The Complete Mitochondrial Genomes of Two Rock Scallops (Bivalvia: Spondylidae) Indicate Extensive Gene Rearrangements and Adaptive Evolution Compared with Pectinidae
Abstract
:1. Introduction
2. Results and Discussion
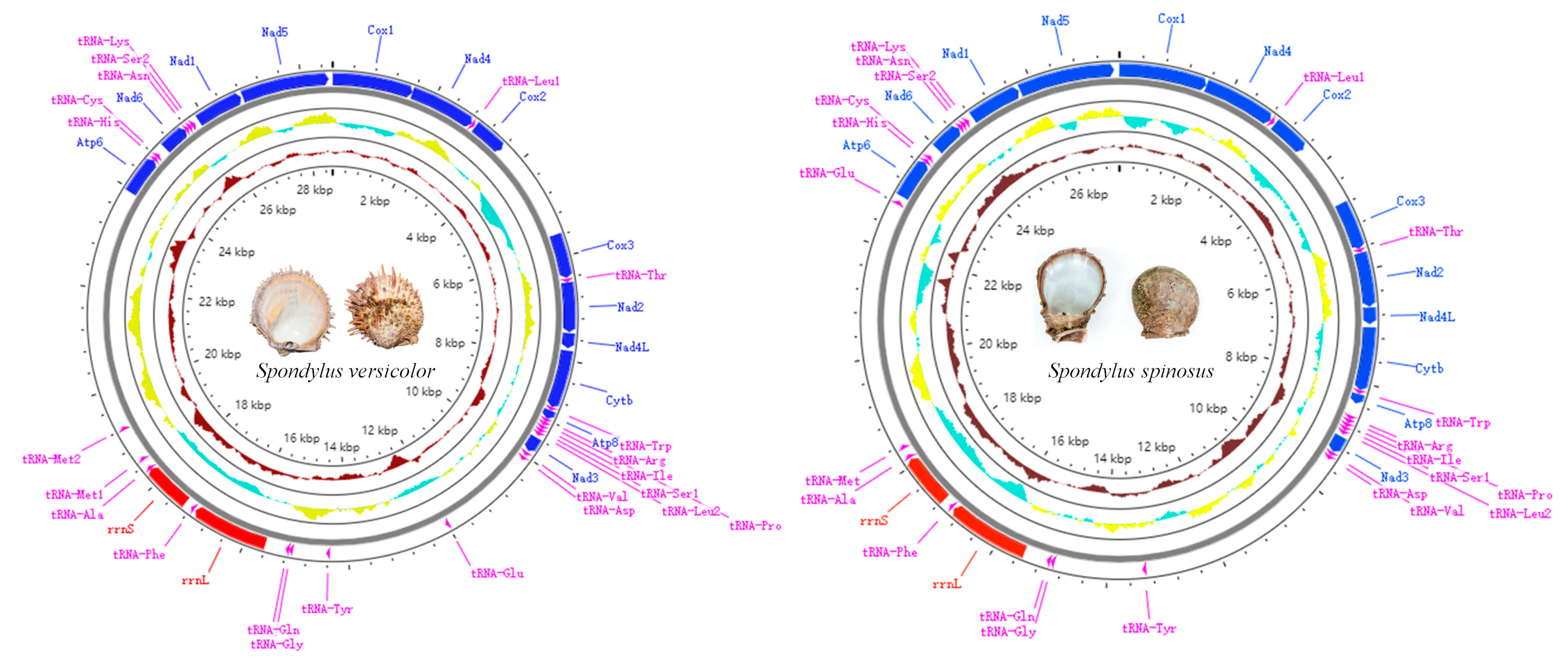
2.1. Mitogenomic Structure and Composition
2.2. Protein-Coding Genes, Transfer RNA, and Ribosomal RNA Genes
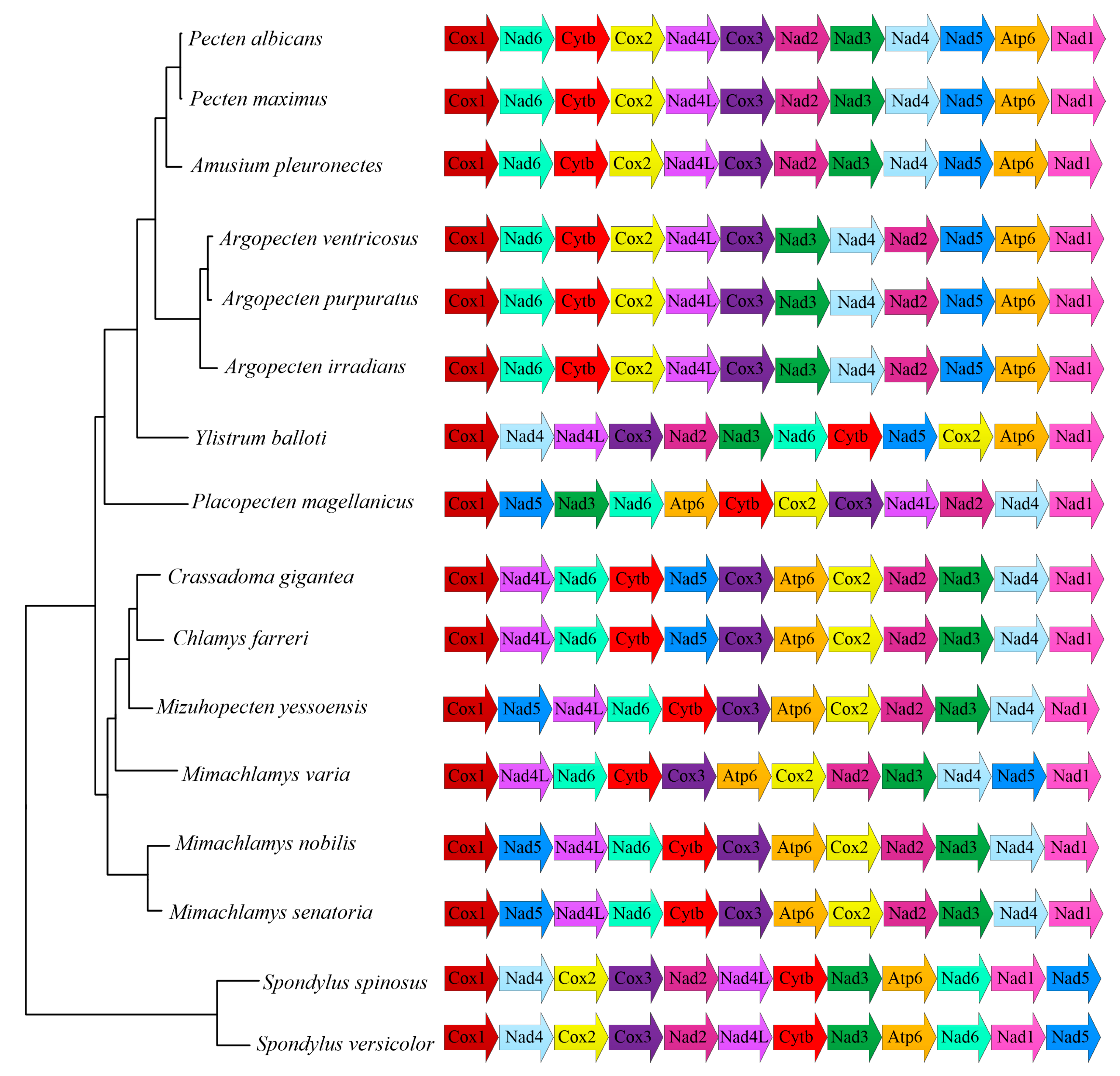
2.3. Phylogenetic Analysis
2.4. Gene Rearrangement
2.5. Positive Selection Analysis
3. Materials and Methods
3.1. Sample Collection and DNA Extraction
3.2. DNA Sequencing, Mitogenome Assembly and Annotation
3.3. Phylogenetic Analysis
3.4. Gene order Comparison and Positive Selection Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Healy, J.M.; Buckland-Nicks, J.A.; Jamieson, B.G. Spermatozoal ultrastructure of spiny oysters (Spondylidae, Bivalvia) including a comparison with other bivalves. Invertebr. Reprod. Dev. 2001, 40, 27–37. [Google Scholar] [CrossRef]
- Lamprell, K.L. Spondylus–Spiny Oyster Shells of the World; Brill Academic Press: Leiden, The Netherlands, 1987. [Google Scholar]
- MolluscaBase. Spondylus Linnaeus, 1758. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=138518 (accessed on 12 April 2023).
- Villalejo-Fuerte, M.; Camacho-Mondragón, M.A.; Ceballos-Vázquez, B.P.; López-Villegas, E.O.; Uría-Galicia, E.; Arellano-Martínez, M. Ultrastructure of sperm development and mature sperm morphology in Spondylus calcifer and S. princeps (Bivalvia: Spondylidae). Malacologia 2018, 62, 1–10. [Google Scholar] [CrossRef]
- Mackensen, A.K.; Brey, T.; Sonnenholzner, S. The fate of Spondylus stocks (Bivalvia: Spondylidae) in Ecuador: Is recovery likely? J. Shellfish Res. 2011, 30, 115–121. [Google Scholar] [CrossRef]
- Healy, J.M.; Keys, J.L.; Daddow, L.Y. Comparative sperm ultrastructure in pteriomorphian bivalves with special reference to phylogenetic and taxonomic implications. In The Evolutionary Biology of the BIVALVIA; Harper, E.M., Taylor, J.D., Crame, J.A., Eds.; Special Publications: London, UK, 2000; pp. 169–190. [Google Scholar]
- Waller, T.R. Phylogeny of families in the Pectinoidea (Mollusca: Bivalvia): Importance of the fossil record. Zool. J. Linn. Soc-Lond. 2006, 148, 313–342. [Google Scholar] [CrossRef]
- Abalde, S.; Tenorio, M.J.; Afonso, C.M.; Uribe, J.E.; Echeverry, A.M.; Zardoya, R. Phylogenetic relationships of cone snails endemic to Cabo Verde based on mitochondrial genomes. BMC Evol. Biol. 2017, 17, 231. [Google Scholar] [CrossRef]
- Bernt, M.; Bleidorn, C.; Braband, A.; Dambach, J.; Donath, A.; Fritzsch, G.; Golombek, A.; Hadrys, H.; Jühling, F.; Meusemann, K.; et al. A comprehensive analysis of bilaterian mitochondrial genomes and phylogeny. Mol. Phylogenet. Evol. 2013, 69, 352–364. [Google Scholar] [CrossRef]
- Uribe, J.E.; Colgan, D.; Castro, L.R.; Kano, Y.; Zardoya, R. Phylogenetic relationships among superfamilies of Neritimorpha (Mollusca: Gastropoda). Mol. Phylogenet. Evol. 2016, 104, 21–31. [Google Scholar] [CrossRef]
- Xu, T.; Qi, L.; Kong, L.; Li, Q. Mitogenomics reveals phylogenetic relationships of Patellogastropoda (Mollusca, Gastropoda) and dynamic gene rearrangements. Zool. Scr. 2022, 51, 147–160. [Google Scholar] [CrossRef]
- Brown, W.M.; George, M., Jr.; Wilson, A.C. Rapid evolution of animal mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1979, 76, 1967–1971. [Google Scholar] [CrossRef]
- Burzyński, A.; Zbawicka, M.; Skibinski, D.O.; Wenne, R. Evidence for recombination of mtDNA in the marine mussel Mytilus trossulus from the Baltic. Mol. Biol. Evol. 2003, 20, 388–392. [Google Scholar] [CrossRef]
- Ghiselli, F.; Gomes-dos-Santos, A.; Adema, C.M.; Lopes-Lima, M.; Sharbrough, J.; Boore, J.L. Molluscan mitochondrial genomes break the rules. Philos. T. R. Soc. B. 2021, 376, 20200159. [Google Scholar] [CrossRef] [PubMed]
- Labecka, A.M.; Domagala, J. Two pathways for spermatogenesis in Sinanodonta woodiana (Lea, 1834) (Bivalvia: Unionidae). J. Mollus. Stud. 2019, 85, 300–310. [Google Scholar] [CrossRef]
- Da Fonseca, R.R.; Johnson, W.E.; O’Brien, S.J.; Ramos, M.J.; Antunes, A. The adaptive evolution of the mammalian mitochondrial genome. BMC Genom. 2008, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Liu, J.; Zhang, H. The first complete mitochondrial genome of the Mariana Trench Freyastera benthophila (Asteroidea: Brisingida: Brisingidae) allows insights into the deep-sea adaptive evolution of Brisingida. Ecol. Evol. 2018, 8, 10673–10686. [Google Scholar] [CrossRef]
- Zhao, B.; Gao, S.; Zhao, M.; Lv, H.; Song, J.; Wang, H.; Zeng, Q.; Liu, J. Mitochondrial genomic analyses provide new insights into the “missing” atp8 and adaptive evolution of Mytilidae. BMC Genom. 2022, 23, 738. [Google Scholar] [CrossRef]
- Sun, S.; Hui, M.; Wang, M.; Sha, Z. The complete mitochondrial genome of the alvinocaridid shrimp Shinkaicaris leurokolos (Decapoda, Caridea): Insight into the mitochondrial genetic basis of deep-sea hydrothermal vent adaptation in the shrimp. Comp. Biochem. Phys. D 2018, 25, 42–52. [Google Scholar] [CrossRef]
- Alejandrino, A.; Puslednik, L.; Serb, J.M. Convergent and parallel evolution in life habit of the scallops (Bivalvia: Pectinidae). BMC Evol. Biol. 2011, 11, 164. [Google Scholar] [CrossRef]
- Kong, L.; Li, Y.; Kocot, K.M.; Yang, Y.; Qi, L.; Li, Q.; Halanych, K.M. Mitogenomics reveals phylogenetic relationships of Arcoida (Mollusca, Bivalvia) and multiple independent expansions and contractions in mitochondrial genome size. Mol. Phylogenet. Evol. 2020, 150, 106857. [Google Scholar] [CrossRef]
- Li, F.; Fan, M.; Wang, S.; Gu, Z.; Wang, A.; Liu, C.; Yang, Y.; Liu, S. The Complete mitochondrial genome of Hyotissa hyotis (Bivalvia: Gryphaeidae) reveals a unique gene order within Ostreoidea. Fishes 2022, 7, 317. [Google Scholar] [CrossRef]
- Uribe, J.E.; Sei, M.; Harasewych, M.G. The mitogenome of the sunken wood limpet Notocrater youngi: Insights into mitogenome evolution in Lepetellida (Gastropoda: Vetigastropoda). J. Mollus. Stud. 2022, 88, eyac009. [Google Scholar] [CrossRef]
- Malkócs, T.; Viricel, A.; Becquet, V.; Evin, L.; Dubillot, E.; Pante, E. Complex mitogenomic rearrangements within the Pectinidae (Mollusca: Bivalvia). BMC Ecol. Evol. 2022, 22, 29. [Google Scholar] [CrossRef]
- Li, F.; Zheng, J.; Ma, Q.; Gu, Z.; Wang, A.; Yang, Y.; Liu, C. Phylogeny of Strombidae (Gastropoda) based on mitochondrial genomes. Front. Mar. Sci. 2022, 9, 930910. [Google Scholar] [CrossRef]
- Ojala, D.; Montoya, J.; Attardi, G. tRNA punctuation model of RNA processing in human mitochondria. Nature 1981, 290, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Higgs, P.G. Codon usage in mitochondrial genomes: Distinguishing context-dependent mutation from translational selection. Mol. Biol. Evol. 2008, 25, 339–351. [Google Scholar] [CrossRef]
- Ren, J.; Shen, X.; Jiang, F.; Liu, B. The mitochondrial genomes of two scallops, Argopecten irradians and Chlamys farreri (Mollusca: Bivalvia): The most highly rearranged gene order in the family Pectinidae. J. Mol. Evol. 2010, 70, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Kanno, M.; Yu, H.; Li, Q.; Kijima, A. Complete mitochondrial DNA sequence and phylogenetic analysis of Zhikong scallop Chlamys farreri (Bivalvia: Pectinidae). Mol. Biol. Rep. 2011, 38, 3067–3074. [Google Scholar] [CrossRef] [PubMed]
- Danic-Tchaleu, G.; Heurtebise, S.; Morga, B.; Lapègue, S. Complete mitochondrial DNA sequence of the European flat oyster Ostrea edulis confirms Ostreidae classification. BMC Res. Notes 2011, 4, 400. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, X.; Yu, Z. Comparative studies of the complete mitochondrial genomes of four Paphia clams and reconsideration of subgenus Neotapes (Bivalvia: Veneridae). Gene 2012, 494, 17–23. [Google Scholar] [CrossRef]
- Waller, T.R. Morphology, morphoclines and a new classification of the Pteriomorphia (Mollusca: Bivalvia). Philos. Trans. R. Soc. Lond. B Biol. Sci. 1978, 284, 345–365. [Google Scholar]
- Waller, T.R. Evolutionary relationships among commercial scallops (Mollusca: Bivalvia: Pectinidae). In Scallops: Biology, Ecology and Aquaculture; Shumway, S.E., Ed.; Elseiver: New York, NY, USA, 1991; pp. 1–73. [Google Scholar]
- Matsumoto, M. Phylogenetic analysis of the subclass Pteriomorphia (Bivalvia) from mtDNA COI sequences. Mol. Phylogenet. Evol. 2003, 27, 429–440. [Google Scholar] [CrossRef]
- Smedley, G.D.; Audino, J.A.; Grula, C.; Porath-Krause, A.; Pairett, A.N.; Alejandrino, A.; Lacey, L.; Masters, F.; Duncan, P.F.; Strong, E.E.; et al. Molecular phylogeny of the Pectinoidea (Bivalvia) indicates Propeamussiidae to be a non-monophyletic family with one clade sister to the scallops (Pectinidae). Mol. Phylogenet. Evol. 2019, 137, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Puslednik, L.; Serb, J.M. Molecular phylogenetics of the Pectinidae (Mollusca: Bivalvia) and effect of increased taxon sampling and outgroup selection on tree topology. Mol. Phylogenet. Evol. 2008, 48, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Irwin, A.R.; Strong, E.E.; Kano, Y.; Harper, E.M.; Williams, S.T. Eight new mitogenomes clarify the phylogenetic relationships of Stromboidea within the caenogastropod phylogenetic framework. Mol. Phylogenet. Evol. 2021, 158, 107081. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, C.; Wang, H.; Xu, Z. Diversity and evolution of living oysters. J. Shellfish Res. 2018, 37, 755–771. [Google Scholar] [CrossRef]
- Tempestini, A.; Massamba-N’Siala, G.; Vermandele, F.; Beaudreau, N.; Mortz, M.; Dufresne, F.; Calosi, P. Extensive gene rearrangements in the mitogenomes of congeneric annelid species and insights on the evolutionary history of the genus Ophryotrocha. BMC Genom. 2020, 21, 815. [Google Scholar] [CrossRef]
- Botero-Castro, F.; Tilak, M.K.; Justy, F.; Catzeflis, F.; Delsuc, F.; Douzery, E.J. In cold blood: Compositional bias and positive selection drive the high evolutionary rate of vampire bats mitochondrial genomes. Genome Biol. Evol. 2018, 10, 2218–2239. [Google Scholar] [CrossRef]
- Tomasco, I.H.; Lessa, E.P. The evolution of mitochondrial genomes in subterranean caviomorph rodents: Adaptation against a background of purifying selection. Mol. Phylogenet. Evol. 2011, 61, 64–70. [Google Scholar] [CrossRef]
- Romero, P.E.; Weigand, A.M.; Pfenninger, M. Positive selection on panpulmonate mitogenomes provide new clues on adaptations to terrestrial life. BMC Evol. Biol. 2016, 16, 164. [Google Scholar] [CrossRef]
- Zhao, D.; Guo, Y.; Gao, Y. Natural selection drives the evolution of mitogenomes in Acrossocheilus. PLoS ONE 2022, 17, e0276056. [Google Scholar] [CrossRef]
- Yang, M.; Dong, D.; Li, X. The complete mitogenome of Phymorhynchus sp. (Neogastropoda, Conoidea, Raphitomidae) provides insights into the deep-sea adaptive evolution of Conoidea. Ecol. Evol. 2021, 11, 7518–7531. [Google Scholar] [CrossRef]
- Stanley, S.M. Relation of shell form to life habits of the Bivalvia (Mollusca); Geological Society of America: Boulder, CO, USA, 1970; pp. 1–296. [Google Scholar]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Laslett, D.; Canbäck, B. ARWEN: A program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics 2008, 24, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.R.; Stothard, P. The CGView Server: A comparative genomics tool for circular genomes. Nucleic Acids Res. 2008, 36, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Xia, X. DAMBE7: New and improved tools for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2018, 35, 1550–1552. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Won, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [PubMed]

| Newly Sequenced Mitochondrial Genome | ||||
|---|---|---|---|---|
| Family | Species | Length (bp) | Sampling Time | Accession No. |
| Spondylidae | Spondylus versicolor | 28,600 | May, 2022 | OR167109 |
| Spondylidae | Spondylus spinosus | 27,566 | May, 2022 | OR167110 |
| GenBank Mitochondrial Genomes | ||||
| Family | Species | Length (bp) | Accession No. | |
| Pectinidae | Pecten albicans (Schröter, 1802) | 16,653 | KP900974 | |
| Pectinidae | Pecten maximus (Linnaeus, 1758) | 17,252 | KP900975 | |
| Pectinidae | Amusium pleuronectes (Linnaeus, 1758) | 18,044 | MT419374 | |
| Pectinidae | Argopecten ventricosus (G. B. Sowerby II, 1842) | 16,079 | KT161261 | |
| Pectinidae | Argopecten purpuratus (Lamarck, 1819) | 16,270 | KT161260 | |
| Pectinidae | Argopecten irradians (Lamarck, 1819) | 16,212 | KU589290 | |
| Pectinidae | Ylistrum balloti (Bernardi, 1861) | 19,484 | ON041136 | |
| Pectinidae | Placopecten magellanicus (Gmelin, 1791) | 32,115 | DQ088274 | |
| Pectinidae | Crassadoma gigantea (J. E. Gray, 1825) | 18,495 | MH016739 | |
| Pectinidae | Chlamys farreri (K. H. Jones and Preston, 1904) | 20,889 | EF473269 | |
| Pectinidae | Mizuhopecten yessoensis (Jay, 1857) | 20,964 | FJ595959 | |
| Pectinidae | Mimachlamys varia (Linnaeus, 1758) | 20,400 | MZ520326 | |
| Pectinidae | Mimachlamys nobilis (Reeve, 1852) | 17,935 | FJ595958 | |
| Pectinidae | Mimachlamys senatoria (Gmelin, 1791) | 17,383 | KF214684 | |
| Ostreidae | Crassostrea gigas (Thunberg, 1793) | 18,225 | EU672831 | |
| Ostreidae | Ostrea edulis (Linnaeus, 1758) | 16,320 | JF274008 | |
| Branch-Specific Models | ||||
|---|---|---|---|---|
| Model | lnL | Estimates of parameters | Model compared | 2ΔlnL |
| M0 | −100,792.0493 | ω = 0.03351 | ||
| Two-ratio | −100,751.3274 | ω1 = 1.84124, ω0 = 0.03263 | Two-ratio versus M0 | 81.4439 ** |
| Free-ratio | −100,397.5439 | Free-ratio versus M0 | 789.0108 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Zhang, Y.; Zhong, T.; Heng, X.; Ao, T.; Gu, Z.; Wang, A.; Liu, C.; Yang, Y. The Complete Mitochondrial Genomes of Two Rock Scallops (Bivalvia: Spondylidae) Indicate Extensive Gene Rearrangements and Adaptive Evolution Compared with Pectinidae. Int. J. Mol. Sci. 2023, 24, 13844. https://doi.org/10.3390/ijms241813844
Li F, Zhang Y, Zhong T, Heng X, Ao T, Gu Z, Wang A, Liu C, Yang Y. The Complete Mitochondrial Genomes of Two Rock Scallops (Bivalvia: Spondylidae) Indicate Extensive Gene Rearrangements and Adaptive Evolution Compared with Pectinidae. International Journal of Molecular Sciences. 2023; 24(18):13844. https://doi.org/10.3390/ijms241813844
Chicago/Turabian StyleLi, Fengping, Yu Zhang, Tao Zhong, Xin Heng, Tiancheng Ao, Zhifeng Gu, Aimin Wang, Chunsheng Liu, and Yi Yang. 2023. "The Complete Mitochondrial Genomes of Two Rock Scallops (Bivalvia: Spondylidae) Indicate Extensive Gene Rearrangements and Adaptive Evolution Compared with Pectinidae" International Journal of Molecular Sciences 24, no. 18: 13844. https://doi.org/10.3390/ijms241813844






