Large HBV Surface Protein-Induced Unfolded Protein Response Dynamically Regulates p27 Degradation in Hepatocellular Carcinoma Progression
Abstract
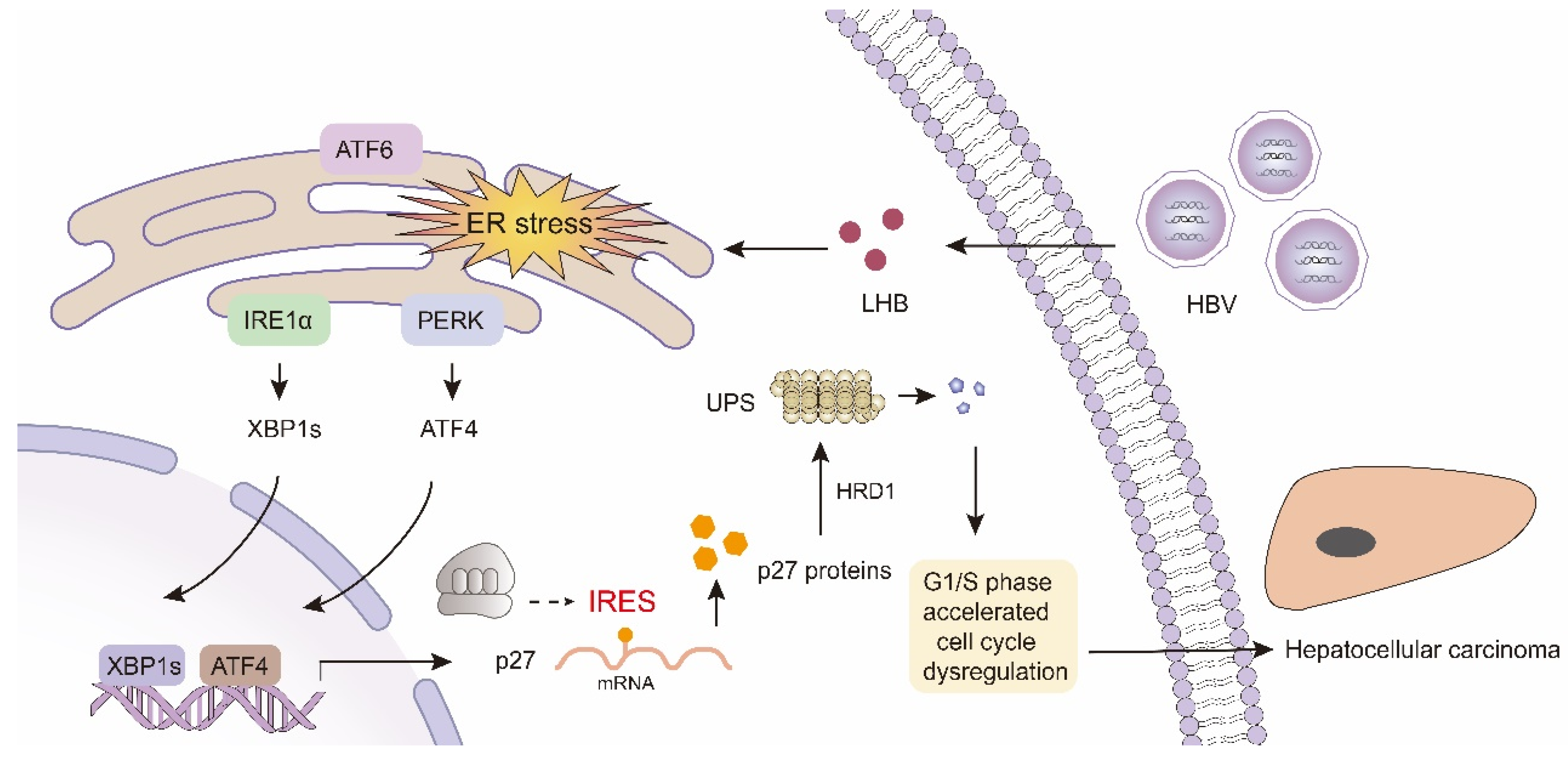
:1. Introduction
2. Results
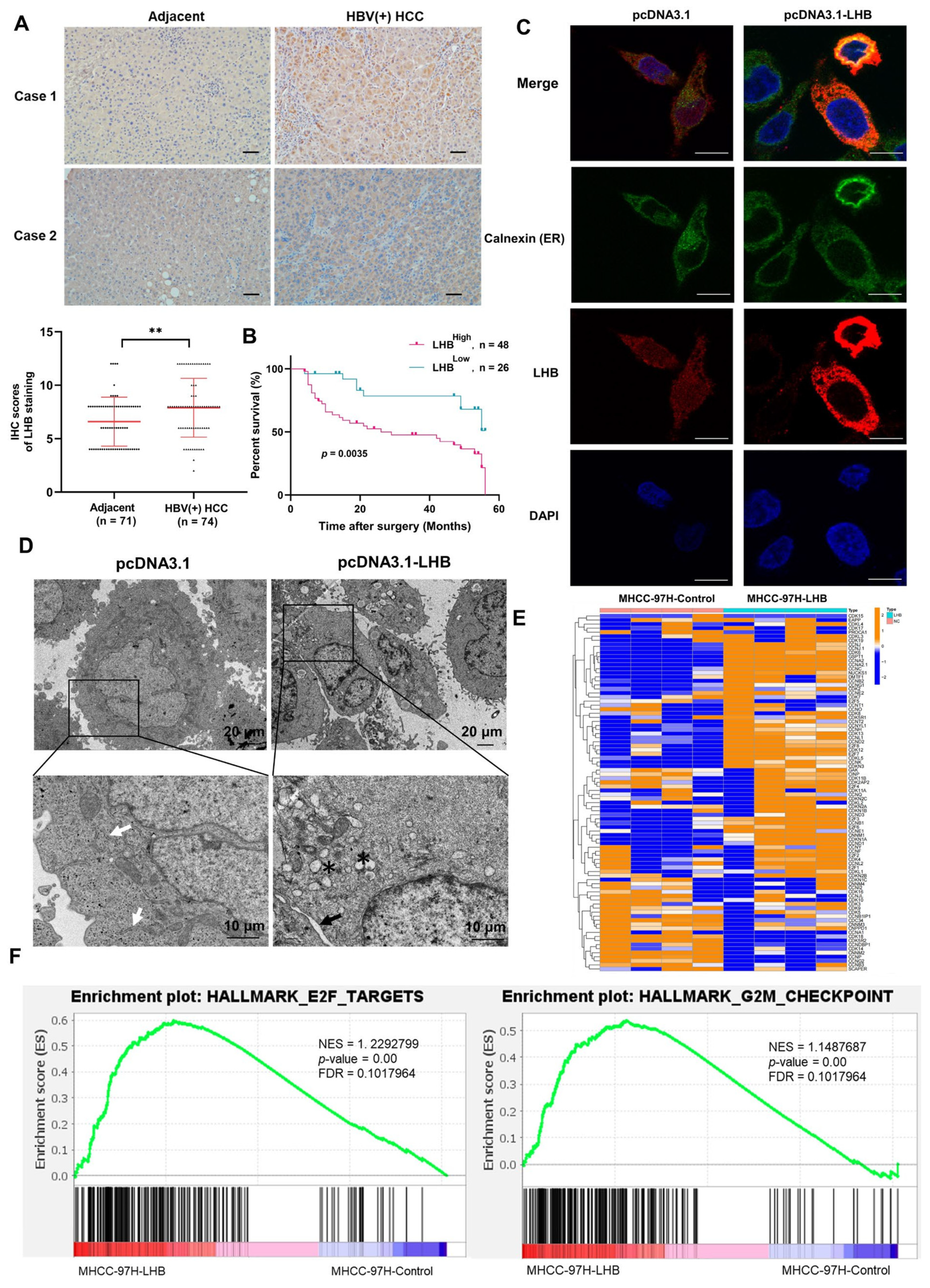
2.1. LHB Is Upregulated in Human HBV(+) HCC Tissues and Associated with Poor Prognosis
2.2. Intracellular Retention of LHB Leads to Morphological Changes of ER and Affects the Cell Cycle of HCC
2.3. LHB Induces ER Stress and Promotes Tumor Formation by Regulating the Cell Cycle
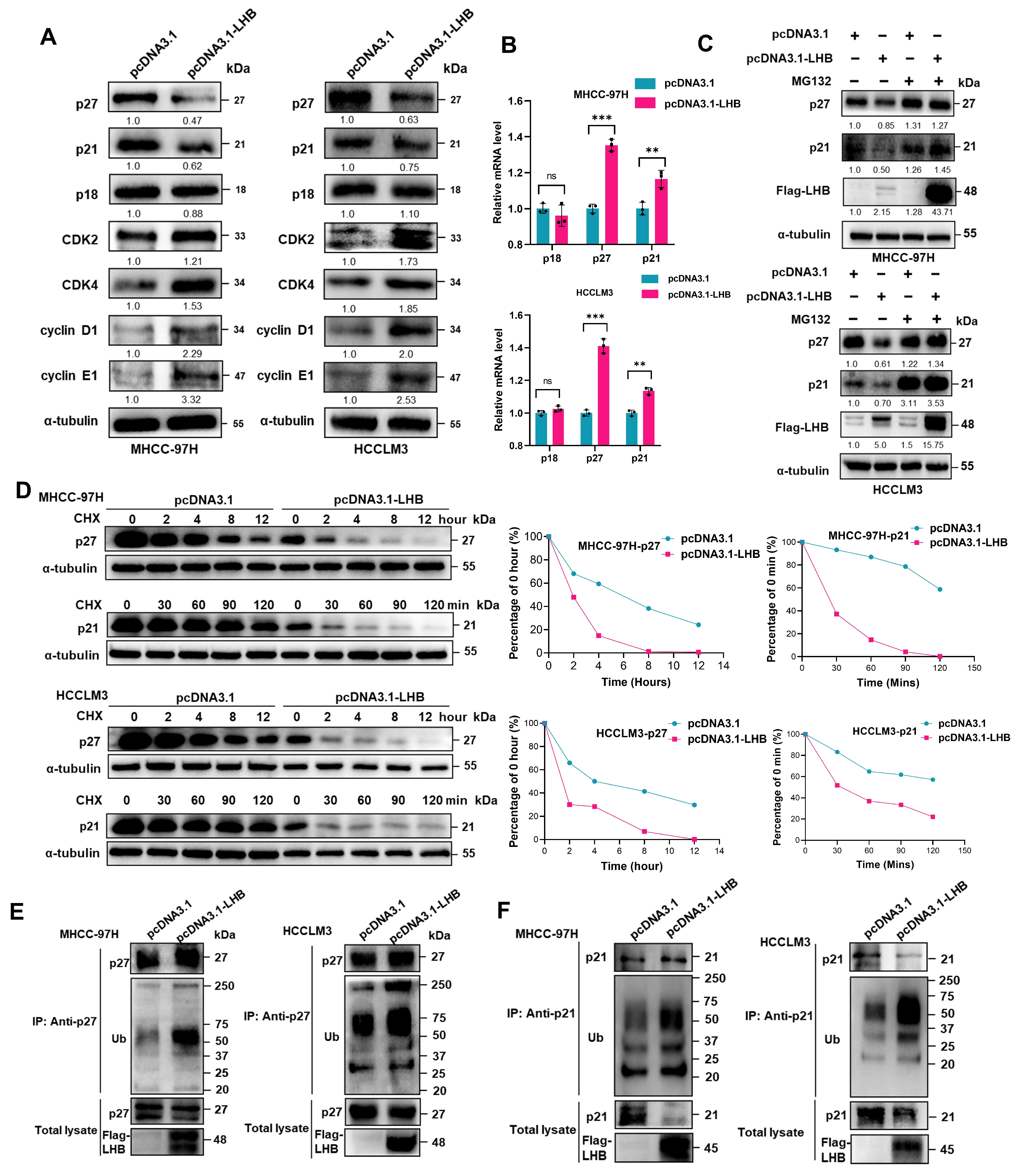
2.4. Overexpression of LHB Regulates Cell Cycle Progression by Enhancing p27 Ubiquitination
2.5. LHB-Induced ER Stress Regulates p27 mRNA Transcription and Enhances the Selective Translation of p27
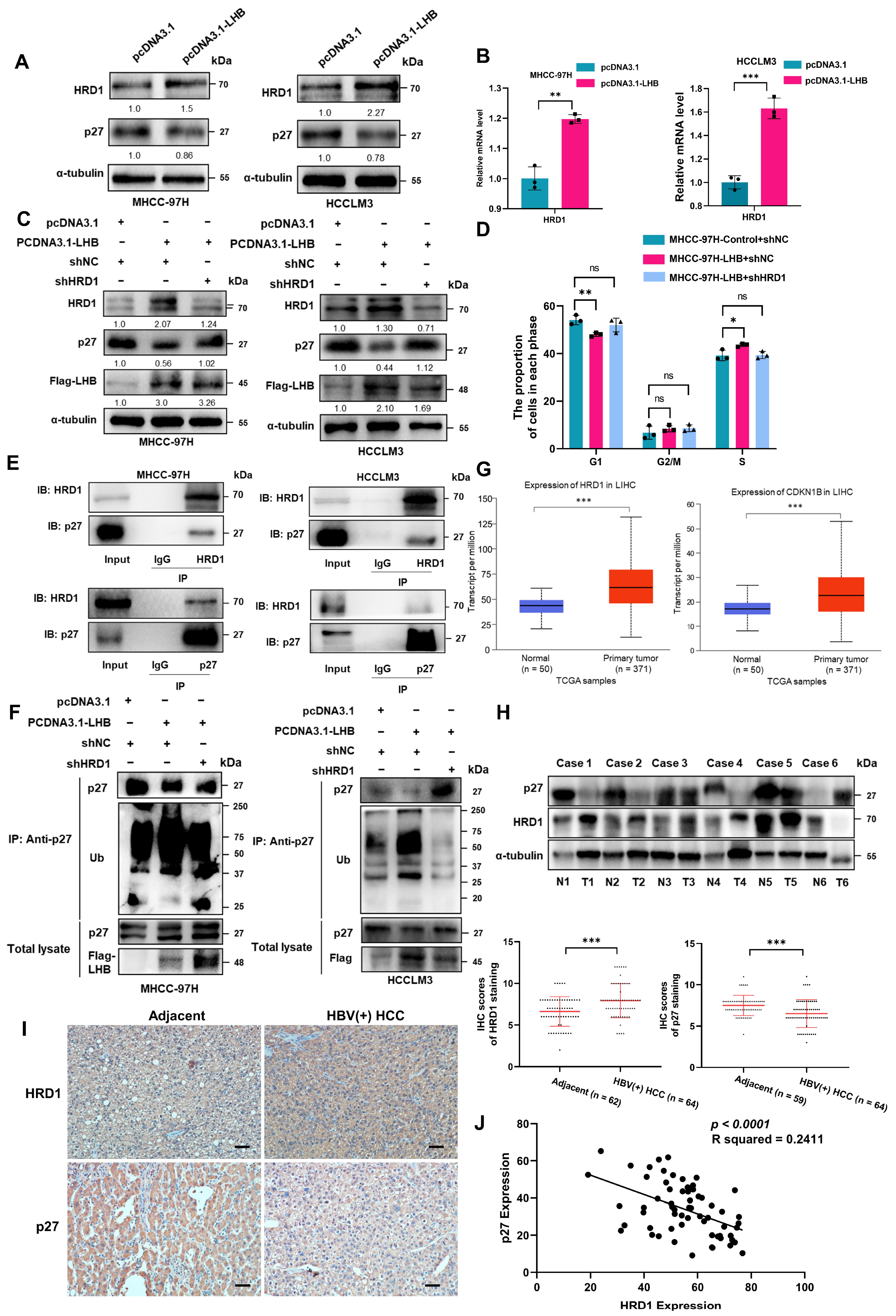
2.6. p27 Degradation by the E3 Ubiquitin Ligase HRD1 Occurs in the Presence of LHB-Induced ER Stress
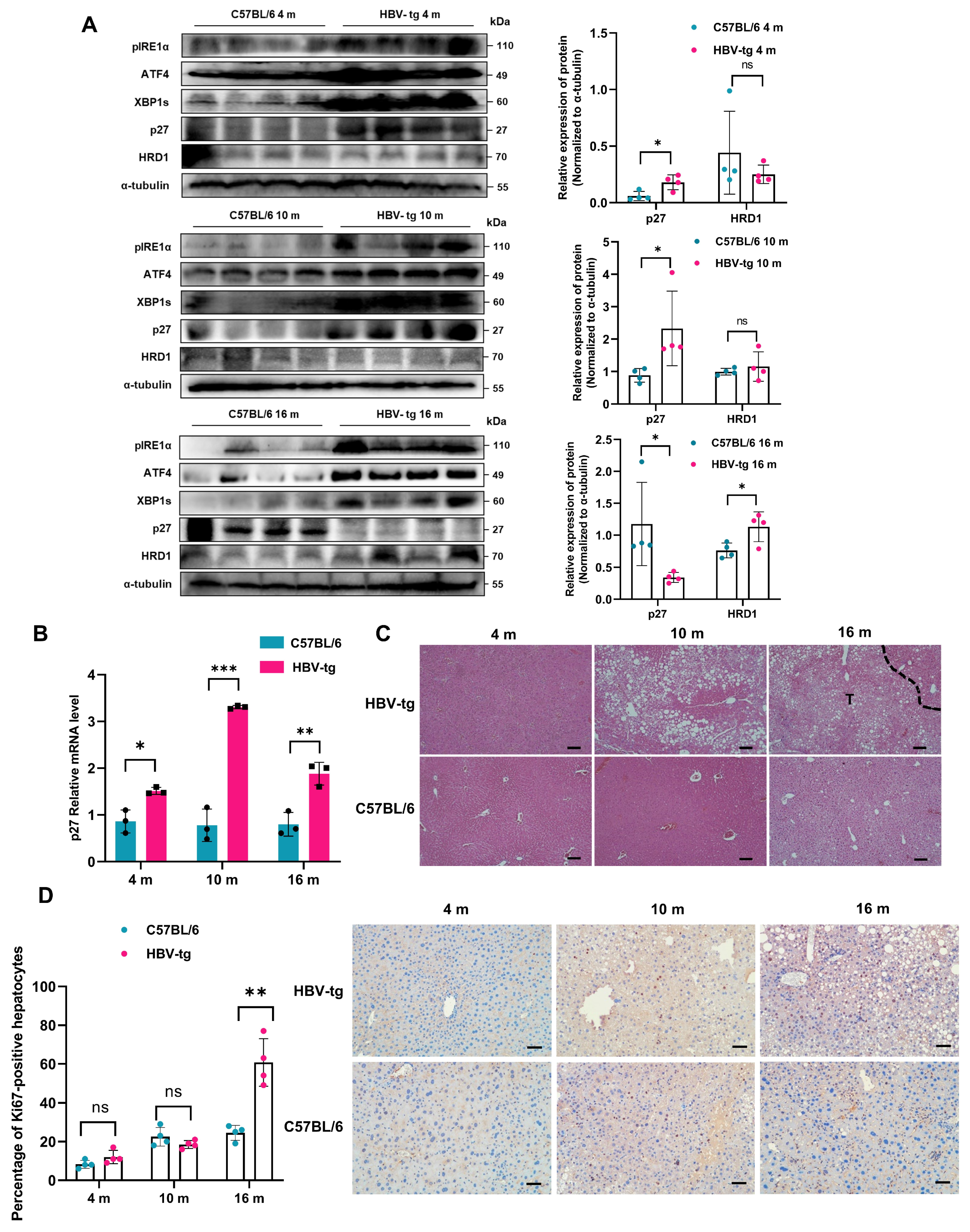
2.7. p27 Expression Is Regulated by LHB-Induced ER Stress In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Transfection
4.2. Immunohistochemistry
4.3. Transgenic Mice Model
4.4. In Vivo Xenograft Mouse Mode
4.5. Plasmid Construction
4.6. Reagents
4.7. Real-Time qPCR
4.8. Western Blotting
4.9. RNA-Seq
4.10. Cell Proliferation and Cell Cycle Assays
4.11. Immunofluorescence
4.12. Luciferase Reporter Assay
4.13. Chromatin Immunoprecipitation (ChIP)
4.14. Immunoprecipitation and Coimmunoprecipitation
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Jeng, W.J.; Papatheodoridis, G.V.; Lok, A.S.F. Hepatitis B. Lancet 2023, 401, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Iwamoto, M.; Yun, J.H.; Uchikubo-Kamo, T.; Son, D.; Jin, Z.; Yoshida, H.; Ohki, M.; Ishimoto, N.; Mizutani, K.; et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 2022, 606, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jensen, G.; Yen, T.S. Activation of hepatitis B virus S promoter by the viral large surface protein via induction of stress in the endoplasmic reticulum. J. Virol. 1997, 71, 7387–7392. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Gu, C.; Yin, J.; He, Y.; Xie, J.; Cao, G. Associations between hepatitis B virus mutations and the risk of hepatocellular carcinoma: A meta-analysis. J. Natl. Cancer. Inst. 2009, 101, 1066–1082. [Google Scholar] [CrossRef]
- Liang, Y.J.; Teng, W.; Chen, C.L.; Sun, C.P.; Teng, R.D.; Huang, Y.H.; Liang, K.H.; Chen, Y.W.; Lin, C.C.; Su, C.W.; et al. Clinical Implications of HBV PreS/S Mutations and the Effects of PreS2 Deletion on Mitochondria, Liver Fibrosis, and Cancer Development. Hepatology 2021, 74, 641–655. [Google Scholar] [CrossRef]
- Huang, S.N.; Chisari, F.V. Strong, sustained hepatocellular proliferation precedes hepatocarcinogenesis in hepatitis B surface antigen transgenic mice. Hepatology 1995, 21, 620–626. [Google Scholar] [PubMed]
- Hadziyannis, S.; Raimondo, G.; Papaioannou, C.; Anastassakos, C.; Wong, D.; Sninsky, J.; Shafritz, D. Expression of pre-S gene-encoded proteins in liver and serum during chronic hepatitis B virus infection in comparison to other markers of active virus replication. J. Hepatol. 1987, 5, 253–259. [Google Scholar] [CrossRef]
- Lefkowitch, J.H.; Lobritto, S.J.; Brown, R.S., Jr.; Emond, J.C.; Schilsky, M.L.; Rosenthal, L.A.; George, D.M.; Cairo, M.S. Ground-glass, polyglucosan-like hepatocellular inclusions: A "new" diagnostic entity. Gastroenterology 2006, 131, 713–718. [Google Scholar] [CrossRef]
- Chisari, F.V.; Klopchin, K.; Moriyama, T.; Pasquinelli, C.; Dunsford, H.A.; Sell, S.; Pinkert, C.A.; Brinster, R.L.; Palmiter, R.D. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell 1989, 59, 1145–1156. [Google Scholar] [CrossRef]
- Chisari, F.V.; Filippi, P.; McLachlan, A.; Milich, D.R.; Riggs, M.; Lee, S.; Palmiter, R.D.; Pinkert, C.A.; Brinster, R.L. Expression of hepatitis B virus large envelope polypeptide inhibits hepatitis B surface antigen secretion in transgenic mice. J. Virol. 1986, 60, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Chisari, F.V.; Filippi, P.; Buras, J.; McLachlan, A.; Popper, H.; Pinkert, C.A.; Palmiter, R.D.; Brinster, R.L. Structural and pathological effects of synthesis of hepatitis B virus large envelope polypeptide in transgenic mice. Proc. Natl. Acad. Sci. USA 1987, 84, 6909–6913. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.W.; Lin, Y.J.; Lin, P.W.; Wu, H.C.; Hsu, K.H.; Yen, C.J.; Chan, S.H.; Huang, W.; Su, I.J. A clustered ground-glass hepatocyte pattern represents a new prognostic marker for the recurrence of hepatocellular carcinoma after surgery. Cancer 2011, 117, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.T.; Tillmann, H.L.; Manns, M.P.; Trautwein, C. The pre-S region determines the intracellular localization and appearance of hepatitis B virus. Hepatology 1999, 30, 517–525. [Google Scholar] [CrossRef]
- Bruss, V. Revisiting the cytopathic effect of hepatitis B virus infection. Hepatology 2002, 36, 1327–1329. [Google Scholar] [CrossRef]
- Yen, C.J.; Ai, Y.L.; Tsai, H.W.; Chan, S.H.; Yen, C.S.; Cheng, K.H.; Lee, Y.P.; Kao, C.W.; Wang, Y.C.; Chen, Y.L.; et al. Hepatitis B virus surface gene pre-S(2) mutant as a high-risk serum marker for hepatoma recurrence after curative hepatic resection. Hepatology 2018, 68, 815–826. [Google Scholar] [CrossRef]
- Wang, H.C.; Huang, W.; Lai, M.D.; Su, I.J. Hepatitis B virus pre-S mutants, endoplasmic reticulum stress and hepatocarcinogenesis. Cancer. Sci. 2006, 97, 683–688. [Google Scholar] [CrossRef]
- Pollicino, T.; Cacciola, I.; Saffioti, F.; Raimondo, G. Hepatitis B virus PreS/S gene variants: Pathobiology and clinical implications. J. Hepatol. 2014, 61, 408–417. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Z.Y.; Ye, S.L.; Liu, Y.K.; Chen, J.; Xue, Q.; Chen, J.; Gao, D.M.; Bao, W.H. Establishment of cell clones with different metastatic potential from the metastatic hepatocellular carcinoma cell line MHCC97. World. J. Gastroenterol. 2001, 7, 630–636. [Google Scholar] [CrossRef]
- Ding, S.J.; Li, Y.; Shao, X.X.; Zhou, H.; Zeng, R.; Tang, Z.Y.; Xia, Q.C. Proteome analysis of hepatocellular carcinoma cell strains, MHCC97-H and MHCC97-L, with different metastasis potentials. Proteomics 2004, 4, 982–994. [Google Scholar] [CrossRef]
- Senft, D.; Ronai, Z.A. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends. Biochem. Sci. 2015, 40, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Spriggs, K.A.; Bushell, M.; Mitchell, S.A.; Willis, A.E. Internal ribosome entry segment-mediated translation during apoptosis: The role of IRES-trans-acting factors. Cell Death Differ. 2005, 12, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Kranzusch, P.J.; Cate, J.H. eIF3 targets cell-proliferation messenger RNAs for translational activation or repression. Nature 2015, 522, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Harada, B.T.; Lu, D.; Ma, R.; Gao, B.; Xu, Y.; Montauti, E.; Mani, N.; Chaudhuri, S.M.; Gregory, S.; et al. HRD1-mediated METTL14 degradation regulates m(6)A mRNA modification to suppress ER proteotoxic liver disease. Mol. Cell 2021, 81, 5052–5065.e5056. [Google Scholar] [CrossRef]
- Wolf, L.M.; Lambert, A.M.; Haenlin, J.; Boutros, M. EVI/WLS function is regulated by ubiquitylation and is linked to ER-associated degradation by ERLIN2. J. Cell. Sci. 2021, 134, 257790. [Google Scholar] [CrossRef]
- Zhang, R.; Real, C.I.; Liu, C.; Baba, H.A.; Gerken, G.; Lu, M.; Broering, R. Hepatic expression of oncogenes Bmi1 and Dkk1 is up-regulated in hepatitis B virus surface antigen-transgenic mice and can be induced by treatment with HBV particles or lipopolysaccharides in vitro. Int. J. Cancer 2017, 141, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, R.; Schefczyk, S.; Liang, Y.; Lin, S.S.; Liu, S.; Baba, H.A.; Lange, C.M.; Wedemeyer, H.; Lu, M.; et al. Nuclear translocation of YAP drives BMI-associated hepatocarcinogenesis in hepatitis B virus infection. Liver. Int. 2023, 43, 2002–2016. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.Y.; Wong, D.K.; Seto, W.K.; Mak, L.Y.; Cheung, T.T.; Yuen, M.F. Tumor suppressive role of mitochondrial sirtuin 4 in induction of G2/M cell cycle arrest and apoptosis in hepatitis B virus-related hepatocellular carcinoma. Cell Death Discov. 2021, 7, 88. [Google Scholar] [CrossRef]
- Lazar, C.; Uta, M.; Branza-Nichita, N. Modulation of the unfolded protein response by the human hepatitis B virus. Front. Microbiol. 2014, 5, 433. [Google Scholar] [CrossRef]
- Chen, C.H.; Hung, C.H.; Lee, C.M.; Hu, T.H.; Wang, J.H.; Wang, J.C.; Lu, S.N.; Changchien, C.S. Pre-S deletion and complex mutations of hepatitis B virus related to advanced liver disease in HBeAg-negative patients. Gastroenterology 2007, 133, 1466–1474. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Su, I.J.; Wang, H.C.; Tsai, J.H.; Huang, Y.J.; Chang, W.W.; Lai, M.D.; Lei, H.Y.; Huang, W. Hepatitis B virus pre-S2 mutant surface antigen induces degradation of cyclin-dependent kinase inhibitor p27Kip1 through c-Jun activation domain-binding protein 1. Mol. Cancer Res. 2007, 5, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Chang, W.T.; Chang, W.W.; Wu, H.C.; Huang, W.; Lei, H.Y.; Lai, M.D.; Fausto, N.; Su, I.J. Hepatitis B virus pre-S2 mutant upregulates cyclin A expression and induces nodular proliferation of hepatocytes. Hepatology 2005, 41, 761–770. [Google Scholar] [CrossRef]
- Klenovsky, L.; Odehnalova, M. Relation between the effect of therapy and the course of neuroses in a day care sanatorium. Ceskoslovenska Psychiatr. 1990, 86, 190–194. [Google Scholar]
- Cubillos-Ruiz, J.R.; Bettigole, S.E.; Glimcher, L.H. Tumorigenic and Immunosuppressive Effects of Endoplasmic Reticulum Stress in Cancer. Cell 2017, 168, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Qi, L. Quality Control in the Endoplasmic Reticulum: Crosstalk between ERAD and UPR pathways. Trends Biochem. Sci. 2018, 43, 593–605. [Google Scholar] [CrossRef]
- Gao, Q.; Zhu, H.; Dong, L.; Shi, W.; Chen, R.; Song, Z.; Huang, C.; Li, J.; Dong, X.; Zhou, Y.; et al. Integrated Proteogenomic Characterization of HBV-Related Hepatocellular Carcinoma. Cell 2019, 179, 1240. [Google Scholar] [CrossRef]
- Susaki, E.; Nakayama, K.I. Functional similarities and uniqueness of p27 and p57: Insight from a knock-in mouse model. Cell Cycle 2009, 8, 2497–2501. [Google Scholar] [CrossRef]
- Uddin, M.S.; Yu, W.S.; Lim, L.W. Exploring ER stress response in cellular aging and neuroinflammation in Alzheimer’s disease. Ageing Res. Rev. 2021, 70, 101417. [Google Scholar] [CrossRef]
- Madhusudhan, T.; Wang, H.; Dong, W.; Ghosh, S.; Bock, F.; Thangapandi, V.R.; Ranjan, S.; Wolter, J.; Kohli, S.; Shahzad, K.; et al. Defective podocyte insulin signalling through p85-XBP1 promotes ATF6-dependent maladaptive ER-stress response in diabetic nephropathy. Nat. Commun. 2015, 6, 6496. [Google Scholar] [CrossRef]
- Luedde, T.; Rodriguez, M.E.; Tacke, F.; Xiong, Y.; Brenner, D.A.; Trautwein, C. p18(INK4c) collaborates with other CDK-inhibitory proteins in the regenerating liver. Hepatology 2003, 37, 833–841. [Google Scholar] [CrossRef]
- Zezula, J.; Casaccia-Bonnefil, P.; Ezhevsky, S.A.; Osterhout, D.J.; Levine, J.M.; Dowdy, S.F.; Chao, M.V.; Koff, A. p21cip1 is required for the differentiation of oligodendrocytes independently of cell cycle withdrawal. EMBO Rep. 2001, 2, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Weingarten-Gabbay, S.; Elias-Kirma, S.; Nir, R.; Gritsenko, A.A.; Stern-Ginossar, N.; Yakhini, Z.; Weinberger, A.; Segal, E. Comparative genetics. Systematic discovery of cap-independent translation sequences in human and viral genomes. Science 2016, 351, 4939. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Tsai, C.T.; Huangfu, C.A.; Huang, W.Y.; Lei, H.Y.; Lin, C.F.; Su, I.J.; Chang, W.T.; Wu, P.H.; Chen, Y.T.; et al. ACSL3 and GSK-3beta are essential for lipid upregulation induced by endoplasmic reticulum stress in liver cells. J. Cell. Biochem. 2011, 112, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.X.; Ye, S.S.; Hong, Y.X.; Chen, Y.; Wang, B.; Lin, X.J.; Lin, X. Hepatitis B Virus Small Envelope Protein Promotes Hepatocellular Carcinoma Angiogenesis via Endoplasmic Reticulum Stress Signaling to Upregulate the Expression of Vascular Endothelial Growth Factor A. J. Virol. 2022, 96, e0197521. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.L.; Zhang, R.Y.; Liu, Z.K.; Wei, D.; Shang, Y.K.; Wu, J.; Zhang, Z.Y.; Li, C.; Chen, Z.N.; Bian, H. Gamma-secretase complex-dependent intramembrane proteolysis of CD147 regulates the Notch1 signaling pathway in hepatocellular carcinoma. J. Pathol. 2019, 249, 255–267. [Google Scholar] [CrossRef] [PubMed]


| Clinicopathological | LHB Protein Express | p Value | |
|---|---|---|---|
| Value | High (n = 48) | Low (n = 26) | |
| Gender | |||
| Male | 43 | 24 | 0.7022 |
| Female | 5 | 2 | |
| Age | |||
| <50 years | 24 | 11 | 0.5269 |
| ≥50 years | 24 | 15 | |
| Serum AFP | |||
| <20 (ng/mL) | 16 | 11 | 0.4439 |
| ≥20 (ng/mL) | 32 | 15 | |
| Tumor number | |||
| Single | 37 | 24 | 0.1337 |
| Multiple | 10 | 2 | |
| Tumor size | |||
| <5 cm | 29 | 20 | 0.1518 |
| ≥5 cm | 19 | 6 | |
| TNM stage | |||
| I | 28 | 20 | 0.1098 |
| II–IV | 20 | 6 | |
| Tumor differentiation | |||
| I–II | 17 | 18 | 0.0054 |
| III–IV | 31 | 8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Shao, J.; Zhang, R.; Han, M.; Kong, L.; Liu, Z.; Li, H.; Wei, D.; Lu, M.; Zhang, S.; et al. Large HBV Surface Protein-Induced Unfolded Protein Response Dynamically Regulates p27 Degradation in Hepatocellular Carcinoma Progression. Int. J. Mol. Sci. 2023, 24, 13825. https://doi.org/10.3390/ijms241813825
Guo Y, Shao J, Zhang R, Han M, Kong L, Liu Z, Li H, Wei D, Lu M, Zhang S, et al. Large HBV Surface Protein-Induced Unfolded Protein Response Dynamically Regulates p27 Degradation in Hepatocellular Carcinoma Progression. International Journal of Molecular Sciences. 2023; 24(18):13825. https://doi.org/10.3390/ijms241813825
Chicago/Turabian StyleGuo, Yixiao, Jie Shao, Renyu Zhang, Mingwei Han, Lingmin Kong, Zekun Liu, Hao Li, Ding Wei, Meng Lu, Shuai Zhang, and et al. 2023. "Large HBV Surface Protein-Induced Unfolded Protein Response Dynamically Regulates p27 Degradation in Hepatocellular Carcinoma Progression" International Journal of Molecular Sciences 24, no. 18: 13825. https://doi.org/10.3390/ijms241813825





