PDT-Induced Activation Enhanced by Hormone Response to Treatment
Abstract
:1. Introduction
1.1. Photodynamic Therapy-Mechanism
1.2. Reactive Oxygen Species
2. Results
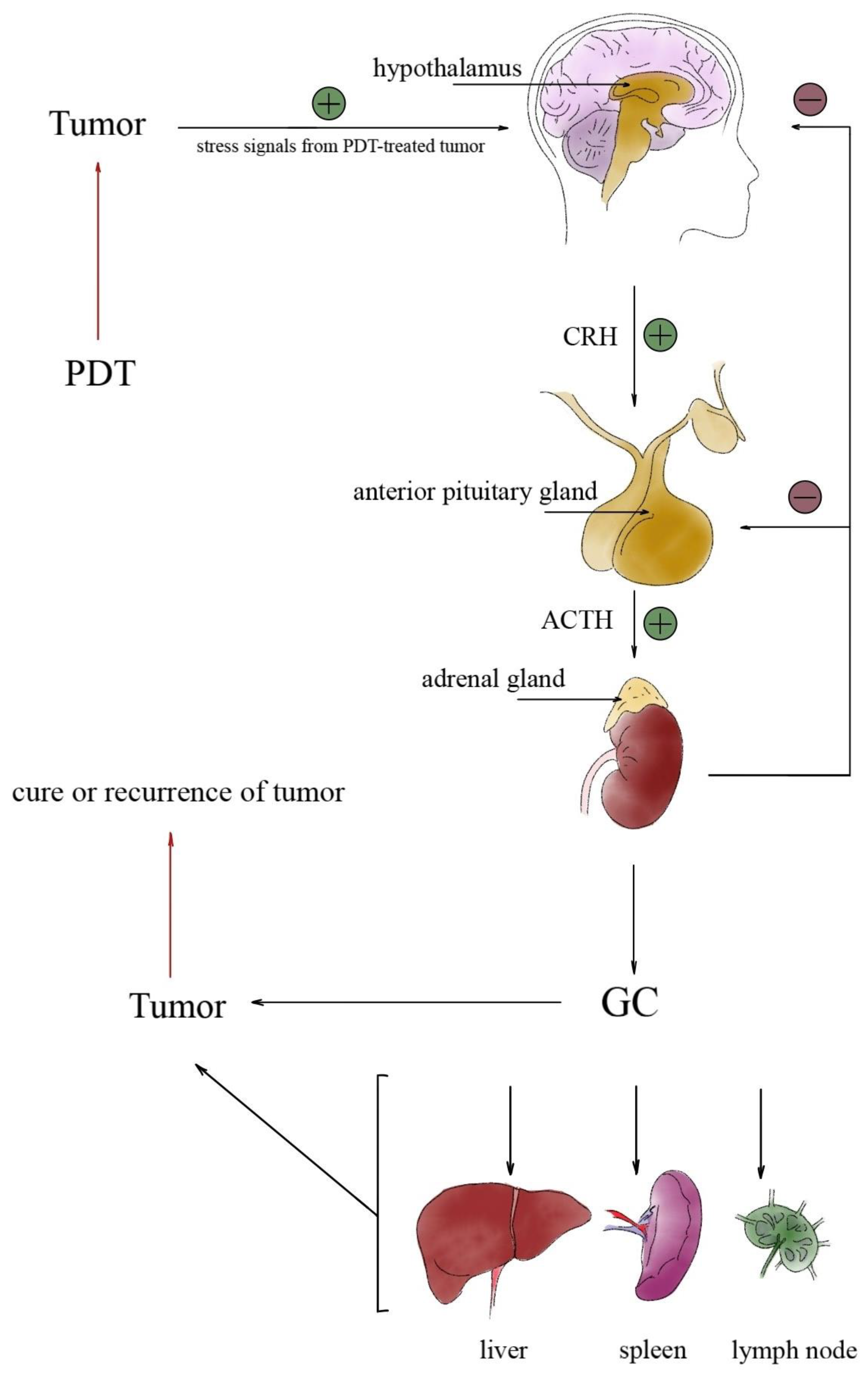
2.1. Involvement of the Hypothalamic-Pituitary-Brain Axis during PDT Treatment
2.2. PDT-Induced Hormonal Activation
2.3. The Role of Glucocorticoids in Tumor Response to PDT
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fojo, T.; Grady, C. How Much Is Life Worth: Cetuximab, Non-Small Cell Lung Cancer, and the $440 Billion Question. J. Natl. Cancer Inst. 2009, 101, 1044–1048. [Google Scholar] [CrossRef]
- Bergh, J. Quo vadis with targeted drugs in the 21st century? J. Clin. Oncol. 2009, 27, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Huang, X.; Gao, L.; Zhou, G.; Xie, F. The application of photodynamic therapy in plastic and reconstructive surgery. Front. Chem. 2022, 10, 967312. [Google Scholar] [CrossRef] [PubMed]
- Lotan, Y. Promises and challenges of fluorescence cystoscopy. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Saberi, S.; Hakimiha, N.; Alaeddini, M.; Etemad-Moghadam, S.; Roudbari, P.; Shahabi, S. In Vitro Anti-tumor Effects of Photodynamic Therapy on Oral Squamous Cell Carcinoma: A Review. J. Lasers Med. Sci. 2022, 13, e49. [Google Scholar] [CrossRef]
- Donohoe, C.; Senge, M.O.; Arnaut, L.G.; Gomes-Da-Silva, L.C. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2019, 1872, 188308. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.; Dharan, A.; PV, J.; Pal, S.; Nair, B.G.; Kar, R.; Mishra, N. Paraptosis: A unique cell death mode for targeting cancer. Front. Pharmacol. 2023, 14, 1159409. [Google Scholar] [CrossRef]
- Van Straten, D.; Mashayekhi, V.; De Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer—A Review of the Current Clinical Status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, J.; Jiao, J.; Qin, W.; Yang, X. Photodynamic therapy for prostate cancer: Recent advances, challenges and opportunities. Front. Oncol. 2022, 12, 980239. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Grumezescu, A.M. Photodynamic Therapy—An Up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Mušković, M.; Pokrajac, R.; Malatesti, N. Combination of Two Photosensitisers in Anticancer, Antimicrobial and Upconversion Photodynamic Therapy. Pharmaceuticals 2023, 16, 613. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, X.; Jin, L.; Lin, L.; Lin, H.; Yang, Z.; Huang, W. Strategic Design of Conquering Hypoxia in Tumor for Advanced Photodynamic Therapy. Adv. Heal. Mater. 2023, 1, e2300530. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, N.; Samadani, A.A. Implications of photodynamic cancer therapy: An overview of PDT mechanisms basically and practically. J. Egypt. Natl. Cancer Inst. 2021, 33, 34. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, T.; Balalaeva, I.; Gorokhova, A.; Vedunova, M.; Krysko, D.V. Which cell death modality wins the contest for photodynamic therapy of cancer? Cell Death Dis. 2022, 13, 455. [Google Scholar] [CrossRef]
- Ostańska, E.; Aebisher, D.; Bartusik-Aebisher, D. The potential of photodynamic therapy in current breast cancer treatment methodologies. Biomed. Pharmacother. 2021, 137, 111302. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Wei, M.; Yang, B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics 2022, 12, 434–458. [Google Scholar] [CrossRef]
- Kubrak, T.; Karakuła, M.; Czop, M.; Kawczyk-Krupka, A.; Aebisher, D. Advances in Management of Bladder Cancer—The Role of Photodynamic Therapy. Molecules 2022, 27, 731. [Google Scholar] [CrossRef]
- Liu, W.-T.; Wang, H.-T.; Yeh, Y.-H.; Wong, T.-W. An Update on Recent Advances of Photodynamic Therapy for Primary Cutaneous Lymphomas. Pharmaceutics 2023, 15, 1328. [Google Scholar] [CrossRef] [PubMed]
- Kulbacka, J. Nanosecond pulsed electric fields (nsPEFs) impact and enhanced Photofrin II® delivery in photodynamic reaction in cancer and normal cells. Photodiagn. Photodyn. Ther. 2015, 12, 621–629. [Google Scholar] [CrossRef]
- Lee, C.-N.; Hsu, R.; Chen, S.; Wong, T.-W. Daylight Photodynamic Therapy: An Update. Molecules 2020, 25, 5195. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Huang, Y. Imaging in Photodynamic Therapy; Taylor & Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Fitzgerald, F. Photodynamic Therapy (PDT): Principles, Mechanisms and Applications; Nova Science Publishers, Inc.: New York, NY, USA, 2017. [Google Scholar]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Kari, S.; Subramanian, K.; Altomonte, I.A.; Murugesan, A.; Yli-Harja, O.; Kandhavelu, M. Programmed cell death detection methods: A systematic review and a categorical comparison. Apoptosis 2022, 27, 482–508. [Google Scholar] [CrossRef]
- Lin, L.; Song, X.; Dong, X.; Li, B. Nano-photosensitizers for enhanced photodynamic therapy. Photodiagn. Photodyn. Ther. 2021, 36, 102597. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photodynamic therapy for cancer: What’s past is prologue. Photochem. Photobiol. 2020, 96, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef]
- Agarwal, M.L.; Clay, M.E.; Harvey, E.J.; Evans, H.H.; Antunez, A.R.; Oleinick, N.L. Photodynamic therapy induces rapid cell death by apoptosis in L5178Y mouse lymphoma cells. Cancer Res. 1991, 51, 5993–5996. [Google Scholar]
- Chiu, S.M.; Xue, L.Y.; Lam, M.; Rodriguez, M.E.; Zhang, P.; Kenney, M.E.; Nieminen, A.L.; Oleinick, N.L. A requirement for bid for induction of apoptosis by photodynamic therapy with a lysosome- but not a mitochondrion-targeted photosensitizer. Photochem. Photobiol. 2010, 86, 1161–1173. [Google Scholar] [CrossRef]
- Azad, M.B.; Chen, Y.; Gibson, S.B. Regulation of autophagy by reactive oxygen species (ROS): Implications for cancer progression and treatment. Antioxid. Redox Signal. 2009, 11, 777–790. [Google Scholar] [CrossRef]
- Galluzzi, L.; Bravo-San Pedro, J.M.; Demaria, S.; Formenti, S.C.; Kroemer, G. Activating autophagy to potentiate immunogenic chemotherapy and radiation therapy. Nature reviews. Clin. Oncol. 2017, 14, 247–258. [Google Scholar]
- Dąbrowski, J.M. Chapter nine- reactive species in photodynamic therapy; mechanisms of their generation and potentiation. Adv. Inorg. Chem. 2017, 70, 343–394. [Google Scholar]
- Kreuz, S.; Fischle, W. Oxidative stress signaling to chromatin in health and disease. Epigenomics 2016, 8, 843–862. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Roychoudhury, S. Reactive Oxygen Species in the Reproductive System: Sources and Physiological Roles. In Oxidative Stress and Toxicity in Reproductive Biology and Medicine: A Comprehensive Update on Male Infertility—Volume One; Springer: Cham, Switzerland, 2022; Volume 1358, pp. 9–40. [Google Scholar] [CrossRef]
- Dayem, A.A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
- Ingenbosch, K.N.; Quint, S.; Dyllick-Brenzinger, M.; Wunschik, D.S.; Kiebist, J.; Süss, P.; Liebelt, U.; Zuhse, R.; Menyes, U.; Scheibner, K.; et al. Singlet-Oxygen Generation by Peroxidases and Peroxygenases for Chemoenzymatic Synthesis. ChemBioChem 2020, 22, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, P.S.; Bhattarai, H.K. Singlet Oxygen, Photodynamic Therapy, and Mechanisms of Cancer Cell Death. J. Oncol. 2022, 2022, 7211485. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R.; George, S. Structure and functions of Aggregation-Induced Emission-Photosensitizers in anticancer and antimicrobial theranostics. Front. Chem. 2022, 10, 984268. [Google Scholar] [CrossRef]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front. Cell Dev. Biol. 2021, 9, 181. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.-H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free. Radic. Biol. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef]
- Dryden, M. Reactive oxygen species: A novel antimicrobial. Int. J. Antimicrob. Agents 2018, 51, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial photodynamic therapy: Overview of a promising approach to fight antibiotic-resistant bacterial infections. J. Clin. Transl. Res. 2015, 1, 140–167. [Google Scholar] [PubMed]
- Segal, L.M.; Wilson, R.A. Reactive oxygen species metabolism and plant-fungal interactions. Fungal Genet. Biol. 2018, 110, 1–9. [Google Scholar] [CrossRef]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Gul, M.; Alzweiri, M.; Ishaqat, A.; ALSalamat, H.A.; Bashatwah, R.M. Reactive Oxygen Species: The Dual Role in Physiological and Pathological Conditions of the Human Body. Eurasian J. Med. 2018, 50, 193–201. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wan, J.; Zhang, Z.; Wang, F.; Guo, J.; Wang, C. Localized Fe(II)-Induced Cytotoxic Reactive Oxygen Species Generating Nanosystem for Enhanced Anticancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 4439–4449. [Google Scholar] [CrossRef] [PubMed]
- Falk-Mahapatra, R.; Gollnick, S.O. Photodynamic Therapy and Immunity: An Update. Photochem. Photobiol. 2020, 96, 550–559. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889. [Google Scholar] [CrossRef]
- Carruth, J.A. Clinical applications of photodynamic therapy. Int. J. Clin. Pract. 1998, 52, 39–42. [Google Scholar] [CrossRef]
- Bartusik, D.; Aebisher, D.; Ghogare, A.; Ghosh, G.; Abramova, I.; Hasan, T.; Greer, A. A Fiberoptic (Photodynamic Therapy Type) Device with a Photosensitizer and Singlet Oxygen Delivery Probe Tip for Ovarian Cancer Cell Killing. Photochem. Photobiol. 2013, 89, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Domka, W.; Bartusik-Aebisher, D.; Mytych, W.; Dynarowicz, K.; Aebisher, D. The Use of Photodynamic Therapy for Head, Neck, and Brain Diseases. Int. J. Mol. Sci. 2023, 24, 11867. [Google Scholar] [CrossRef] [PubMed]
- Bartusik-Aebisher, D.; Żołyniak, A.; Barnaś, E.; Machorowska-Pieniążek, A.; Oleś, P.; Kawczyk-Krupka, A.; Aebisher, D. The Use of Photodynamic Therapy in the Treatment of Brain Tumors—A Review of the Literature. Molecules 2022, 27, 6847. [Google Scholar] [CrossRef] [PubMed]
- Międzybrodzka, A.; Bartusik-Aebisher, D.; Aebisher, D.; Cieślar, G.; Kawczyk-Krupka, A. A review of preferential photosensitizers in brain cancer photodiagnosis and photodynamic therapy. Acta Pol. Pharma. 2021, 78, 457–465. [Google Scholar]
- Kalka, K.; Merk, H.; Mukhtar, H. Photodynamic therapy in dermatology. J. Am. Acad. Dermatol. 2000, 42, 389–413. [Google Scholar] [CrossRef]
- Kessel, D. Apoptosis, Paraptosis and Autophagy: Death and Survival Pathways Associated with Photodynamic Therapy. Photochem. Photobiol. 2018, 95, 119–125. [Google Scholar] [CrossRef]
- Wang, Y.; An, R.; Umanah, G.K.; Park, H.; Nambiar, K.; Eacker, S.M.; Kim, B.; Bao, L.; Harraz, M.M.; Chang, C.; et al. A nuclease that mediates cell death induced by DNA damage and poly(ADP-ribose) polymerase-1. Science 2016, 354, 6308. [Google Scholar] [CrossRef]
- Vantieghem, A.; Xu, Y.; Assefa, Z.; Piette, J.; Vandenheede, J.R.; Merlevede, W.; De Witte, P.A.; Agostinis, P. Phosphorylation of Bcl-2 in G2/M phase-arrested cells following photodynamic therapy with hypericin involves a CDK1-mediated signal and delays the onset of apoptosis. J. Biol. Chem. 2002, 277, 37718–37731. [Google Scholar] [CrossRef]
- Kimura, M.; Yoshioka, T.; Saio, M.; Banno, Y.; Nagaoka, H.; Okano, Y. Mitotic catastrophe and cell death induced by depletion of centrosomal proteins. Cell Death Dis. 2013, 4, e603. [Google Scholar] [CrossRef]
- Mascaraque, M.; Delgado-Wicke, P.; Damian, A.; Lucena, S.R.; Carrasco, E.; Juarranz, Á. Mitotic Catastrophe Induced in HeLa Tumor Cells by Photodynamic Therapy with Methyl-aminolevulinate. Int. J. Mol. Sci. 2019, 20, 1229. [Google Scholar] [CrossRef]
- Vanaja, S.K.; Rathinam, V.A.; Fitzgerald, K.A. Mechanisms of inflammasome activation: Recent advances and novel insights. Trends Cell Biol. 2015, 25, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheung, Y.K.; Ng, D.K.; Fong, W.P. Immunogenic necroptosis in the antitumor photodynamic action of BAM-SiPc, a silicon(IV) phthalocyanine-based photosensitizer. Cancer Immunol. Immunother. 2021, 70, 485–495. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Fingar, V.H.; Dougherty, T.J.; Korbelik, M. Vascular Effects of Photodynamic Therapy. J. Clin. Laser Med. Surg. 1996, 14, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Sirotkina, M.A.; Moiseev, A.A.; Matveev, L.A.; Zaitsev, V.Y.; Elagin, V.V.; Kuznetsov, S.S.; Gelikonov, G.V.; Ksenofontov, S.Y.; Zagaynova, E.V.; Feldchtein, F.I.; et al. Accurate early prediction of tumour response to PDT using optical coherence angiography. Sci. Rep. 2019, 9, 6492. [Google Scholar] [CrossRef]
- Huis in ‘t Veld, R.V.; Heuts, J.; Ma, S.; Cruz, L.J.; Ossendorp, F.A.; Jager, M.J. Current Challenges and Opportunities of Photodynamic Therapy against Cancer. Pharmaceutics 2023, 15, 330. [Google Scholar] [CrossRef]
- Jin, H.; Liao, S.; Yao, F.; Li, J.; Xu, Z.; Zhao, K.; Xu, X.; Sun, S. Insight into the Crosstalk between Photodynamic Therapy and Immunotherapy in Breast Cancer. Cancers 2023, 15, 1532. [Google Scholar] [CrossRef]
- Bartusik-Aebisher, D.; Mielnik, M.; Cieślar, G.; Chodurek, E.; Kawczyk-Krupka, A.; Aebisher, D. Photon Upconversion in Small Molecules. Molecules 2022, 27, 5874. [Google Scholar] [CrossRef]
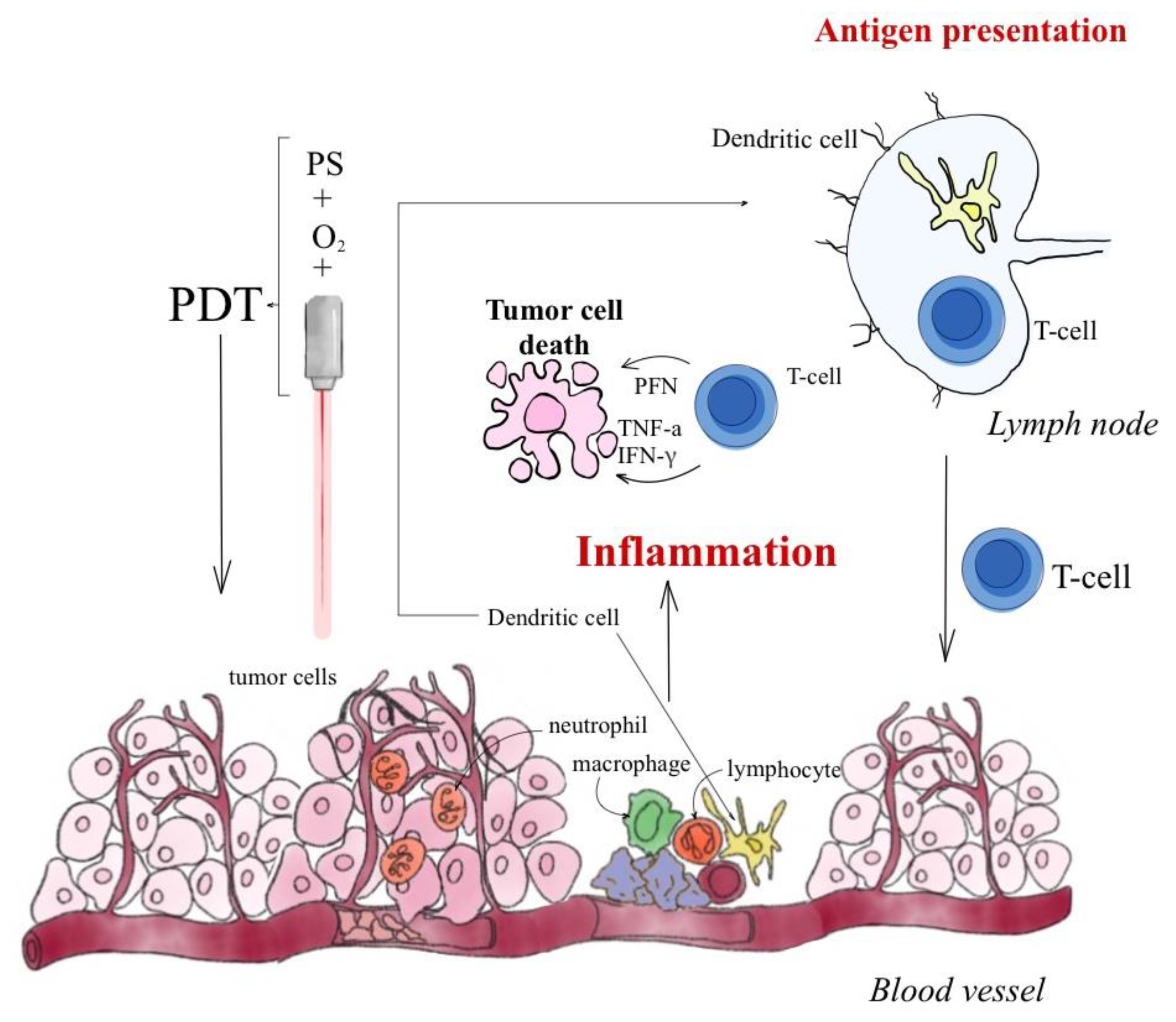
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and antitumor immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [PubMed]
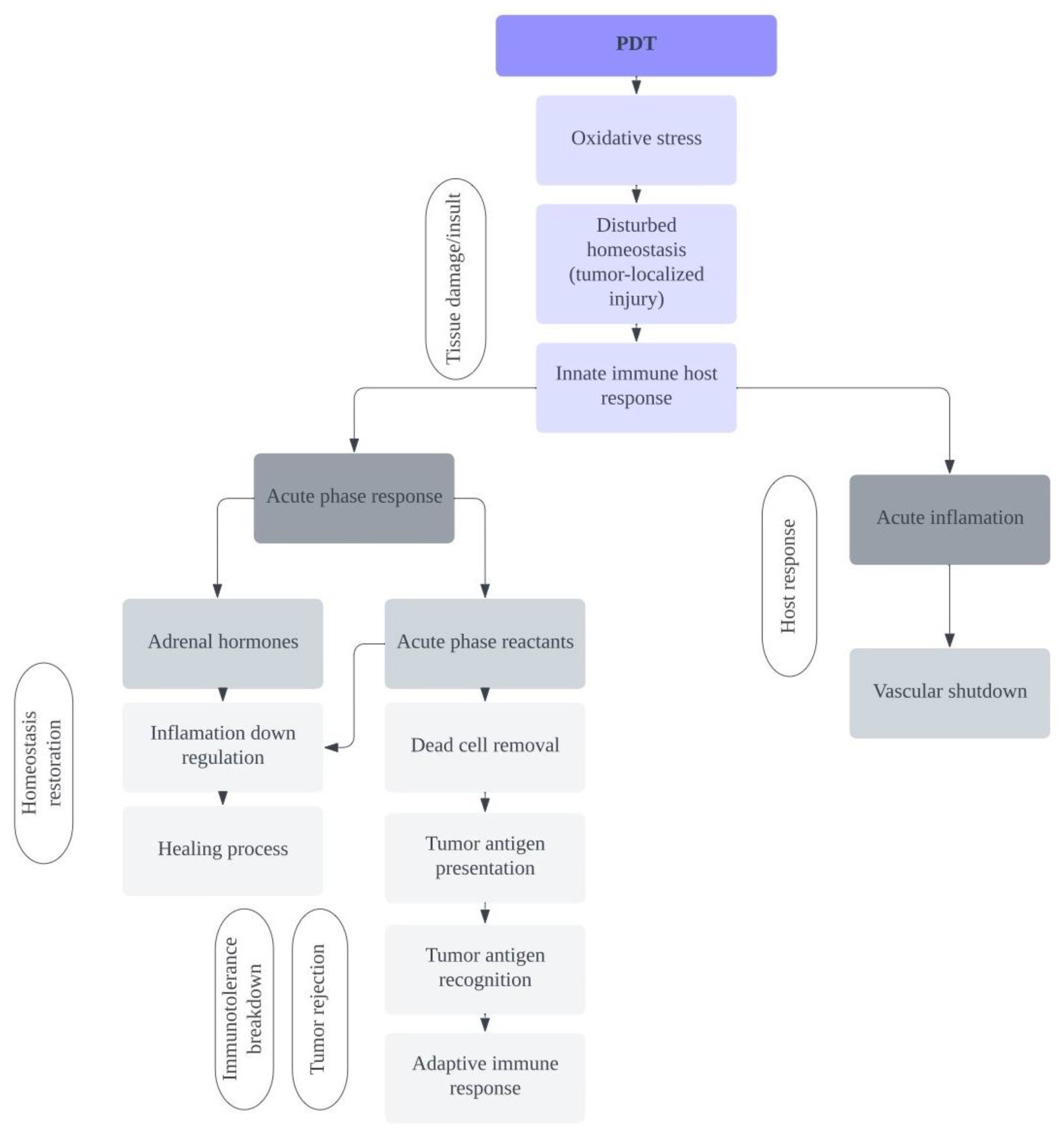
- Korbelik, M. PDT-associated host response and its role in the therapy outcome. Lasers Surg. Med. 2006, 38, 500–508. [Google Scholar] [CrossRef]
- Korbelik, M. PDT and inflammation. In Advances in Photodynamic Therapy: Basic, Translational and Clinical; Hamblin, M.R., Mroz, P., Eds.; Artech House: Boston, MA, USA; London, UK, 2008. [Google Scholar]
- Baumann, H.; Gauldie, J. The acute phase response. Immunol. Today 1994, 15, 74–80. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-Phase Proteins and Other Systemic Responses to Inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Kushner, I.; Rzewnicki, D. Acute phase response. In Inflammation: Basic Principles and Clinical Correlations; Galin, J.I., Snyderman, R., Eds.; Lippincottt Williams & Wilkins: Philadelphia, PA, USA, 1999; pp. 317–329. [Google Scholar]
- Korbelik, M.; Cecic, I.; Merchant, S.; Sun, J. Acute phase response induction by cancer treatment with photodynamic therapy. Int. J. Cancer 2007, 122, 1411–1417. [Google Scholar] [CrossRef]
- Lamberti, M.J.; Nigro, A.; Mentucci, F.M.; Vittar, N.B.R.; Casolaro, V.; Col, J.D. Dendritic Cells and Immunogenic Cancer Cell Death: A Combination for Improving Antitumor Immunity. Pharmaceutics 2020, 12, 256. [Google Scholar] [CrossRef] [PubMed]
- Ti, D.; Yan, X.; Wei, J.; Wu, Z.; Wang, Y.; Han, W. Inducing immunogenic cell death in immuno-oncological therapies. Chin. J. Cancer Res. 2022, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, M.; Łysenko, L.; Gerber, H.; Nowak, R. Cell reactions and immune responses to photodynamic therapy in oncology. Postępy Hig. I Med. Doświadczalnej 2016, 70, 735–742. [Google Scholar] [CrossRef]
- Tan, H.Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef]
- Bartusik, D.; Aebisher, D.; Ghosh, G.; Minnis, M.; Greer, A. Fluorine end-capped optical fibers for photosensitizer release and singlet oxygen production. J. Org. Chem. 2012, 77, 4557–4565. [Google Scholar] [CrossRef]
- Korbelik, M.; Merchant, S. Hormonal component of tumor photodynamic therapy response. In Biophotonics and Immune Responses III; SPIE: Bellingham, WA, USA, 2008; Volume 6857, p. 685705. [Google Scholar] [CrossRef]
- Remigante, A.; Morabito, R.; Marino, A. Band 3 protein function and oxidative stress in erythrocytes. J. Cell. Physiol. 2021, 236, 6225–6234. [Google Scholar] [CrossRef] [PubMed]
- Desborough, J. The stress response to trauma and surgery. Br. J. Anaesth. 2000, 85, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P. The hypothalamic-pituitary-adrenal axis and immune-related inflammation. N. Engl. J. Med. 1995, 332, 1351–1362. [Google Scholar] [CrossRef]
- Silverman, M.N.; Pearce, B.D.; Biron, C.A.; Miller, A.H.; Fernandes, C.A.; Nóbrega, Y.K.; Tosta, C.E.; Abdul-Careem, M.; Hunter, B.; Sarson, A.; et al. Immune Modulation of the Hypothalamic-Pituitary-Adrenal (HPA) Axis during Viral Infection. Viral Immunol. 2005, 18, 41–78. [Google Scholar] [CrossRef]
- Bellavance, M.A.; Rivest, S. The HPA—Immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front. Immunol. 2014, 5, 136. [Google Scholar] [CrossRef]
- Löwenberg, M.; Verhaar, A.P.; Brink, G.R.v.D.; Hommes, D.W. Glucocorticoid signaling: A nongenomic mechanism for T-cell immunosuppression. Trends Mol. Med. 2007, 13, 158–163. [Google Scholar] [CrossRef]
- Franchimont, D. Overview of the Actions of Glucocorticoids on the Immune Response: A Good Model to Characterize New Pathways of Immunosuppression for New Treatment Strategies. Ann. N. Y. Acad. Sci. 2004, 1024, 124–137. [Google Scholar] [CrossRef]
- Cecic, I.; Stott, B.; Korbelik, M. Acute phase response-associated systemic neutrophil mobilization in mice bearing tumors treated by photodynamic therapy. Int. Immunopharmacol. 2006, 6, 1259–1266. [Google Scholar] [CrossRef]
- Merchant, S.; Korbelik, M. Heat shock protein 70 is acute phase reactant: Response elicited by tumor treatment with photodynamic therapy. Cell Stress Chaperones 2011, 16, 153–162. [Google Scholar] [CrossRef]
- Belvisi, M.G.; Wicks, S.L.; Battram, C.H.; Bottoms, S.E.; Redford, J.E.; Woodman, P.; Brown, T.J.; Webber, S.E.; Foster, M.L. Therapeutic benefits of dissociated glucocortcosteroids and the relevance of in vitro separation of transrepression from transactivation. J. Immunol. 2001, 166, 1975–1982. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Y.; Ma, H.; Sun, Y.; Cao, J. How to improve photodynamic therapy-induced antitumor immunity for cancer treatment? Theranostics 2022, 12, 4629–4655. [Google Scholar] [CrossRef]
- Hernández, I.B.; Yu, Y.; Ossendorp, F.; Korbelik, M.; Oliveira, S. Preclinical and Clinical Evidence of Immune Responses Triggered in Oncologic Photodynamic Therapy: Clinical Recommendations. J. Clin. Med. 2020, 9, 333. [Google Scholar] [CrossRef]
- Grossman, C.E.; Carter, S.L.; Czupryna, J.; Wang, L.; Putt, M.E.; Busch, T.M. Fluence Rate Differences in Photodynamic Therapy Efficacy and Activation of Epidermal Growth Factor Receptor after Treatment of the Tumor-Involved Murine Thoracic Cavity. Int. J. Mol. Sci. 2016, 17, 101. [Google Scholar] [CrossRef]
- Qiang, Y.-G.; Yow, C.M.; Huang, Z. Combination of photodynamic therapy and immunomodulation: Current status and future trends. Med. Res. Rev. 2007, 28, 632–644. [Google Scholar] [CrossRef]
- Osiecka, B.; Jurczyszyn, K.; Ziółkowski, P. The application of Levulan®-based photodynamic therapy with imiquimod in the treatment of recurrent basal cell carcinoma. Experiment 2012, 18, PI5–PI9. [Google Scholar] [CrossRef]
- Serra-Guillén, C.; Nagore, E.; Guillén, C. Terapia fotodinámica versus imiquimod. Actas Dermosifiliogr. 2012, 103, 488–501. [Google Scholar] [CrossRef]
- Nath, S.; Obaid, G.; Hasan, T. The Course of Immune Stimulation by Photodynamic Therapy: Bridging Fundamentals of Photochemically Induced Immunogenic Cell Death to the Enrichment of T-Cell Repertoire. Photochem. Photobiol. 2019, 95, 1288–1305. [Google Scholar] [CrossRef]
- Hargus, J.A.; Fronczek, F.R.; Vicente, M.G.; Smith, K.M. Mono-(L)-aspartylchlorin-e6. Photochem. Photobiol. 2007, 83, 1006–1015. [Google Scholar] [CrossRef]
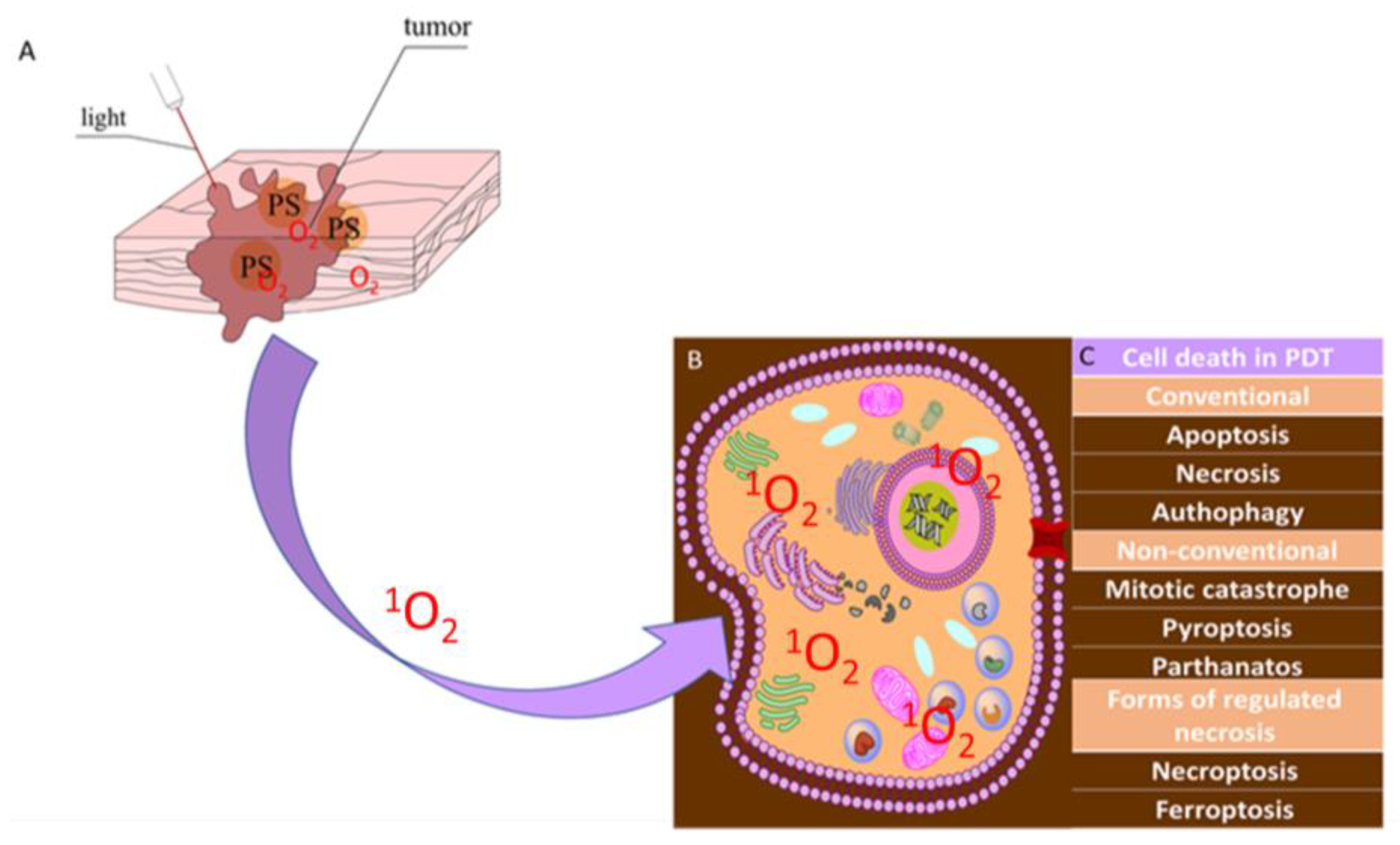
| Cell Death | Mechanisms | References |
|---|---|---|
| paraptosis | Paraptosis occurs independently of caspase activation and is characterized by vacuolation of the cytoplasm, with the formation of vacuoles joined by a single rather than a double membrane and swelling of the mitochondria. In the process of paraptosis, chromatin condensation and cell fragmentation do not occur. | [61] |
| parthanatos | Parthanatos is the cell’s response to DNA damage and is a caspase-independent process. Cell death is caused by the AIF/MIF complex, which is formed from the AIF factor released from the mitochondria and the MIF macrophage migration inhibitory factor [b]. | [62] |
| mitotic catastrophe | Mitotic catastrophe is a disruption of microtubule organization. The proteins AuroraA, ninein, TOG, and TACC3 enhance aberrant spindle formation and result in apoptotic-like cell death. | [63,64,65] |
| pyroptosis | Pyroptosis is accompanied by DNA fragmentation and chromatin condensation. Pyroptosis is associated with the activation of one or more caspases. | [66] |
| necroptosis | Necroptosis can be triggered by multiple stimuli, including surface-associated death receptors, e.g., tumor necrosis factor receptor 1 (TNFR1), DR4/5, and the FAS receptor; by pattern-recognition receptors such as Toll-like receptor 3 (TLR3), TLR4, and Z-DNA binding protein 1 (ZBP1; and by other stimuli that are well described in previous reviews. | [67] |
| ferroptosis | Ferroptosis is a form of regulated necrotic cell death associated with iron-dependent oxidative modification of phospholipid membranes [h]. | [68] |
| Type | Mechanism |
|---|---|
| Regulation of the acute-phase response | In the early phase, they promote and downregulate the acute-phase response through a negative feedback loop and regulate it in the late phase after PDT. |
| Regulation of expression of acute-phase response reagents | They control the generation of acute-phase reagents in the PDT response. |
| Neutrophil regulation | They participate in the regulation of PDT-induced tumor blood neutrophilia as one of many mediators. |
| Promoting the progression of induced inflammation | They have a pro-inflammatory effect in the initial stage of Toll-like receptor mRNA stabilization of some proteins, and accelerate its resolution in the advanced stage (as intermediaries in the removal of cells killed by transactivating genes of scavenger receptors, metalloproteinases, IL-10, and TGF-alpha) |
| Regulation of apoptosis | They have the ability to initiate eosinophils’ apoptosis by regulating their function (preventing their degranulation and releasing cytotoxic proteins) [96]. |
| Influence on tissue remodeling and healing | In the area subjected to PDT, they affect, among other things, the expressions of TGF-alpha, angiogenic factors, fibronectins, growth factors, and other things and promote healing and tissue remodeling. |
| Alleviating the intensity of the immune response | They increase the expression of genes mediating immunosuppression, which leads to alleviation of the intensity of the anti-cancer adaptive immune response. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domka, W.; Bartusik-Aebisher, D.; Przygoda, M.; Dynarowicz, K.; Tomik, J.; Aebisher, D. PDT-Induced Activation Enhanced by Hormone Response to Treatment. Int. J. Mol. Sci. 2023, 24, 13917. https://doi.org/10.3390/ijms241813917
Domka W, Bartusik-Aebisher D, Przygoda M, Dynarowicz K, Tomik J, Aebisher D. PDT-Induced Activation Enhanced by Hormone Response to Treatment. International Journal of Molecular Sciences. 2023; 24(18):13917. https://doi.org/10.3390/ijms241813917
Chicago/Turabian StyleDomka, Wojciech, Dorota Bartusik-Aebisher, Maria Przygoda, Klaudia Dynarowicz, Jerzy Tomik, and David Aebisher. 2023. "PDT-Induced Activation Enhanced by Hormone Response to Treatment" International Journal of Molecular Sciences 24, no. 18: 13917. https://doi.org/10.3390/ijms241813917
APA StyleDomka, W., Bartusik-Aebisher, D., Przygoda, M., Dynarowicz, K., Tomik, J., & Aebisher, D. (2023). PDT-Induced Activation Enhanced by Hormone Response to Treatment. International Journal of Molecular Sciences, 24(18), 13917. https://doi.org/10.3390/ijms241813917









