Mitigation of Dextran-Sodium-Sulfate-Induced Colitis in Mice through Oral Administration of Microbiome-Derived Inosine and Its Underlying Mechanisms
Abstract
:1. Introduction
2. Results
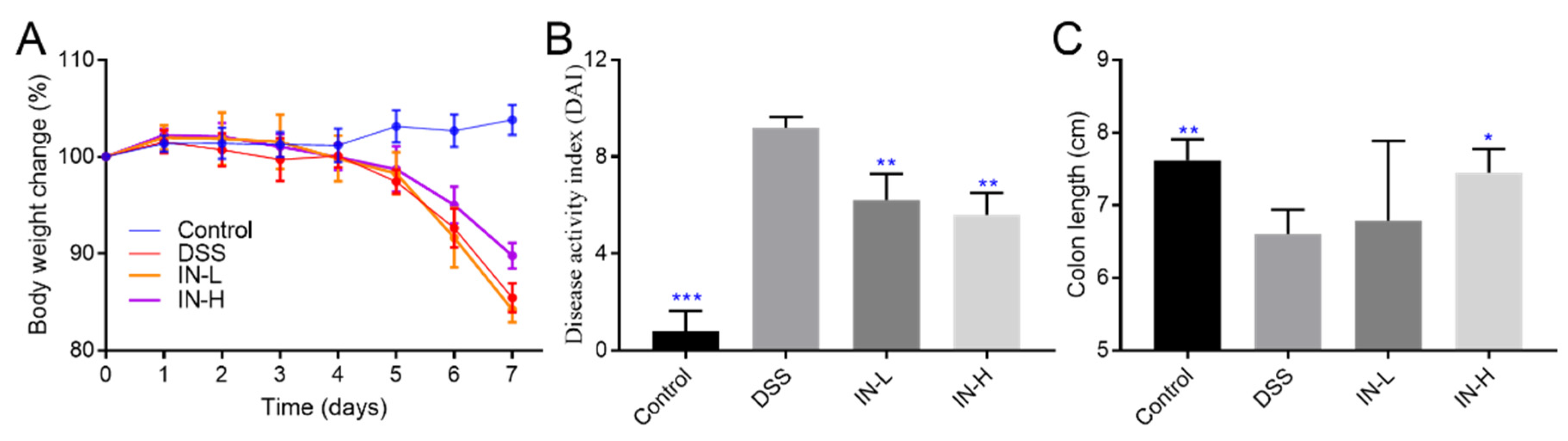
2.1. Inosine Alleviated DSS-Induced Body Weight Loss, DAI Score, and Colon Shortening in Mice
2.2. Inosine Alleviated DSS-Induced Inflammatory Cytokines in Mice
2.3. Inosine Elevated the Colonic Antioxidant Abilities in Mice with Colitis
2.4. Inosine Prevented DSS-Induced Loss of Tight Junctional Proteins in Mice
2.5. Effects of Inosine on the Concentrations of Cecal SCFAs
2.6. Inosine Regulated the Transcription Levels of Genes Related to Inflammation and Oxidative Stress in Mice with Colitis
2.7. Inosine Prevented DSS-Induced Gut Microbiota Disorder
2.8. The Associations between Colitis-Related Parameters and Key Microbiota
3. Discussion
3.1. Inosine Improved Colonic Inflammatory Responses by Suppressing the NF-κB Pathway
3.2. Inosine Suppressed the Oxidative Stress by Activating the Nrf2 Pathway
3.3. Inosine Prevented the Loss of Tight Junction Proteins Induced by DSS
3.4. Inosine Induced the Inhibition of Pathogenic Bacteria and the Elevation of Potential Beneficial Bacteria in Mice
4. Materials and Methods
4.1. Materials
4.2. Animal Experiment Design
4.3. Evaluation of the Disease Activity
4.4. Histopathology Analysis
4.5. Measurement of the Concentrations of Inflammatory Factors
4.6. Measurement of the Activity of Antioxidant Enzyme
4.7. Measurement of Cecal SCFA Concentrations
4.8. Quantitative Real-Time PCR Analysis
4.9. High-Throughput Sequencing
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Dahlhamer, J.M.; Zammitti, E.P.; Ward, B.W.; Wheaton, A.G.; Croft, J.B. Prevalence of inflammatory bowel disease among adults aged >/=18 years-united states, 2015. MMWR-Morb. Mortal. Wkly. Rep. 2016, 65, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- White, B.A.; Ramos, G.P.; Kane, S. The impact of alcohol in inflammatory bowel diseases. Inflamm. Bowel Dis. 2022, 28, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Mao, B.; Cui, S.; Tang, X.; Zhang, Q.; Zhao, J.; Zhang, H. Protective effects of a novel probiotic Bifidobacterium pseudolongum on the intestinal barrier of colitis mice via modulating the Pparγ/STAT3 pathway and intestinal microbiota. Foods 2022, 11, 1551. [Google Scholar] [CrossRef]
- Guan, F.; Luo, H.; Wu, J.; Li, M.; Chen, L.; Huang, N.; Wei, G.; Nie, J.; Chen, B.; Su, Z.; et al. Andrographolide sodium bisulfite ameliorates dextran sulfate sodium-induced colitis and liver injury in mice via inhibiting macrophage proinflammatory polarization from the gut-liver axis. Int. Immunopharmacol. 2022, 110, 109007. [Google Scholar] [CrossRef]
- Singh, S.; Fumery, M.; Sandborn, W.J.; Murad, M.H. Systematic review with network meta-analysis: First- and second-line pharmacotherapy for moderate-severe ulcerative colitis. Aliment. Pharmacol. Ther. 2018, 47, 162–175. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vazquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef]
- Guo, W.; Cui, S.; Tang, X.; Zhang, Q.; Zhao, J.; Mao, B.; Zhang, H. Intestinal microbiomics and metabolomics insights into the hepatoprotective effects of Lactobacillus paracasei CCFM1222 against the acute liver injury in mice. Probiotics Antimicrob. Proteins 2022. [Google Scholar] [CrossRef]
- Guo, W.L.; Guo, J.B.; Liu, B.Y.; Lu, J.Q.; Chen, M.; Liu, B.; Bai, W.D.; Rao, P.F.; Ni, L.; Lv, X.C. Ganoderic acid A from Ganoderma lucidum ameliorates lipid metabolism and alters gut microbiota composition in hyperlipidemic mice fed a high-fat diet. Food Funct. 2020, 11, 6818–6833. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Feng, Y.; Tian, M.; Ji, J.; Hu, X.; Chen, F. Gut microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPARγ signaling activation. Microbiome 2021, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Xiang, Q.; Mao, B.; Tang, X.; Cui, S.; Li, X.; Zhao, J.; Zhang, H.; Chen, W. Protective Effects of microbiome-derived inosine on lipopolysaccharide-induced acute liver damage and inflammation in mice via mediating the TLR4/NF-κB Pathway. J. Agric. Food Chem. 2021, 69, 7619–7628. [Google Scholar] [CrossRef] [PubMed]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guo, W.; Cui, S.; Tang, X.; Zhang, Q.; Lu, W.; Jin, Y.; Zhao, J.; Mao, B.; Chen, W. Broccoli seed extract rich in polysaccharides and glucoraphanin ameliorates DSS-induced colitis via intestinal barrier protection and gut microbiota modulation in mice. J. Sci. Food Agric. 2023, 103, 1749–1760. [Google Scholar] [CrossRef]
- Li, P.; Xiao, N.; Zeng, L.; Xiao, J.; Huang, J.; Xu, Y.; Chen, Y.; Ren, Y.; Du, B. Structural characteristics of a mannoglucan isolated from Chinese yam and its treatment effects against gut microbiota dysbiosis and DSS-induced colitis in mice. Carbohydr. Polym. 2020, 250, 116958. [Google Scholar] [CrossRef]
- Wan, P.; Peng, Y.; Chen, G.; Xie, M.; Dai, Z.; Huang, K.; Dong, W.; Zeng, X.; Sun, Y. Dicaffeoylquinic acids from Ilex kudingcha attenuate dextran sulfate sodium-induced colitis in C57BL/6 mice in association with the modulation of gut microbiota. J. Funct. Foods 2019, 61, 103468. [Google Scholar] [CrossRef]
- Yang, H.; Bocchetta, M.; Kroczynska, B.; Elmishad, A.G.; Chen, Y.; Liu, Z.; Bubici, C.; Mossman, B.T.; Pass, H.I.; Testa, J.R.; et al. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-κB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 10397–10402. [Google Scholar] [CrossRef]
- Marini, M.; Bamias, G.; Rivera-Nieves, J.; Moskaluk, C.A.; Hoang, S.B.; Ross, W.G.; Pizarro, T.T.; Cominelli, F. TNF-alpha neutralization ameliorates the severity of murine Crohn’s-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 8366–8371. [Google Scholar] [CrossRef]
- Tang, X.; Marciano, D.L.; Leeman, S.E.; Amar, S. LPS induces the interaction of a transcription factor, LPS-induced TNF-alpha factor, and STAT6(B) with effects on multiple cytokines. Proc. Natl. Acad. Sci. USA 2005, 102, 5132–5137. [Google Scholar] [CrossRef]
- Xu, P.; Luo, S.; Song, J.; Dai, Z.; Li, D.; Wu, C.E. Effect of sodium alginate-based hydrogel loaded with lutein on gut microbiota and inflammatory response in DSS-induced colitis mice. Food Sci. Hum. Wellness 2023, 12, 2428–2439. [Google Scholar] [CrossRef]
- Li, L.; Qiu, N.; Meng, Y.; Wang, C.; Mine, Y.; Keast, R.; Guyonnet, V. Preserved egg white alleviates DSS-induced colitis in mice through the reduction of oxidative stress, modulation of infl ammatory cytokines, NF-κB, MAPK and gut microbiota composition. Food Sci. Hum. Wellness 2023, 12, 312–323. [Google Scholar] [CrossRef]
- Guo, W.; Mao, B.; Tang, X.; Zhang, Q.; Zhao, J.; Cui, S.; Zhang, H. Lactobacillus paracasei CCFM1223 Protects against lipopolysaccharide-induced acute liver injury in mice by regulating the “gut-liver” axis. Microorganisms 2022, 10, 1321. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zeng, X.; Liu, Y.; Wu, Z.; Zheng, X.; Zhang, X. Inhibitory effect of Dendrobium officinale polysaccharide on oxidative damage of glial cells in aging mice by regulating gut microbiota. Int. J. Biol. Macromol. 2023, 247, 125787. [Google Scholar] [CrossRef]
- Abdelkader, N.F.; Ibrahim, S.M.; Moustafa, P.E.; Elbaset, M.A. Inosine mitigated diabetic peripheral neuropathy via modulating GLO1/AGEs/RAGE/NF-κB/Nrf2 and TGF-β/PKC/TRPV1 signaling pathways. Biomed Pharmacother. 2022, 145, 112395. [Google Scholar] [CrossRef]
- Cavalu, S.; Sharaf, H.; Saber, S.; Youssef, M.E.; Abdelhamid, A.M.; Mourad, A.; Ibrahim, S.; Allam, S.; Elgharabawy, R.M.; El-Ahwany, E.; et al. Ambroxol, a mucolytic agent, boosts HO-1, suppresses NF-kappaB, and decreases the susceptibility of the inflamed rat colon to apoptosis: A new treatment option for treating ulcerative colitis. FASEB J. 2022, 36, e22496. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, Y.; Yin, H.; Jin, Z.; Yuan, J.; Shang, H.; Song, H. Hericium caput-medusae (Bull.: Fr.) Pers. Fermentation concentrate polysaccharide ameliorate diarrhea in DSS-induced early colitis by modulating ion channel. J. Funct. Foods 2023, 100, 105390. [Google Scholar] [CrossRef]
- Guillemot, L.; Schneider, Y.; Brun, P.; Castagliuolo, I.; Pizzuti, D.; Martines, D.; Jond, L.; Bongiovanni, M.; Citi, S. Cingulin is dispensable for epithelial barrier function and tight junction structure, and plays a role in the control of claudin-2 expression and response to duodenal mucosa injury. J. Cell Sci. 2012, 125, 5005–5014. [Google Scholar] [CrossRef]
- Poritz, L.S.; Garver, K.I.; Green, C.; Fitzpatrick, L.; Ruggiero, F.; Koltun, W.A. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J. Surg. Res. 2007, 140, 12–19. [Google Scholar] [CrossRef]
- Kuo, W.T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The tight junction protein ZO-1 is dispensable for barrier function but critical for effective mucosal repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef]
- Rubsam, M.; Mertz, A.F.; Kubo, A.; Marg, S.; Jungst, C.; Goranci-Buzhala, G.; Schauss, A.C.; Horsley, V.; Dufresne, E.R.; Moser, M.; et al. E-cadherin integrates mechanotransduction and EGFR signaling to control junctional tissue polarization and tight junction positioning. Nat. Commun. 2017, 8, 1250. [Google Scholar] [CrossRef]
- Nie, H.; Li, Y.; Lu, X.L.; Yan, J.; Liu, X.R.; Yin, Q. Prodigiosin derived from chromium-resistant Serratia sp. prevents inflammation and modulates gut microbiota homeostasis in DSS-induced colitis mice. Int. Immunopharmacol. 2023, 116, 109800. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.P.; Li, C.Y.; Peng, X.; Wangensteen, H.; Inngjerdingen, K.T.; Zou, Y.F. Pectic polysaccharides from Aconitum carmichaelii leaves protects against DSS-induced ulcerative colitis in mice through modulations of metabolism and microbiota composition. Biomed. Pharmacother. 2022, 155, 113767. [Google Scholar] [CrossRef]
- Wang, C.; Bai, J.; Wang, B.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Suo, H.; Chen, W.; Zhai, Q. Stachyose modulates gut microbiota and alleviates DSS-induced ulcerative colitis in mice. Food Sci. Hum. Wellness 2023, 12, 2211–2220. [Google Scholar] [CrossRef]
- Fu, W.; Duan, P.; Wang, Q.; Song, J.; Wang, Y.; Zhang, Z.; Wang, P.; Jiang, H.; Zhang, X.; Song, G.; et al. Effect of Pseudomonas stutzeri F2 on rearing water quality and growth, innate immunity, visceral morphology and gut microbiota structure of juvenile spotted seabass (Lateolabrax maculatus). Aquacult. Rep. 2023, 30, 101536. [Google Scholar] [CrossRef]
- Amorim, A.M.; Nascimento, J.D. Acinetobacter: An underrated foodborne pathogen? J. Infect. Dev. Ctries. 2017, 11, 111–114. [Google Scholar] [CrossRef]
- Liu, A.; Lv, H.; Wang, H.; Yang, H.; Li, Y.; Qian, J. Aging increases the severity of colitis and the related changes to the gut barrier and gut microbiota in humans and mice. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2020, 75, 1284–1292. [Google Scholar] [CrossRef]
- Han, D.; Wu, W.; Liu, P.; Yang, Y.; Hsu, H.; Kuo, C.; Wu, M.; Wang, T. Differences in the gut microbiome and reduced fecal butyrate in elders with low skeletal muscle mass. Clin. Nutr. 2022, 41, 1491–1500. [Google Scholar] [CrossRef]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef]
- Ye, X.; Liu, Y.; Hu, J.; Gao, Y.; Ma, Y.; Wen, D. Chlorogenic acid-induced gut microbiota improves metabolic endotoxemia. Front. Endocrinol. 2021, 12, 762691. [Google Scholar] [CrossRef]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Shen, Q.; Lyu, W.; Lv, L.; Wang, W.; Yu, M.; Yang, H.; Tao, S.; Xiao, Y. Clostridium butyricum and its derived extracellular vesicles modulate gut homeostasis and ameliorate acute experimental colitis. Microbiol. Spectr. 2022, 10, e136822. [Google Scholar] [CrossRef] [PubMed]
- Abdulqadir, R.F.; Al-Sadi, R.; Ma, T.Y. Sa1215 Bifidobacterium bifidum causes an enhancement of the intestinal epithelial tight junction barrier is mediated by TLR-2-dependent upregulation of occludin expression. Gastroenterology 2023, 164, S-329. [Google Scholar] [CrossRef]
- Wang, S.; Deng, W.; Li, F.; Xiang, L.; Lv, P.; Chen, Y. Treatment with butyrate alleviates dextran sulfate sodium and Clostridium difficile-induced colitis by preventing activity of Th17 cells via regulation of SIRT1/mTOR in mice. J. Nutr. Biochem. 2023, 111, 109155. [Google Scholar] [CrossRef]







| Groups | SCFAs | Acetic Acid | Propanoic Acid | Isobutyric Acid | Butyric Acid | Valeric Acid | Isovaleric Acid |
|---|---|---|---|---|---|---|---|
| NC | 147.23 ± 32.32 * | 105.78 ± 11.89 * | 27.35 ± 3.83 | 3.81 ± 0.38 * | 3.72 ± 0.51 * | 2.30 ± 0.77 | 4.26 ± 0.67 ** |
| DSS | 118.21 ± 15.43 | 84.05 ± 15.93 | 24.00 ± 2.77 | 3.09 ± 0.45 | 2.42 ± 0.47 | 2.27 ± 0.50 | 2.37 ± 0.56 |
| IN-L | 153.32 ± 22.62 * | 110.49 ± 22.38 * | 29.03 ± 4.31 | 3.94 ± 1.01 | 3.64 ± 0.87 | 2.74 ± 0.75 | 3.09 ± 0.60 * |
| IN-H | 122.62 ± 42.85 | 83.34 ± 34.45 | 25.89 ± 9.07 | 3.56 ± 1.59 | 4.34 ± 2.48 | 2.89 ± 1.79 | 2.60 ± 1.16 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Tang, X.; Zhang, Q.; Zhao, J.; Mao, B.; Zhang, H.; Cui, S. Mitigation of Dextran-Sodium-Sulfate-Induced Colitis in Mice through Oral Administration of Microbiome-Derived Inosine and Its Underlying Mechanisms. Int. J. Mol. Sci. 2023, 24, 13852. https://doi.org/10.3390/ijms241813852
Guo W, Tang X, Zhang Q, Zhao J, Mao B, Zhang H, Cui S. Mitigation of Dextran-Sodium-Sulfate-Induced Colitis in Mice through Oral Administration of Microbiome-Derived Inosine and Its Underlying Mechanisms. International Journal of Molecular Sciences. 2023; 24(18):13852. https://doi.org/10.3390/ijms241813852
Chicago/Turabian StyleGuo, Weiling, Xin Tang, Qiuxiang Zhang, Jianxin Zhao, Bingyong Mao, Hao Zhang, and Shumao Cui. 2023. "Mitigation of Dextran-Sodium-Sulfate-Induced Colitis in Mice through Oral Administration of Microbiome-Derived Inosine and Its Underlying Mechanisms" International Journal of Molecular Sciences 24, no. 18: 13852. https://doi.org/10.3390/ijms241813852
APA StyleGuo, W., Tang, X., Zhang, Q., Zhao, J., Mao, B., Zhang, H., & Cui, S. (2023). Mitigation of Dextran-Sodium-Sulfate-Induced Colitis in Mice through Oral Administration of Microbiome-Derived Inosine and Its Underlying Mechanisms. International Journal of Molecular Sciences, 24(18), 13852. https://doi.org/10.3390/ijms241813852






