R.; Xu, C.-Q. Dopamine D2 Receptor Stimulation Inhibits Angiotensin II-Induced Hypertrophy in Cultured Neonatal Rat Ventricular Myocytes. Clin. Exp. Pharmacol. Physiol. 2009, 36, 312–318. [Google Scholar] [CrossRef]
- Li, H.; Guo, J.; Gao, J.; Han, L.; Jiang, C.; Li, H.; Bai, S.; Zhang, W.; Li, G.; Wang, L.; et al. Role of Dopamine D2 Receptors in Ischemia/Reperfusion Induced Apoptosis of Cultured Neonatal Rat Cardiomyocytes. J. Biomed. Sci. 2011, 18, 18. [Google Scholar] [CrossRef]
- Aguayo-Cerón, K.A.; Calzada-Mendoza, C.C.; Méndez-Bolaina, E.; Romero-Nava, R.; Ocharan-Hernández, M.E. The Regulatory Effect of Bromocriptine on Cardiac Hypertrophy by Prolactin and D2 Receptor Modulation. Clin. Exp. Hypertens. 2020, 42, 675–679. [Google Scholar] [CrossRef]
- Gupta, V.; Goyal, R.; Sharma, P.L. Preconditioning Offers Cardioprotection in Hyperlipidemic Rat Hearts: Possible Role of Dopamine (D2) Signaling. BMC Cardiovasc. Disord. 2015, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, W.-L.; Xu, J.-J.; Zhu, S.-Q.; Long, X.; Che, J.-P. D2 Dopamine Receptor Antagonist Raclopride Induces Non-Canonical Autophagy in Cardiac Myocytes. J. Cell Biochem. 2013, 114, 103–110. [Google Scholar] [CrossRef]
- Li, H.; Wei, C.; Gao, J.; Bai, S.; Li, H.; Zhao, Y.; Li, H.; Han, L.; Tian, Y.; Yang, G.; et al. Mediation of Dopamine D2 Receptors Activation in Post-Conditioning-Attenuated Cardiomyocyte Apoptosis. Exp. Cell Res. 2014, 323, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Guo, J.; Li, H.; Bai, S.; Li, H.; Wu, B.; Wang, L.; Xi, Y.; Tian, Y.; Yang, G.; et al. Involvement of Dopamine D2 Receptors Activation in Ischemic Post-Conditioning-Induced Cardioprotection through Promoting PKC-ε Particulate Translocation in Isolated Rat Hearts. Mol. Cell Biochem. 2013, 379, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Gaweda, G.; Iyer, R.P.; Shaver, P.R.; Grilo, G.A.; Dinkins, M.-L.; Stoffel, H.J.; Clemens, S.; de Castro Brás, L.E. Dopamine Receptor D3 Agonist (Pramipexole) Reduces Morphine-Induced Cardiac Fibrosis. Biochem. Biophys. Res. Commun. 2020, 529, 1080–1085. [Google Scholar] [CrossRef]
- Johnson, T.L.; Tulis, D.A.; Keeler, B.E.; Virag, J.A.; Lust, R.M.; Clemens, S. The Dopamine D3 Receptor Knockout Mouse Mimics Aging-Related Changes in Autonomic Function and Cardiac Fibrosis. PLoS ONE 2013, 8, e74116. [Google Scholar] [CrossRef]
- Liu, X.-S.; Zeng, J.; Yang, Y.-X.; Qi, C.-L.; Xiong, T.; Wu, G.-Z.; Zeng, C.-Y.; Wang, D.-X. DRD4 Mitigates Myocardial Ischemia/Reperfusion Injury in Association With PI3K/AKT Mediated Glucose Metabolism. Front. Pharmacol. 2020, 11, 619426. [Google Scholar] [CrossRef]
- Niewiarowska-Sendo, A.; Kozik, A.; Guevara-Lora, I. Influence of Bradykinin B2 Receptor and Dopamine D2 Receptor on the Oxidative Stress, Inflammatory Response, and Apoptotic Process in Human Endothelial Cells. PLoS ONE 2018, 13, e0206443. [Google Scholar] [CrossRef]
- Kang, H.; Yu, H.; Fan, J.; Cao, G. Rotigotine Protects against Oxidized Low-Density Lipoprotein(Ox-LDL)-Induced Damages in Human Umbilical Vein Endothelial Cells(HUVECs). Bioengineered 2021, 12, 10568–10579. [Google Scholar] [CrossRef]
- Sookhai, S.; Wang, J.H.; Winter, D.; Power, C.; Kirwan, W.; Redmond, H.P. Dopamine Attenuates the Chemoattractant Effect of Interleukin-8: A Novel Role in the Systemic Inflammatory Response Syndrome. Shock 2000, 14, 295–299. [Google Scholar] [CrossRef]
- Kapper, S.; Beck, G.; Riedel, S.; Prem, K.; Haak, M.; van der Woude, F.J.; Yard, B.A. Modulation of chemokine production and expression of adhesion molecules in renal tubular epithelial and endothelial cells by catecholamines. Transplantation 2002, 74, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Borcherding, D.C.; Hugo, E.R.; Idelman, G.; De Silva, A.; Richtand, N.W.; Loftus, J.; Ben-Jonathan, N. Dopamine Receptors in Human Adipocytes: Expression and Functions. PLoS ONE 2011, 6, e25537. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.; Ribeiro, L. Dopaminergic Pathways in Obesity-Associated Inflammation. J. Neuroimmune Pharmacol. 2020, 15, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Villar, V.A.; Tiu, A.; Upadhyay, K.K.; Cuevas, S. Dopamine D2 Receptor Upregulates Leptin and IL-6 in Adipocytes. J. Lipid Res. 2018, 59, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Kohlie, R.; Perwitz, N.; Resch, J.; Schmid, S.M.; Lehnert, H.; Klein, J.; Iwen, K.A. Dopamine Directly Increases Mitochondrial Mass and Thermogenesis in Brown Adipocytes. J. Mol. Endocrinol. 2017, 58, 57–66. [Google Scholar] [CrossRef]
- Raffaelli, F.-M.; Resch, J.; Oelkrug, R.; Iwen, K.A.; Mittag, J. Dopamine Receptor D1- and D2-Agonists Do Not Spark Brown Adipose Tissue Thermogenesis in Mice. Sci. Rep. 2020, 10, 20203. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, M.; Asico, L.D.; Eisner, G.M.; Jose, P.A. The Dopaminergic System in Hypertension. Clin. Sci. 2007, 112, 583–597. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Y.; Zhu, M.; Gu, Y.; Zhang, W.; Shao, H.; Wang, Y.; Ping, Z.; Hu, X.; Wang, L.; et al. Inhibition of Titanium-Particle-Induced Inflammatory Osteolysis after Local Administration of Dopamine and Suppression of Osteoclastogenesis via D2-like Receptor Signaling Pathway. Biomaterials 2016, 80, 1–10. [Google Scholar] [CrossRef]
- Lu, J.-H.; Liu, Y.-Q.; Deng, Q.-W.; Peng, Y.-P.; Qiu, Y.-H. Dopamine D2 Receptor Is Involved in Alleviation of Type II Collagen-Induced Arthritis in Mice. Biomed. Res. Int. 2015, 2015, 496759. [Google Scholar] [CrossRef]
- Puyó, A.M.; Levin, G.M.; Armando, I.; Barontini, M.B. Free and Conjugated Plasma Catecholamines in Pheochromocytoma Patients with and without Sustained Hypertension. Acta Endocrinol. 1986, 113, 111–117. [Google Scholar] [CrossRef]
- Da Prada, M.; Zürcher, M. Simultaneous Radioenzymatic Determination of Plasma and Tissue Adrenaline, Noradrenaline and Dopamine within the Femtomole Range. Life Sci. 1976, 19, 1161–1174. [Google Scholar] [CrossRef]
- Baines, A.D. Effects of Salt Intake and Renal Denervation on Catecholamine Catabolism and Excretion. Kidney Int. 1982, 21, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Grossman, E.; Armando, I.; Wolfovitz, E.; Folio, C.J.; Holmes, C.; Keiser, H.R. Correlates of Urinary Excretion of Catechols in Humans. Biog. Amines 1993, 10, 3–17. [Google Scholar]
- Lee, M.R. Dopamine and the Kidney: Ten Years On. Clin. Sci. 1993, 84, 357–375. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Siragy, H.M.; Felder, R.A.; Carey, R.M. Intrarenal Dopamine Production and Distribution in the Rat. Physiological Control of Sodium Excretion. Hypertension 1997, 29, 228–234. [Google Scholar] [CrossRef]
- Bell, C. Dopamine Release from Sympathetic Nerve Terminals. Prog. Neurobiol. 1988, 30, 193–208. [Google Scholar] [CrossRef]
- Dinerstein, R.J.; Vannice, J.; Henderson, R.C.; Roth, L.J.; Goldberg, L.I.; Hoffmann, P.C. Histofluorescence Techniques Provide Evidence for Dopamine-Containing Neuronal Elements in Canine Kidney. Science 1979, 205, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Adam, W.R.; Adams, B.A. Production and Excretion of Dopamine by the Isolated Perfused Rat Kidney. Ren. Physiol. 1985, 8, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Akama, H.; Noshiro, T.; Sano, N.; Watanabe, T.; Trigg, L.; Kotsonis, P.; Majewski, H.; McGrath, B.P.; Miura, Y.; Abe, K. Effects of Isotonic Saline Loading on Renal Tubular and Neurogenic Dopamine Release in Conscious Rabbits. Clin. Exp. Pharmacol. Physiol. 1995, 22, 469–471. [Google Scholar] [CrossRef]
- Berndt, T.J.; Khraibi, A.A.; Thothathri, V.; Dousa, T.P.; Tyce, G.M.; Knox, F.G. Effect of Increased Dietary Phosphate Intake on Dopamine Excretion in the Presence and Absence of the Renal Nerves. Miner. Electrolyte Metab. 1994, 20, 158–162. [Google Scholar] [PubMed]
- Hegde, S.S.; Lokhandwala, M.F. Stimulation of Renal Dopamine Production during Acute Volume Expansion Requires the Presence of Intact Vagi but Not Renal Nerves. Clin. Exp. Hypertens. A 1992, 14, 1169–1187. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, R.K.; Sole, M.J.; Baines, A.D. Neural and Extraneural Catecholamine Production by Rat Kidneys. Am. J. Physiol. 1982, 242, F261–F266. [Google Scholar] [CrossRef]
- Ball, S.G.; Gunn, I.G.; Douglas, I.H. Renal Handling of Dopa, Dopamine, Norepinephrine, and Epinephrine in the Dog. Am. J. Physiol. 1982, 242, F56–F62. [Google Scholar] [CrossRef]
- Boren, D.R.; Henry, D.P.; Selkurt, E.E.; Weinberger, M.H. Renal Modulation of Urinary Catecholamine Excretion during Volume Expansion in the Dog. Hypertension 1980, 2, 383–389. [Google Scholar] [CrossRef]
- Grossman, E.; Hoffman, A.; Armando, I.; Abassi, Z.; Kopin, I.J.; Goldstein, D.S. Sympathoadrenal Contribution to Plasma Dopa (3,4-Dihydroxyphenylalanine) in Rats. Clin. Sci. 1992, 83, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Nakane, H.; Kawamura, M.; Yoshizawa, M.; Takeshita, E.; Saruta, T. Excretion and Metabolism of Dopa and Dopamine by Isolated Perfused Rat Kidney. Am. J. Physiol. 1984, 247, E285–E290. [Google Scholar] [CrossRef] [PubMed]
- Wolfovitz, E.; Grossman, E.; Folio, C.J.; Keiser, H.R.; Kopin, I.J.; Goldstein, D.S. Derivation of Urinary Dopamine from Plasma Dihydroxyphenylalanine in Humans. Clin. Sci. 1993, 84, 549–557. [Google Scholar] [CrossRef]
- Zimlichman, R.; Levinson, P.D.; Kelly, G.; Stull, R.; Keiser, H.R.; Goldstein, D.S. Derivation of Urinary Dopamine from Plasma Dopa. Clin. Sci. 1988, 75, 515–520. [Google Scholar] [CrossRef]
- Baines, A.D.; Chan, W. Production of Urine Free Dopamine from DOPA; a Micropuncture Study. Life Sci. 1980, 26, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Baines, A.D.; Drangova, R.; Hatcher, C. Dopamine Production by Isolated Glomeruli and Tubules from Rat Kidneys. Can. J. Physiol. Pharmacol. 1985, 63, 155–158. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Goldstein, D.S.; Ropchak, T.G.; Kopin, I.J. Source and Physiological Significance of Plasma 3,4-Dihydroxyphenylalanine in the Rat. J. Neurochem. 1988, 51, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Eldrup, E.; Hetland, M.L.; Christensen, N.J. Increase in Plasma 3,4-Dihydroxyphenylalanine (DOPA) Appearance Rate after Inhibition of DOPA Decarboxylase in Humans. Eur. J. Clin. Investig. 1994, 24, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Holmes, C.; Cannon, R.O.; Eisenhofer, G.; Kopin, I.J. Sympathetic Cardioneuropathy in Dysautonomias. N. Engl. J. Med. 1997, 336, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Udelsman, R.; Eisenhofer, G.; Stull, R.; Keiser, H.R.; Kopin, I.J. Neuronal Source of Plasma Dihydroxyphenylalanine. J. Clin. Endocrinol. Metab. 1987, 64, 856–861. [Google Scholar] [CrossRef]
- Pinho, M.J.; Serrão, M.P.; Gomes, P.; Hopfer, U.; Jose, P.A.; Soares-da-Silva, P. Over-expression of renal LAT1 and LAT2 and enhanced L-DOPA uptake in SHR immortalized renal proximal tubular cells. Kidney Int. 2004, 66, 216–226. [Google Scholar] [CrossRef]
- Pinho, M.J.; Gomes, P.; Serrão, M.P.; Bonifácio, M.J.; Soares-da-Silva, P. Organ-specific overexpression of renal LAT2 and enhanced tubular L-DOPA uptake precede the onset of hypertension. Hypertension. 2003, 42, 613–618. [Google Scholar] [CrossRef]
- Wu, Y.; Yin, Q.; Lin, S.; Huang, X.; Xia, Q.; Chen, Z.; Zhang, X.; Yang, D. Increased SLC7A8 expression mediates L-DOPA uptake by renal tubular epithelial cells. Mol. Med. Rep. 2017, 16, 887–893. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Yang, Y.; Yang, J.; Asico, L.D.; Chen, W.; Felder, R.A.; Armando, I.; Jose, P.A.; Yang, Z. Gastrin stimulates renal dopamine production by increasing the renal tubular uptake of l-DOPA. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E1–E10. [Google Scholar] [CrossRef]
- Hoeger, S.; Reisenbuechler, A.; Gottmann, U.; Doyon, F.; Braun, C.; Kaya, Z.; Seelen, M.A.; van Son, W.J.; Waldherr, R.; Schnuelle, P.; et al. Donor Dopamine Treatment in Brain Dead Rats Is Associated with an Improvement in Renal Function Early after Transplantation and a Reduction in Renal Inflammation. Transpl. Int. 2008, 21, 1072–1080. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Yao, B.; Wang, S.; Fan, X.; Wu, G.; Yang, H.; Yin, H.; Yang, S.; Harris, R.C. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J. Clin. Investig. 2011, 121, 2845–2854. [Google Scholar] [CrossRef]
- Yang, S.; Yao, B.; Zhou, Y.; Yin, H.; Zhang, M.-Z.; Harris, R.C. Intrarenal Dopamine Modulates Progressive Angiotensin II-Mediated Renal Injury. Am. J. Physiol. Ren. Physiol. 2012, 302, F742–F749. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Yao, B.; Yang, S.; Yang, H.; Wang, S.; Fan, X.; Yin, H.; Fogo, A.B.; Moeckel, G.W.; Harris, R.C. Intrarenal dopamine inhibits progression of diabetic nephropathy. Diabetes 2012, 61, 2575–2584. [Google Scholar] [CrossRef]
- Baradaran, A.; Nasri, H.; Rafieian-Kopaei, M. Oxidative Stress and Hypertension: Possibility of Hypertension Therapy with Antioxidants. J. Res. Med. Sci. 2014, 19, 358–367. [Google Scholar] [PubMed]
- Rodrigo, R.; González, J.; Paoletto, F. The Role of Oxidative Stress in the Pathophysiology of Hypertension. Hypertens. Res. 2011, 34, 431–440. [Google Scholar] [CrossRef]
- Sedeek, M.; Hébert, R.L.; Kennedy, C.R.; Burns, K.D.; Touyz, R.M. Molecular Mechanisms of Hypertension: Role of Nox Family NADPH Oxidases. Curr. Opin. Nephrol. Hypertens. 2009, 18, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Santillo, M.; Colantuoni, A.; Mondola, P.; Guida, B.; Damiano, S. NOX Signaling in Molecular Cardiovascular Mechanisms Involved in the Blood Pressure Homeostasis. Front. Physiol. 2015, 6, 194. [Google Scholar] [CrossRef]
- Farooqui, Z.; Mohammad, R.S.; Lokhandwala, M.F.; Banday, A.A. Nrf2 inhibition induces oxidative stress, renal inflammation and hypertension in mice. Clin. Exp. Hypertens. 2021, 43, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Banday, A.A.; Fazili, F.R.; Lokhandwala, M.F. Oxidative stress causes renal dopamine D1 receptor dysfunction and hypertension via mechanisms that involve nuclear factor-kappaB and protein kinase C. J. Am. Soc. Nephrol. 2007, 18, 1446–1457. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Góngora, M.C.; Harrison, D.G.; Lambeth, J.D.; Dikalov, S.; Griendling, K.K. Upregulation of Nox1 in Vascular Smooth Muscle Leads to Impaired Endothelium-Dependent Relaxation via ENOS Uncoupling. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H673–H679. [Google Scholar] [CrossRef]
- Wind, S.; Beuerlein, K.; Armitage, M.E.; Taye, A.; Kumar, A.H.S.; Janowitz, D.; Neff, C.; Shah, A.M.; Wingler, K.; Schmidt, H.H.H.W. Oxidative Stress and Endothelial Dysfunction in Aortas of Aged Spontaneously Hypertensive Rats by NOX1/2 Is Reversed by NADPH Oxidase Inhibition. Hypertension 2010, 56, 490–497. [Google Scholar] [CrossRef]
- Armando, I.; Wang, X.; Villar, V.A.M.; Jones, J.E.; Asico, L.D.; Escano, C.; Jose, P.A. Reactive Oxygen Species-Dependent Hypertension in Dopamine D2 Receptor-Deficient Mice. Hypertension 2007, 49, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Cuevas, S.; Villar, V.A.; Escano, C.; Asico, L.; Yu, P.; Grandy, D.K.; Felder, R.A.; Armando, I.; et al. Paraoxonase 2 decreases renal reactive oxygen species production, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of NADPH oxidase. Free Radic. Biol. Med. 2012, 53, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cuevas, S.; Yang, S.; Villar, V.A.; Escano, C.; Asico, L.; Yu, P.; Jiang, X.; Weinman, E.J.; Armando, I.; et al. Sestrin2 decreases renal oxidative stress, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of reactive oxygen species production. Hypertension 2014, 64, 825–832. [Google Scholar] [CrossRef]
- Yasunari, K.; Kohno, M.; Kano, H.; Minami, M.; Yoshikawa, J. Dopamine as a Novel Antioxidative Agent for Rat Vascular Smooth Muscle Cells through Dopamine D(1)-like Receptors. Circulation 2000, 101, 2302–2308. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Han, W.; Villar, V.A.M.; Li, H.; Arnaldo, F.B.; Concepcion, G.P.; Felder, R.A.; Quinn, M.T.; Jose, P.A. Dopamine D1 Receptor-Mediated Inhibition of NADPH Oxidase Activity in Human Kidney Cells Occurs via Protein Kinase A-Protein Kinase C Cross Talk. Free Radic. Biol. Med. 2011, 50, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Asico, L.D.; Yu, P.; Wang, Z.; Jones, J.E.; Bai, R.-K.; Sibley, D.R.; Felder, R.A.; Jose, P.A. D5 Dopamine Receptor Regulation of Phospholipase D. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H55–H61. [Google Scholar] [CrossRef]
- Yang, Z.; Asico, L.D.; Yu, P.; Wang, Z.; Jones, J.E.; Escano, C.S.; Wang, X.; Quinn, M.T.; Sibley, D.R.; Romero, G.G.; et al. D5 Dopamine Receptor Regulation of Reactive Oxygen Species Production, NADPH Oxidase, and Blood Pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R96–R104. [Google Scholar] [CrossRef] [PubMed]
- Amatya, B.; Yang, S.; Yu, P.; Vaz de Castro, P.A.S.; Armando, I.; Zeng, C.; Felder, R.A.; Asico, L.D.; Jose, P.A.; Lee, H. Peroxiredoxin-4 and dopamine D5 receptor interact to reduce oxidative stress and inflammation in the kidney. Antioxid. Redox Signal. 2023, 38, 1150–1166. [Google Scholar] [CrossRef]
- Choi, M.R.; Correa, A.H.; del Valle Turco, V.; Garcia, F.A.; Fernández, B.E. Angiotensin II Regulates Extraneuronal Dopamine Uptake in the Kidney. Nephron Physiol. 2006, 104, 136–143. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Yao, L.; Sanada, H.; Ozono, R.; Mouradian, M.M.; Jose, P.A.; Carey, R.M.; Felder, R.A. Dopamine D1A receptors and renin release in rat juxtaglomerular cells. Hypertension 1997, 29, 962–9688. [Google Scholar] [CrossRef]
- Asico, L.D.; Ladines, C.; Fuchs, S.; Accili, D.; Carey, R.M.; Semeraro, C.; Pocchiari, F.; Felder, R.A.; Eisner, G.M.; Jose, P.A. Disruption of the Dopamine D3 Receptor Gene Produces Renin-Dependent Hypertension. J. Clin. Investig. 1998, 102, 493–498. [Google Scholar] [CrossRef]
- Asico, L.; Zhang, X.; Jiang, J.; Cabrera, D.; Escano, C.S.; Sibley, D.R.; Wang, X.; Yang, Y.; Mannon, R.; Jones, J.E.; et al. Lack of Renal Dopamine D5 Receptors Promotes Hypertension. J. Am. Soc. Nephrol. 2011, 22, 82–89. [Google Scholar] [CrossRef]
- Zeng, C.; Luo, Y.; Asico, L.D.; Hopfer, U.; Eisner, G.M.; Felder, R.A.; Jose, P.A. Perturbation of D1 Dopamine and AT1 Receptor Interaction in Spontaneously Hypertensive Rats. Hypertension 2003, 42, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.F.; Becker, B.N.; Harris, R.C. Dopamine Decreases Expression of Type-1 Angiotensin II Receptors in Renal Proximal Tubule. J. Clin. Investig. 1996, 97, 2745–2752. [Google Scholar] [CrossRef]
- Bek, M.J.; Wang, X.; Asico, L.D.; Jones, J.E.; Zheng, S.; Li, X.; Eisner, G.M.; Grandy, D.K.; Carey, R.M.; Soares-da-Silva, P.; et al. Angiotensin-II Type 1 Receptor-Mediated Hypertension in D4 Dopamine Receptor-Deficient Mice. Hypertension 2006, 47, 288–295. [Google Scholar] [CrossRef]
- Zeng, C.; Liu, Y.; Wang, Z.; He, D.; Huang, L.; Yu, P.; Zheng, S.; Jones, J.E.; Asico, L.D.; Hopfer, U.; et al. Activation of D3 Dopamine Receptor Decreases Angiotensin II Type 1 Receptor Expression in Rat Renal Proximal Tubule Cells. Circ. Res. 2006, 99, 494–500. [Google Scholar] [CrossRef]
- Banday, A.A.; Diaz, A.D.; Lokhandwala, M. Kidney Dopamine D1-like Receptors and Angiotensin 1-7 Interaction Inhibits Renal Na+ Transporters. Am. J. Physiol. Renal Physiol. 2019, 317, F949–F956. [Google Scholar] [CrossRef]
- Zhang, Y.; Cuevas, S.; Asico, L.D.; Escano, C.; Yang, Y.; Pascua, A.M.; Wang, X.; Jones, J.E.; Grandy, D.; Eisner, G.; et al. Deficient Dopamine D2 Receptor Function Causes Renal Inflammation Independently of High Blood Pressure. PLoS ONE 2012, 7, e38745. [Google Scholar] [CrossRef] [PubMed]
- Konkalmatt, P.R.; Asico, L.D.; Zhang, Y.; Yang, Y.; Drachenberg, C.; Zheng, X.; Han, F.; Jose, P.A.; Armando, I. Renal Rescue of Dopamine D2 Receptor Function Reverses Renal Injury and High Blood Pressure. JCI Insight 2016, 1, e85888. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Konkalmatt, P.; Asico, L.D.; Hunt, J.; Latham, P.; Jose, P.A.; Armando, I. Dopamine D2 Receptor Specific Deletion in the Renal Proximal Tubules Increases Blood Pressure in Males but Not Female Mice. Circulation 2019, 140 (Suppl. S1), A15988. [Google Scholar]
- Wang, Y.; Tay, Y.C.; Harris, D.C. Proximal tubule cells stimulated by lipopolysaccharide inhibit macrophage activation. Kidney Int. 2004, 66, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Guijarro, C.; Egido, J. Transcription factor kappa B (NF-kappa B) and renal disease. Kidney Int. 2001, 59, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Therrien, F.J.; Agharazii, M.; Lebel, M.; Larivière, R. Neutralization of tumor necrosis factor-alpha reduces renal fibrosis and hypertension in rats with renal failure. Am. J. Nephrol. 2012, 36, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, X.; Qin, C.; Cuevas, S.; Jose, P.A.; Armando, I. Dopamine D2 Receptors’ Effects on Renal Inflammation Are Mediated by Regulation of PP2A Function. Am. J. Physiol. Renal Physiol. 2016, 310, F128–F134. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Gainetdinov, R.R. The physiology, signaling and pharmacology of dopamine receptors. Pharm. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.M.; Gainetdinov, R.R.; Caron, M.G. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol. Sci. 2007, 28, 166172. [Google Scholar] [CrossRef]
- Han, F.; Konkalmatt, P.; Mokashi, C.; Kumar, M.; Zhang, Y.; Ko, A.; Farino, Z.J.; Asico, L.D.; Xu, G.; Gildea, J.; et al. Dopamine D2 Receptor Modulates Wnt Expression and Control of Cell Proliferation. Sci. Rep. 2019, 9, 16861. [Google Scholar] [CrossRef]
- Fang, Y.J.; Thomas, G.N.; Xu, Z.L.; Fang, J.Q.; Critchley, J.A.; Tomlinson, B. An affected pedigree member analysis of linkage between the dopamine D2 receptor gene TaqI polymorphism and obesity and hypertension. Int. J. Cardiol. 2005, 102, 111–116. [Google Scholar] [CrossRef]
- Thompson, J.; Thomas, N.; Singleton, A.; Piggott, M.; Lloyd, S.; Perry, E.K.; Morris, C.M.; Perry, R.H.; Ferrier, I.N.; Court, J.A. D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: Reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics 1997, 7, 479–484. [Google Scholar] [CrossRef]
- Jönsson, E.G.; Nöthen, M.M.; Grünhage, F.; Farde, L.; Nakashima, Y.; Propping, P.; Sedvall, G.C. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol. Psychiatry 1999, 4, 290–296. [Google Scholar] [CrossRef]
- Duan, J.; Wainwright, M.S.; Comeron, J.M.; Saitou, N.; Sanders, A.R.; Gelernter, J.; Gejman, P.V. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum. Mol. Genet. 2003, 12, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.P.; Blum, K.; Ritchie, T.; Montgomery, A.; Sheridan, P.J. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch. Gen. Psychiatry 1991, 48, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Rajeevan, H.; Soundararajan, U.; Kidd, J.R.; Pakstis, A.J.; Kidd, K.K. ALFRED: An allele frequency resource for research and teaching. Nucleic Acids Res. 2012, 40, D1010–D1015. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Konkalmatt, P.; Yang, Y.; Gildea, J.; Jones, J.E.; Cuevas, S.; Felder, R.A.; Jose, P.A.; Armando, I. Single-Nucleotide Polymorphisms of the Dopamine D2 Receptor Increase Inflammation and Fibrosis in Human Renal Proximal Tubule Cells. Hypertension 2014, 63, e74–e80. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Konkalmatt, P.; Chen, J.; Gildea, J.; Felder, R.A.; Jose, P.A.; Armando, I. MiR-217 Mediates the Protective Effects of the Dopamine D2 Receptor on Fibrosis in Human Renal Proximal Tubule Cells. Hypertension 2015, 65, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Kumar, K.M.; Ammini, A.C.; Gupta, A.; Gupta, R.; Thelma, B.K. Association of dopaminergic pathway gene polymorphisms with chronic renal insufficiency among Asian Indians with type-2 diabetes. BMC Genet. 2008, 9, 26. [Google Scholar] [CrossRef]
- O’Seaghdha, C.M.; Fox, C.S. Genetics of chronic kidney disease. Nephron Clin. Pract. 2011, 118, c55–c63. [Google Scholar] [CrossRef]
- McKnight, A.J.; Currie, D.; Maxwell, A.P. Unravelling the genetic basis of renal diseases; from single gene to multifactorial disorders. J. Pathol. 2010, 220, 198–216. [Google Scholar] [CrossRef]
- Garrett, M.R.; Pezzolesi, M.G.; Korstanje, R. Integrating human and rodent data to identify the genetic factors involved in chronic kidney disease. J. Am. Soc. Nephrol. 2010, 21, 398–405. [Google Scholar] [CrossRef]
- Wetmore, J.B.; Hung, A.M.; Lovett, D.H.; Sen, S.; Quershy, O.; Johansen, K.L. Interleukin-1 gene cluster polymorphisms predict risk of ESRD. Kidney Int. 2005, 68, 278–284. [Google Scholar] [CrossRef]
- Doi, K.; Noiri, E.; Nakao, A.; Fujita, T.; Kobayashi, S.; Tokunaga, K. Functional polymorphisms in the vascular endothelial growth factor gene are associated with development of end-stage renal disease in males. J. Am. Soc. Nephrol. 2006, 17, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Lira, S.S.; Ahammad, I. A comprehensive in silico investigation into the nsSNPs of Drd2 gene predicts significant functional consequences in dopamine signaling and pharmacotherapy. Sci. Rep. 2021, 11, 23212. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, T.; Noble, E.P. Association of Seven Polymorphisms of the D2 Dopamine Receptor Gene with Brain Receptor-Binding Characteristics. Neurochem. Res. 2003, 28, 73–82. [Google Scholar] [CrossRef]
- Pohjalainen, T.; Rinne, J.O.; Någren, K.; Lehikoinen, P.; Anttila, K.; Syvälahti, E.K.; Hietala, J. The A1 Allele of the Human D2 Dopamine Receptor Gene Predicts Low D2 Receptor Availability in Healthy Volunteers. Mol. Psychiatry 1998, 3, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Mullally, J.A.; Chung, W.K.; LeDuc, C.A.; Reid, T.J.; Febres, G.; Holleran, S.; Ramakrishnan, R.; Korner, J. Weight-loss response to naltrexone/bupropion is modulated by the Taq1A genetic variant near DRD2 (rs1800497): A pilot study. Diabetes Obes. Metab. 2021, 23, 850–853. [Google Scholar] [CrossRef]
- Thomas, G.N.; Tomlinson, B.; Critchley, J.A. Modulation of blood pressure and obesity with the dopamine D2 receptor gene TaqI polymorphism. Hypertension 2000, 36, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Comings, D.E.; Gade, R.; MacMurray, J.P.; Mulhalman, D.; Peters, W.R. Genetic variants of the human obesity (OB) gene: Association with body mass index in young women, psychiatric symptoms, and interaction with the dopamine D2 receptor (DRD2) gene. Mol. Psychiatry 1996, 1, 325–335. [Google Scholar]
- Noble, E.P.; Noble, R.E.; Ritchie, T.; Grandy, D.K.; Sparkes, R.S. Allelic association of the human D2 dopamine receptor gene with obesity. Int. J. Eat. Disord. 1994, 15, 205–217. [Google Scholar] [CrossRef]
- Spitz, M.R.; Detry, M.A.; Pillow, P.; Hu, Y.H.; Amos, C.I.; Hong, W.K.; Wu, X.F. Variant alleles of the D2 dopamine receptor gene and obesity. Nutrition Res. 2000, 20, 371–380. [Google Scholar] [CrossRef]
- Barnard, N.D.; Noble, E.P.; Ritchie, T.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Green, A.A.; Ferdowsian, H. D2 dopamine receptor Taq1A polymorphism, body weight, and dietary intake in type 2 diabetes. Nutrition 2009, 25, 58–65. [Google Scholar] [CrossRef]
- Hirvonen, M.M.; Lumme, V.; Hirvonen, J.; Pesonen, U.; Någren, K.; Vahlberg, T.; Scheinin, H.; Hietala, J. C957T Polymorphism of the Human Dopamine D2 Receptor Gene Predicts Extrastriatal Dopamine Receptor Availability in vivo. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, M.M.; Laakso, A.; Någren, K.; Rinne, J.O.; Pohjalainen, T.; Hietala, J. C957T Polymorphism of Dopamine D2 Receptor Gene Affects Striatal DRD2 in Vivo Availability by Changing the Receptor Affinity. Synapse 2009, 63, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, M.; Laakso, A.; Någren, K.; Rinne, J.O.; Pohjalainen, T.; Hietala, J. C957T Polymorphism of the Dopamine D2 Receptor (DRD2) Gene Affects Striatal DRD2 Availability in Vivo. Mol. Psychiatry 2004, 9, 1060–1061. [Google Scholar] [CrossRef] [PubMed]
- Lawford, B.R.; Barnes, M.; Morris, C.P.; Noble, E.P.; Nyst, P.; Heslop, K.; Young, R.M.; Voisey, J.; Connor, J.P. Dopamine 2 Receptor Genes Are Associated with Raised Blood Glucose in Schizophrenia. Can. J. Psychiatry 2016, 61, 291–297. [Google Scholar] [CrossRef]
- Gildea, J.J.; Xu, P.; Schiermeyer, K.A.; Yue, W.; Carey, R.M.; Jose, P.A.; Felder, R.A. Inverse Salt Sensitivity of Blood Pressure Is Associated with an Increased Renin-Angiotensin System Activity. Biomedicines 2022, 10, 2811. [Google Scholar] [CrossRef]
- Amin, M.; Wu, R.; Postolache, T.T.; Gragnoli, C. Linkage and association of novel DRD2 variants to the comorbidity of type 2 diabetes and depression. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8370–8375. [Google Scholar] [CrossRef]
- Li, T.; Arranz, M.; Aitchison, K.J.; Bryant, C.; Liu, X.; Kerwin, R.W.; Murray, R.; Sham, P.; Collier, D.A. Case-Control, Haplotype Relative Risk and Transmission Disequilibrium Analysis of a Dopamine D2 Receptor Functional Promoter Polymorphism in Schizophrenia. Schizophr. Res. 1998, 32, 87–92. [Google Scholar]
- Moyer, R.A.; Wang, D.; Papp, A.C.; Smith, R.M.; Duque, L.; Mash, D.C.; Sadee, W. Intronic Polymorphisms Affecting Alternative Splicing of Human Dopamine D2 Receptor Are Associated with Cocaine Abuse. Neuropsychopharmacology 2011, 36, 753–762. [Google Scholar] [CrossRef]
- Kaalund, S.S.; Newburn, E.N.; Ye, T.; Tao, R.; Li, C.; Deep-Soboslay, A.; Herman, M.M.; Hyde, T.M.; Weinberger, D.R.; Lipska, B.K.; et al. Contrasting Changes in DRD1 and DRD2 Splice Variant Expression in Schizophrenia and Affective Disorders, and Associations with SNPs in Postmortem Brain. Mol. Psychiatry 2014, 19, 1258–1266. [Google Scholar] [CrossRef]
- Cohen, O.S.; Weickert, T.W.; Hess, J.L.; Paish, L.M.; McCoy, S.Y.; Rothmond, D.A.; Galletly, C.; Liu, D.; Weinberg, D.D.; Huang, X.-F.; et al. A Splicing-Regulatory Polymorphism in DRD2 Disrupts ZRANB2 Binding, Impairs Cognitive Functioning and Increases Risk for Schizophrenia in Six Han Chinese Samples. Mol. Psychiatry 2016, 21, 975–982. [Google Scholar] [CrossRef]
- Bertolino, A.; Fazio, L.; Di Giorgio, A.; Blasi, G.; Romano, R.; Taurisano, P.; Caforio, G.; Sinibaldi, L.; Ursini, G.; Popolizio, T.; et al. Genetically Determined Interaction between the Dopamine Transporter and the D2 Receptor on Prefronto-Striatal Activity and Volume in Humans. J. Neurosci. 2009, 29, 1224–1234. [Google Scholar] [CrossRef]
- Bertolino, A.; Taurisano, P.; Pisciotta, N.M.; Blasi, G.; Fazio, L.; Romano, R.; Gelao, B.; Lo Bianco, L.; Lozupone, M.; Di Giorgio, A.; et al. Genetically Determined Measures of Striatal D2 Signaling Predict Prefrontal Activity during Working Memory Performance. PLoS ONE 2010, 5, e9348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bertolino, A.; Fazio, L.; Blasi, G.; Rampino, A.; Romano, R.; Lee, M.L.; Xiao, T.; Papp, A.; Wang, D.; et al. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc. Natl. Acad. Sci. USA 2007, 104, 20552–20557. [Google Scholar] [CrossRef] [PubMed]
- Cravchik, A.; Sibley, D.R.; Gejman, P.V. Functional Analysis of the Human D2 Dopamine Receptor Missense Variants. J. Biol. Chem. 1996, 271, 26013–26017. [Google Scholar] [CrossRef]
- Doehring, A.; Kirchhof, A.; Lötsch, J. Genetic diagnostics of functional variants of the human dopamine D2 receptor gene. Psychiatr. Genet. 2009, 19, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Laursen, T.M.; Munk-Olsen, T.; Vestergaard, M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr. Opin. Psychiatry 2012, 25, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, N.S.; Hsu, Y.H.; Ho, S.Y.; Kuo, Y.C.; Lee, H.C.; Yin, Y.J.; Chen, H.A.; Chen, W.L.; Chu, W.C.C.; Huang, H.L. Is schizophrenia associated with an increased risk of chronic kidney disease? A nationwide matched cohort study. BMJ Open 2015, 5, e006777. [Google Scholar] [CrossRef]
- Højlund, M.; Lund, L.C.; Herping, J.L.E.; Haastrup, M.B.; Damkier, P.; Henriksen, D.P. Second-generation antipsychotics and the risk of chronic kidney disease: A population-based case-control study. BMJ Open 2020, 10, e038247. [Google Scholar] [CrossRef]
- Jiang, Y.; McCombs, J.S.; Park, S.H. A Retrospective Cohort Study of Acute Kidney Injury Risk Associated with Antipsychotics. CNS Drugs 2017, 31, 319–326. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Melamud, M.M.; Boiko, A.S.; Kamaeva, D.A.; Ivanova, S.A.; Nevinsky, G.A.; Buneva, V.N. Association of Peripheral Inflammatory Biomarkers and Growth Factors Levels with Sex, Therapy and Other Clinical Factors in Schizophrenia and Patient Stratification Based on These Data. Brain Sci. 2023, 13, 836. [Google Scholar] [CrossRef]
- Kluge, M.; Schuld, A.; Schacht, A.; Himmerich, H.; Dalal, M.A.; Wehmeier, P.M.; Hinze-Selch, D.; Kraus, T.; Dittmann, R.W.; Pollmächer, T. Effects of clozapine and olanzapine on cytokine systems are closely linked to weight gain and drug-induced fever. Psychoneuroendocrinology 2009, 34, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Tourjman, V.; Kouassi, E.; Koue, M.-E.; Rocchetti, M.; Fortin-Fournier, S.; Fusar-Poli, P.; Potvin, S. Antipsychotics’ effects on blood levels of cytokines in schizophrenia: A meta-analysis. Schizophr. Res. 2013, 151, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Patlola, S.R.; Donohoe, G.; McKernan, D.P. Anti-inflammatory effects of 2nd generation antipsychotics in patients with schizophrenia: A systematic review and meta-analysis. J. Psychiatr. Res. 2023, 160, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.J.; Dixon, S.N.; Reiss, J.P.; Wald, R.; Parikh, C.R.; Gandhi, S.; Shariff, S.Z.; Pannu, N.; Nash, D.M.; Rehman, F.; et al. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults: A population-based cohort study. Ann. Intern. Med. 2014, 161, 242–248. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Public Health Advisory: Deaths with Antipsychotics in Elderly Patients with Behavioral Disturbances. Available online: www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm053171.htm (accessed on 25 April 2013).
- Burghardt, K.J.; Mando, W.; Seyoum, B.; Yi, Z.; Burghardt, P.R. The effect of antipsychotic treatment on hormonal, inflammatory, and metabolic biomarkers in healthy volunteers: A systematic review and meta-analysis. Pharmacotherapy 2022, 42, 504–513. [Google Scholar] [CrossRef]
- Klemettila, J.P.; Kampman, O.; Seppala, N.; Viikki, M.; Hamalainen, M.; Moilanen, E.; Leinonen, E. Cytokine and adipokine alterations in patients with schizophrenia treated with clozapine. Psychiatry Res. 2014, 218, 277–283. [Google Scholar] [CrossRef]
- Correll, C.U.; Detraux, J.; De Lepeleire, J.; De Hert, M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015, 14, 119–136. [Google Scholar] [CrossRef]
- Meyer, J.M.; Correll, C.U. Increased Metabolic Potential, Efficacy, and Safety of Emerging Treatments in Schizophrenia. CNS Drugs 2023, 37, 545–570. [Google Scholar] [CrossRef]
- De Hert, M.; Detraux, J.; van Winkel, R.; Yu, W.; Correll, C.U. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat. Rev. Endocrinol. 2012, 8, 114–126. [Google Scholar] [CrossRef]
- Correll, C.U.; Lencz, T.; Malhotra, A.K. Antipsychotic drugs and obesity. Trends Mol. Med. 2011, 17, 97–107. [Google Scholar] [CrossRef]
- Coccurello, R.; Moles, A. Potential mechanisms of atypical antipsychotic-induced metabolic derangement: Clues for understanding obesity and novel drug design. Pharmacol. Ther. 2010, 127, 210–251. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, S.C.; Vaz de Castro, P.A.S.; Yaqub, D.; Jose, P.A.; Armando, I. Anti-Inflammatory Effects of Peripheral Dopamine. Int. J. Mol. Sci. 2023, 24, 13816. https://doi.org/10.3390/ijms241813816
Moore SC, Vaz de Castro PAS, Yaqub D, Jose PA, Armando I. Anti-Inflammatory Effects of Peripheral Dopamine. International Journal of Molecular Sciences. 2023; 24(18):13816. https://doi.org/10.3390/ijms241813816
Chicago/Turabian StyleMoore, Shaun C., Pedro A. S. Vaz de Castro, Daniel Yaqub, Pedro A. Jose, and Ines Armando. 2023. "Anti-Inflammatory Effects of Peripheral Dopamine" International Journal of Molecular Sciences 24, no. 18: 13816. https://doi.org/10.3390/ijms241813816
APA StyleMoore, S. C., Vaz de Castro, P. A. S., Yaqub, D., Jose, P. A., & Armando, I. (2023). Anti-Inflammatory Effects of Peripheral Dopamine. International Journal of Molecular Sciences, 24(18), 13816. https://doi.org/10.3390/ijms241813816






