Methacrylated Gelatin as a Scaffold for Mechanically Isolated Stromal Vascular Fraction for Cutaneous Wound Repair
Abstract
:1. Introduction
2. Results
2.1. Patients
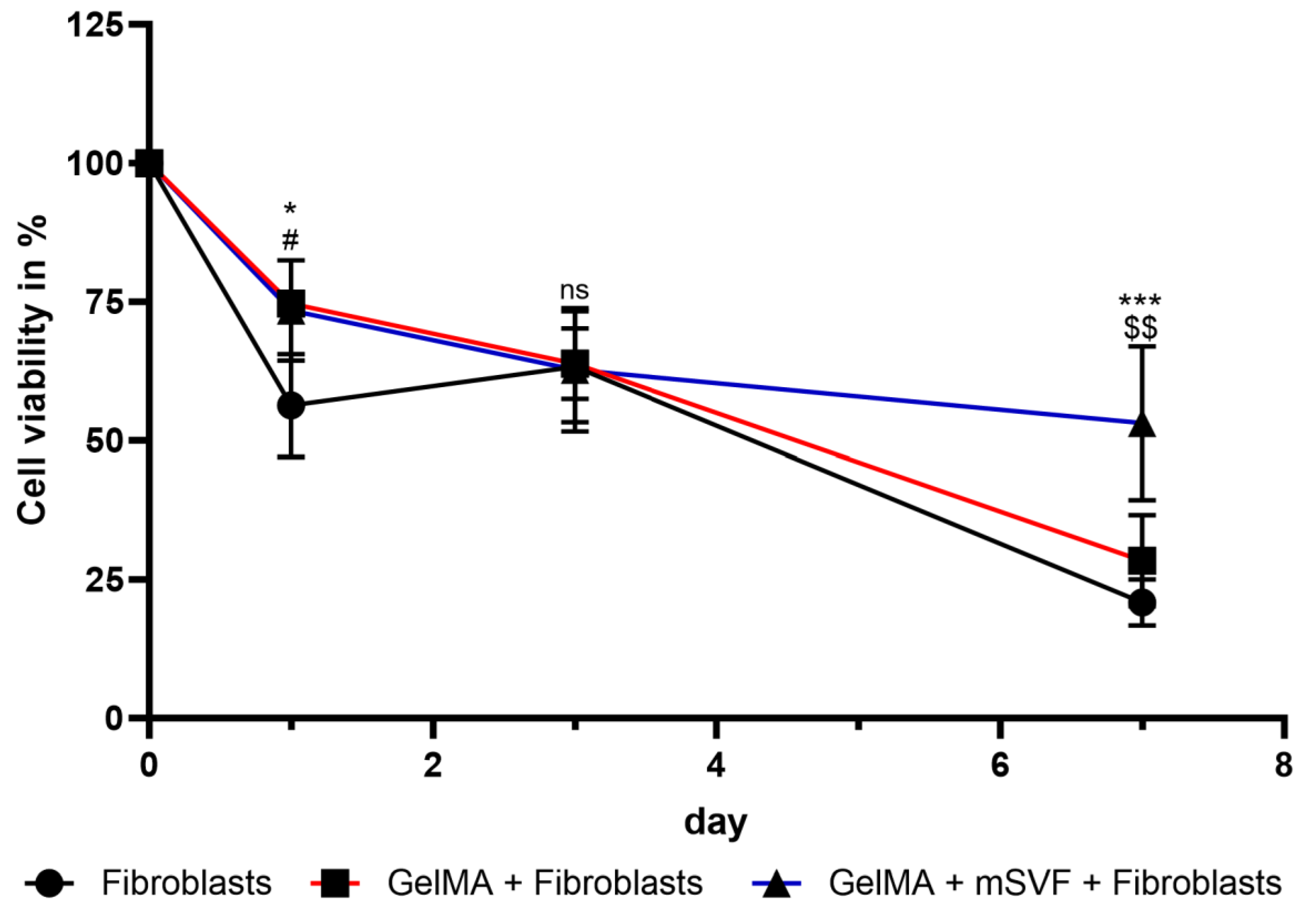
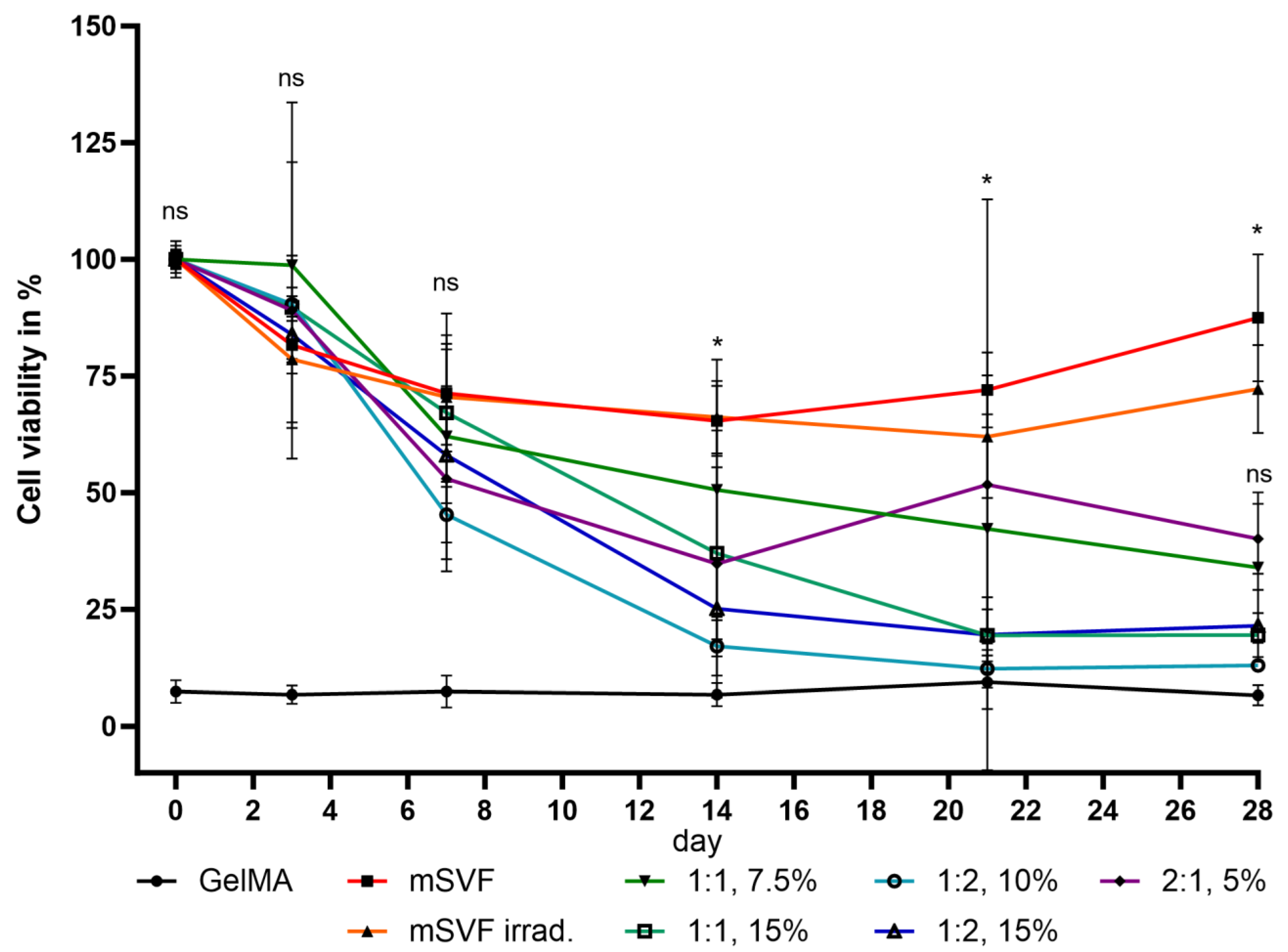
2.2. Viability Assay
2.3. ELISA
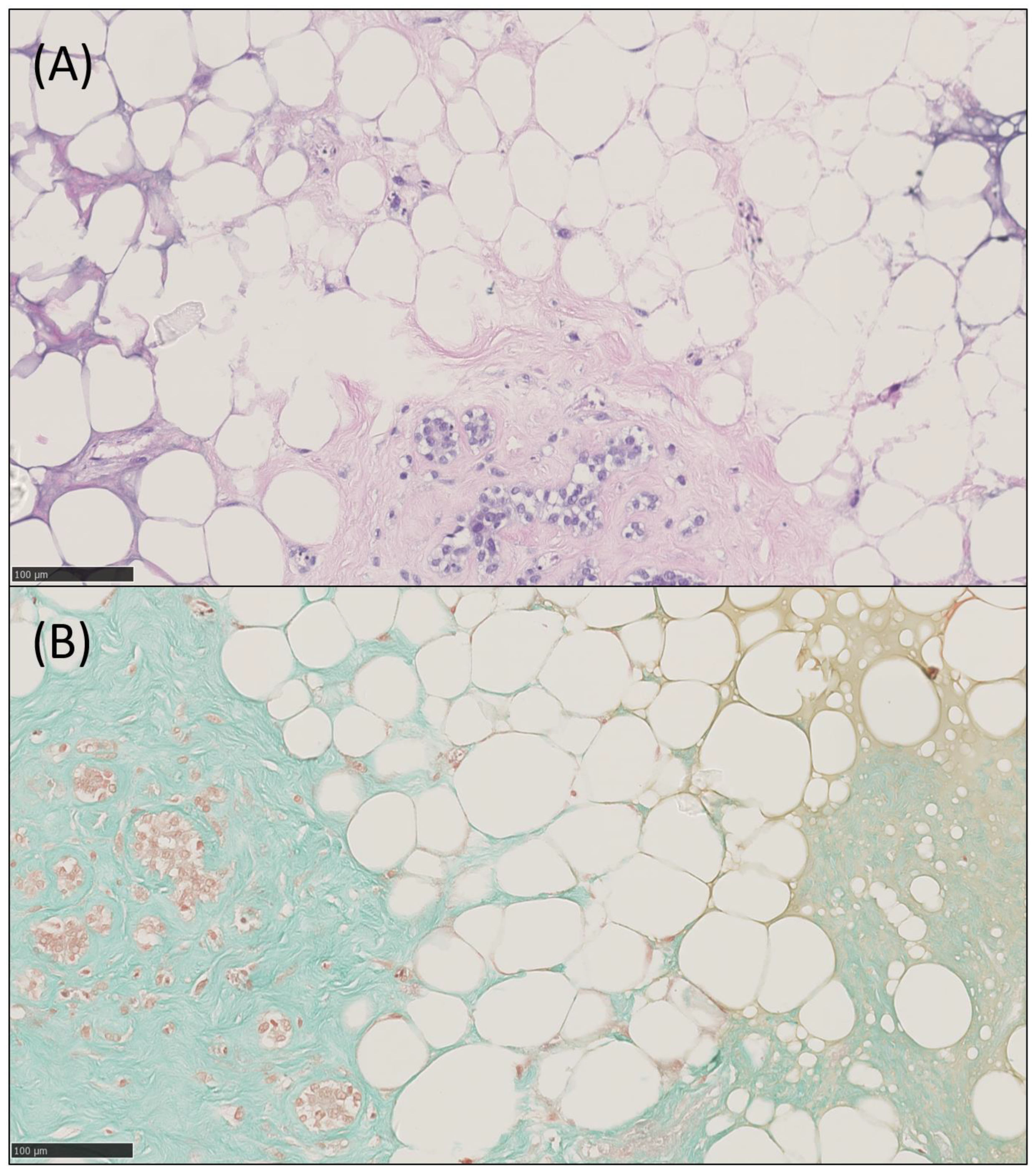
2.4. Histology, IHC and IF
2.5. Co-Culture Assay
3. Discussion
4. Materials and Methods
4.1. Tissue Collection
4.2. Sample Collection
4.3. Protocol for mSVF Isolation
4.4. Synthesis of GelMA and LAP
4.5. Spectroscopic Analysis of GelMA
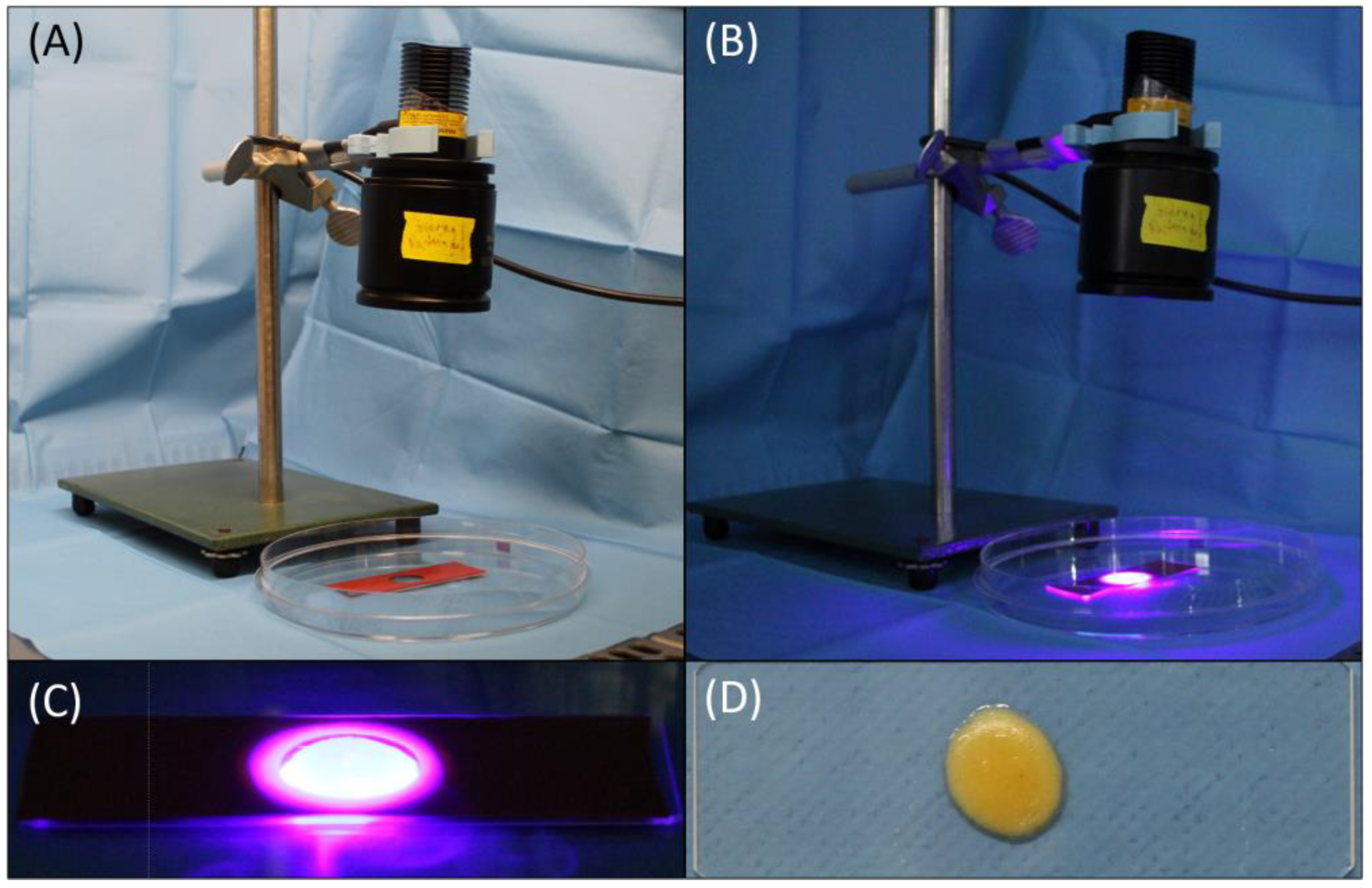
4.6. Protocol Establishment and Preliminary Experiments of GelMA-mSVF Mixture and Irradiation Time
4.7. GelMA-mSVF Mixture Protocol and UV Exposure Time
4.8. Viability Assay
4.9. ELISA
4.10. Histology, Immunohistochemistry (IHC) and Immunofluorescence (IF)
4.11. Dermal Fibroblast Isolation and Co-Culture Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, B., II; Janis, J.E.; Attinger, C.E. Wound Healing: An Overview. Plast. Reconstr. Surg. 2006, 117, 1e-S–32e-S. [Google Scholar] [CrossRef]
- Janis, J.E.; Harrison, B. Wound Healing: Part I. Basic Science. Plast. Reconstr. Surg. 2016, 138, 9S–17S. [Google Scholar] [CrossRef]
- Kim, B.-S.; Pallua, N.; Bernhagen, J.; Bucala, R. The macrophage migration inhibitory factor protein superfamily in obesity and wound repair. Exp. Mol. Med. 2015, 47, e161. [Google Scholar] [CrossRef] [PubMed]
- Ennis, W.J.; Lee, C.; Gellada, K.; Corbiere, T.F.; Koh, T.J. Advanced Technologies to Improve Wound Healing: Electrical Stimulation, Vibration Therapy, and Ultrasound-What Is the Evidence? Plast. Reconstr. Surg. 2016, 138, 94S–104S. [Google Scholar] [CrossRef] [PubMed]
- Deptuła, M.; Brzezicka, A.; Skoniecka, A.; Zieliński, J.; Pikuła, M. Adipose-derived stromal cells for nonhealing wounds: Emerging opportunities and challenges. Med. Res. Rev. 2021, 41, 2130–2171. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Li, H.; Zhang, C.; Mao, Y.; Nie, F.; Xing, Y.; Sha, W.; Wang, X.; Irwin, D.M.; Tan, H. Stromal vascular fraction promotes migration of fibroblasts and angiogenesis through regulation of extracellular matrix in the skin wound healing process. Stem Cell Res. Ther. 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Sterodimas, A.; Calabrese, C.; Garcovich, S. Systematic review: Advances of fat tissue engineering as bioactive scaffold, bioactive material, and source for adipose-derived mesenchymal stem cells in wound and scar treatment. Stem Cell Res. Ther. 2021, 12, 318. [Google Scholar] [CrossRef]
- Moon, K.-C.; Suh, H.-S.; Kim, K.-B.; Han, S.-K.; Young, K.-W.; Lee, J.-W.; Kim, M.-H. Potential of Allogeneic Adipose-Derived Stem Cell–Hydrogel Complex for Treating Diabetic Foot Ulcers. Diabetes 2019, 68, 837–846. [Google Scholar] [CrossRef]
- Pallua, N.M.; Grasys, J.; Kim, B.-S.M. Enhancement of Progenitor Cells by Two-Step Centrifugation of Emulsified Lipoaspirates. Plast. Reconstr. Surg. 2018, 142, 99–109. [Google Scholar] [CrossRef]
- Huang, Q.; Zou, Y.; Arno, M.C.; Chen, S.; Wang, T.; Gao, J.; Dove, A.P.; Du, J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017, 46, 6255–6275. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Alge, D.L.; Anseth, K.S. Bioactive hydrogels: Lighting the way. Nat. Mater. 2013, 12, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Abdollahiyan, P.; Oroojalian, F.; Mokhtarzadeh, A. The triad of nanotechnology, cell signalling, and scaffold implantation for the successful repair of damaged organs: An overview on soft-tissue engineering. J. Control. Release 2021, 332, 460–492. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chan-Park, M.B. A biomimetic hydrogel based on methacrylated dextran-graft-lysine and gelatin for 3D smooth muscle cell culture. Biomaterials 2010, 31, 1158–1170. [Google Scholar] [CrossRef] [PubMed]
- Kurian, A.G.; Singh, R.K.; Patel, K.D.; Lee, J.-H.; Kim, H.-W. Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics. Bioact. Mater. 2022, 8, 267–295. [Google Scholar] [CrossRef]
- Tiryaki, K.T.; Cohen, S.; Kocak, P.; Turkay, S.C.; Hewett, S. In-Vitro Comparative Examination of the Effect of Stromal Vascular Fraction Isolated by Mechanical and Enzymatic Methods on Wound Healing. Aesthetic Surg. J. 2020, 40, 1232–1240. [Google Scholar] [CrossRef]
- Tiryaki, T.; Condé-Green, A.; Cohen, S.R.; Canikyan, S.B.; Kocak, P. A 3-step Mechanical Digestion Method to Harvest Adipose-derived Stromal Vascular Fraction. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2652. [Google Scholar] [CrossRef]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Li, P.; Dou, X.; Feng, C.; Schönherr, H. Enhanced cell adhesion on a bio-inspired hierarchically structured polyester modified with gelatin-methacrylate. Biomater. Sci. 2018, 6, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Asim, S.; Tabish, T.A.; Liaqat, U.; Ozbolat, I.T.; Rizwan, M. Advances in Gelatin Bioinks to Optimize Bioprinted Cell Functions. Adv. Healthc. Mater. 2023, 12, e2203148. [Google Scholar] [CrossRef] [PubMed]
- Guzzi, E.A.; Bischof, R.; Dranseikiene, D.; Deshmukh, D.V.; Wahlsten, A.; Bovone, G.; Bernhard, S.; Tibbitt, M.W. Hierarchical biomaterials via photopatterning-enhanced direct ink writing. Biofabrication 2021, 13, 044105. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-Hydrogel: A Hybrid Biomaterial System for Localized Drug Delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Fukuda, K.; Sato, Y.; Hattori, H. Biomaterials as cell carriers for augmentation of adipose tissue-derived stromal cell transplantation. Bio-Med. Mater. Eng. 2018, 29, 567–585. [Google Scholar] [CrossRef] [PubMed]
- Kessler, L.; Gehrke, S.; Winnefeld, M.; Huber, B.; Hoch, E.; Walter, T.; Wyrwa, R.; Schnabelrauch, M.; Schmidt, M.; Kückelhaus, M.; et al. Methacrylated gelatin/hyaluronan-based hydrogels for soft tissue engineering. J. Tissue Eng. 2017, 8. [Google Scholar] [CrossRef]
- Kim, B.-S.; Chen, S.-H.; Vasella, M.; Guidi, M.; Gousopoulos, E.; Lindenblatt, N.; Kao, H.-K. In Vivo Evaluation of Mechanically Processed Stromal Vascular Fraction in a Chamber Vascularized by an Arteriovenous Shunt. Pharmaceutics 2022, 14, 417. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, B.T.; Al-Ghadban, S.; Ives, C.J.; L’Ecuyer, M.P.; Monjure, T.A.; Romero-Lopez, M.; Li, Z.; Goodman, S.B.; Lin, H.; Tuan, R.S.; et al. Adipose Tissue-Derived Stem Cells Retain Their Adipocyte Differentiation Potential in Three-Dimensional Hydrogels and Bioreactors. Biomolecules 2020, 10, 1070. [Google Scholar] [CrossRef]
- Li, S.; Sun, J.; Yang, J.; Yang, Y.; Ding, H.; Yu, B.; Ma, K.; Chen, M. Gelatin methacryloyl (GelMA) loaded with concentrated hypoxic pretreated adipose-derived mesenchymal stem cells(ADSCs) conditioned medium promotes wound healing and vascular regeneration in aged skin. Biomater. Res. 2023, 27, 11. [Google Scholar] [CrossRef]
- Yoshinoya, Y.; Böcker, A.H.; Ruhl, T.; Siekmann, U.; Pallua, N.; Beier, J.P.; Kim, B.-S. The Effect of Hyperbaric Oxygen Therapy on Human Adipose-Derived Stem Cells. Plast. Reconstr. Surg. 2020, 146, 309–320. [Google Scholar] [CrossRef]
- Colle, J.; Blondeel, P.; De Bruyne, A.; Bochar, S.; Tytgat, L.; Vercruysse, C.; Van Vlierberghe, S.; Dubruel, P.; Declercq, H. Bioprinting predifferentiated adipose-derived mesenchymal stem cell spheroids with methacrylated gelatin ink for adipose tissue engineering. J. Mater. Sci. Mater. Med. 2020, 31, 36. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.A.; Marre, D.; Grinsell, D.; Batty, A.; Trost, N.; O’Connor, A.J. Creation of a Large Adipose Tissue Construct in Humans Using a Tissue-engineering Chamber: A Step Forward in the Clinical Application of Soft Tissue Engineering. EBioMedicine 2016, 6, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, D.; Tan, F.; Yin, W.; Li, Z. Umbilical cord derived mesenchymal stem cell-GelMA microspheres for accelerated wound healing. Biomed. Mater. 2023, 18, 015019. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.; Borchers, K.; Tovar, G.E.; Kluger, P.J. Methacrylated gelatin and mature adipocytes are promising components for adipose tissue engineering. J. Biomater. Appl. 2016, 30, 699–710. [Google Scholar] [CrossRef]
- Wittmann, K.; Dietl, S.; Ludwig, N.; Berberich, O.; Hoefner, C.; Storck, K.; Blunk, T.; Bauer-Kreisel, P. Engineering Vascularized Adipose Tissue Using the Stromal-Vascular Fraction and Fibrin Hydrogels. Tissue Eng. Part A 2015, 21, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Liu, K.; Jiang, T.; Li, S.; Chen, J.; Wu, Z.; Li, W.; Tan, R.; Wei, W.; Yang, X.; et al. GelMA/PEGDA microneedles patch loaded with HUVECs-derived exosomes and Tazarotene promote diabetic wound healing. J. Nanobiotechnol. 2022, 20, 147. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.-R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast Growth Factors: Biology, Function, and Application for Tissue Regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef]
- Rehman, S.R.U.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Tariq, M.; Hasan, A. Reduced Graphene Oxide Incorporated GelMA Hydrogel Promotes Angiogenesis for Wound Healing Applications. Int. J. Nanomed. 2019, 14, 9603–9617. [Google Scholar] [CrossRef]
- Deshmukh, D.V.; Reichert, P.; Zvick, J.; Labouesse, C.; Künzli, V.; Dudaryeva, O.; Bar-Nur, O.; Tibbitt, M.W.; Dual, J. Continuous Production of Acoustically Patterned Cells within Hydrogel Fibers for Musculoskeletal Tissue Engineering. Adv. Funct. Mater. 2022, 32, 2113038. [Google Scholar] [CrossRef]
- Xia, W.; Lai, G.; Li, Y.; Zeng, C.; Sun, C.; Zhang, P.; Zhu, G.; Li, L.; Wu, L. Photo-crosslinked adhesive hydrogel loaded with extracellular vesicles promoting hemostasis and liver regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1170212. [Google Scholar] [CrossRef]
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat Grafting. Plast. Reconstr. Surg. 2013, 132, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Ott, V.; Boecker, A.H.; Stromps, J.-P.; Paul, N.E.; Alharbi, Z.M.; Cakmak, E.; Bernhagen, J.; Bucala, R.M.; Pallua, N.M. The Effect of Antiseptics on Adipose-Derived Stem Cells. Plast. Reconstr. Surg. 2017, 139, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.M.; Suschek, C.V.; Lin, Q.; Bork, S.; Goergens, M.; Joussen, S.; Pallua, N.; Ho, A.D.; Zenke, M.; Wagner, W. Specific Age-Associated DNA Methylation Changes in Human Dermal Fibroblasts. PLoS ONE 2011, 6, e16679. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Maffulli, N.; Burgos-Alonso, N. Stromal vascular fraction technologies and clinical applications. Expert Opin. Biol. Ther. 2019, 19, 1289–1305. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Sun, H.M.; Hwang, K.-C.; Kim, S.-W. Adipose-Derived Stromal Vascular Fraction Cells: Update on Clinical Utility and Efficacy. Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Girard, P.; Dulong, J.; Duisit, J.; Mocquard, C.; Le Gallou, S.; Chaput, B.; Lupon, E.; Watier, E.; Varin, A.; Tarte, K.; et al. Modified nanofat grafting: Stromal vascular fraction simple and efficient mechanical isolation technique and perspectives in clinical recellularization applications. Front. Bioeng. Biotechnol. 2022, 10, 895735. [Google Scholar] [CrossRef] [PubMed]
- Tiryaki, T.; Cohen, S.R.; Turkay, S.C.; Kocak, P.; Sterodimas, A.M.; Schlaudraff, K.-U.; Demir, I.A.; Agovino, A.; Kul, Y.B. Hybrid Stromal Vascular Fraction (Hybrid-SVF): A New Paradigm in Mechanical Regenerative Cell Processing. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4702. [Google Scholar] [CrossRef] [PubMed]
- Vargel, I.; Tuncel, A.; Baysal, N.; Hartuç-Çevik, I.; Korkusuz, F. Autologous Adipose-Derived Tissue Stromal Vascular Fraction (AD-tSVF) for Knee Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 13517. [Google Scholar] [CrossRef]
- Ramakrishnan, V.M.; Boyd, N.L. The Adipose Stromal Vascular Fraction as a Complex Cellular Source for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2018, 24, 289–299. [Google Scholar] [CrossRef]





| Patient # | Age | Gender | BMI |
|---|---|---|---|
| 1 | 40 | Female | 28.0 |
| 2 | 52 | Female | 30.9 |
| 3 | 18 | Female | 25.2 |
| 4 | 27 | Female | 31.2 |
| 5 | 48 | Female | 29.7 |
| 6 | 44 | Female | 21.6 |
| 7 | 34 | Female | 29.2 |
| 8 | 64 | Female | 34.6 |
| 9 | 65 | Male | 22.8 |
| 10 | 40 | Female | 25.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasella, M.; Arnke, K.; Dranseikiene, D.; Guzzi, E.; Melega, F.; Reid, G.; Klein, H.J.; Schweizer, R.; Tibbitt, M.W.; Kim, B.-S. Methacrylated Gelatin as a Scaffold for Mechanically Isolated Stromal Vascular Fraction for Cutaneous Wound Repair. Int. J. Mol. Sci. 2023, 24, 13944. https://doi.org/10.3390/ijms241813944
Vasella M, Arnke K, Dranseikiene D, Guzzi E, Melega F, Reid G, Klein HJ, Schweizer R, Tibbitt MW, Kim B-S. Methacrylated Gelatin as a Scaffold for Mechanically Isolated Stromal Vascular Fraction for Cutaneous Wound Repair. International Journal of Molecular Sciences. 2023; 24(18):13944. https://doi.org/10.3390/ijms241813944
Chicago/Turabian StyleVasella, Mauro, Kevin Arnke, Dalia Dranseikiene, Elia Guzzi, Francesca Melega, Gregory Reid, Holger Jan Klein, Riccardo Schweizer, Mark W. Tibbitt, and Bong-Sung Kim. 2023. "Methacrylated Gelatin as a Scaffold for Mechanically Isolated Stromal Vascular Fraction for Cutaneous Wound Repair" International Journal of Molecular Sciences 24, no. 18: 13944. https://doi.org/10.3390/ijms241813944





