Comparison of the Formation of Plant–Microbial Interface in Pisum sativum L. and Medicago truncatula Gaertn. Nitrogen-Fixing Nodules
Abstract
:1. Introduction
2. Results
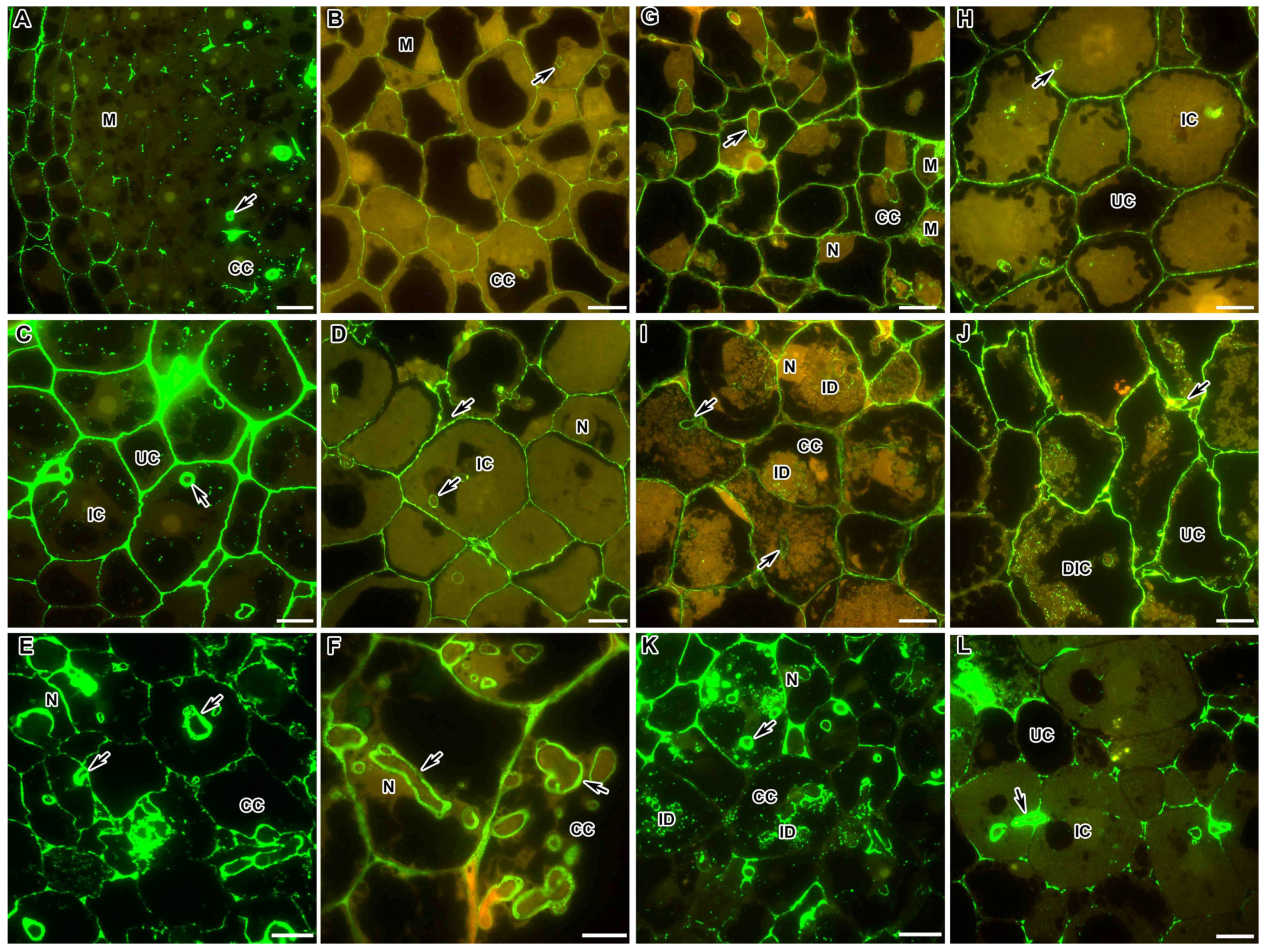
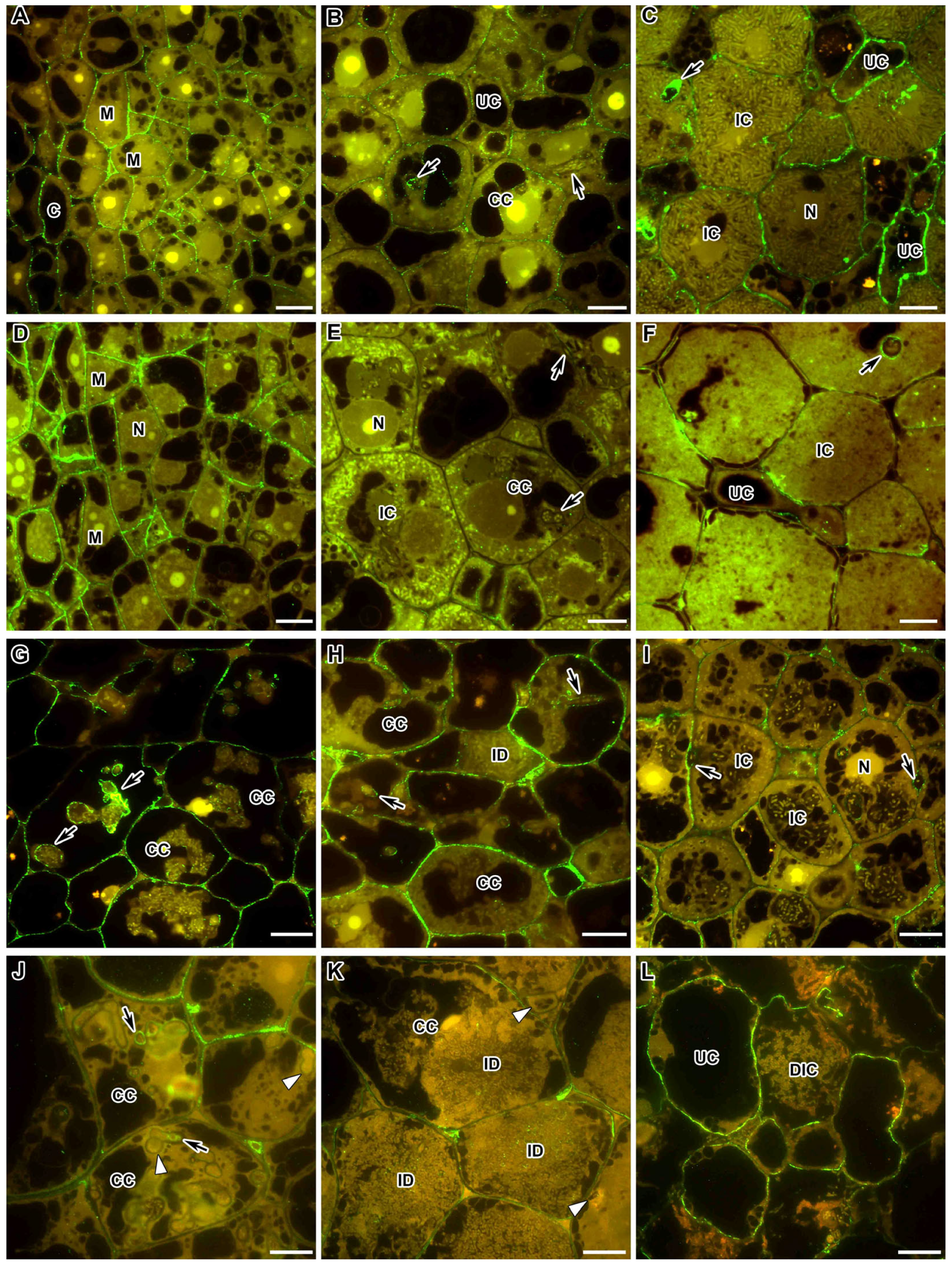
2.1. Methylesterified Pectin
2.2. Demethylesterified Pectin
2.3. Pectin Cross-Linked by Ca2+ Ions
2.4. Rhamnogalacturonan I (Unbranched) Backbone
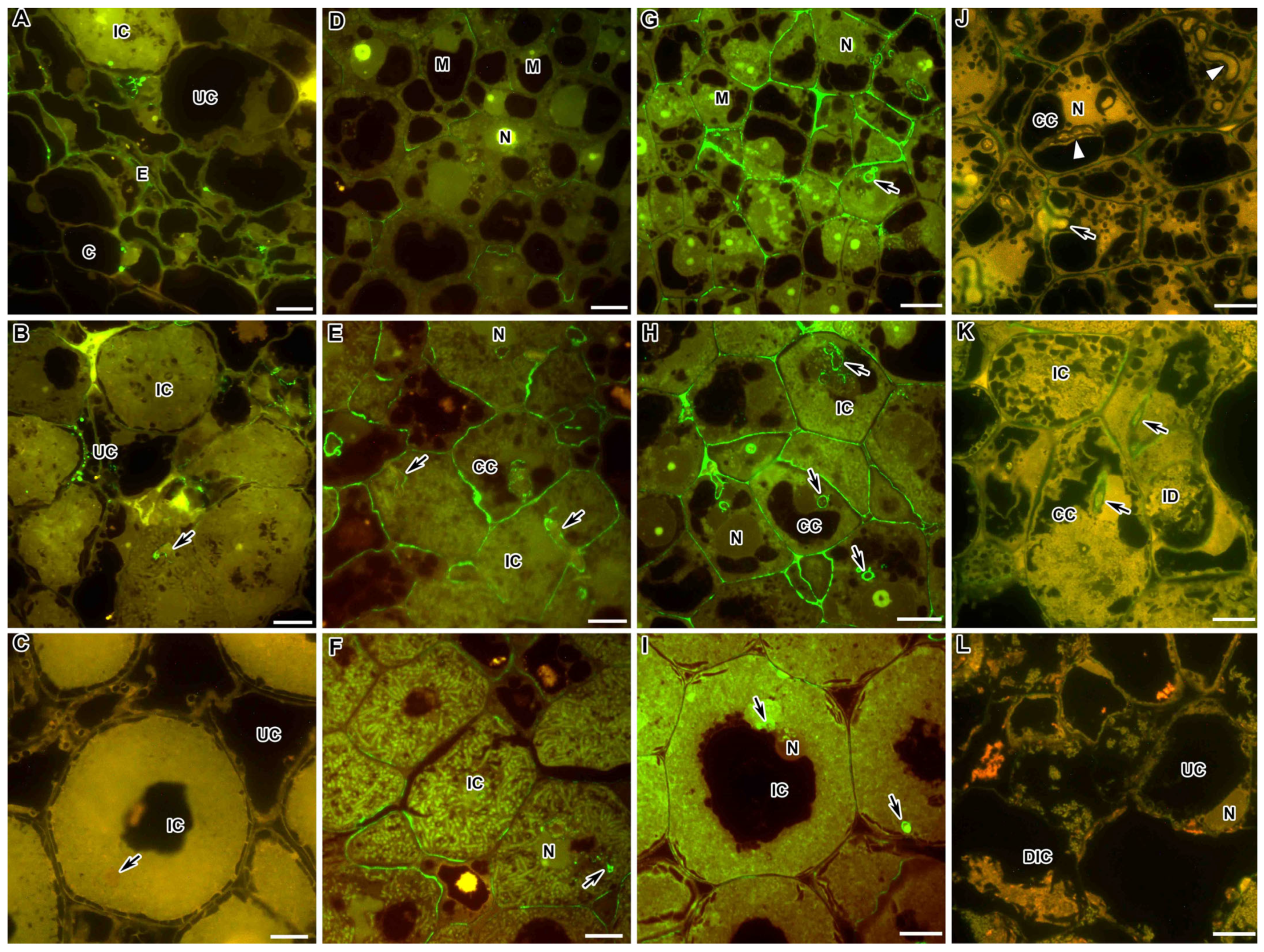
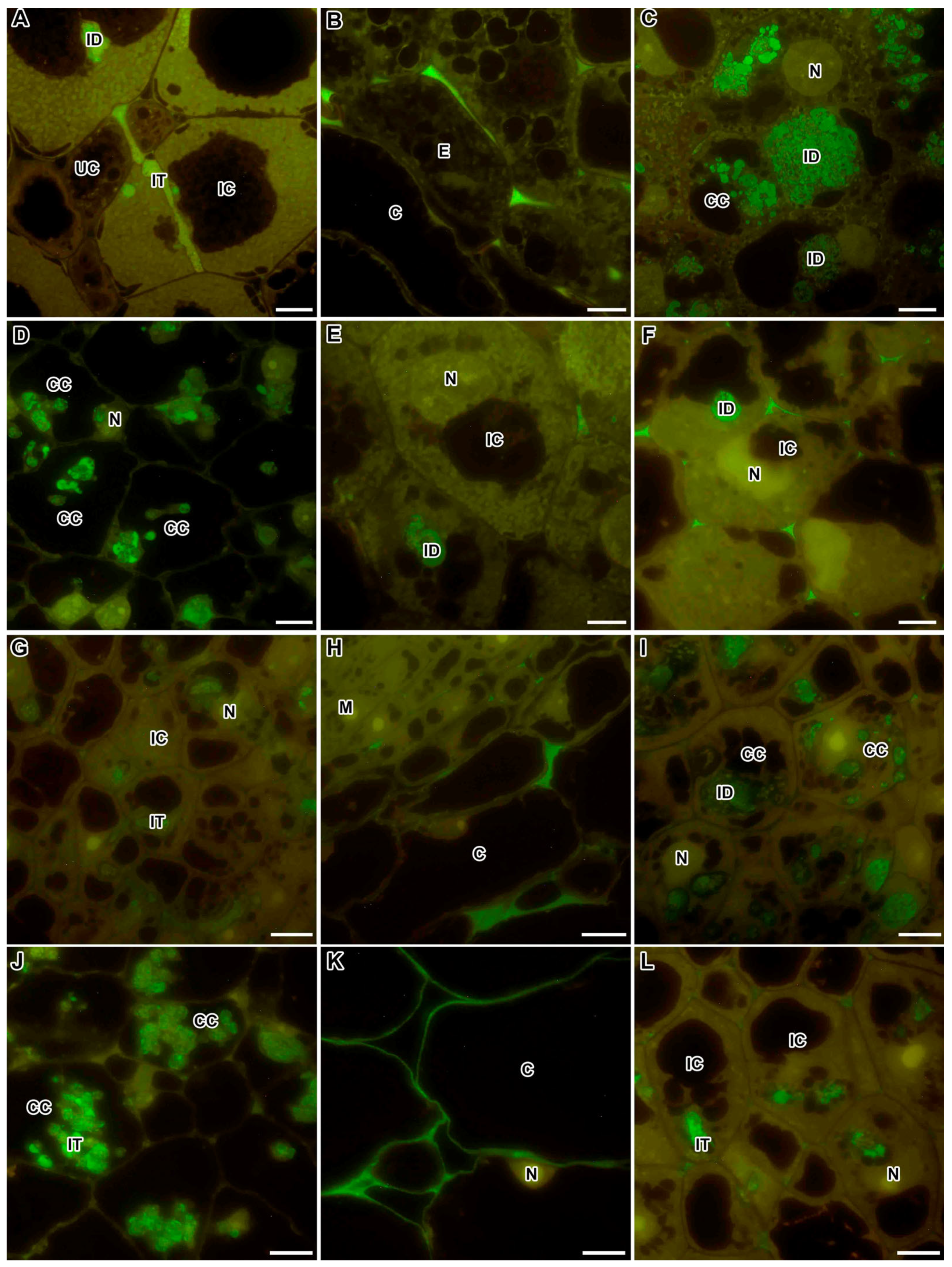
2.5. Rhamnogalacturonan I (Linear (1-5)-α-L-Arabinan)
2.6. Xylogalacturonan
2.7. Feruloylated-(1→4)-β-D-Arabinan
2.8. Fucosylated Xyloglucan
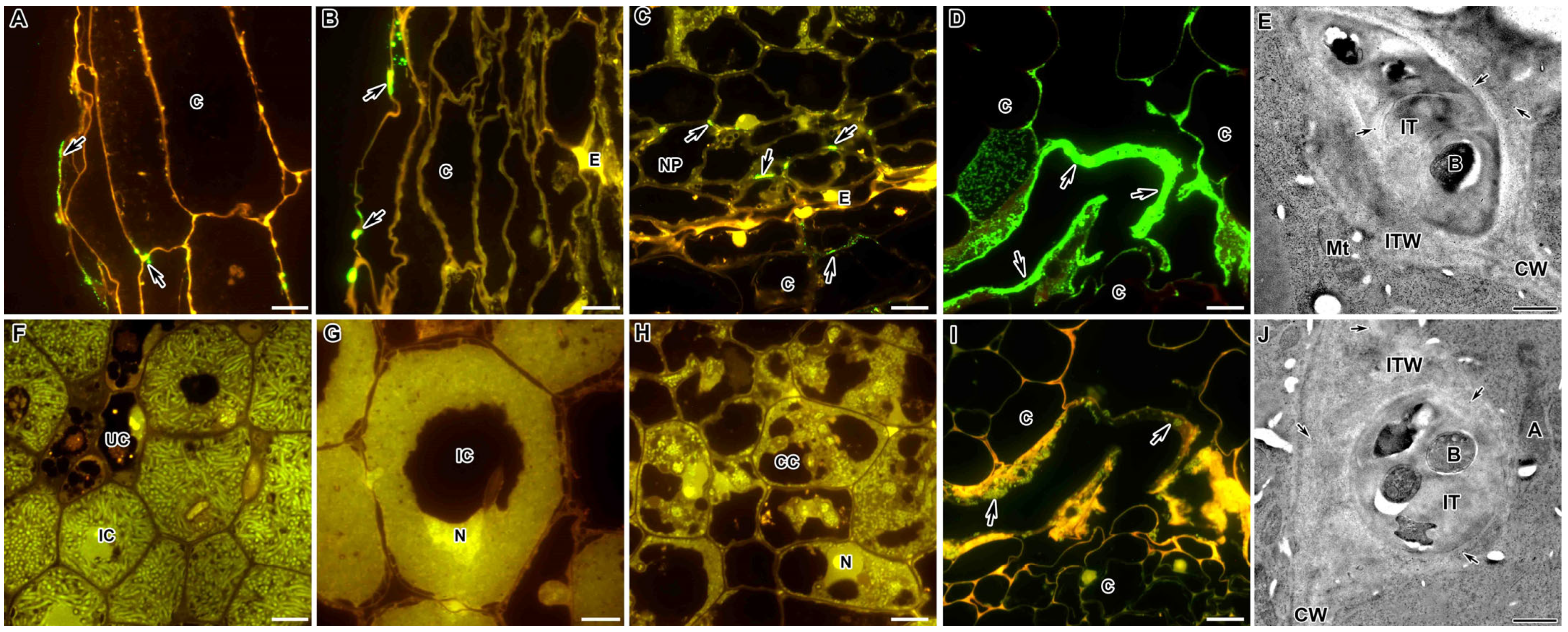
2.9. Arabinogalactan Protein
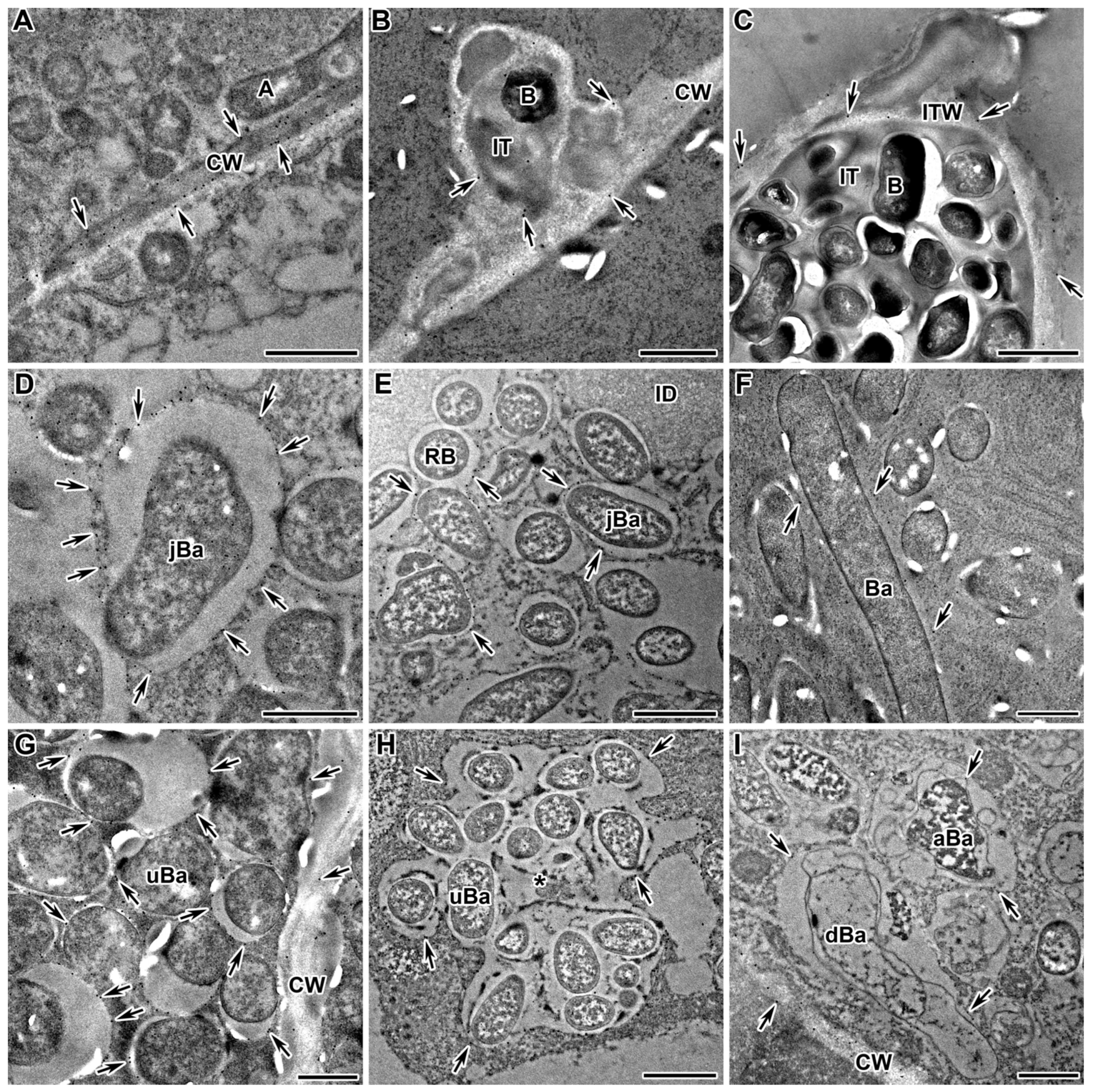
2.10. Extensin
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Bacterial Strains, Inoculation and Plant Growth Conditions
4.3. Sample Preparation
4.4. Fluorescence Microscopy
4.5. Transmission Electron Microscopy
4.6. Antibodies
4.7. Controls of the Specificity of the Immunolabelling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sprent, J.I. Evolving ideas of legume evolution and diversity: A taxonomic perspective on the occurrence of nodulation. New Phytol. 2007, 174, 11–25. [Google Scholar] [CrossRef]
- Van Rhijn, P.; Vanderleyden, J. The Rhizobium-plant symbiosis. Microbiol. Rev. 1995, 59, 124–142. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E.D.; Murray, J.D.; Poole, P.S.; Downie, J.A. The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 2011, 45, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Guinel, F.C. Getting around the legume nodule: I. The structure of the peripheral zone in four nodule types. Botany 2009, 87, 1117–1138. [Google Scholar] [CrossRef]
- Rich, M.K.; Schorderet, M.; Reinhardt, D. The role of the cell wall compartment in mutualistic symbioses of plants. Front. Plant Sci. 2014, 5, 238. [Google Scholar] [CrossRef]
- Venado, R.E.; Liang, J.; Marín, M. Rhizobia infection, a journey to the inside of plant cells. In Advances in Botanical Research; Frendo, P., Frugier, F., Masson-Boivin, C., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 94, pp. 97–118. [Google Scholar]
- Bozsoki, Z.; Gysel, K.; Hansen, S.B.; Lironi, D.; Krönauer, C.; Feng, F.; de Jong, N.; Vinther, M.; Kamble, M.; Thygesen, M.B.; et al. Ligand-recognizing motifs in plant LysM receptors are major determinants of specificity. Science 2020, 369, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Mbengue, M.D.; Hervé, C.; Debellé, F. Nod factor signaling in symbiotic nodulation. In Advances in Botanical Research; Frendo, P., Frugier, F., Masson-Boivin, C., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 94, pp. 1–39. [Google Scholar]
- Oldroyd, G.E.D.; Leyser, O. A plant’s diet, surviving in a variable nutrient environment. Science 2020, 368, eaba0196. [Google Scholar] [CrossRef] [PubMed]
- Perotto, S.; Vandenbosch, K.A.; Butcher, G.W.; Brewin, N.J. Molecular composition and development of the plant with the peribacteroid membrane of pea root nodules. Development 1991, 112, 763–773. [Google Scholar] [CrossRef]
- Brewin, N.J. Plant cell wall remodelling in the Rhizobium–legume symbiosis. Crit. Rev. Plant Sci. 2004, 23, 293–316. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Seliverstova, E.V.; Brewin, N.J.; Tsyganov, V.E. Comparative analysis of remodelling of the plant–microbe interface in Pisum sativum and Medicago truncatula symbiotic nodules. Protoplasma 2019, 256, 983–996. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Tsyganov, V.E.; Findlay, K.C.; Borisov, A.Y.; Tikhonovich, I.A.; Brewin, N.J. Distribution of legume arabinogalactan protein-extensin (AGPE) glycoproteins in symbiotically defective pea mutants with abnormal infection threads. Cell Tissue Biol. 2009, 3, 93–102. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Walker, J.L.; Guillon, F.; Scheller, H.V. A comparative study of sample preparation for staining and immunodetection of plant cell walls by light microscopy. Front. Plant Sci. 2017, 8, 1505. [Google Scholar] [CrossRef] [PubMed]
- Rydahl, M.G.; Hansen, A.R.; Kračun, S.K.; Mravec, J. Report on the current inventory of the toolbox for plant cell wall analysis: Proteinaceous and small molecular probes. Front. Plant Sci. 2018, 9, 581. [Google Scholar] [CrossRef] [PubMed]
- de Faria, S.M.; Ringelberg, J.J.; Gross, E.; Koenen, E.J.M.; Cardoso, D.; Ametsitsi, G.K.D.; Akomatey, J.; Maluk, M.; Tak, N.; Gehlot, H.S.; et al. The innovation of the symbiosome has enhanced the evolutionary stability of nitrogen fixation in legumes. New Phytol. 2022, 235, 2365–2377. [Google Scholar] [CrossRef] [PubMed]
- Limpens, E.; Ivanov, S.; van Esse, W.; Voets, G.; Fedorova, E.; Bisseling, T. Medicago N2-fixing symbiosomes acquire the endocytic identity marker Rab7 but delay the acquisition of vacuolar identity. Plant Cell 2009, 21, 2811–2828. [Google Scholar] [CrossRef] [PubMed]
- Grefen, C.; Honsbein, A.; Blatt, M.R. Ion transport, membrane traffic and cellular volume control. Curr. Opin. Plant Biol. 2011, 14, 332–339. [Google Scholar] [CrossRef]
- Gavrin, A.; Kaiser, B.N.; Geiger, D.; Tyerman, S.D.; Wen, Z.; Bisseling, T.; Fedorova, E.E. Adjustment of host cells for accommodation of symbiotic bacteria: Vacuole defunctionalization, HOPS suppression, and TIP1g retargeting in Medicago. Plant Cell 2014, 26, 3809–3822. [Google Scholar] [CrossRef]
- Gavrin, A.; Kulikova, O.; Bisseling, T.; Fedorova, E.E. Interface symbiotic membrane formation in root nodules of Medicago truncatula: The role of synaptotagmins MtSyt1, MtSyt2 and MtSyt3. Front. Plant Sci. 2017, 8, 201. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Brewin, N.J.; Tsyganov, V.E. Structure and development of the legume-rhizobial symbiotic interface in infection threads. Cells 2021, 10, 1050. [Google Scholar] [CrossRef]
- Hernandez, L.E.; Perotto, S.; Brewin, N.J.; Drobak, B.K. A novel inositol-lipid in plant-bacteria symbiosis. Biochem. Soc. Trans. 1995, 23, 582S. [Google Scholar] [CrossRef]
- Nguema-Ona, E.; Vicré-Gibouin, M.; Cannesan, M.-A.; Driouich, A. Arabinogalactan proteins in root–microbe interactions. Trends Plant Sci. 2013, 18, 440–449. [Google Scholar] [CrossRef]
- Saeki, K. Rhizobial measures to evade host defense strategies and endogenous threats to persistent symbiotic nitrogen fixation: A focus on two legume-rhizobium model systems. Cell. Mol. Life Sci. 2011, 68, 1327–1339. [Google Scholar] [CrossRef]
- Berrabah, F.; Bourcy, M.; Eschstruth, A.; Cayrel, A.; Guefrachi, I.; Mergaert, P.; Wen, J.; Jean, V.; Mysore, K.S.; Gourion, B.; et al. A nonRD receptor-like kinase prevents nodule early senescence and defense-like reactions during symbiosis. New Phytol. 2014, 203, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Berrabah, F.; Ratet, P.; Gourion, B. Legume nodules: Massive infection in the absence of defense induction. Mol. Plant Microbe Interact. 2019, 32, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Høgslund, N.; Radutoiu, S.; Krusell, L.; Voroshilova, V.; Hannah, M.A.; Goffard, N.; Sanchez, D.H.; Lippold, F.; Ott, T.; Sato, S.; et al. Dissection of symbiosis and organ development by integrated transcriptome analysis of Lotus japonicus mutant and wild-type plants. PLoS ONE 2009, 4, e6556. [Google Scholar] [CrossRef] [PubMed]
- Resendis-Antonio, O.; Hernández, M.; Salazar, E.; Contreras, S.; Batallar, G.M.; Mora, Y.; Encarnación, S. Systems biology of bacterial nitrogen fixation: High-throughput technology and its integrative description with constraint-based modeling. BMC Syst. Biol. 2011, 5, 120. [Google Scholar] [CrossRef]
- Takanashi, K.; Takahashi, H.; Sakurai, N.; Sugiyama, A.; Suzuki, H.; Shibata, D.; Nakazono, M.; Yazaki, K. Tissue-specific transcriptome analysis in nodules of Lotus japonicus. Mol. Plant Microbe Interact. 2012, 25, 869–876. [Google Scholar] [CrossRef]
- Sakamoto, K.; Ogiwara, N.; Kaji, T.; Sugimoto, Y.; Ueno, M.; Sonoda, M.; Matsui, A.; Ishida, J.; Tanaka, M.; Totoki, Y.; et al. Transcriptome analysis of soybean (Glycine max) root genes differentially expressed in rhizobial, arbuscular mycorrhizal, and dual symbiosis. J. Plant Res. 2019, 132, 541–568. [Google Scholar] [CrossRef]
- Kusakin, P.G.; Serova, T.A.; Gogoleva, N.E.; Gogolev, Y.V.; Tsyganov, V.E. Laser microdissection of Pisum sativum L. nodules followed by RNA-Seq analysis revealed crucial transcriptomic changes during infected cell differentiation. Agronomy 2021, 11, 2504. [Google Scholar] [CrossRef]
- Roy, S.; Liu, W.; Nandety, R.S.; Crook, A.; Mysore, K.S.; Pislariu, C.I.; Frugoli, J.; Dickstein, R.; Udvardi, M.K. Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. Plant Cell 2020, 32, 15–41. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Tsyganova, A.V. Symbiotic regulatory genes controlling nodule development in Pisum sativum L. Plants 2020, 9, 1741. [Google Scholar] [CrossRef]
- Ivanova, K.A.; Tsyganova, A.V.; Brewin, N.J.; Tikhonovich, I.A.; Tsyganov, V.E. Induction of host defences by Rhizobium during ineffective nodulation of pea (Pisum sativum L.) carrying symbiotically defective mutations sym40 (PsEFD), sym33 (PsIPD3/PsCYCLOPS) and sym42. Protoplasma 2015, 252, 1505–1517. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Seliverstova, E.V.; Brewin, N.J.; Tsyganov, V.E. Bacterial release is accompanied by ectopic accumulation of cell wall material around the vacuole in nodules of Pisum sativum sym33-3 allele encoding transcription factor PsCYCLOPS/PsIPD3. Protoplasma 2019, 256, 1449–1453. [Google Scholar] [CrossRef] [PubMed]
- Puhlmann, J.; Bucheli, E.; Swain, M.J.; Dunning, N.; Albersheim, P.; Darvill, A.G.; Hahn, M.G. Generation of monoclonal antibodies against plant cell-wall polysaccharides. I. Characterization of a monoclonal antibody to a terminal α-(1→2)-linked fucosyl-containing epitope. Plant Physiol. 1994, 104, 699–710. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Keegstra, K. Plant cell walls. Plant Physiol. 2010, 154, 483–486. [Google Scholar] [CrossRef]
- Anderson, C.T. We be jammin’: An update on pectin biosynthesis, trafficking and dynamics. J. Exp. Bot. 2015, 67, 495–502. [Google Scholar] [CrossRef]
- Liu, Z.; Persson, S.; Sánchez-Rodríguez, C. At the border: The plasma membrane–cell wall continuum. J. Exp. Bot. 2015, 66, 1553–1563. [Google Scholar] [CrossRef]
- Wang, T.; Park, Y.B.; Caporini, M.A.; Rosay, M.; Zhong, L.; Cosgrove, D.J.; Hong, M. Sensitivity-enhanced solid-state NMR detection of expansin’s target in plant cell walls. Proc. Natl. Acad. Sci. USA 2013, 110, 16444–16449. [Google Scholar] [CrossRef]
- Palin, R.; Geitmann, A. The role of pectin in plant morphogenesis. Biosystems 2012, 109, 397–402. [Google Scholar] [CrossRef]
- Wolf, S.; Greiner, S. Growth control by cell wall pectins. Protoplasma 2012, 249, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Vincken, J.-P.; Schols, H.A.; Oomen, R.J.; McCann, M.C.; Ulvskov, P.; Voragen, A.G.; Visser, R.G. If homogalacturonan were a side chain of rhamnogalacturonan I. Implications for cell wall architecture. Plant Physiol. 2003, 132, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef] [PubMed]
- Atmodjo, M.A.; Hao, Z.; Mohnen, D. Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 2013, 64, 747–779. [Google Scholar] [CrossRef] [PubMed]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Tsyganov, V.E. Plant cell wall in symbiotic interactions. Pectins. Sel’skokhozyaistvennaya Biol. Agric. Biol. 2019, 54, 446–457. [Google Scholar] [CrossRef]
- Wolf, S.; Mouille, G.; Pelloux, J. Homogalacturonan methyl-esterification and plant development. Mol. Plant 2009, 2, 851–860. [Google Scholar] [CrossRef]
- Su, C. Pectin modifications at the symbiotic interface. New Phytol. 2023, 238, 25–32. [Google Scholar] [CrossRef]
- Su, C.; Zhang, G.; Rodriguez-Franco, M.; Hinnenberg, R.; Wietschorke, J.; Liang, P.; Yang, W.; Uhler, L.; Li, X.; Ott, T. Transcellular progression of infection threads in Medicago truncatula roots is associated with locally confined cell wall modifications. Curr. Biol. 2023, 33, 533–542.e535. [Google Scholar] [CrossRef]
- Levesque-Tremblay, G.; Pelloux, J.; Braybrook, S.A.; Müller, K. Tuning of pectin methylesterification: Consequences for cell wall biomechanics and development. Planta 2015, 242, 791–811. [Google Scholar] [CrossRef]
- Gavrin, A.; Chiasson, D.; Ovchinnikova, E.; Kaiser, B.N.; Bisseling, T.; Fedorova, E.E. VAMP721a and VAMP721d are important for pectin dynamics and release of bacteria in soybean nodules. New Phytol. 2016, 210, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Peaucelle, A.; Braybrook, S.A.; Le Guillou, L.; Bron, E.; Kuhlemeier, C.; Höfte, H. Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr. Biol. 2011, 21, 1720–1726. [Google Scholar] [CrossRef]
- Rae, A.L.; Bonfante-Fasolo, P.; Brewin, N.J. Structure and growth of infection threads in the legume symbiosis with Rhizobium leguminosarum. Plant J. 1992, 2, 385–395. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Morzhina, E.V.; Stefanov, S.Y.; Borisov, A.Y.; Lebsky, V.K.; Tikhonovich, I.A. The pea (Pisum sativum L.) genes sym33 and sym40 control infection thread formation and root nodule function. Mol. Gen. Genet. 1998, 259, 491–503. [Google Scholar] [CrossRef]
- Hocq, L.; Pelloux, J.; Lefebvre, V. Connecting homogalacturonan-type pectin remodeling to acid growth. Trends Plant Sci. 2017, 22, 20–29. [Google Scholar] [CrossRef]
- Willats, W.G.T.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef]
- Cabrera, J.C.; Boland, A.; Messiaen, J.; Cambier, P.; Van Cutsem, P. Egg box conformation of oligogalacturonides: The time-dependent stabilization of the elicitor-active conformation increases its biological activity. Glycobiology 2008, 18, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Wormit, A.; Usadel, B. The multifaceted role of pectin methylesterase inhibitors (PMEIs). Int. J. Mol. Sci. 2018, 19, 2878. [Google Scholar] [CrossRef]
- Lionetti, V.; Cervone, F.; Bellincampi, D. Methyl esterification of pectin plays a role during plant–pathogen interactions and affects plant resistance to diseases. J. Plant Physiol. 2012, 169, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, V.; Fabri, E.; De Caroli, M.; Hansen, A.R.; Willats, W.G.T.; Piro, G.; Bellincampi, D. Three pectin methylesterase inhibitors protect cell wall integrity for Arabidopsis immunity to Botrytis. Plant Physiol. 2017, 173, 1844–1863. [Google Scholar] [CrossRef]
- Pogorelko, G.; Lionetti, V.; Bellincampi, D.; Zabotina, O. Cell wall integrity: Targeted post-synthetic modifications to reveal its role in plant growth and defense against pathogens. Plant Signal. Behav. 2013, 8, e25435. [Google Scholar] [CrossRef] [PubMed]
- Sujkowska-Rybkowska, M.; Borucki, W. Pectins esterification in the apoplast of aluminum-treated pea root nodules. J. Plant Physiol. 2015, 184, 1–7. [Google Scholar] [CrossRef]
- Komalavilas, P.; Mort, A.J. The acetylation of O-3 of galacturonic acid in the rhamnose-rich portion of pectins. Carbohydr. Res. 1989, 189, 261–272. [Google Scholar] [CrossRef]
- Silva, I.R.; Jers, C.; Meyer, A.S.; Mikkelsen, J.D. Rhamnogalacturonan I modifying enzymes: An update. New Biotechnol. 2016, 33, 41–54. [Google Scholar] [CrossRef]
- Zablackis, E.; Huang, J.; Muller, B.; Darvill, A.G.; Albersheim, P. Characterization of the cell-wall polysaccharides of Arabidopsis thaliana leaves. Plant Physiol. 1995, 107, 1129–1138. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Marcus, S.E.; Haeger, A.; Ordaz-Ortiz, J.J.; Knox, J.P. An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr. Res. 2009, 344, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.; Steele-King, C.G.; Marcus, S.E.; Knox, J.P. Side chains of pectic polysaccharides are regulated in relation to cell proliferation and cell differentiation. Plant J. 1999, 20, 619–628. [Google Scholar] [CrossRef] [PubMed]
- McCartney, L.; Ormerod, A.P.; Gidley, M.J.; Knox, J.P. Temporal and spatial regulation of pectic (1→4)-β-D-galactan in cell walls of developing pea cotyledons: Implications for mechanical properties. Plant J. 2000, 22, 105–113. [Google Scholar] [CrossRef]
- McCartney, L.; Steele-King, C.G.; Jordan, E.; Knox, J.P. Cell wall pectic (1→4)-β-D-galactan marks the acceleration of cell elongation in the Arabidopsis seedling root meristem. Plant J. 2003, 33, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.T.; Marcus, S.E.; Knox, J.P. Generation of a monoclonal antibody specific to (1→5)-α-L-arabinan. Carbohydr. Res. 1998, 308, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Showalter, A.M. Arabinogalactan-proteins: Structure, expression and function. Cell. Mol. Life Sci. 2001, 58, 1399–1417. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.; Egelund, J.; Schultz, C.J.; Bacic, A. Arabinogalactan-proteins (AGPs): Key regulators at the cell surface? Plant Physiol. 2010, 153, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Saffer, A.M. Expanding roles for pectins in plant development. J. Integr. Plant Biol. 2018, 60, 910–923. [Google Scholar] [CrossRef]
- Willats, W.G.T.; McCartney, L.; Steele-King, C.G.; Marcus, S.E.; Mort, A.; Huisman, M.; van Alebeek, G.-J.; Schols, H.A.; Voragen, A.G.J.; Le Goff, A.; et al. A xylogalacturonan epitope is specifically associated with plant cell detachment. Planta 2004, 218, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Waldron, K.W.; Smith, A.C.; Parr, A.J.; Ng, A.; Parker, M.L. New approaches to understanding and controlling cell separation in relation to fruit and vegetable texture. Trends Food Sci. Technol. 1997, 8, 213–221. [Google Scholar] [CrossRef]
- Ng, A.; Harvey, A.J.; Parker, M.L.; Smith, A.C.; Waldron, K.W. Effect of oxidative coupling on the thermal stability of texture and cell wall chemistry of beet root (Beta vulgaris). J. Agric. Food Chem. 1998, 46, 3365–3370. [Google Scholar] [CrossRef]
- Waldron, K.W.; Brett, C.T. The role of polymer cross-linking in intercellular adhesion. In Plant Cell Separation and Adhesion; Roberts, J.A., Gonzalez-Carranza, Z., Eds.; Blackwell Publishing: Oxford, UK, 2007; pp. 183–204. [Google Scholar] [CrossRef]
- Zamil, M.S.; Geitmann, A. The middle lamella—More than a glue. Phys. Biol. 2017, 14, 015004. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Eberhard, S.; Albersheim, P.; Darvill, A.G. Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 2001, 294, 846–849. [Google Scholar] [CrossRef]
- Reiter, W.-D.; Chapple, C.C.S.; Somerville, C.R. Altered growth and cell walls in a fucose-deficient mutant of Arabidopsis. Science 1993, 261, 1032–1035. [Google Scholar] [CrossRef]
- Freshour, G.; Bonin, C.P.; Reiter, W.-D.; Albersheim, P.; Darvill, A.G.; Hahn, M.G. Distribution of fucose-containing xyloglucans in cell walls of the mur1 mutant of Arabidopsis. Plant Physiol. 2003, 131, 1602–1612. [Google Scholar] [CrossRef]
- Cassab, G.I. Plant cell wall proteins. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 281–309. [Google Scholar] [CrossRef]
- Showalter, A.M.; Basu, D. Extensin and arabinogalactan-protein biosynthesis: Glycosyltransferases, research challenges, and biosensors. Front. Plant Sci. 2016, 7, 814. [Google Scholar] [CrossRef] [PubMed]
- Klepsch, M.M.; Schmitt, M.; Knox, J.P.; Jansen, S. The chemical identity of intervessel pit membranes in Acer challenges hydrogel control of xylem hydraulic conductivity. AoB Plants 2016, 8, plw052. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Takáč, T.; Li, X.; Chen, H.; Wang, Y.; Xu, E.; Xie, L.; Su, Z.; Šamaj, J.; Xu, C. Variable content and distribution of arabinogalactan proteins in banana (Musa spp.) under low temperature stress. Front. Plant Sci. 2015, 6, 353. [Google Scholar] [CrossRef]
- Knox, J.P.; Linstead, P.J.; Cooper, J.P.C.; Roberts, K. Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant J. 1991, 1, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Parra, R.; Paredes, M.A.; Labrador, J.; Nunes, C.; Coimbra, M.A.; Fernandez-Garcia, N.; Olmos, E.; Gallardo, M.; Gomez-Jimenez, M.C. Cell wall composition and ultrastructural immunolocalization of pectin and arabinogalactan protein during Olea europaea L. fruit abscission. Plant Cell Physiol. 2020, 61, 814–825. [Google Scholar] [CrossRef]
- Nguema-Ona, E.; Bannigan, A.; Chevalier, L.; Baskin, T.I.; Driouich, A. Disruption of arabinogalactan proteins disorganizes cortical microtubules in the root of Arabidopsis thaliana. Plant J. 2007, 52, 240–251. [Google Scholar] [CrossRef]
- Dahiya, P.; Brewin, N.J. Immunogold localization of callose and other cell wall components in pea nodule transfer cells. Protoplasma 2000, 214, 210–218. [Google Scholar] [CrossRef]
- Cheng, J.-S.; Lei, C.; Wu, J.-C.; Yuan, Y.-J. Expression of arabinogalactan proteins involved in Taxol production by immobilized Taxus cuspidata cells. J. Biotech. 2008, 133, 96–102. [Google Scholar] [CrossRef]
- Rae, A.; Perotto, S.; Knox, J.; Kannenberg, E.; Brewin, N. Expression of extracellular glycoproteins in the uninfected cells of developing pea nodule tissue. Mol. Plant Microbe Interact. 1991, 4, 563–570. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Brewin, N.; Tsyganov, V.E. Analysis of epitope distribution of arabinogalactan protein-extensins in pea (Pisum sativum) nodules of wild-type and mutants impaired in infection thread growth. Ekol. Genet. 2019, 17, 5–12. [Google Scholar] [CrossRef]
- Sujkowska-Rybkowska, M.; Borucki, W. Accumulation and localization of extensin protein in apoplast of pea root nodule under aluminum stress. Micron 2014, 67, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, M.; Martin, H.; Knox, J.P. An epitope of rice threonine- and hydroxyproline-rich glycoprotein is common to cell wall and hydrophobic plasma-membrane glycoproteins. Planta 1995, 196, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Chen, X.-M.; Zhang, Y.; Cho, Y.-H.; Wang, A.-R.; Yeung, E.C.; Zeng, X.; Guo, S.-X.; Lee, Y.-I. Immunolocalization and changes of hydroxyproline-rich glycoproteins during symbiotic germination of Dendrobium officinale. Front. Plant Sci. 2018, 9, 552. [Google Scholar] [CrossRef] [PubMed]
- Kosterin, O.E.; Rozov, S.M. Mapping of the new mutation blb and the problem of integrity of linkage group I. Pisum Genet. 1993, 25, 27–31. [Google Scholar]
- Tsyganov, V.E.; Borisov, A.Y.; Rozov, S.M.; Tikhonovich, I.A. New symbiotic mutants of pea obtained after mutagenesis of laboratory line SGE. Pisum Genet. 1994, 26, 36–37. [Google Scholar]
- Voroshilova, V.A.; Boesten, B.; Tsyganov, V.E.; Borisov, A.Y.; Tikhonovich, I.A.; Priefer, U.B. Effect of mutations in Pisum sativum L. genes blocking different stages of nodule development on the expression of late symbiotic genes in Rhizobium leguminosarum bv. viciae. Mol. Plant Microbe Interact. 2001, 14, 471–476. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Voroshilova, V.A.; Borisov, A.Y.; Tikhonovich, I.A.; Rozov, S.M. Four more symbiotic mutants obtained using EMS mutagenesis of line SGE. Pisum Genet. 2000, 32, 63. [Google Scholar]
- Serova, T.A.; Tsyganova, A.V.; Tsyganov, V.E. Early nodule senescence is activated in symbiotic mutants of pea (Pisum sativum L.) forming ineffective nodules blocked at different nodule developmental stages. Protoplasma 2018, 255, 1443–1459. [Google Scholar] [CrossRef]
- Borisov, A.Y.; Rozov, S.; Tsyganov, V.; Kulikova, O.; Kolycheva, A.; Yakobi, L.; Ovtsyna, A.; Tikhonovich, I. Identification of symbiotic genes in pea (Pisum sativum L.) by means of experimental mutagenesis. Sov. Genet. 1994, 30, 1484–1494. [Google Scholar]
- Borisov, A.Y.; Rozov, S.M.; Tsyganov, V.E.; Morzhina, E.V.; Lebsky, V.K.; Tikhonovich, I.A. Sequential functioning of Sym-13 and Sym-31, two genes affecting symbiosome development in root nodules of pea (Pisum sativum L). Mol. Gen. Genet. 1997, 254, 592–598. [Google Scholar] [CrossRef]
- Vernié, T.; Moreau, S.; de Billy, F.; Plet, J.; Combier, J.-P.; Rogers, C.; Oldroyd, G.; Frugier, F.; Niebel, A.; Gamas, P. EFD is an ERF transcription factor involved in the control of nodule number and differentiation in Medicago truncatula. Plant Cell 2008, 20, 2696–2713. [Google Scholar] [CrossRef]
- Maunoury, N.; Redondo-Nieto, M.; Bourcy, M.; Van de Velde, W.; Alunni, B.; Laporte, P.; Durand, P.; Agier, N.; Marisa, L.; Vaubert, D.; et al. Differentiation of symbiotic cells and endosymbionts in Medicago truncatula nodulation are coupled to two transcriptome-switches. PLoS ONE 2010, 5, e9519. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, E.; Journet, E.-P.; Chabaud, M.; Cosson, V.; Ratet, P.; Duc, G.; Fedorova, E.; Liu, W.; den Camp, R.O.; Zhukov, V.; et al. IPD3 controls the formation of nitrogen-fixing symbiosomes in pea and Medicago spp. Mol. Plant Microbe Interact. 2011, 24, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, W.; Zehirov, G.; Szatmari, A.; Debreczeny, M.; Ishihara, H.; Kevei, Z.; Farkas, A.; Mikulass, K.; Nagy, A.; Tiricz, H.; et al. Plant peptides govern terminal differentiation of bacteria in symbiosis. Science 2010, 327, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Griffitts, J.; Starker, C.; Fedorova, E.; Limpens, E.; Ivanov, S.; Bisseling, T.; Long, S. A nodule-specific protein secretory pathway required for nitrogen-fixing symbiosis. Science 2010, 327, 1126–1129. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Seliverstova, E.V.; Voroshilova, V.A.; Tsyganova, A.V.; Pavlova, Z.B.; Lebskii, V.K.; Borisov, A.Y.; Brewin, N.J.; Tikhonovich, I.A. Double mutant analysis of sequential functioning of pea (Pisum sativum L.) genes Sym13, Sym33, and Sym40 during symbiotic nodule development. Russ. J. Genet. Appl. Res. 2011, 1, 343. [Google Scholar] [CrossRef]
- Glenn, A.R.; Poole, P.S.; Hudman, J.F. Succinate uptake by free-living and bacteroid forms of Rhizobium leguminosarum. Microbiology 1980, 119, 267–271. [Google Scholar] [CrossRef]
- Cheng, H.-P.; Walker, G.C. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J. Bacteriol. 1998, 180, 5183–5191. [Google Scholar] [CrossRef]
- Kitaeva, A.B.; Demchenko, K.N.; Tikhonovich, I.A.; Timmers, A.C.J.; Tsyganov, V.E. Comparative analysis of the tubulin cytoskeleton organization in nodules of Medicago truncatula and Pisum sativum: Bacterial release and bacteroid positioning correlate with characteristic microtubule rearrangements. New Phytol. 2016, 210, 168–183. [Google Scholar] [CrossRef]
- Fåhraeus, G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J. Gen. Microbiol. 1957, 16, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Liners, F.; Letesson, J.-J.; Didembourg, C.; Van Cutsem, P. Monoclonal antibodies against pectin: Recognition of a conformation induced by calcium. Plant Physiol. 1989, 91, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Liners, F.; Thibault, J.-F.; Van Cutsem, P. Influence of the degree of polymerization of oligogalacturonates and of esterification pattern of pectin on their recognition by monoclonal antibodies. Plant Physiol. 1992, 99, 1099–1104. [Google Scholar] [CrossRef]
- Pattathil, S.; Avci, U.; Baldwin, D.; Swennes, A.G.; McGill, J.A.; Popper, Z.; Bootten, T.; Albert, A.; Davis, R.H.; Chennareddy, C.; et al. A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol. 2010, 153, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Cornuault, V.; Buffetto, F.; Marcus, S.E.; Crépeau, M.-J.; Guillon, F.; Ralet, M.-C.; Knox, J.P. LM6-M: A high avidity rat monoclonal antibody to pectic α-1,5-L-arabinan. bioRxiv 2017, 161604. [Google Scholar] [CrossRef]
- Pedersen, H.L.; Fangel, J.U.; McCleary, B.; Ruzanski, C.; Rydahl, M.G.; Ralet, M.-C.; Farkas, V.; von Schantz, L.; Marcus, S.E.; Andersen, M.C.F.; et al. Versatile high resolution oligosaccharide microarrays for plant glycobiology and cell wall research. J. Biol. Chem. 2012, 287, 39429–39438. [Google Scholar] [CrossRef]
- Smallwood, M.; Beven, A.; Donovan, N.; Neill, S.J.; Peart, J.; Roberts, K.; Knox, J.P. Localization of cell wall proteins in relation to the developmental anatomy of the carrot root apex. Plant J. 1994, 5, 237–246. [Google Scholar] [CrossRef]
- Newcomb, W. A correlated light and electron microscopic study of symbiotic growth and differentiation in Pisum sativum root nodules. Can. J. Bot. 1976, 54, 2163–2186. [Google Scholar] [CrossRef]




| Nodule Zone, Tissue, Cell Type or Cell Structure | P. sativum | M. truncatula | |
|---|---|---|---|
| Endodermis | Galactan RG-I [12] Arabinan RG-I AGP Extensin | Galactan RG-I [12] Arabinan RG-I AGP Extensin RG-I backbone | |
| Meristem | HM HG Galactan RG-I [12] Arabinan RG-I XG RG-I backbone | HM HG Galactan RG-I [12] Arabinan RG-I XG | |
| Infection zone | colonised cells | HM HG LM HG RG-I backbone Arabinan RG-I Ca2+-bound HG | HM HG LM HG RG-I backbone Arabinan RG-I XG |
| infection thread walls | HM HG LM HG Ca2+-bound HG RG-I backbone Arabinan RG-I Galactan RG-I [12] | HM HG LM HG Ca2+-bound HG RG-I backbone Arabinan RG-I XG | |
| juvenile symbiosomes | Arabinan RG-I AGP with GPI [12] | Arabinan RG-I | |
| Nitrogen fixation zone | infected cells | HM HG LM HG Ca2+-bound HG Arabinan RG-I RG-I backbone | HM HG LM HG Ca2+-bound HG Arabinan RG-I |
| infection thread walls | HM HG LM HG XG RG-I backbone Arabinan RG-I Galactan RG-I [12] | HM HG LM HG XG RG-I backbone Arabinan RG-I Ca2+-bound HG | |
| mature symbiosomes | AGP with GPI [12] ↑ | – | |
| Uninfected cells | HM HG LM HG Arabinan RG-I Galactan RG-I [12] | HM HG LM HG Arabinan RG-I Galactan RG-I [12] XG | |
| Intercellular space | Extensin | Extensin | |
| Infection thread matrix | Extensin | Extensin | |
| Lines | Phenotype | References |
|---|---|---|
| P. sativum | ||
| SGE | Wild type | [56,98] |
| SGEFix−-1 (sym40-1) a | Hypertrophied infection droplets and infection threads, abnormal bacteroids | [56] |
| SGEFix−-2 (sym33-3) b | Abnormal infection thread growth inside the nodule, no bacterial release c | [56,99,100] |
| SGEFix−-3 (sym26) | Early senescence | [101,102] |
| Sprint-2 | Wild type | [103] |
| Sprint-2Fix− (sym31) | Undifferentiated bacteroids | [103,104] |
| M. truncatula | ||
| cv Jemalong A17 | Wild type | |
| efd–1 | Hypertrophied infection droplets and infection threads, abnormal bacteroids | [105] |
| TR3 (ipd3) | Abnormal infection thread growth inside the nodule, no bacterial release | [106,107] |
| dnf1–1 | Undifferentiated bacteroids | [108,109] |
| Antibody | Dilution FM/IGL | Antigen/Epitope | Reference/Source |
|---|---|---|---|
| Pectins | |||
| LM19 | 1:10/1:10, 1:25 | Low methylesterified HG/α-GalA-(1→4)(4) | [68]; https://www.agrisera.com (accessed on 29 August 2023) |
| LM20 | 1:10/1:10, 1:25 | High methylesterified HG/α-MeGalA-(1→4)(4) | [68]; https://www.agrisera.com (accessed on 29 August 2023) |
| 2F4 | 1:10/1:200 | Dimeric association of pectic chains through calcium ions | [115,116]; https://carbosource.uga.edu (accessed on 28 August 2023) |
| CCRC-M35 | 1:10/1:10, 1:25 | Rhamnogalacturonan I (backbone)/Rha-(1→4)-GalA-(1→2)-Rha-(1→4)-GalA-(1→2)-Rha-(1→4) Requires at least two unbranched disaccharide repeats | [117]; https://www.agrisera.com (accessed on 28 August 2023) |
| CCRC-M36 | 1:10/1:10, 1:25 | Rhamnogalacturonan I (backbone)/Rha-(1→4)-GalA-(1→2)-Rha-(1→4)-GalA-(1→2)-Rha-(1→4)-GalA-(1→2) Requires at least three unbranched disaccharide repeats | [117]; https://www.agrisera.com (accessed on 28 August 2023) |
| LM6-M | 1:10/1:100 | Rhamnogalacturonan-I/linear pentasaccharide in (1→5)-α-L-arabinans | [72,118]; https://www.agrisera.com (accessed on 28 August 2023) |
| LM8 | 1:10/1:10, 1:25 | Xylogalacturonan/n/a | [76]; https://www.agrisera.com (accessed on 28 August 2023) |
| Xyloglucan | |||
| CCRC-M1 | 1:10/1:10, 1:25 | Fucosylated xyloglucan/α-Fuc-(1→2)-β-Gal | [117]; https://www.agrisera.com (accessed on 28 August 2023) |
| Other polysaccharides | |||
| LM12 | 1:10/1:10, 1:25 | Ferulated polysaccharides/n/a | [119]; https://www.agrisera.com (accessed on 28 August 2023) |
| Arabinogalactan protein | |||
| JIM13 | 1:10/1:10, 1:25 | Arabinogalactan protein/Β-linked glucuronic acid | [88]; https://www.agrisera.com (accessed on 28 August 2023) |
| Extensin | |||
| JIM11 | 1:10/1:10, 1:25 | Extensin glycoprotein/n/a | [120]; https://www.agrisera.com (accessed on 28 August 2023) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsyganova, A.V.; Seliverstova, E.V.; Tsyganov, V.E. Comparison of the Formation of Plant–Microbial Interface in Pisum sativum L. and Medicago truncatula Gaertn. Nitrogen-Fixing Nodules. Int. J. Mol. Sci. 2023, 24, 13850. https://doi.org/10.3390/ijms241813850
Tsyganova AV, Seliverstova EV, Tsyganov VE. Comparison of the Formation of Plant–Microbial Interface in Pisum sativum L. and Medicago truncatula Gaertn. Nitrogen-Fixing Nodules. International Journal of Molecular Sciences. 2023; 24(18):13850. https://doi.org/10.3390/ijms241813850
Chicago/Turabian StyleTsyganova, Anna V., Elena V. Seliverstova, and Viktor E. Tsyganov. 2023. "Comparison of the Formation of Plant–Microbial Interface in Pisum sativum L. and Medicago truncatula Gaertn. Nitrogen-Fixing Nodules" International Journal of Molecular Sciences 24, no. 18: 13850. https://doi.org/10.3390/ijms241813850







