Emerging Roles for DNA 6mA and RNA m6A Methylation in Mammalian Genome
Abstract
:1. Introduction
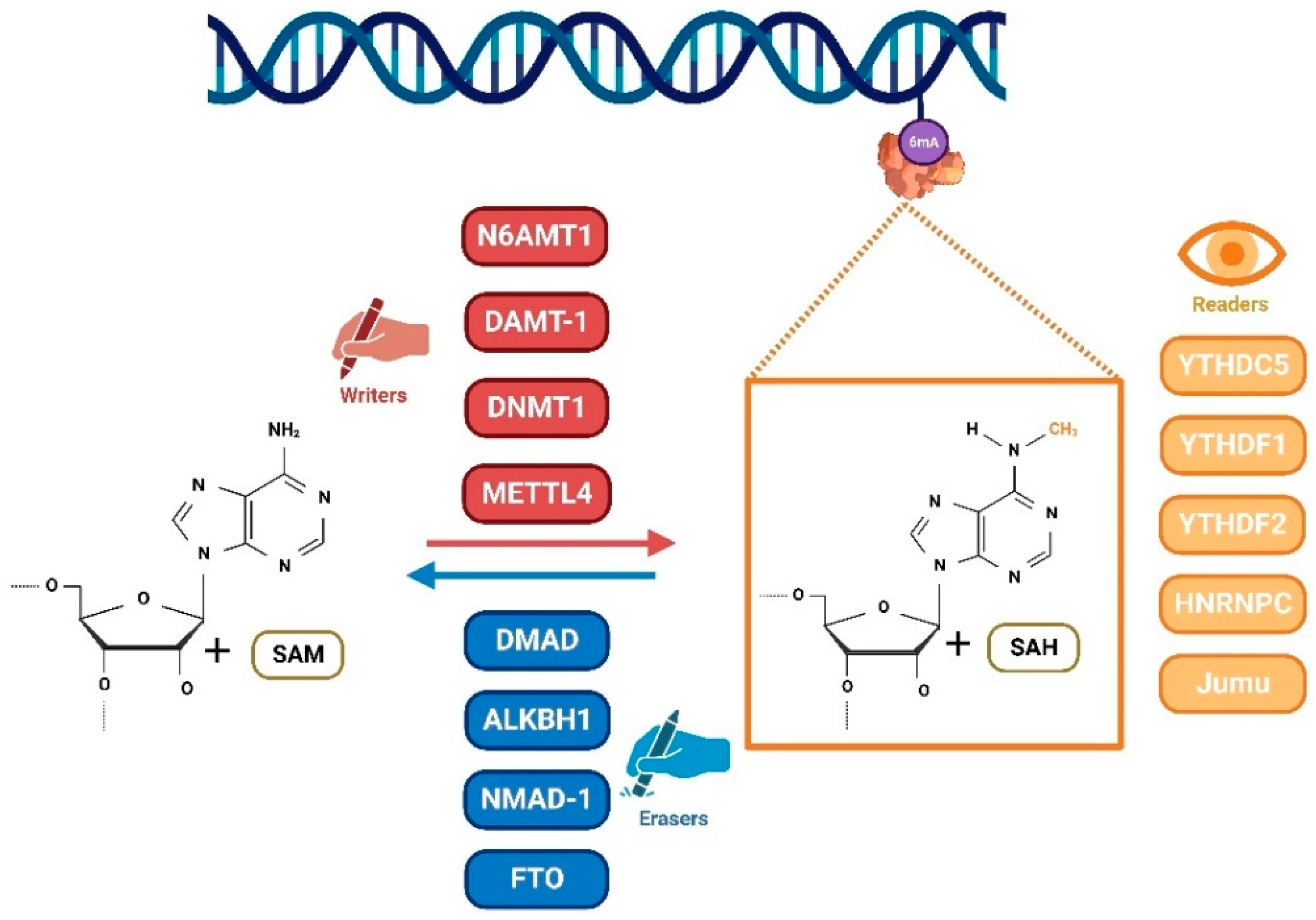
2. DNA 6mA Methylation and Its Writers, Erasers, and Readers
3. The Potential Functions of DNA 6mA Methylation
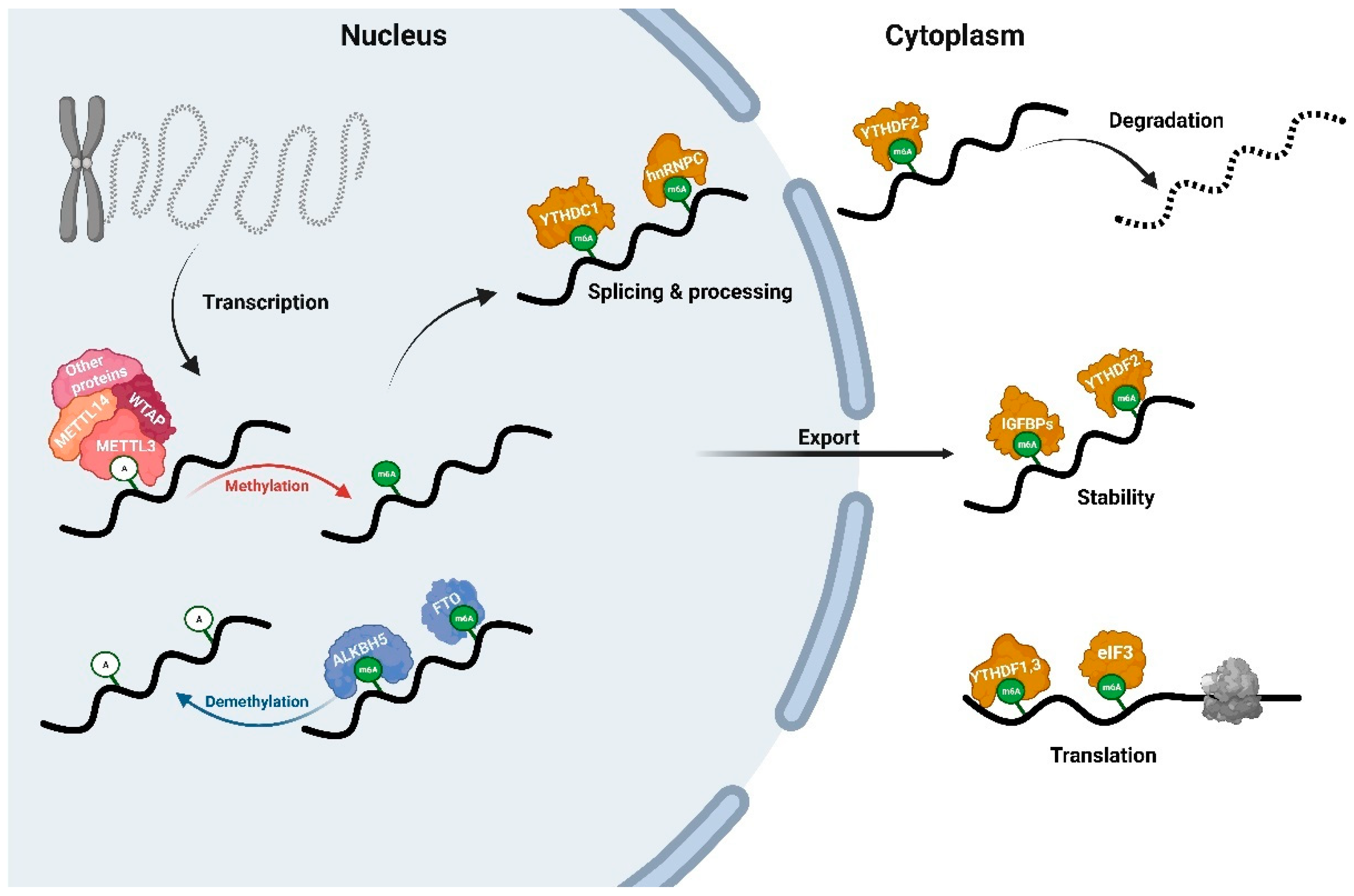
4. RNA m6A Methylation and Its Writers, Erasers, and Readers
5. The Potential Functions of RNA m6A Methylation
6. RNA m6A in Neurodegenerative Diseases
7. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3′UTR | mRNA 3′ untranslated regions | METTL6 | Methyltransferase-like protein 6 |
| 5hmC | 5-hydroxymethylcytosine | mHTT | Mutated huntingtin protein |
| 5mC | 5-methylcytosine | miRNA | MicroRNA |
| 6mA | N6-methyldeoxyadenosine | Mn | Manganese |
| AD | Alzheimer’s disease | MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| ALKBH | AlkB homolog | mRNA | Messenger RNA |
| ALKBH5 | AlkB homolog 5 | mTOR | Mammalian target of rapamycin |
| ALS | Amyotrophic lateral sclerosis | MZT | Maternal to zygotic transition |
| aNSC | Adult neural stem cell | N6AMT1 | N-6 adenine-specific DNA methyltransferase 1 |
| APP | Amyloid protein precursor | NDUFA10 | NADH:ubiquinone oxidoreductase subunit A10 |
| ARC | Activity-regulated cytoskeleton-associated protein | NFT | Neurofibrillary tangle |
| ATAT1 | Alpha-tubulin acetyltransferase 1 | NMAD-1 | N6-methyladenine demethylase 1 |
| Aβ | β-amyloid | NMDA | N-methyl-D-aspartate |
| CAMK II | Calcium/calmodulin dependent protein kinase II | Nurr1 | Nuclear receptor subfamily 4 group A member 2 |
| CBLL1 | Cbl proto-oncogene like 1 | NXF1 | Nuclear RNA export factor 1 |
| Cd | Cadmium | NXT1 | Nuclear transport factor 2 like export factor 1 |
| CDS | Coding sequence | Olig2 | Oligodendrocyte transcription factor 2 |
| CEBPZ | CCAAT enhancer binding protein zeta | Pb | Lead |
| CNS | Central nervous system | PD | Parkinson’s disease |
| Co | Cobalt | PFC | Prefrontal cortex |
| Cp | Ceruloplasmin | Pitx3 | Paired-like homeodomain transcription factor 3 |
| DA | Dopamine | PRRC2A | Proline-rich coiled-coil 2A |
| DAMT-1 | DNA N6 adenine methyltransferase 1 | PSEN1 | Presenilin-1 |
| DMAD | 6mA demethylase | PSEN2 | Presenilin-2 |
| DNMT | DNA methyltransferase | RBM15 | RNA binding motif protein 15 |
| ECM receptor | Extracellular matrix-receptor | RBM15B | RNA binding motif protein 15B |
| eIF3 | Eukaryotic initiation factor 3 | rRNA | Ribosomal RNA |
| ELAVL1 | ELAV like RNA binding protein 1 | SAM | S-adenosyl-L-methionine |
| EN1 | Engrailed homeobox 1 | SMRT-sequencing | Single-molecule real-time sequencing |
| ESC | Embryonic stem cell | SNCA | α-synuclein |
| FMRP | Fragile X mental retardation protein | snRNA | Small nuclear RNA |
| FTO | Fat mass and obesity-associated protein | SSBP1 | Single-stranded DNA-binding protein 1 |
| Gbp11 | Guanylate-binding protein 11 | TDP-43 | TAR-DNA-binding protein of 43 kDa |
| GCT | Germ cell tumor | TET | Ten-eleven translocation |
| GLUA1 | Glutamate ionotropic receptor AMPA type subunit 1 | TRMT112 | tRNA methyltransferase activator subunit 11-2 |
| HD | Huntington’s disease | tRNA | Transfer RNA |
| HNRNP | Heterogeneous nuclear ribonucleoprotein | TSS | Transcription start site |
| IEG | Immediate early gene | UTR | Untranslated region |
| IGF2BP | Insulin-like growth factor 2 mRNA-binding protein | UVB | Ultraviolet B |
| IGF2BP1 | Insulin like growth factor 2 mRNA-binding protein 1 | VIRMA | Vir like m6A methyltransferase associated |
| IGF2BP2 | Insulin-like growth factor 2 mRNA-binding protein 2 | VSMC | Vascular smooth muscle cell |
| lncRNA | Long non-coding RNA | WTAP | Wilms’ tumor 1-associating protein |
| LPS | Lipopolysaccharide | YTHDC | YTH domain-containing protein |
| m6A | N6-methyladenosine | YTHDF | YTH domain family |
| MeRIP-seq | Methylated RNA immunoprecipitation sequencing | ZC3H13 | Zinc finger CCCH-type containing 13 |
| METTL14 | Methyltransferase-like protein 14 | ZFP217 | Zinc finger protein 217 |
| METTL3 | Methyltransferase-like protein 3 |
References
- Meyer, K.D.; Saletore, Y.; Zumbo, P.; Elemento, O.; Mason, C.E.; Jaffrey, S.R. Comprehensive Analysis of MRNA Methylation Reveals Enrichment in 3′ UTRs and near Stop Codons. Cell 2012, 149, 1635–1646. [Google Scholar] [CrossRef]
- Edwards, J.R.; Yarychkivska, O.; Boulard, M.; Bestor, T.H. DNA Methylation and DNA Methyltransferases. Epigenet. Chromatin 2017, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Breiling, A.; Lyko, F. Epigenetic Regulatory Functions of DNA Modifications: 5-Methylcytosine and Beyond. Epigenet. Chromatin 2015, 8, 24. [Google Scholar] [CrossRef]
- Kumar, S.; Chinnusamy, V.; Mohapatra, T. Epigenetics of Modified DNA Bases: 5-Methylcytosine and Beyond. Front. Genet. 2018, 9, 640. [Google Scholar] [CrossRef]
- Jones, P.A. Functions of DNA Methylation: Islands, Start Sites, Gene Bodies and Beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Koziol, M.J.; Bradshaw, C.R.; Allen, G.E.; Costa, A.S.H.; Frezza, C.; Gurdon, J.B. Identification of Methylated Deoxyadenosines in Vertebrates Reveals Diversity in DNA Modifications. Nat. Struct. Mol. Biol. 2016, 23, 24–30. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Q.; Wei, W.; Lin, Q.; Magnan, C.; Emami, M.R.; Wearick-Silva, L.E.; Viola, T.W.; Marshall, P.R.; Yin, J.; et al. The DNA Modification N6-Methyl-2′-Deoxyadenosine (M6dA) Drives Activity-Induced Gene Expression and Is Required for Fear Extinction. Nat. Neurosci. 2019, 22, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Douvlataniotis, K.; Bensberg, M.; Lentini, A.; Gylemo, B.; Nestor, C.E. No Evidence for DNA N 6-Methyladenine in Mammals. Sci. Adv. 2020, 6, eaay3335. [Google Scholar] [CrossRef]
- Feng, X.; He, C. Mammalian DNA N6-Methyladenosine: Challenges and New Insights. Mol. Cell 2023, 83, 343–351. [Google Scholar] [CrossRef]
- Motorin, Y.; Helm, M. RNA Nucleotide Methylation. Wiley Interdiscip. Rev. RNA 2011, 2, 611–631. [Google Scholar] [CrossRef]
- Boccaletto, P.; Machnicka, M.A.; Purta, E.; Piatkowski, P.; Baginski, B.; Wirecki, T.K.; de Crécy-Lagard, V.; Ross, R.; Limbach, P.A.; Kotter, A.; et al. MODOMICS: A Database of RNA Modification Pathways. 2017 Update. Nucleic Acids. Res. 2018, 46, D303–D307. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Li, H.-B.; Yin, Z.; Flavell, R.A. Recent Advances in Dynamic M6A RNA Modification. Open Biol. 2016, 6, 160003. [Google Scholar] [CrossRef]
- Chang, M.; Lv, H.; Zhang, W.; Ma, C.; He, X.; Zhao, S.; Zhang, Z.-W.; Zeng, Y.-X.; Song, S.; Niu, Y.; et al. Region-Specific RNA M6A Methylation Represents a New Layer of Control in the Gene Regulatory Network in the Mouse Brain. Open Biol. 2017, 7, 170166. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, X.; Qi, Z.; Sang, Y.; Liu, Y.; Xu, B.; Liu, W.; Xu, Z.; Deng, Y. The Role of MRNA M6A Methylation in the Nervous System. Cell Biosci. 2019, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Widagdo, J.; Anggono, V. The M6A-Epitranscriptomic Signature in Neurobiology: From Neurodevelopment to Brain Plasticity. J. Neurochem. 2018, 147, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Gz, L.; He, C. DNA N6-Methyladenine in Metazoans: Functional Epigenetic Mark or Bystander? Nat. Struct. Mol. Biol. 2017, 24, 503–506. [Google Scholar] [CrossRef]
- An, Y.; Duan, H. The Role of M6A RNA Methylation in Cancer Metabolism. Mol. Cancer 2022, 21, 14. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, G.-Z.; Chen, K.; Deng, X.; Yu, M.; Han, D.; Hao, Z.; Liu, J.; Lu, X.; Dore, L.C.; et al. N6-Methyldeoxyadenosine Marks Active Transcription Start Sites in Chlamydomonas. Cell 2015, 161, 879–892. [Google Scholar] [CrossRef]
- Greer, E.L.; Blanco, M.A.; Gu, L.; Sendinc, E.; Liu, J.; Aristizábal-Corrales, D.; Hsu, C.-H.; Aravind, L.; He, C.; Shi, Y. DNA Methylation on N6-Adenine in C. Elegans. Cell 2015, 161, 868–878. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, H.; Liu, D.; Cheng, Y.; Liu, X.; Zhang, W.; Yin, R.; Zhang, D.; Zhang, P.; Liu, J.; et al. N6-Methyladenine DNA Modification in Drosophila. Cell 2015, 161, 893–906. [Google Scholar] [CrossRef]
- Liang, Z.; Riaz, A.; Chachar, S.; Ding, Y.; Du, H.; Gu, X. Epigenetic Modifications of MRNA and DNA in Plants. Mol. Plant 2020, 13, 14–30. [Google Scholar] [CrossRef]
- Song, J.; Rechkoblit, O.; Bestor, T.H.; Patel, D.J. Structure of DNMT1-DNA Complex Reveals a Role for Autoinhibition in Maintenance DNA Methylation. Science 2011, 331, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, Q.; Tan, S.; You, J.; Lyu, C.; Zhang, Y.; Han, M.; Chen, Z.; Li, J.; Wang, H.; et al. SET8 Prevents Excessive DNA Methylation by Methylation-Mediated Degradation of UHRF1 and DNMT1. Nucleic Acids Res. 2019, 47, 9053–9068. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, C.; Kretzmer, H.; Riemenschneider, C.; Kumar, A.S.; Mattei, A.L.; Bailly, N.; Gottfreund, J.; Giesselmann, P.; Weigert, R.; Brändl, B.; et al. Dnmt1 Has de Novo Activity Targeted to Transposable Elements. Nat. Struct. Mol. Biol. 2021, 28, 594–603. [Google Scholar] [CrossRef]
- Liu, J.; Yue, Y.; Han, D.; Wang, X.; Fu, Y.; Zhang, L.; Jia, G.; Yu, M.; Lu, Z.; Deng, X.; et al. A METTL3-METTL14 Complex Mediates Mammalian Nuclear RNA N6-Adenosine Methylation. Nat. Chem. Biol. 2014, 10, 93–95. [Google Scholar] [CrossRef]
- Iyer, L.M.; Abhiman, S.; Aravind, L. Natural History of Eukaryotic DNA Methylation Systems. Prog. Mol. Biol. Transl. Sci. 2011, 101, 25–104. [Google Scholar] [CrossRef]
- Bokar, J.A.; Shambaugh, M.E.; Polayes, D.; Matera, A.G.; Rottman, F.M. Purification and CDNA Cloning of the AdoMet-Binding Subunit of the Human MRNA (N6-Adenosine)-Methyltransferase. RNA 1997, 3, 1233–1247. [Google Scholar]
- Xiao, C.-L.; Zhu, S.; He, M.; Chen, D.; Zhang, Q.; Chen, Y.; Yu, G.; Liu, J.; Xie, S.-Q.; Luo, F.; et al. N6-Methyladenine DNA Modification in the Human Genome. Mol. Cell 2018, 71, 306–318.e7. [Google Scholar] [CrossRef]
- Yao, B.; Li, Y.; Wang, Z.; Chen, L.; Poidevin, M.; Zhang, C.; Lin, L.; Wang, F.; Bao, H.; Jiao, B.; et al. Active N6-Methyladenine Demethylation by DMAD Regulates Gene Expression by Coordinating with Polycomb Protein in Neurons. Mol. Cell 2018, 71, 848–857.e6. [Google Scholar] [CrossRef]
- Wang, S.Y.; Mao, H.; Shibuya, H.; Uzawa, S.; O’Brown, Z.K.; Wesenberg, S.; Shin, N.; Saito, T.T.; Gao, J.; Meyer, B.J.; et al. The Demethylase NMAD-1 Regulates DNA Replication and Repair in the Caenorhabditis Elegans Germline. PLoS Genet. 2019, 15, e1008252. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.P.; Wang, T.; Seetin, M.G.; Lai, Y.; Zhu, S.; Lin, K.; Liu, Y.; Byrum, S.D.; Mackintosh, S.G.; Zhong, M.; et al. DNA Methylation on N(6)-Adenine in Mammalian Embryonic Stem Cells. Nature 2016, 532, 329–333. [Google Scholar] [CrossRef]
- Xie, Q.; Wu, T.P.; Gimple, R.C.; Li, Z.; Prager, B.C.; Wu, Q.; Yu, Y.; Wang, P.; Wang, Y.; Gorkin, D.U.; et al. N6-Methyladenine DNA Modification in Glioblastoma. Cell 2018, 175, 1228–1243.e20. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, N.; Wang, Y.; Xia, S.; Zhu, Y.; Xing, C.; Tian, X.; Du, Y. DNA N6-Methyladenine Modification in Eukaryotic Genome. Front. Genet. 2022, 13, 914404. [Google Scholar] [CrossRef]
- Nettersheim, D.; Berger, D.; Jostes, S.; Kristiansen, G.; Lochnit, G.; Schorle, H. N6-Methyladenosine Detected in RNA of Testicular Germ Cell Tumors Is Controlled by METTL3, ALKBH5, YTHDC1/F1/F2, and HNRNPC as Writers, Erasers, and Readers. Andrology 2019, 7, 498–506. [Google Scholar] [CrossRef]
- Koh, C.W.Q.; Goh, Y.T.; Toh, J.D.W.; Neo, S.P.; Ng, S.B.; Gunaratne, J.; Gao, Y.-G.; Quake, S.R.; Burkholder, W.F.; Goh, W.S.S. Single-Nucleotide-Resolution Sequencing of Human N6-Methyldeoxyadenosine Reveals Strand-Asymmetric Clusters Associated with SSBP1 on the Mitochondrial Genome. Nucleic Acids Res. 2018, 46, 11659–11670. [Google Scholar] [CrossRef]
- Shen, C.; Wang, K.; Deng, X.; Chen, J. DNA N6-Methyldeoxyadenosine in Mammals and Human Disease. Trends Genet. 2022, 38, 454–467. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, G.; Wang, J.; Gao, Y.; Sun, R.; Cao, Z.; Chen, Z.; Zheng, X.; Yuan, J.; Luo, Y.; et al. 6mA-DNA-Binding Factor Jumu Controls Maternal-to-Zygotic Transition Upstream of Zelda. Nat. Commun. 2019, 10, 2219. [Google Scholar] [CrossRef]
- Cui, H.; Rong, W.; Ma, J.; Zhu, Q.; Jiang, B.; Zhang, L.; Li, C.; Zhuo, Z.; Chen, M. DNA N6-Adenine Methylation in HBV-Related Hepatocellular Carcinoma. Gene 2022, 822, 146353. [Google Scholar] [CrossRef]
- Marinus, M.G.; Morris, N.R. Biological Function for 6-Methyladenine Residues in the DNA of Escherichia Coli K12. J. Mol. Biol. 1974, 85, 309–322. [Google Scholar] [CrossRef]
- Boulias, K.; Greer, E.L. Means, Mechanisms and Consequences of Adenine Methylation in DNA. Nat. Rev. Genet. 2022, 23, 411–428. [Google Scholar] [CrossRef]
- Pukkila, P.J.; Peterson, J.; Herman, G.; Modrich, P.; Meselson, M. Effects of High Levels of DNA Adenine Methylation on Methyl-Directed Mismatch Repair in Escherichia Coli. Genetics 1983, 104, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Sternglanz, H.; Bugg, C.E. Conformation of N6-Methyladenine, a Base Involved in DNA Modification: Restriction Processes. Science 1973, 182, 833–834. [Google Scholar] [CrossRef] [PubMed]
- Mondo, S.J.; Dannebaum, R.O.; Kuo, R.C.; Louie, K.B.; Bewick, A.J.; LaButti, K.; Haridas, S.; Kuo, A.; Salamov, A.; Ahrendt, S.R.; et al. Widespread Adenine N6-Methylation of Active Genes in Fungi. Nat. Genet. 2017, 49, 964–968. [Google Scholar] [CrossRef]
- Marinus, M.G.; Casadesus, J. Roles of DNA Adenine Methylation in Host-Pathogen Interactions: Mismatch Repair, Transcriptional Regulation, and More. FEMS Microbiol. Rev. 2009, 33, 488–503. [Google Scholar] [CrossRef]
- Fernandes, S.B.; Grova, N.; Roth, S.; Duca, R.C.; Godderis, L.; Guebels, P.; Mériaux, S.B.; Lumley, A.I.; Bouillaud-Kremarik, P.; Ernens, I.; et al. N6-Methyladenine in Eukaryotic DNA: Tissue Distribution, Early Embryo Development, and Neuronal Toxicity. Front. Genet. 2021, 12, 657171. [Google Scholar] [CrossRef]
- Guo, Y.; Pei, Y.; Li, K.; Cui, W.; Zhang, D. DNA N6-Methyladenine Modification in Hypertension. Aging 2020, 12, 6276–6291. [Google Scholar] [CrossRef]
- Luo, L.; Liu, Y.; Nizigiyimana, P.; Ye, M.; Xiao, Y.; Guo, Q.; Su, T.; Luo, X.; Huang, Y.; Zhou, H. DNA 6mA Demethylase ALKBH1 Orchestrates Fatty Acid Metabolism and Suppresses Diet-Induced Hepatic Steatosis. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 1213–1233. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhuang, Y.; Wang, P.; Ning, J.; Liu, W.; Huang, Y.; Lin, X.; Peng, L.; Zhang, D. Reducing N6AMT1-Mediated 6mA DNA Modification Promotes Breast Tumor Progression via Transcriptional Repressing Cell Cycle Inhibitors. Cell Death Dis. 2022, 13, 216. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wong, C.C.; Chen, H.; Fu, K.; Shi, L.; Su, H.; Guo, S.; Gou, H.; Hu, X.; Zhang, L.; et al. The N6-Methyladenine DNA Demethylase ALKBH1 Promotes Gastric Carcinogenesis by Disrupting NRF1 Binding Capacity. Cell Rep. 2023, 42, 112279. [Google Scholar] [CrossRef]
- Xiong, J.; Ye, T.-T.; Ma, C.-J.; Cheng, Q.-Y.; Yuan, B.-F.; Feng, Y.-Q. N 6-Hydroxymethyladenine: A Hydroxylation Derivative of N6-Methyladenine in Genomic DNA of Mammals. Nucleic Acids Res. 2019, 47, 1268–1277. [Google Scholar] [CrossRef]
- Yao, B.; Cheng, Y.; Wang, Z.; Li, Y.; Chen, L.; Huang, L.; Zhang, W.; Chen, D.; Wu, H.; Tang, B.; et al. DNA N6-Methyladenine Is Dynamically Regulated in the Mouse Brain Following Environmental Stress. Nat. Commun. 2017, 8, 1122. [Google Scholar] [CrossRef]
- Uchida, S.; Hara, K.; Kobayashi, A.; Otsuki, K.; Yamagata, H.; Hobara, T.; Suzuki, T.; Miyata, N.; Watanabe, Y. Epigenetic Status of Gdnf in the Ventral Striatum Determines Susceptibility and Adaptation to Daily Stressful Events. Neuron 2011, 69, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.; Manashirov, S.; Zwang, R.; Gil, S.; Tsoory, M.; Shemesh, Y.; Chen, A. Dnmt3a in the Medial Prefrontal Cortex Regulates Anxiety-Like Behavior in Adult Mice. J. Neurosci. 2016, 36, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Pan, B.; Wei, F.; Wang, Y.; Gao, S. Case Study of the Response of N6-Methyladenine DNA Modification to Environmental Stressors in the Unicellular Eukaryote Tetrahymena Thermophila. mSphere 2021, 6, e0120820. [Google Scholar] [CrossRef]
- Wu, K.-J. The Epigenetic Roles of DNA N6-Methyladenine (6mA) Modification in Eukaryotes. Cancer Lett. 2020, 494, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Weng, H.; Chen, J. M6A Modification in Coding and Non-Coding RNAs: Roles and Therapeutic Implications in Cancer. Cancer Cell 2020, 37, 270–288. [Google Scholar] [CrossRef]
- Lei, K.; Lin, S.; Yuan, Q. N6-Methyladenosine (M6A) Modification of Ribosomal RNAs (RRNAs): Critical Roles in MRNA Translation and Diseases. Genes Dis. 2023, 10, 126–134. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Y.; Shu, Y.; He, J.; Gao, W. Interaction between N6-Methyladenosine (M6A) Modification and Noncoding RNAs in Cancer. Mol. Cancer 2020, 19, 94. [Google Scholar] [CrossRef]
- Xiao, S.; Cao, S.; Huang, Q.; Xia, L.; Deng, M.; Yang, M.; Jia, G.; Liu, X.; Shi, J.; Wang, W.; et al. The RNA N6-Methyladenosine Modification Landscape of Human Fetal Tissues. Nat. Cell Biol. 2019, 21, 651–661. [Google Scholar] [CrossRef]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M.; et al. Topology of the Human and Mouse M6A RNA Methylomes Revealed by M6A-Seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef]
- Liu, J.; Li, K.; Cai, J.; Zhang, M.; Zhang, X.; Xiong, X.; Meng, H.; Xu, X.; Huang, Z.; Peng, J.; et al. Landscape and Regulation of M6A and M6Am Methylome across Human and Mouse Tissues. Mol. Cell 2020, 77, 426–440.e6. [Google Scholar] [CrossRef]
- Malovic, E.; Pandey, S.C. N6-Methyladenosine (M6A) Epitranscriptomics in Synaptic Plasticity and Behaviors. Neuropsychopharmacology 2023, 48, 221–222. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.-L.; Wang, X.; An, R.; Cassin, J.; Vissers, C.; Liu, Y.; Liu, Y.; Xu, T.; Wang, X.; Wong, S.Z.H.; et al. Epitranscriptomic M6A Regulation of Axon Regeneration in the Adult Mammalian Nervous System. Neuron 2018, 97, 313–325.e6. [Google Scholar] [CrossRef] [PubMed]
- Koranda, J.L.; Dore, L.; Shi, H.; Patel, M.J.; Vaasjo, L.O.; Rao, M.N.; Chen, K.; Lu, Z.; Yi, Y.; Chi, W.; et al. Mettl14 Is Essential for Epitranscriptomic Regulation of Striatal Function and Learning. Neuron 2018, 99, 283–292.e5. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, M.; Xie, D.; Huang, Z.; Zhang, L.; Yang, Y.; Ma, D.; Li, W.; Zhou, Q.; Yang, Y.-G.; et al. METTL3-Mediated N6-Methyladenosine MRNA Modification Enhances Long-Term Memory Consolidation. Cell Res. 2018, 28, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Li, Q.; Wang, M.; Zhao, X.; Wu, J.; Liu, D.; Hong, S.; Yang, Y.; Shu, Q.; Li, X. M6A Modification Involves in Enriched Environment-Induced Neurogenesis and Cognition Enhancement. Front. Cell Dev. Biol. 2022, 10, 903179. [Google Scholar] [CrossRef]
- Dunn, D.B.; Smith, J.D. Occurrence of a New Base in the Deoxyribonucleic Acid of a Strain of Bacterium Coli. Nature 1955, 175, 336–337. [Google Scholar] [CrossRef]
- Scutenaire, J.; Plassard, D.; Matelot, M.; Villa, T.; Zumsteg, J.; Libri, D.; Séraphin, B. The S. cerevisiae M6A-Reader Pho92 Promotes Timely Meiotic Recombination by Controlling Key Methylated Transcripts. Nucleic Acids Res. 2023, 51, 517–535. [Google Scholar] [CrossRef]
- Bodi, Z.; Button, J.D.; Grierson, D.; Fray, R.G. Yeast Targets for MRNA Methylation. Nucleic Acids Res. 2010, 38, 5327–5335. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, B.; Gu, L.; Chen, Y.; Mora, M.; Zhu, M.; Noory, E.; Wang, Q.; Lin, C. A Photoregulatory Mechanism of the Circadian Clock in Arabidopsis. Nat. Plants 2021, 7, 1397–1408. [Google Scholar] [CrossRef]
- Shen, L. Functional Interdependence of N6-Methyladenosine Methyltransferase Complex Subunits in Arabidopsis. Plant Cell 2023, 35, 1901–1916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, Y.; Sun, B.; Wang, L.; Yang, Y.; Ma, D.; Lv, J.; Heng, J.; Ding, Y.; Xue, Y.; et al. M6A Modulates Haematopoietic Stem and Progenitor Cell Specification. Nature 2017, 549, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, X.; Ma, X.; Xiao, W.; Zhang, J. Mapping the M1A, M5C, M6A and M7G Methylation Atlas in Zebrafish Brain under Hypoxic Conditions by MeRIP-Seq. BMC Genom. 2022, 23, 105. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Y.; Wang, X.; Zhu, Y.; Wang, L.; Zhang, W.; Wang, Y.; Gao, Y.; Wu, X.; Cheng, Y.; et al. Dynamic FMR1 Granule Phase Switch Instructed by M6A Modification Contributes to Maternal RNA Decay. Nat. Commun. 2022, 13, 859. [Google Scholar] [CrossRef]
- Perlegos, A.E.; Shields, E.J.; Shen, H.; Liu, K.F.; Bonini, N.M. Mettl3-Dependent M6A Modification Attenuates the Brain Stress Response in Drosophila. Nat. Commun. 2022, 13, 5387. [Google Scholar] [CrossRef]
- Desrosiers, R.; Friderici, K.; Rottman, F. Identification of Methylated Nucleosides in Messenger RNA from Novikoff Hepatoma Cells. Proc. Natl. Acad. Sci. USA 1974, 71, 3971–3975. [Google Scholar] [CrossRef]
- Xiao, W.; Adhikari, S.; Dahal, U.; Chen, Y.-S.; Hao, Y.-J.; Sun, B.-F.; Sun, H.-Y.; Li, A.; Ping, X.-L.; Lai, W.-Y.; et al. Nuclear m(6)A Reader YTHDC1 Regulates MRNA Splicing. Mol. Cell 2016, 61, 507–519. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.; Sun, B.-F.; Shi, Y.; Yang, X.; Xiao, W.; Hao, Y.-J.; Ping, X.-L.; Chen, Y.-S.; Wang, W.-J.; et al. FTO-Dependent Demethylation of N6-Methyladenosine Regulates MRNA Splicing and Is Required for Adipogenesis. Cell Res. 2014, 24, 1403–1419. [Google Scholar] [CrossRef] [PubMed]
- Uzonyi, A.; Dierks, D.; Nir, R.; Kwon, O.S.; Toth, U.; Barbosa, I.; Burel, C.; Brandis, A.; Rossmanith, W.; Le Hir, H.; et al. Exclusion of M6A from Splice-Site Proximal Regions by the Exon Junction Complex Dictates M6A Topologies and MRNA Stability. Mol. Cell 2023, 83, 237–251.e7. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Z.; Gomez, A.; Hon, G.C.; Yue, Y.; Han, D.; Fu, Y.; Parisien, M.; Dai, Q.; Jia, G.; et al. N6-Methyladenosine-Dependent Regulation of Messenger RNA Stability. Nature 2014, 505, 117–120. [Google Scholar] [CrossRef]
- Berulava, T.; Buchholz, E.; Elerdashvili, V.; Pena, T.; Islam, M.R.; Lbik, D.; Mohamed, B.A.; Renner, A.; von Lewinski, D.; Sacherer, M.; et al. Changes in M6A RNA Methylation Contribute to Heart Failure Progression by Modulating Translation. Eur. J. Heart Fail. 2020, 22, 54–66. [Google Scholar] [CrossRef]
- Zhao, B.S.; Roundtree, I.A.; He, C. Post-Transcriptional Gene Regulation by MRNA Modifications. Nat. Rev. Mol. Cell Biol. 2017, 18, 31–42. [Google Scholar] [CrossRef]
- Narayan, P.; Rottman, F.M. An in Vitro System for Accurate Methylation of Internal Adenosine Residues in Messenger RNA. Science 1988, 242, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Doxtader, K.A.; Nam, Y. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol. Cell 2016, 63, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.-X.; Zuo, R.; Anastassiadis, K.; Klungland, A.; Marr, C.; Filipczyk, A. N6-Methyladenosine (M6A) Depletion Regulates Pluripotency Exit by Activating Signaling Pathways in Embryonic Stem Cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2105192118. [Google Scholar] [CrossRef] [PubMed]
- Aguilo, F.; Zhang, F.; Sancho, A.; Fidalgo, M.; Di Cecilia, S.; Vashisht, A.; Lee, D.-F.; Chen, C.-H.; Rengasamy, M.; Andino, B.; et al. Coordination of m(6)A MRNA Methylation and Gene Transcription by ZFP217 Regulates Pluripotency and Reprogramming. Cell Stem Cell 2015, 17, 689–704. [Google Scholar] [CrossRef]
- Geula, S.; Moshitch-Moshkovitz, S.; Dominissini, D.; Mansour, A.A.; Kol, N.; Salmon-Divon, M.; Hershkovitz, V.; Peer, E.; Mor, N.; Manor, Y.S.; et al. Stem Cells. M6A MRNA Methylation Facilitates Resolution of Naïve Pluripotency toward Differentiation. Science 2015, 347, 1002–1006. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.-C.; Huang, C.; Shen, H.; Sun, B.; Cheng, X.; Zhang, Y.-J.; Yang, Y.-G.; Shu, Q.; Yang, Y.; et al. M6A Regulates Neurogenesis and Neuronal Development by Modulating Histone Methyltransferase Ezh2. Genom. Proteom. Bioinform. 2019, 17, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, J.R.; Heynen, A.J.; Shuler, M.G.; Bear, M.F. Learning Induces Long-Term Potentiation in the Hippocampus. Science 2006, 313, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Costa-Mattioli, M.; Gobert, D.; Stern, E.; Gamache, K.; Colina, R.; Cuello, C.; Sossin, W.; Kaufman, R.; Pelletier, J.; Rosenblum, K.; et al. EIF2alpha Phosphorylation Bidirectionally Regulates the Switch from Short- to Long-Term Synaptic Plasticity and Memory. Cell 2007, 129, 195–206. [Google Scholar] [CrossRef]
- Smolen, P.; Zhang, Y.; Byrne, J.H. The Right Time to Learn: Mechanisms and Optimization of Spaced Learning. Nat. Rev. Neurosci. 2016, 17, 77–88. [Google Scholar] [CrossRef]
- Sun, X.; Lin, Y. Npas4: Linking Neuronal Activity to Memory. Trends Neurosci. 2016, 39, 264–275. [Google Scholar] [CrossRef]
- Ping, X.-L.; Sun, B.-F.; Wang, L.; Xiao, W.; Yang, X.; Wang, W.-J.; Adhikari, S.; Shi, Y.; Lv, Y.; Chen, Y.-S.; et al. Mammalian WTAP Is a Regulatory Subunit of the RNA N6-Methyladenosine Methyltransferase. Cell Res. 2014, 24, 177–189. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, G.; Jiang, X.; Li, X.; Sun, K.; Shi, Y.; Yang, Z.; Zhu, X. Loss of Wtap Results in Cerebellar Ataxia and Degeneration of Purkinje Cells. J. Genet. Genom. 2022, 49, 847–858. [Google Scholar] [CrossRef] [PubMed]
- ALKBH1 Promotes Lung Cancer by Regulating M6A RNA Demethylation. Biochem. Pharmacol. 2021, 189, 114284. [CrossRef]
- Fu, Y.; Jia, G.; Pang, X.; Wang, R.N.; Wang, X.; Li, C.J.; Smemo, S.; Dai, Q.; Bailey, K.A.; Nobrega, M.A.; et al. FTO-Mediated Formation of N6-Hydroxymethyladenosine and N6-Formyladenosine in Mammalian RNA. Nat. Commun. 2013, 4, 1798. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, J.; He, C. RNA N6-Methyladenosine Methylation in Post-Transcriptional Gene Expression Regulation. Genes. Dev. 2015, 29, 1343–1355. [Google Scholar] [CrossRef]
- Gerken, T.; Girard, C.A.; Tung, Y.-C.L.; Webby, C.J.; Saudek, V.; Hewitson, K.S.; Yeo, G.S.H.; McDonough, M.A.; Cunliffe, S.; McNeill, L.A.; et al. The Obesity-Associated FTO Gene Encodes a 2-Oxoglutarate-Dependent Nucleic Acid Demethylase. Science 2007, 318, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, M.; Huang, H.; Zhu, J.; Song, H.; Zhu, J.; Park, J.; Ji, S.-J. Dynamic M6A Modification Regulates Local Translation of MRNA in Axons. Nucleic Acids Res. 2018, 46, 1412–1423. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, S.; Li, J.; Wen, Y.; Cui, R.; Zhang, K.; Liu, Y.; Yang, X.; Zhang, L.; Xu, B.; et al. Protective Role of MRNA Demethylase FTO on Axon Guidance Molecules of Nigro-Striatal Projection System in Manganese-Induced Parkinsonism. J. Hazard. Mater. 2022, 426, 128099. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Feng, Y.; Hu, Y.; Liu, J.; Shi, H.; Zhao, R. Exposure to Constant Light Impairs Cognition with FTO Inhibition and M6A-Dependent TrκB Repression in Mouse Hippocampus. Environ. Pollut. 2021, 283, 117037. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhuang, Y.; Chen, J.; Xu, W.; Shou, Y.; Huang, X.; Shu, Q.; Li, X. Dynamic Effects of Fto in Regulating the Proliferation and Differentiation of Adult Neural Stem Cells of Mice. Hum. Mol. Genet. 2020, 29, 727–735. [Google Scholar] [CrossRef]
- Li, L.; Zang, L.; Zhang, F.; Chen, J.; Shen, H.; Shu, L.; Liang, F.; Feng, C.; Chen, D.; Tao, H.; et al. Fat Mass and Obesity-Associated (FTO) Protein Regulates Adult Neurogenesis. Hum. Mol. Genet. 2017, 26, 2398–2411. [Google Scholar] [CrossRef]
- Du, T.; Li, G.; Yang, J.; Ma, K. RNA Demethylase Alkbh5 Is Widely Expressed in Neurons and Decreased during Brain Development. Brain Res. Bull. 2020, 163, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Martinez De La Cruz, B.; Markus, R.; Malla, S.; Haig, M.I.; Gell, C.; Sang, F.; Bellows, E.; Sherif, M.A.; McLean, D.; Lourdusamy, A.; et al. Modifying the M6A Brain Methylome by ALKBH5-Mediated Demethylation: A New Contender for Synaptic Tagging. Mol. Psychiatry 2021, 26, 7141–7153. [Google Scholar] [CrossRef]
- Klungland, A.; Dahl, J.A.; Greggains, G.; Fedorcsak, P.; Filipczyk, A. Reversible RNA Modifications in Meiosis and Pluripotency. Nat. Methods 2016, 14, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Dahl, J.A.; Niu, Y.; Fedorcsak, P.; Huang, C.-M.; Li, C.J.; Vågbø, C.B.; Shi, Y.; Wang, W.-L.; Song, S.-H.; et al. ALKBH5 Is a Mammalian RNA Demethylase That Impacts RNA Metabolism and Mouse Fertility. Mol. Cell 2013, 49, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, B.S.; Roundtree, I.A.; Lu, Z.; Han, D.; Ma, H.; Weng, X.; Chen, K.; Shi, H.; He, C. N(6)-Methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 2015, 161, 1388–1399. [Google Scholar] [CrossRef]
- Li, A.; Chen, Y.-S.; Ping, X.-L.; Yang, X.; Xiao, W.; Yang, Y.; Sun, H.-Y.; Zhu, Q.; Baidya, P.; Wang, X.; et al. Cytoplasmic M6A Reader YTHDF3 Promotes MRNA Translation. Cell Res. 2017, 27, 444–447. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.; Lu, Z.; Zhao, B.S.; Ma, H.; Hsu, P.J.; Liu, C.; He, C. YTHDF3 Facilitates Translation and Decay of N6-Methyladenosine-Modified RNA. Cell Res. 2017, 27, 315–328. [Google Scholar] [CrossRef]
- Lasman, L.; Krupalnik, V.; Viukov, S.; Mor, N.; Aguilera-Castrejon, A.; Schneir, D.; Bayerl, J.; Mizrahi, O.; Peles, S.; Tawil, S.; et al. Context-Dependent Functional Compensation between Ythdf M6A Reader Proteins. Genes Dev. 2020, 34, 1373–1391. [Google Scholar] [CrossRef]
- Roundtree, I.A.; Luo, G.-Z.; Zhang, Z.; Wang, X.; Zhou, T.; Cui, Y.; Sha, J.; Huang, X.; Guerrero, L.; Xie, P.; et al. YTHDC1 Mediates Nuclear Export of N6-Methyladenosine Methylated MRNAs. eLife 2017, 6, e31311. [Google Scholar] [CrossRef]
- Mao, Y.; Dong, L.; Liu, X.-M.; Guo, J.; Ma, H.; Shen, B.; Qian, S.-B. M6A in MRNA Coding Regions Promotes Translation via the RNA Helicase-Containing YTHDC2. Nat. Commun. 2019, 10, 5332. [Google Scholar] [CrossRef]
- Tanabe, A.; Tanikawa, K.; Tsunetomi, M.; Takai, K.; Ikeda, H.; Konno, J.; Torigoe, T.; Maeda, H.; Kutomi, G.; Okita, K.; et al. RNA Helicase YTHDC2 Promotes Cancer Metastasis via the Enhancement of the Efficiency by Which HIF-1α MRNA Is Translated. Cancer Lett. 2016, 376, 34–42. [Google Scholar] [CrossRef]
- Edupuganti, R.R.; Geiger, S.; Lindeboom, R.G.H.; Shi, H.; Hsu, P.J.; Lu, Z.; Wang, S.-Y.; Baltissen, M.P.A.; Jansen, P.W.T.C.; Rossa, M.; et al. N6-Methyladenosine (M6A) Recruits and Repels Proteins to Regulate MRNA Homeostasis. Nat. Struct. Mol. Biol. 2017, 24, 870–878. [Google Scholar] [CrossRef]
- Choi, S.H.; Flamand, M.N.; Liu, B.; Zhu, H.; Hu, M.; Wang, M.; Sewell, J.; Holley, C.L.; Al-Hashimi, H.M.; Meyer, K.D. RBM45 Is an M6A-Binding Protein That Affects Neuronal Differentiation and the Splicing of a Subset of MRNAs. Cell Rep. 2022, 40, 111293. [Google Scholar] [CrossRef]
- Li, M.; Zhao, X.; Wang, W.; Shi, H.; Pan, Q.; Lu, Z.; Perez, S.P.; Suganthan, R.; He, C.; Bjørås, M.; et al. Ythdf2-Mediated M6A MRNA Clearance Modulates Neural Development in Mice. Genome Biol. 2018, 19, 69. [Google Scholar] [CrossRef]
- Louloupi, A.; Ntini, E.; Conrad, T.; Ørom, U.A.V. Transient N-6-Methyladenosine Transcriptome Sequencing Reveals a Regulatory Role of M6A in Splicing Efficiency. Cell Rep. 2018, 23, 3429–3437. [Google Scholar] [CrossRef]
- Schöller, E.; Weichmann, F.; Treiber, T.; Ringle, S.; Treiber, N.; Flatley, A.; Feederle, R.; Bruckmann, A.; Meister, G. Interactions, Localization, and Phosphorylation of the M6A Generating METTL3-METTL14-WTAP Complex. RNA 2018, 24, 499–512. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Q.; Meng, R.; Yi, B.; Xu, Q. METTL3 Regulates Alternative Splicing of MyD88 upon the Lipopolysaccharide-Induced Inflammatory Response in Human Dental Pulp Cells. J. Cell Mol. Med. 2018, 22, 2558–2568. [Google Scholar] [CrossRef]
- Balacco, D.L.; Soller, M. The M6A Writer: Rise of a Machine for Growing Tasks. Biochemistry 2019, 58, 363–378. [Google Scholar] [CrossRef]
- Lesbirel, S.; Viphakone, N.; Parker, M.; Parker, J.; Heath, C.; Sudbery, I.; Wilson, S.A. The M6A-Methylase Complex Recruits TREX and Regulates MRNA Export. Sci. Rep. 2018, 8, 13827. [Google Scholar] [CrossRef]
- Lesbirel, S.; Wilson, S.A. The M6A-methylase Complex and MRNA Export. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 319–328. [Google Scholar] [CrossRef]
- Fustin, J.-M.; Doi, M.; Yamaguchi, Y.; Hida, H.; Nishimura, S.; Yoshida, M.; Isagawa, T.; Morioka, M.S.; Kakeya, H.; Manabe, I.; et al. RNA-Methylation-Dependent RNA Processing Controls the Speed of the Circadian Clock. Cell 2013, 155, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Choe, J.; Du, P.; Triboulet, R.; Gregory, R.I. The m(6)A Methyltransferase METTL3 Promotes Translation in Human Cancer Cells. Mol. Cell 2016, 62, 335–345. [Google Scholar] [CrossRef]
- Choe, J.; Lin, S.; Zhang, W.; Liu, Q.; Wang, L.; Ramirez-Moya, J.; Du, P.; Kim, W.; Tang, S.; Sliz, P.; et al. MRNA Circularization by METTL3-EIF3h Enhances Translation and Promotes Oncogenesis. Nature 2018, 561, 556–560. [Google Scholar] [CrossRef]
- Barbieri, I.; Tzelepis, K.; Pandolfini, L.; Shi, J.; Millán-Zambrano, G.; Robson, S.C.; Aspris, D.; Migliori, V.; Bannister, A.J.; Han, N.; et al. Promoter-Bound METTL3 Maintains Myeloid Leukaemia by M6A-Dependent Translation Control. Nature 2017, 552, 126–131. [Google Scholar] [CrossRef]
- Slobodin, B.; Han, R.; Calderone, V.; Vrielink, J.A.F.O.; Loayza-Puch, F.; Elkon, R.; Agami, R. Transcription Impacts the Efficiency of MRNA Translation via Co-Transcriptional N6-Adenosine Methylation. Cell 2017, 169, 326–337.e12. [Google Scholar] [CrossRef]
- Qi, S.-T.; Ma, J.-Y.; Wang, Z.-B.; Guo, L.; Hou, Y.; Sun, Q.-Y. N6-Methyladenosine Sequencing Highlights the Involvement of MRNA Methylation in Oocyte Meiotic Maturation and Embryo Development by Regulating Translation in Xenopus Laevis. J. Biol. Chem. 2016, 291, 23020–23026. [Google Scholar] [CrossRef]
- Batista, P.J.; Molinie, B.; Wang, J.; Qu, K.; Zhang, J.; Li, L.; Bouley, D.M.; Lujan, E.; Haddad, B.; Daneshvar, K.; et al. M(6)A RNA Modification Controls Cell Fate Transition in Mammalian Embryonic Stem Cells. Cell Stem Cell 2014, 15, 707–719. [Google Scholar] [CrossRef]
- Castro-Hernández, R.; Berulava, T.; Metelova, M.; Epple, R.; Peña Centeno, T.; Richter, J.; Kaurani, L.; Pradhan, R.; Sakib, M.S.; Burkhardt, S.; et al. Conserved Reduction of M6A RNA Modifications during Aging and Neurodegeneration Is Linked to Changes in Synaptic Transcripts. Proc. Natl. Acad. Sci. USA 2023, 120, e2204933120. [Google Scholar] [CrossRef]
- Merkurjev, D.; Hong, W.-T.; Iida, K.; Oomoto, I.; Goldie, B.J.; Yamaguti, H.; Ohara, T.; Kawaguchi, S.-Y.; Hirano, T.; Martin, K.C.; et al. Synaptic N6-Methyladenosine (M6A) Epitranscriptome Reveals Functional Partitioning of Localized Transcripts. Nat. Neurosci. 2018, 21, 1004–1014. [Google Scholar] [CrossRef]
- Walters, B.J.; Mercaldo, V.; Gillon, C.J.; Yip, M.; Neve, R.L.; Boyce, F.M.; Frankland, P.W.; Josselyn, S.A. The Role of The RNA Demethylase FTO (Fat Mass and Obesity-Associated) and MRNA Methylation in Hippocampal Memory Formation. Neuropsychopharmacology 2017, 42, 1502–1510. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, X.; Weng, Y.-L.; Lu, Z.; Liu, Y.; Lu, Z.; Li, J.; Hao, P.; Zhang, Y.; Zhang, F.; et al. M6A Facilitates Hippocampus-Dependent Learning and Memory through YTHDF1. Nature 2018, 563, 249–253. [Google Scholar] [CrossRef]
- Du, B.; Zhang, Y.; Liang, M.; Du, Z.; Li, H.; Fan, C.; Zhang, H.; Jiang, Y.; Bi, X. N6-Methyladenosine (M6A) Modification and Its Clinical Relevance in Cognitive Dysfunctions. Aging 2021, 13, 20716–20737. [Google Scholar] [CrossRef]
- Shafik, A.M.; Zhang, F.; Guo, Z.; Dai, Q.; Pajdzik, K.; Li, Y.; Kang, Y.; Yao, B.; Wu, H.; He, C.; et al. N6-Methyladenosine Dynamics in Neurodevelopment and Aging, and Its Potential Role in Alzheimer’s Disease. Genome Biol. 2021, 22, 17. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, Y.; Lu, M.; Song, M.; Yu, Z.; Wang, J.; Wang, S.; Ren, J.; Yang, Y.-G.; Liu, G.-H.; et al. METTL3 Counteracts Premature Aging via M6A-Dependent Stabilization of MIS12 MRNA. Nucleic Acids Res. 2020, 48, 11083–11096. [Google Scholar] [CrossRef]
- Wu, Z.; Lu, M.; Liu, D.; Shi, Y.; Ren, J.; Wang, S.; Jing, Y.; Zhang, S.; Zhao, Q.; Li, H.; et al. M6A Epitranscriptomic Regulation of Tissue Homeostasis during Primate Aging. Nat. Aging 2023, 3, 705–721. [Google Scholar] [CrossRef]
- Maggipinto, M.; Rabiner, C.; Kidd, G.J.; Hawkins, A.J.; Smith, R.; Barbarese, E. Increased Expression of the MBP MRNA Binding Protein HnRNP A2 during Oligodendrocyte Differentiation. J. Neurosci. Res. 2004, 75, 614–623. [Google Scholar] [CrossRef]
- Xu, H.; Dzhashiashvili, Y.; Shah, A.; Kunjamma, R.B.; Weng, Y.-L.; Elbaz, B.; Fei, Q.; Jones, J.S.; Li, Y.I.; Zhuang, X.; et al. M6A MRNA Methylation Is Essential for Oligodendrocyte Maturation and CNS Myelination. Neuron 2020, 105, 293–309.e5. [Google Scholar] [CrossRef]
- Wu, R.; Li, A.; Sun, B.; Sun, J.-G.; Zhang, J.; Zhang, T.; Chen, Y.; Xiao, Y.; Gao, Y.; Zhang, Q.; et al. A Novel M6A Reader Prrc2a Controls Oligodendroglial Specification and Myelination. Cell Res. 2019, 29, 23–41. [Google Scholar] [CrossRef]
- Li, Q.; Wen, S.; Ye, W.; Zhao, S.; Liu, X. The Potential Roles of M6A Modification in Regulating the Inflammatory Response in Microglia. J. Neuroinflamm. 2021, 18, 149. [Google Scholar] [CrossRef]
- Ding, L.; Wu, H.; Wang, Y.; Li, Y.; Liang, Z.; Xia, X.; Zheng, J.C. M6A Reader Igf2bp1 Regulates the Inflammatory Responses of Microglia by Stabilizing Gbp11 and Cp MRNAs. Front. Immunol. 2022, 13, 872252. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Camats-Perna, J.; Medeiros, R.; Anggono, V.; Widagdo, J. Altered Expression of the M6A Methyltransferase METTL3 in Alzheimer’s Disease. eNeuro 2020, 7, ENEURO.0125-20.2020. [Google Scholar] [CrossRef]
- Zhao, F.; Xu, Y.; Gao, S.; Qin, L.; Austria, Q.; Siedlak, S.L.; Pajdzik, K.; Dai, Q.; He, C.; Wang, W.; et al. METTL3-Dependent RNA M6A Dysregulation Contributes to Neurodegeneration in Alzheimer’s Disease through Aberrant Cell Cycle Events. Mol. Neurodegener. 2021, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xia, Q.; Zhao, X.; Zheng, F.; Xiao, J.; Ge, F.; Wang, D.; Gao, X. The Landscape of M6A Regulators in Multiple Brain Regions of Alzheimer’s Disease. Mol. Neurobiol. 2023, 60, 5184–5198. [Google Scholar] [CrossRef]
- Li, H.; Ren, Y.; Mao, K.; Hua, F.; Yang, Y.; Wei, N.; Yue, C.; Li, D.; Zhang, H. FTO Is Involved in Alzheimer’s Disease by Targeting TSC1-MTOR-Tau Signaling. Biochem. Biophys. Res. Commun. 2018, 498, 234–239. [Google Scholar] [CrossRef]
- Jiang, L.; Lin, W.; Zhang, C.; Ash, P.E.A.; Verma, M.; Kwan, J.; van Vliet, E.; Yang, Z.; Cruz, A.L.; Boudeau, S.; et al. Interaction of Tau with HNRNPA2B1 and N6-Methyladenosine RNA Mediates the Progression of Tauopathy. Mol. Cell 2021, 81, 4209–4227.e12. [Google Scholar] [CrossRef]
- Tang, Z.; Cao, J.; Yao, J.; Fan, X.; Zhao, J.; Zhao, M.; Duan, Q.; Han, B.; Duan, S. KDM1A-Mediated Upregulation of METTL3 Ameliorates Alzheimer’s Disease via Enhancing Autophagic Clearance of p-Tau through M6A-Dependent Regulation of STUB1. Free Radic. Biol. Med. 2023, 195, 343–358. [Google Scholar] [CrossRef]
- Xu, C.; Huang, H.; Zhang, M.; Zhang, P.; Li, Z.; Liu, X.; Fang, M. Methyltransferase-Like 3 Rescues the Amyloid-Beta Protein-Induced Reduction of Activity-Regulated Cytoskeleton Associated Protein Expression via YTHDF1-Dependent N6-Methyladenosine Modification. Front. Aging Neurosci. 2022, 14, 890134. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Ju, Z.; Zheng, M.; Zhang, X.; Zuo, W.; Wang, Y.; Ding, X.; Zhang, X.; Peng, Y.; Li, J.; et al. Loss of the M6A Methyltransferase METTL3 in Monocyte-Derived Macrophages Ameliorates Alzheimer’s Disease Pathology in Mice. PLoS Biol. 2023, 21, e3002017. [Google Scholar] [CrossRef]
- Deng, Y.; Zhu, H.; Xiao, L.; Liu, C.; Liu, Y.-L.; Gao, W. Identification of the Function and Mechanism of M6A Reader IGF2BP2 in Alzheimer’s Disease. Aging 2021, 13, 24086–24100. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Pang, X.; Guo, W.; Zhu, C.; Yu, L.; Song, X.; Wang, K.; Pang, C. An Exploration of the Coherent Effects between METTL3 and NDUFA10 on Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 10111. [Google Scholar] [CrossRef]
- He, H.; Zhang, Q.; Liao, J.; Lei, J.; Luo, M.; Huang, J.; Chen, M.; Shen, Y.; Wang, J.; Xu, P.; et al. METTL14 Is Decreased and Regulates M6 A Modification of α-Synuclein in Parkinson’s Disease. J. Neurochem. 2023, 166, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Hess, M.E.; Hess, S.; Meyer, K.D.; Verhagen, L.A.W.; Koch, L.; Brönneke, H.S.; Dietrich, M.O.; Jordan, S.D.; Saletore, Y.; Elemento, O.; et al. The Fat Mass and Obesity Associated Gene (Fto) Regulates Activity of the Dopaminergic Midbrain Circuitry. Nat. Neurosci. 2013, 16, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, C.; Guo, M.; Zheng, X.; Ali, S.; Huang, H.; Zhang, L.; Wang, S.; Huang, Y.; Qie, S.; et al. Down-Regulation of M6A MRNA Methylation Is Involved in Dopaminergic Neuronal Death. ACS Chem. Neurosci. 2019, 10, 2355–2363. [Google Scholar] [CrossRef]
- Selberg, S.; Yu, L.-Y.; Bondarenko, O.; Kankuri, E.; Seli, N.; Kovaleva, V.; Herodes, K.; Saarma, M.; Karelson, M. Small-Molecule Inhibitors of the RNA M6A Demethylases FTO Potently Support the Survival of Dopamine Neurons. Int. J. Mol. Sci. 2021, 22, 4537. [Google Scholar] [CrossRef]
- Teng, Y.; Liu, Z.; Chen, X.; Liu, Y.; Geng, F.; Le, W.; Jiang, H.; Yang, L. Conditional Deficiency of M6A Methyltransferase Mettl14 in Substantia Nigra Alters Dopaminergic Neuron Function. J. Cell Mol. Med. 2021, 25, 8567–8572. [Google Scholar] [CrossRef]
- Quan, W.; Li, J.; Liu, L.; Zhang, Q.; Qin, Y.; Pei, X.; Chen, J. Influence of N6-Methyladenosine Modification Gene HNRNPC on Cell Phenotype in Parkinson’s Disease. Park. Dis. 2021, 2021, 9919129. [Google Scholar] [CrossRef]
- Yu, Z.; Huang, L.; Xia, Y.; Cheng, S.; Yang, C.; Chen, C.; Zou, Z.; Wang, X.; Tian, X.; Jiang, X.; et al. Analysis of M6A Modification Regulators in the Substantia Nigra and Striatum of MPTP-Induced Parkinson’s Disease Mice. Neurosci. Lett. 2022, 791, 136907. [Google Scholar] [CrossRef]
- McMillan, M.; Gomez, N.; Hsieh, C.; Bekier, M.; Li, X.; Miguez, R.; Tank, E.M.H.; Barmada, S.J. RNA Methylation Influences TDP43 Binding and Disease Pathogenesis in Models of Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Mol. Cell 2023, 83, 219–236.e7. [Google Scholar] [CrossRef]
- Pupak, A.; Singh, A.; Sancho-Balsells, A.; Alcalá-Vida, R.; Espina, M.; Giralt, A.; Martí, E.; Ørom, U.A.V.; Ginés, S.; Brito, V. Altered M6A RNA Methylation Contributes to Hippocampal Memory Deficits in Huntington’s Disease Mice. Cell Mol. Life Sci. 2022, 79, 416. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, S.; Cui, Y.-H.; Wei, J.; Shah, P.; Park, G.; Cui, X.; He, C.; He, Y.-Y. METTL14 Facilitates Global Genome Repair and Suppresses Skin Tumorigenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2025948118. [Google Scholar] [CrossRef]
- Guo, H.; Zeng, H.; Hu, Y.; Jiang, L.; Lei, L.; Hung, J.; Fu, C.; Li, H.; Long, Y.; Chen, J.; et al. UVB Promotes Melanogenesis by Regulating METTL3. J. Cell Physiol. 2023, 10, 1002. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Laurent, B.; Hsu, C.-H.; Nachtergaele, S.; Lu, Z.; Sheng, W.; Xu, C.; Chen, H.; Ouyang, J.; Wang, S.; et al. RNA M6A Methylation Regulates the Ultraviolet-Induced DNA Damage Response. Nature 2017, 543, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, K.; Sui, L.; Nie, J.; Cui, K.; Liu, J.; Zhang, H.; Yang, X.; Lu, K.; Liang, X. Constant Light Exposure Causes Oocyte Meiotic Defects and Quality Deterioration in Mice. Environ. Pollut. 2020, 267, 115467. [Google Scholar] [CrossRef]
- Li, Y.; Ren, J.; Zhang, Z.; Weng, Y.; Zhang, J.; Zou, X.; Wu, S.; Hu, H. Modification and Expression of MRNA M6A in the Lateral Habenular of Rats after Long-Term Exposure to Blue Light during the Sleep Period. Genes 2023, 14, 143. [Google Scholar] [CrossRef]
- Li, T.; Tan, Y.-T.; Chen, Y.-X.; Zheng, X.-J.; Wang, W.; Liao, K.; Mo, H.-Y.; Lin, J.; Yang, W.; Piao, H.-L.; et al. Methionine Deficiency Facilitates Antitumour Immunity by Altering M6A Methylation of Immune Checkpoint Transcripts. Gut 2023, 72, 501–511. [Google Scholar] [CrossRef]
- Kaspi, A.; Khurana, I.; Ziemann, M.; Connor, T.; Spolding, B.; Zimmet, P.; Walder, K.; El-Osta, A. Diet during Pregnancy Is Implicated in the Regulation of Hypothalamic RNA Methylation and Risk of Obesity in Offspring. Mol. Nutr. Food Res. 2018, 62, e1800134. [Google Scholar] [CrossRef]
- Klinge, C.M.; Piell, K.M.; Petri, B.J.; He, L.; Zhang, X.; Pan, J.; Rai, S.N.; Andreeva, K.; Rouchka, E.C.; Wahlang, B.; et al. Combined Exposure to Polychlorinated Biphenyls and High-Fat Diet Modifies the Global Epitranscriptomic Landscape in Mouse Liver. Environ. Epigenet. 2021, 7, dvab008. [Google Scholar] [PubMed]
- Xiong, Y.-W.; Tan, L.-L.; Zhang, J.; Zhu, H.-L.; Zheng, X.-M.; Chang, W.; Gao, L.; Wei, T.; Xu, D.-X.; Wang, H. Combination of High-Fat Diet and Cadmium Impairs Testicular Spermatogenesis in an M6A-YTHDF2-Dependent Manner. Environ. Pollut. 2022, 313, 120112. [Google Scholar] [CrossRef]
- Xu, Z.; Qin, Y.; Lv, B.; Tian, Z.; Zhang, B. Intermittent Fasting Improves High-Fat Diet-Induced Obesity Cardiomyopathy via Alleviating Lipid Deposition and Apoptosis and Decreasing M6A Methylation in the Heart. Nutrients 2022, 14, 251. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, S.; Wang, X.; Yang, X.; Chen, L.; Huang, T.; Zheng, Y.; Zheng, X.; Wu, X.; Sun, Y.; et al. Exercise Mitigates Endothelial Pyroptosis and Atherosclerosis by Downregulating NEAT1 Through N6-Methyladenosine Modifications. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 910–926. [Google Scholar] [CrossRef] [PubMed]
- Winocur, G.; Wojtowicz, J.M.; Huang, J.; Tannock, I.F. Physical Exercise Prevents Suppression of Hippocampal Neurogenesis and Reduces Cognitive Impairment in Chemotherapy-Treated Rats. Psychopharmacology 2014, 231, 2311–2320. [Google Scholar] [CrossRef]
- Xu, Z.; Qin, Y.; Lv, B.; Tian, Z.; Zhang, B. Effects of Moderate-Intensity Continuous Training and High-Intensity Interval Training on Testicular Oxidative Stress, Apoptosis and M6A Methylation in Obese Male Mice. Antioxidants 2022, 11, 1874. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wei, J.-A.; Yang, F.; Wang, M.; Wang, S.; Cheng, T.; Liu, X.; Jia, Y.; So, K.-F.; Zhang, L. Physical Exercise Prevented Stress-Induced Anxiety via Improving Brain RNA Methylation. Adv. Sci. 2022, 9, e2105731. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, R.; Li, M.; Ye, H.; Wu, C.; Wang, C.; Li, S.; Tan, L.; Mai, D.; Li, G.; et al. Excessive MiR-25-3p Maturation via N6-Methyladenosine Stimulated by Cigarette Smoke Promotes Pancreatic Cancer Progression. Nat. Commun. 2019, 10, 1858. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Wu, Y.; Zhao, J.; Cheng, C.; Lin, J.; Yang, Y.; Lu, L.; Xiang, Q.; Bian, T.; Liu, Q. N6-Methyladenosine-Modified CircSAV1 Triggers Ferroptosis in COPD through Recruiting YTHDF1 to Facilitate the Translation of IREB2. Cell Death Differ. 2023, 30, 1293–1304. [Google Scholar] [CrossRef]
- Jin, M.; Li, G.; Liu, W.; Wu, X.; Zhu, J.; Zhao, D.; Zeng, Z.; Xiong, M.; Song, Y.; He, X.; et al. Cigarette Smoking Induces Aberrant N6-Methyladenosine of DAPK2 to Promote Non-Small Cell Lung Cancer Progression by Activating NF-ΚB Pathway. Cancer Lett. 2021, 518, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wu, Y.; Xiao, T.; Xue, J.; Sun, J.; Xia, H.; Ma, H.; Lu, L.; Li, J.; Shi, A.; et al. METTL3-Mediated M6A Modification of ZBTB4 MRNA Is Involved in the Smoking-Induced EMT in Cancer of the Lung. Mol. Ther. Nucleic Acids 2021, 23, 487–500. [Google Scholar] [CrossRef]
- Xia, H.; Wu, Y.; Zhao, J.; Li, W.; Lu, L.; Ma, H.; Cheng, C.; Sun, J.; Xiang, Q.; Bian, T.; et al. The Aberrant Cross-Talk of Epithelium-Macrophages via METTL3-Regulated Extracellular Vesicle MiR-93 in Smoking-Induced Emphysema. Cell Biol. Toxicol. 2022, 38, 167–183. [Google Scholar] [CrossRef]
- Song, J.; Zeng, Y.; Zhu, M.; Zhu, G.; Chen, C.; Jin, M.; Wang, J.; Song, Y. Comprehensive Analysis of Transcriptome-Wide M6A Methylome in the Lung Tissues of Mice with Acute Particulate Matter Exposure. Ecotoxicol. Environ. Saf. 2022, 241, 113810. [Google Scholar] [CrossRef]
- Ji, D.; Hu, C.; Ning, J.; Ying, X.; Zhang, H.; Zhang, B.; Liu, B.; Liu, Q.; Ji, W.; Zhang, R. N6-Methyladenosine Mediates Nrf2 Protein Expression Involved in PM2.5-Induced Pulmonary Fibrosis. Ecotoxicol. Environ. Saf. 2023, 254, 114755. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Tao, Y.; Li, X.; Wang, J.; Chen, J.; Aniagu, S.; Jiang, Y.; Chen, T. AHR-Mediated M6A RNA Methylation Contributes to PM2.5-Induced Cardiac Malformations in Zebrafish Larvae. J. Hazard. Mater. 2023, 457, 131749. [Google Scholar] [CrossRef]
- Liu, H.; Gu, J.; Huang, Z.; Han, Z.; Xin, J.; Yuan, L.; Du, M.; Chu, H.; Wang, M.; Zhang, Z. Fine Particulate Matter Induces METTL3-Mediated M6A Modification of BIRC5 MRNA in Bladder Cancer. J. Hazard. Mater. 2022, 437, 129310. [Google Scholar] [CrossRef]
- Ning, J.; Du, H.; Zhang, Y.; Liu, Q.; Jiang, T.; Pang, Y.; Tian, X.; Yan, L.; Niu, Y.; Zhang, R. N6-Methyladenosine Modification of CDH1 MRNA Promotes PM2.5-Induced Pulmonary Fibrosis via Mediating Epithelial Mesenchymal Transition. Toxicol. Sci. 2022, 185, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Su, Q.; Xu, R.; Peng, J.; Wang, Z.; Zhu, X.; Wei, Y. Effect of Acute PM2.5 Exposure on PTGS2 and RNA M6A Modification. Environ. Pollut. 2023, 335, 122264. [Google Scholar] [CrossRef]
- Ning, J.; Pei, Z.; Wang, M.; Hu, H.; Chen, M.; Liu, Q.; Wu, M.; Yang, P.; Geng, Z.; Zheng, J.; et al. Site-Specific Atg13 Methylation-Mediated Autophagy Regulates Epithelial Inflammation in PM2.5-Induced Pulmonary Fibrosis. J. Hazard. Mater. 2023, 457, 131791. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, L.; Liu, S.; Wang, J.; Liu, Y.; Xiong, A.; Jiang, M.; Luo, L.; Ying, X.; Li, G. Methyltransferase-like 3 Leads to Lung Injury by up-Regulation of Interleukin 24 through N6-Methyladenosine-Dependent MRNA Stability and Translation Efficiency in Mice Exposed to Fine Particulate Matter 2.5. Environ. Pollut. 2022, 308, 119607. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Sun, G.; Zhou, M.; Zhang, H.; Liu, H.; Wang, M.; Zhang, Z.; Chu, H. Linc01515 Regulates PM2.5-Induced Oxidative Stress via Targeting NRF2 in Airway Epithelial Cells. Environ. Pollut. 2023, 331, 121798. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhu, H.; Liu, H.; Wang, M.; Chu, H.; Zhang, Z. METTL3 Regulates PM2.5-Induced Cell Injury by Targeting OSGIN1 in Human Airway Epithelial Cells. J. Hazard. Mater. 2021, 415, 125573. [Google Scholar] [CrossRef]
- Guo, X.; Lin, Y.; Lin, Y.; Zhong, Y.; Yu, H.; Huang, Y.; Yang, J.; Cai, Y.; Liu, F.; Li, Y.; et al. PM2.5 Induces Pulmonary Microvascular Injury in COPD via METTL16-Mediated M6A Modification. Environ. Pollut. 2022, 303, 119115. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, J.; Deng, H. Transcriptome-Wide M6A Modification Mediates Cardiotoxicity in Mice after Chronic Exposure to Microplastics. Chemosphere 2023, 317, 137877. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, J.; Zhou, J.; Song, L.; Ding, J.; Deng, H.P.; Weng, L.; Zhu, Y.; Xu, Z. N6-Methyladenosine Methylation Mediates Non-Coding RNAs Modification in Microplastic-Induced Cardiac Injury. Ecotoxicol. Environ. Saf. 2023, 262, 115174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, X.; Sun, D.; Zhang, Z. Oxidative Stress: One Potential Factor for Arsenite-Induced Increase of N6-Methyladenosine in Human Keratinocytes. Environ. Toxicol. Pharmacol. 2019, 69, 95–103. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, D.; Zhao, M.; Lai, Y.; Liu, Y.; Zhang, Z. N6-Methyladenosine Mediates Arsenite-Induced Human Keratinocyte Transformation by Suppressing P53 Activation. Environ. Pollut. 2020, 259, 113908. [Google Scholar] [CrossRef]
- Cayir, A.; Barrow, T.M.; Guo, L.; Byun, H.-M. Exposure to Environmental Toxicants Reduces Global N6-Methyladenosine RNA Methylation and Alters Expression of RNA Methylation Modulator Genes. Environ. Res. 2019, 175, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, A. Involvement of METTL3 in Arsenite-Induced Skin Lesions by Targeting the SOCS3/STAT3/Krt Signaling Pathway. Environ. Pollut. 2023, 316, 120634. [Google Scholar] [CrossRef]
- Li, S.; Zhou, H.; Liang, Y.; Yang, Q.; Zhang, J.; Shen, W.; Lei, L. Integrated Analysis of Transcriptome-Wide M6A Methylation in a Cd-Induced Kidney Injury Rat Model. Ecotoxicol. Environ. Saf. 2023, 256, 114903. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Zhang, H.; Wang, L.; Jie, S.; Zhao, Q.; Chen, F.; Yue, Y.; Wang, H.; Tian, L.; Xie, J.; et al. Long-Term Cadmium Exposure Impairs Cognitive Function by Activating Lnc-Gm10532/M6A/FIS1 Axis-Mediated Mitochondrial Fission and Dysfunction. Sci. Total Environ. 2023, 858, 159950. [Google Scholar] [CrossRef]
- Li, W.; Tan, M.; Wang, H.; Wang, Z.; Pang, Y.; Yang, R.; Zhong, S.; Pan, X.; Chen, S.; Wang, Q.; et al. METTL3-Mediated M6A MRNA Modification Was Involved in Cadmium-Induced Liver Injury. Environ. Pollut. 2023, 331, 121887. [Google Scholar] [CrossRef]
- Li, L.; Zhou, M.; Chen, B.; Wang, Q.; Pan, S.; Hou, Y.; Xia, J.; Zhou, X. ALKBH5 Promotes Cadmium-Induced Transformation of Human Bronchial Epithelial Cells by Regulating PTEN Expression in an M6A-Dependent Manner. Ecotoxicol. Environ. Saf. 2021, 224, 112686. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, L.; Zhu, J.; Luo, L.; Li, Y.; Zhang, C.; Zhang, W. Cadmium Disrupts Mouse Embryonic Stem Cell Differentiation into Ovarian Granulosa Cells through Epigenetic Mechanisms. Ecotoxicol. Environ. Saf. 2022, 235, 113431. [Google Scholar] [CrossRef]
- Tang, J.; Su, Q.; Guo, Z.; Zhou, J.; Zheng, F.; Yu, G.; Shao, W.; Hu, H.; Wu, S.; Li, H. N6-Methyladenosine(M6A) Demethylase FTO Regulates Cellular Apoptosis Following Cobalt-Induced Oxidative Stress. Environ. Pollut. 2022, 297, 118749. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zheng, F.; Liu, X.; Li, Y.; Guo, Z.; Lin, X.; Zhou, J.; Zhang, Y.; Yu, G.; Hu, H.; et al. Cobalt Induces Neurodegeneration through FTO-Triggered Autophagy Impairment by Targeting TSC1 in an M6A-YTHDF2-Dependent Manner. J. Hazard. Mater. 2023, 453, 131354. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Yu, G.; Su, Q.; Wu, L.; Tang, J.; Lin, X.; Chen, Y.; Guo, Z.; Zheng, F.; Zheng, H.; et al. The Deficiency of N6-Methyladenosine Demethylase ALKBH5 Enhances the Neurodegenerative Damage Induced by Cobalt. Sci. Total Environ. 2023, 881, 163429. [Google Scholar] [CrossRef]
- Tang, J.; Zheng, C.; Zheng, F.; Li, Y.; Wang, Y.-L.; Aschner, M.; Guo, Z.; Yu, G.; Wu, S.; Li, H. Global N6-Methyladenosine Profiling of Cobalt-Exposed Cortex and Human Neuroblastoma H4 Cells Presents Epitranscriptomics Alterations in Neurodegenerative Disease-Associated Genes. Environ. Pollut. 2020, 266, 115326. [Google Scholar] [CrossRef]
- Qi, Z.; Liu, Y.; Yang, H.; Yang, X.; Wang, H.; Liu, B.; Yuan, Y.; Wang, G.; Xu, B.; Liu, W.; et al. Protective Role of M6A Binding Protein YTHDC2 on CCNB2 in Manganese-Induced Spermatogenesis Dysfunction. Chem. Biol. Interact. 2022, 351, 109754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, X.; Su, D.; Liu, C.; Chen, Q.; Qi, Z. An Analysis of Differentially Expressed and Differentially M6A-Modified Transcripts in Soybean Roots Treated with Lead. J. Hazard. Mater. 2023, 453, 131370. [Google Scholar] [CrossRef] [PubMed]
- Ai, S.; Li, D.; Gu, X.; Xu, Y.; Wang, Y.; Wang, H.-L.; Chen, X.-T. Profile of N6-Methyladenosine of Pb-Exposed Neurons Presents Epitranscriptomic Alterations in PI3K-AKT Pathway-Associated Genes. Food Chem. Toxicol. 2023, 178, 113821. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Han, S.; Sun, Y.; Han, M.; Zheng, X.; Li, F.; Wei, Y.; Wang, Y.; Bi, J. Differential Methylation of CircRNA M6A in an APP/PS1 Alzheimer’s Disease Mouse Model. Mol. Med. Rep. 2023, 27, 55. [Google Scholar] [CrossRef]
- Han, M.; Liu, Z.; Xu, Y.; Liu, X.; Wang, D.; Li, F.; Wang, Y.; Bi, J. Abnormality of M6A MRNA Methylation Is Involved in Alzheimer’s Disease. Front. Neurosci. 2020, 14, 98. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the Diagnosis of Parkinson’s Disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, D.; Aarsland, D.; Chaudhuri, K.R.; Dobkin, R.D.; Leentjens, A.F.; Rodriguez-Violante, M.; Schrag, A. The Neuropsychiatry of Parkinson’s Disease: Advances and Challenges. Lancet Neurol. 2022, 21, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Koeglsperger, T.; Rumpf, S.-L.; Schließer, P.; Struebing, F.L.; Brendel, M.; Levin, J.; Trenkwalder, C.; Höglinger, G.U.; Herms, J. Neuropathology of Incidental Lewy Body & Prodromal Parkinson’s Disease. Mol. Neurodegener. 2023, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Min, S.; Shu, L.; Pan, H.; Zhong, J.; Guo, J.; Sun, Q.; Yan, X.; Chen, C.; Tang, B.; et al. Genetic Analysis of N6-Methyladenosine Modification Genes in Parkinson’s Disease. Neurobiol. Aging 2020, 93, 143.e9–143.e13. [Google Scholar] [CrossRef]
| Models | Regulators | m6A Level | Phenotype | References |
|---|---|---|---|---|
| Aging | METTL3 ↑ | Increase | METTL3 expression and global m6A levels increase during aging in mice and human. | [59,72] |
| METTL3 ↓ | Decrease | METTL3 reduction decreases NPNT expression and m6A levels in human pluripotent stem cell-derived myotubes, leading to senescence and apoptosis. | [138] | |
| METTL3 KD | Decrease | Accelerates hMSC senescence. | [137] | |
| METTL3 OE | Increase | Rescues hMSC senescence. | ||
| AD | METTL3 ↓, RBM15B ↑ | - | Downregulation of METTL3 and upregulation of RBM15 were detected in AD human hippocampus. | [144] |
| METTL3 ↓, METTL14 ↓, WTAP ↓, FTO ↓, YTHDF1 ↓ | Decrease | Reduces expression of METTL3, METTL14, FTO, and YTHDF1, and reduces m6A levels and abnormality of METTL3/14 nuclear localization in AD patient’s pyramidal neurons. | [145] | |
| METTL3 KD | Decrease | Promotes neuronal death in the hippocampus, Aβ oligomer induced cognitive and memory impairments in mice. | ||
| METTL3 OE | Increase | Rescues the effects of METTL3 KD. | ||
| RBM15 ↓, FTO ↓, ELAVL1 ↓, YTHDF2 ↓ | - | Downregulation of RBM15 and FTO and upregulation of ELAVL1 and YTHDF2 in AD hippocampus affects memory and cognition. | [146] | |
| FTO ↓ | Increase | Reduces the phosphorylation level of tau protein in AD mice (may decrease Tsc1 mRNA m6A level and its stability). | [147] | |
| FTO OE | Decrease | Rescues the effects of FTO KD. | ||
| HNRNPA2B ↓ | - | Reduces oligomeric tau-induced neurotoxicity in mice. | [148] | |
| METTL3 ↑ | Increase | Promotes autophagic clearance of phosphorylated tau. | [149] | |
| METTL3 ↓ | Decrease | Reduces ARC protein expression. | [150] | |
| METTL3 ↑ | Increase | Rescues the effects of METTL3 knockdown. | ||
| METTL3 KD | Decrease | Downregulates ATAT1, decreases α-tubulin, and promotes Aβ clearance. | [151] | |
| IGF2BP2 ↑ | - | IGF2BP2 shows an increased level in AD patients. | [152] | |
| METTL3 ↓ | Decrease | Reduction in METTL3 leads to decreased expression of NDUFA10, affecting electronic respiratory chain function. | [153] | |
| PD | METTL3 ↓, METTL14 ↓, YTHDF2 ↓ | Decrease | METTL3, METTL14, YTHDF2, and m6A levels were lower in PD patients. | [154] |
| METTL14 OE | Increase | Increases α-synuclein mRNA m6A level and decreases its stability. | ||
| FTO ↓ | Increase | Mn decreases FTO level and causes dopaminergic neuron projection damage and movement disorders. | [100] | |
| FTO OE | Decrease | FTO and ephrin-B2 overexpression increases survival rate of neurons. | ||
| FTO ↓ | Increase | Increased methylation of mRNAs associated with DA signaling pathways in the midbrain and striatum. | [155] | |
| FTO ↑ | Decrease | Decreases expression of N-methyl-D-aspartate (NMDA) receptor 1, increases oxidative stress, Ca2+ influx, and apoptosis of dopaminergic neurons. | [156] | |
| FTO ↓ | Increase | Small molecule inhibitor targeting FTO promotes survival of dopaminergic neurons. | [157] | |
| METTL14 cKO | Decrease | Downregulates Nurr1, pitx3, and EN1 expression in the substantia nigra region. | [158] | |
| HNRNPC OE | - | Inhibits apoptosis and immune inflammation in PC2 cells. | [159] | |
| ALKBH5 ↑, IGF2BP2 ↑, YTHDF1↓, METTL3↓, FMR ↑, CBLL1 ↑, RBM15 ↓ | Decrease | m6A-related proteins showed differential expression levels in SN and striatum in MPTP-induced PD mouse model. | [160] | |
| ALS | YTHDF2 KD | - | YTHDF2 knockdown attenuates TDP43-associated neurotoxicity and prolongs survival. | [161] |
| HD | FTO KD | Increase | FTO knockdown in the hippocampal CA1 region of HD mouse model (Hdh+/Q111 mouse) improved hippocampal spatial and recognition memories | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Zhang, X.; Xie, J.; Xu, Y.; Li, X.-J.; Lin, L. Emerging Roles for DNA 6mA and RNA m6A Methylation in Mammalian Genome. Int. J. Mol. Sci. 2023, 24, 13897. https://doi.org/10.3390/ijms241813897
Xie L, Zhang X, Xie J, Xu Y, Li X-J, Lin L. Emerging Roles for DNA 6mA and RNA m6A Methylation in Mammalian Genome. International Journal of Molecular Sciences. 2023; 24(18):13897. https://doi.org/10.3390/ijms241813897
Chicago/Turabian StyleXie, Leijie, Xiaosong Zhang, Jiaxiang Xie, Yanru Xu, Xiao-Jiang Li, and Li Lin. 2023. "Emerging Roles for DNA 6mA and RNA m6A Methylation in Mammalian Genome" International Journal of Molecular Sciences 24, no. 18: 13897. https://doi.org/10.3390/ijms241813897
APA StyleXie, L., Zhang, X., Xie, J., Xu, Y., Li, X.-J., & Lin, L. (2023). Emerging Roles for DNA 6mA and RNA m6A Methylation in Mammalian Genome. International Journal of Molecular Sciences, 24(18), 13897. https://doi.org/10.3390/ijms241813897





