Phylogeny of PmCCD Gene Family and Expression Analysis of Flower Coloration and Stress Response in Prunus mume
Abstract
:1. Introduction
2. Results
2.1. Identification of CCD Gene Family Members in P. mume
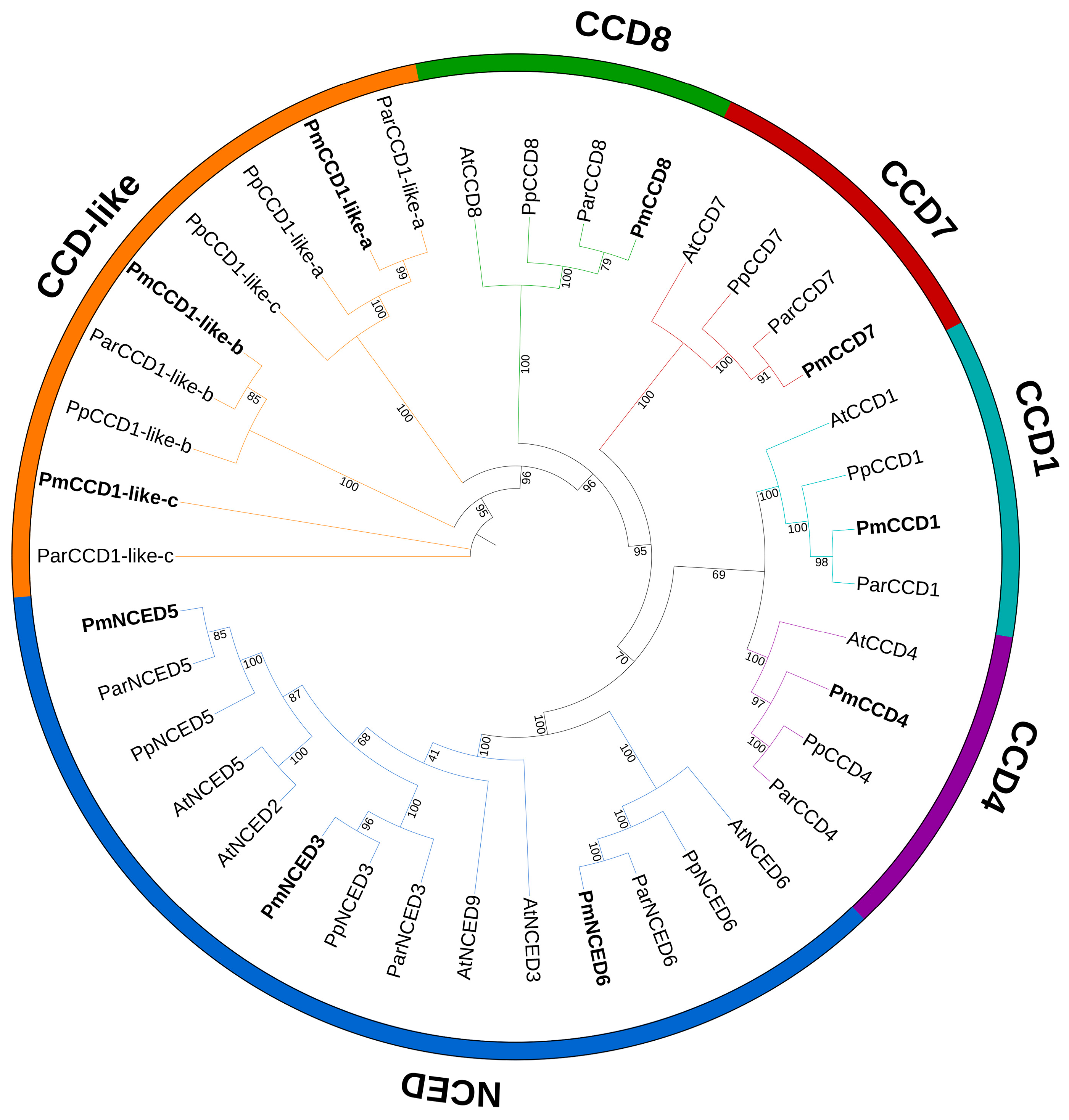
2.2. Phylogenetic Analysis and Protein Sequence Alignment
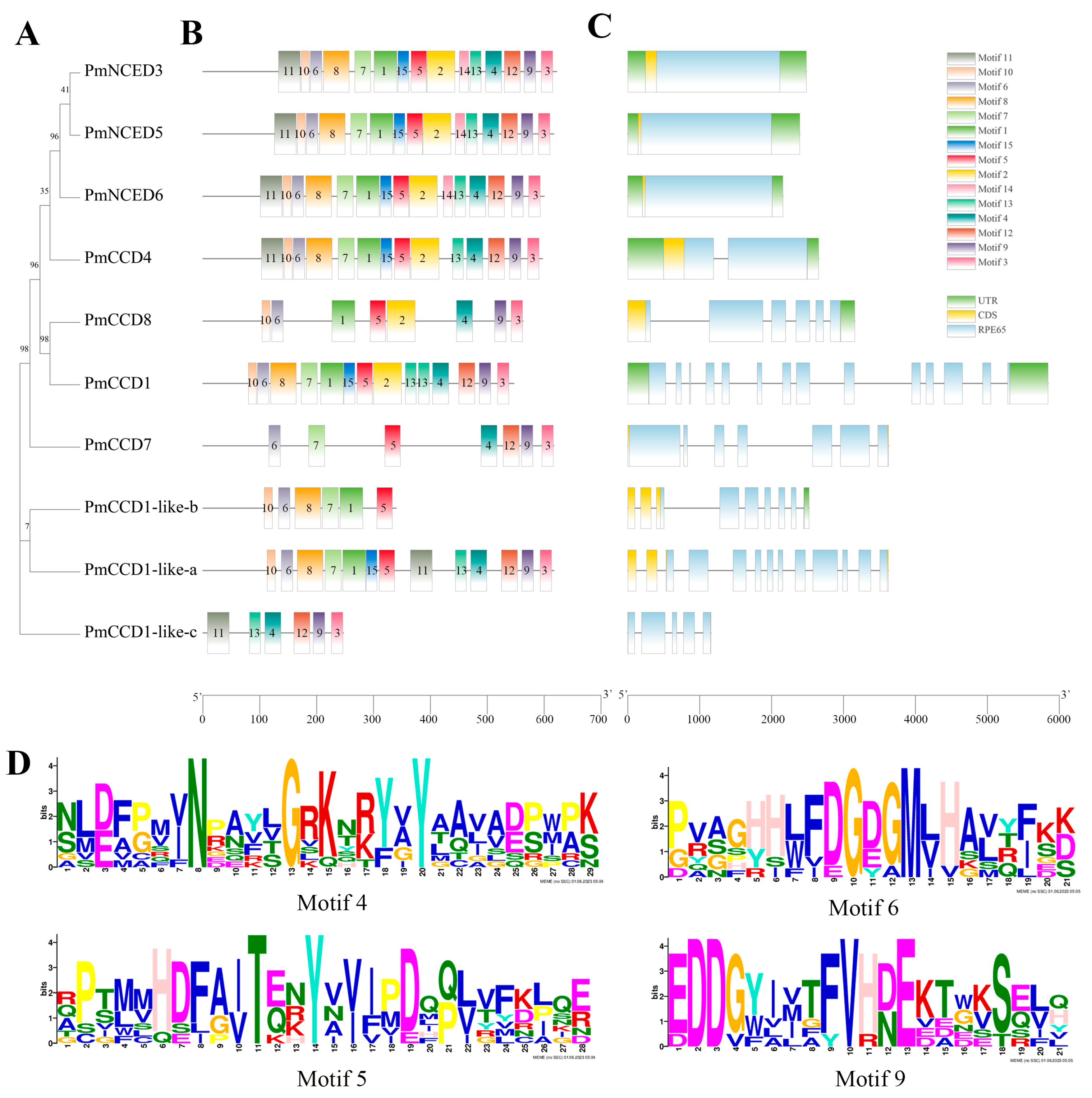
2.3. Gene Structure and Conserved Motif
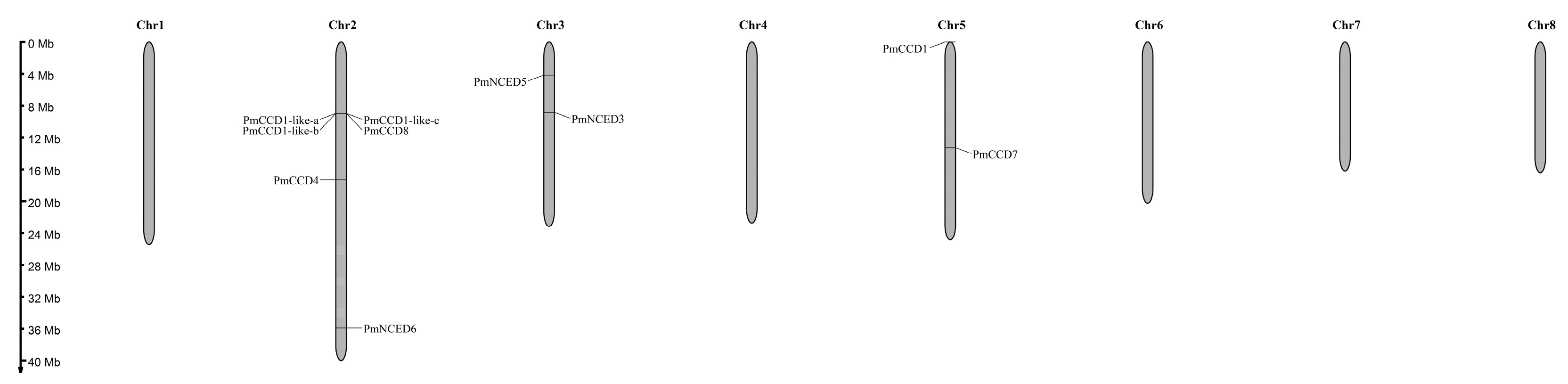
2.4. Chromosomal Distribution and Collinearity Analysis
2.5. Promoter Cis-Acting Element Analysis
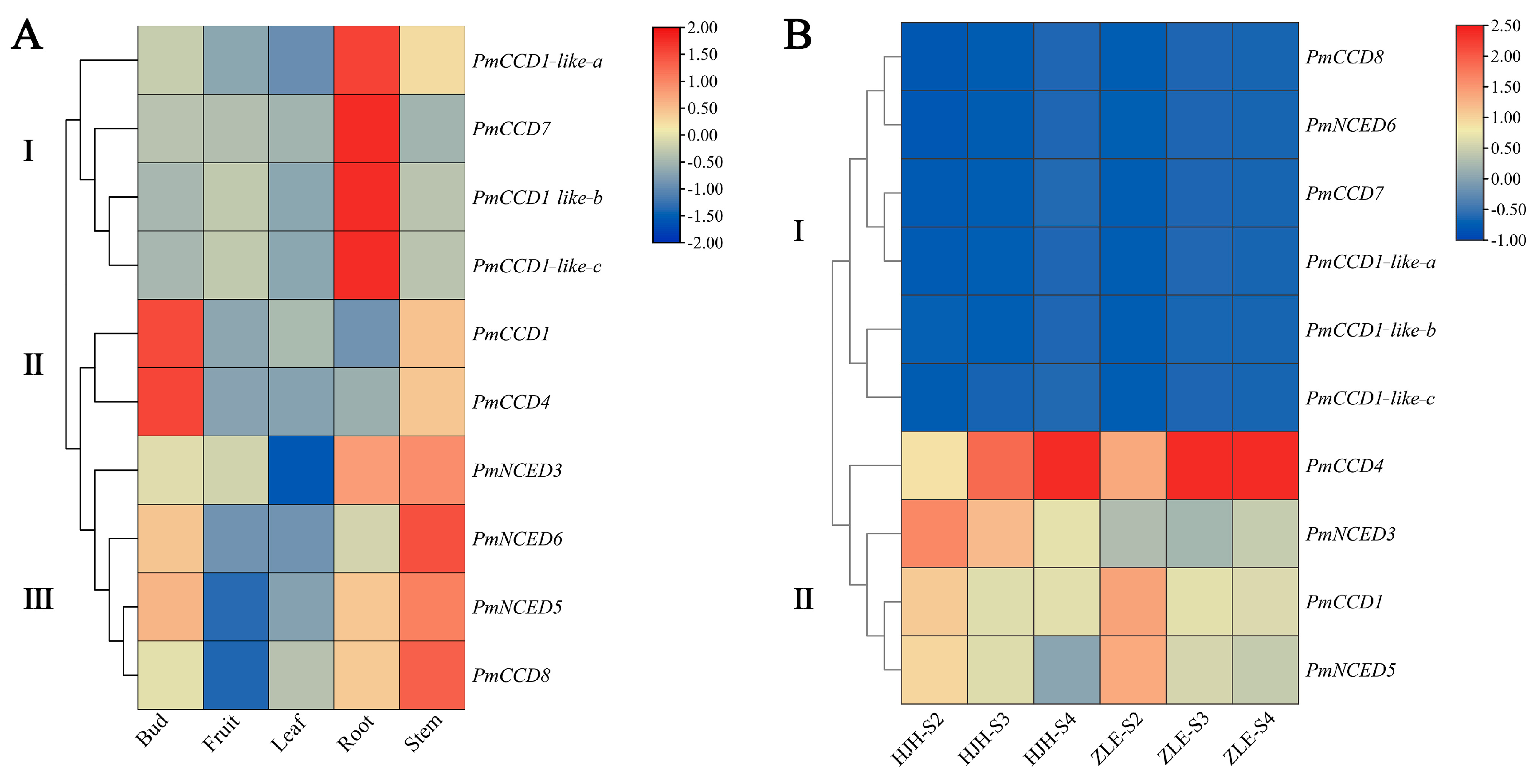
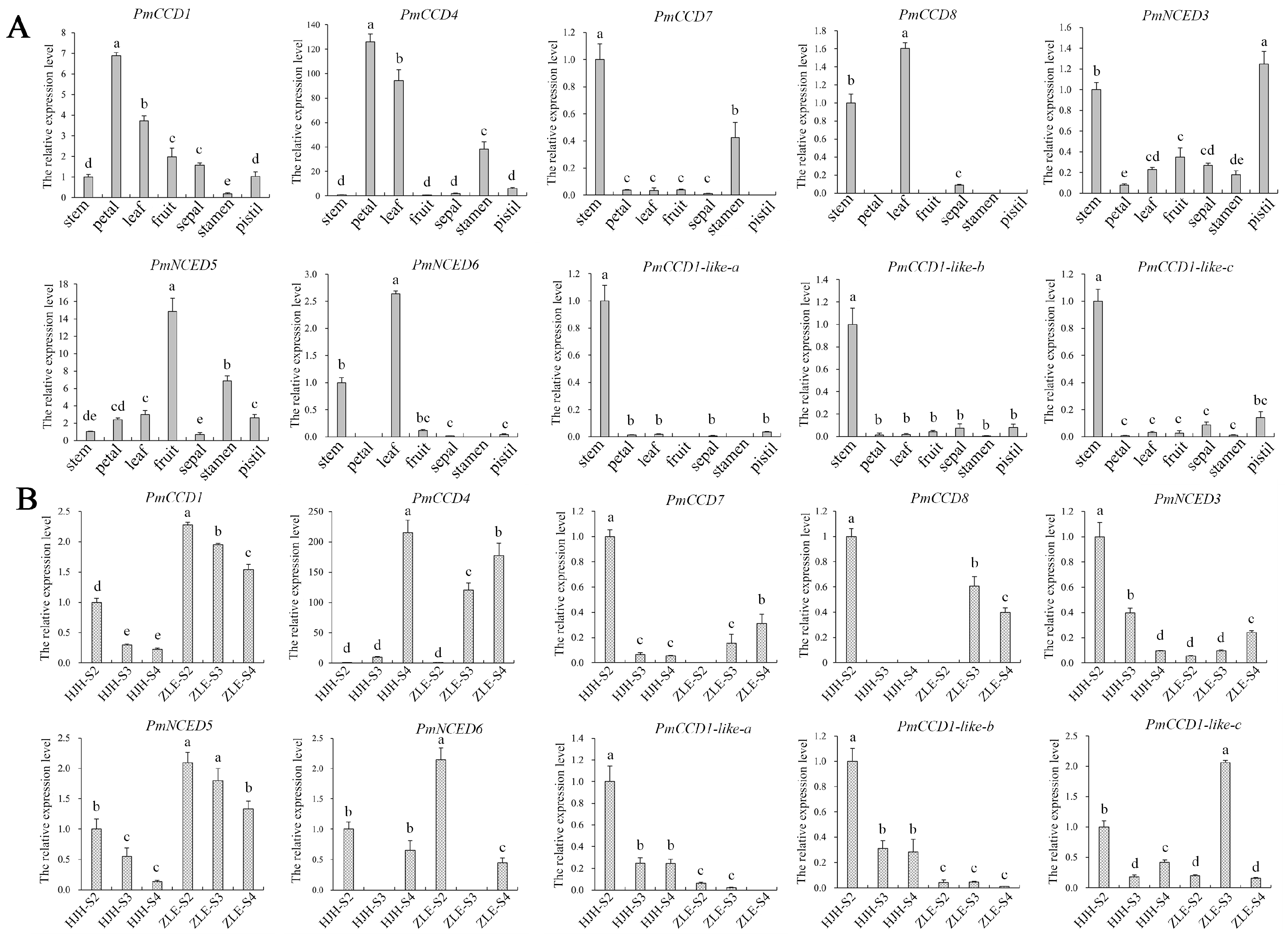
2.6. Expression Patterns of PmCCD Genes in Various Tissues and Different Varieties
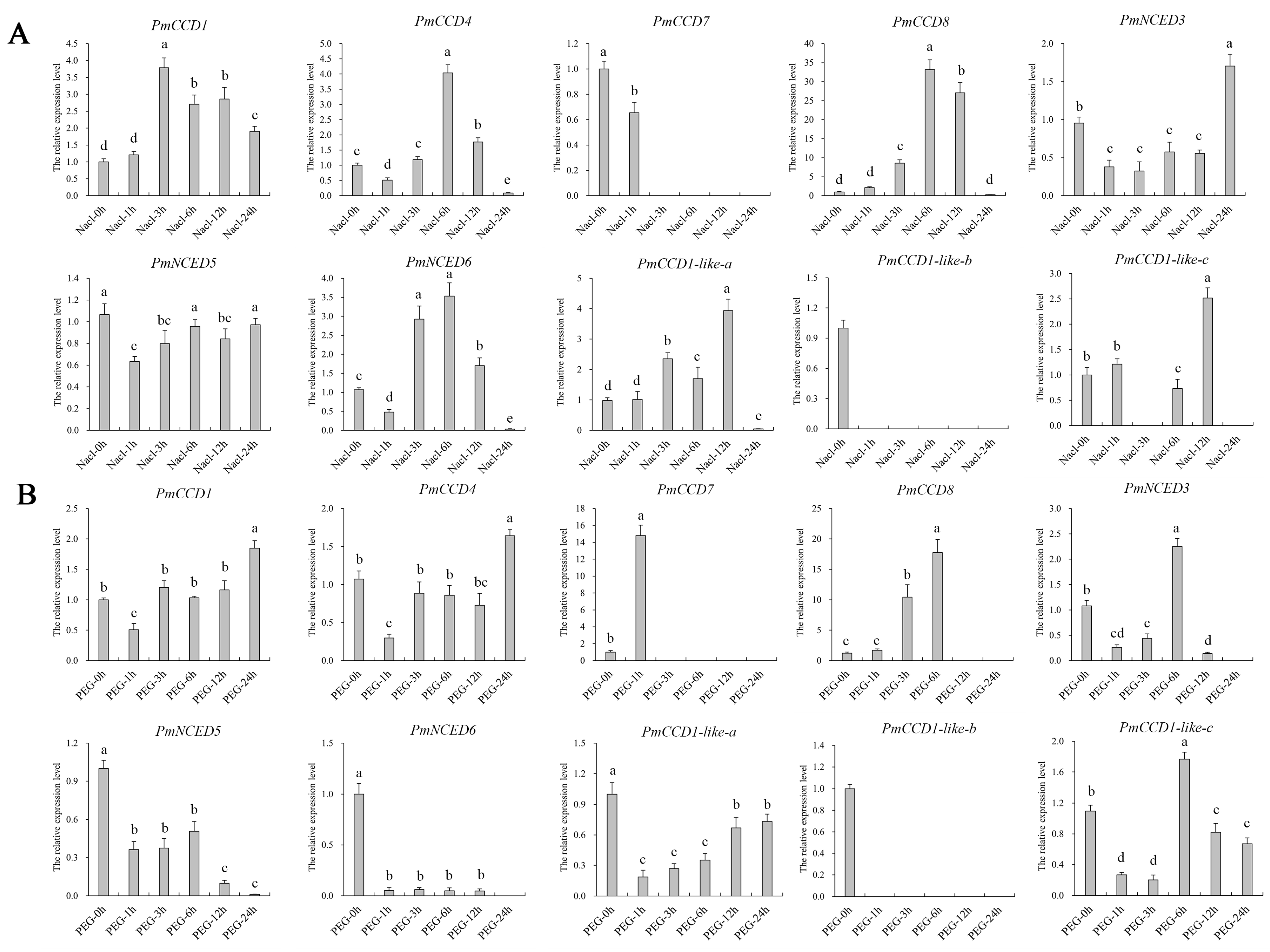
2.7. Expression Analysis of PmCCD Genes in Abiotic Stress Treatments
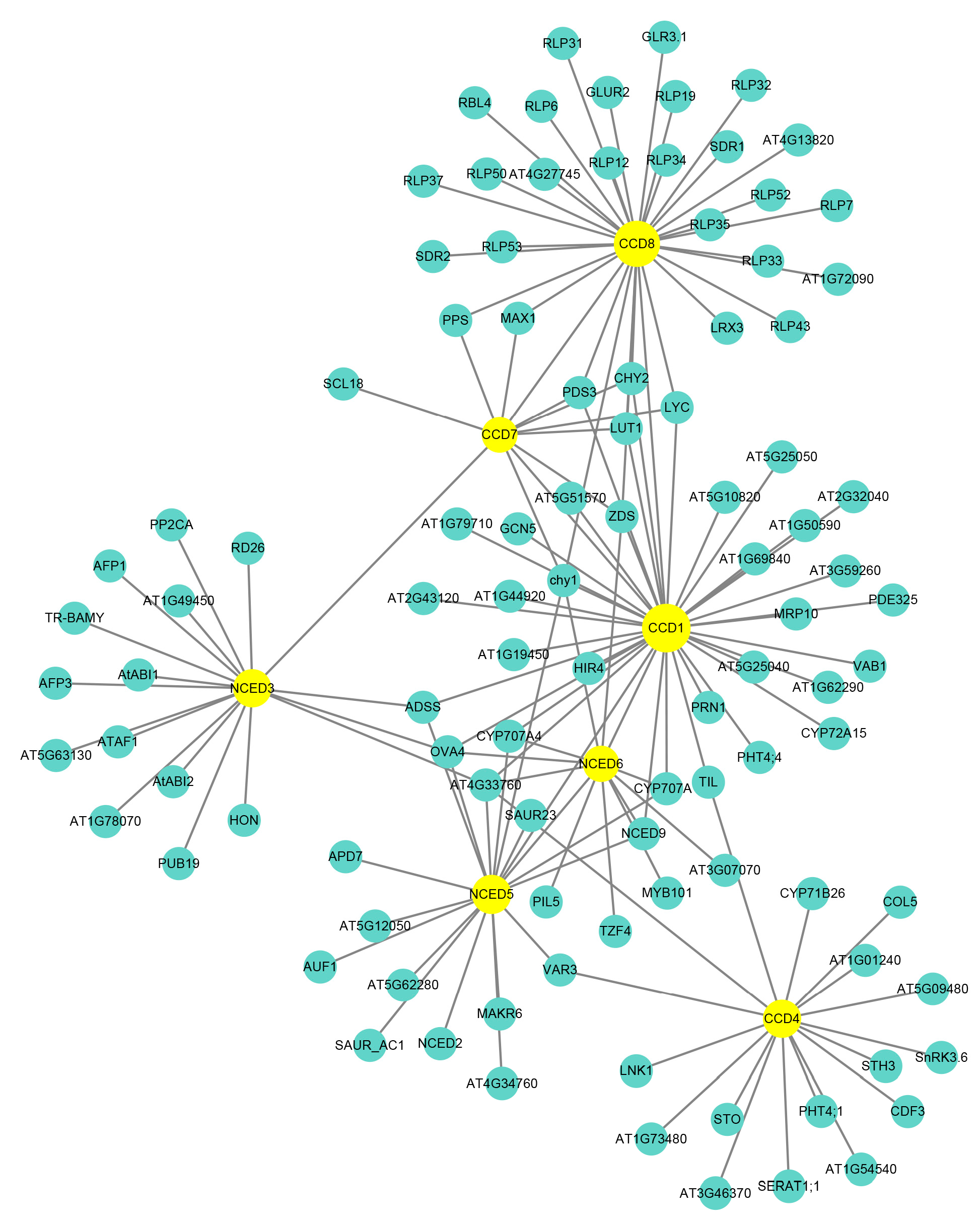
2.8. Protein Interaction Network Analysis
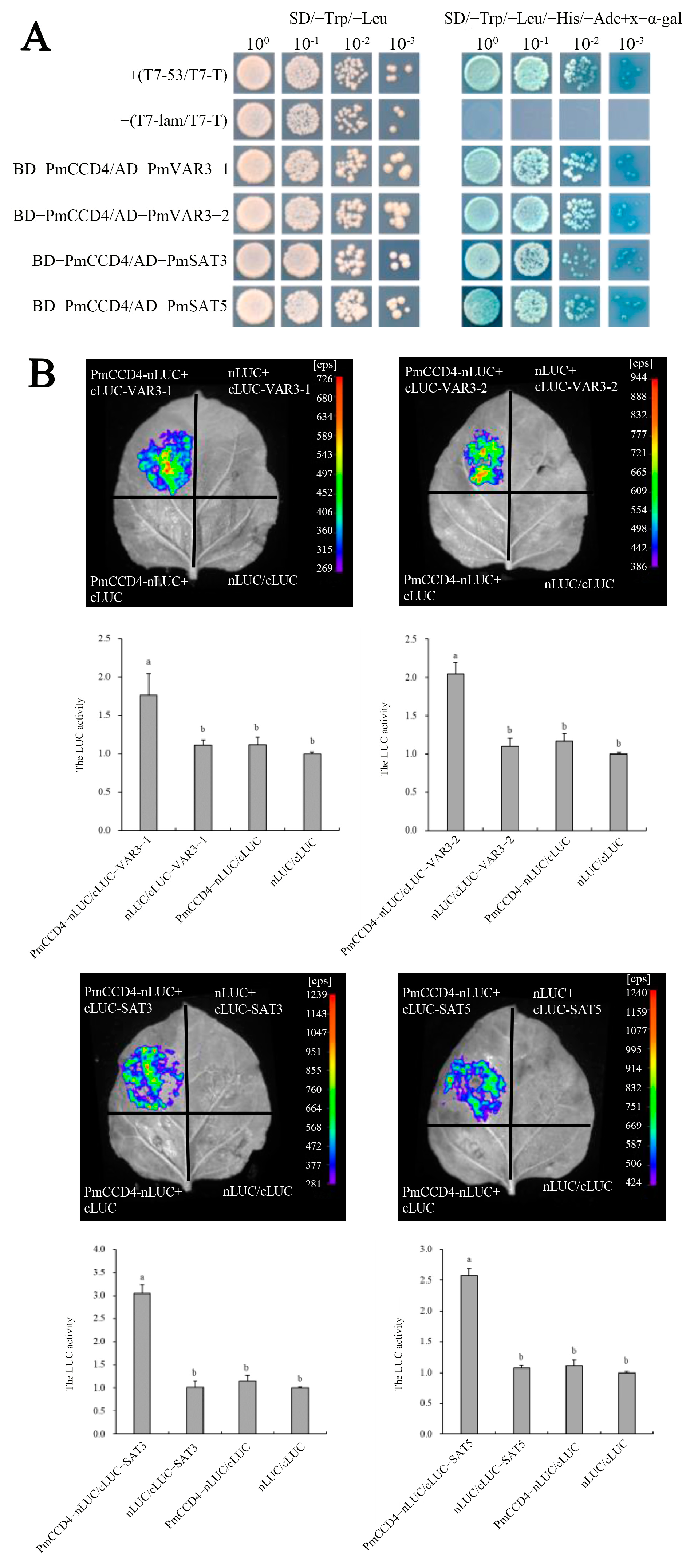
2.9. Interaction of PmCCD4 with Other Proeins
3. Discussion
4. Materials and Methods
4.1. Identification of PmCCDs
4.2. Phylogenetic Tree Analysis and Protein Sequence Alignment of PmCCD Proteins
4.3. Gene Structure and Conservation Motif Analysis of PmCCD Sequences
4.4. Chromosome Localization and Collinearity Analysis of PmCCDs
4.5. Analysis of PmCCDs Expression Pattern
4.6. RNA Extraction and qRT-PCR Analysis
4.7. Analysis of PmCCD Promoter Element and Protein Interaction Network
4.8. Yeast Two-Hybrid System
4.9. Luciferase Complementation Experiment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frank, H.A.; Cogde, I.P.R.J. Carotenoids in photosynthesis. Photochem. Photobiol. 1996, 3, 257–264. [Google Scholar] [CrossRef]
- Qin, X.; Zeevaart, J.A. The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc. Natl. Acad. Sci. USA 1999, 96, 15354–15361. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Baz, L.; Al-Babili, S. From carotenoids to strigolactones. J. Exp. Bot. 2018, 69, 2189–2204. [Google Scholar] [CrossRef] [PubMed]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef]
- Walter, M.H.; Strack, D. ChemInform Abstract: Carotenoids and Their Cleavage Products: Biosynthesis and Functions. ChemInform 2011, 28, 663–692. [Google Scholar] [CrossRef]
- Tan, B.C.; Schwartz, S.H.; Zeevaart, J.A.; McCarty, D.R. Genetic Control of Abscisic Acid Biosynthesis in Maize. Proc. Natl. Acad. Sci. USA 1997, 94, 12235–12240. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Joseph, L.M.; Deng, W.; Liu, L.; Li, Q.; Cline, K.; McCarty, D.R. Molecular characterization of the Arabidopsis 9-cis-epoxycarotenoid dioxygenase gene family. Plant J. 2003, 35, 44–56. [Google Scholar] [CrossRef]
- Vallabhaneni, R.; Bradbury, L.M.T.; Wurtzel, E.T. The carotenoid dioxygenase gene family in maize, sorghum, and rice. Arch. Biochem. Biophys. 2010, 504, 104–111. [Google Scholar] [CrossRef]
- Wei, Y.; Wan, H.; Wu, Z.; Wang, R.; Ruan, M.; Ye, Q.; Li, Z.; Zhou, G.; Yao, Z.; Yang, Y. A Comprehensive Analysis of Carotenoid Cleavage Dioxygenases Genes in Solanum L. Plant Mol. Biol. Rep. 2016, 34, 512–523. [Google Scholar] [CrossRef]
- Chen, H.; Zuo, X.; Shao, H.; Fan, S.; Ma, J.; Zhang, D.; Zhao, C.; Yan, X.; Liu, X.; Han, M. Genome-wide analysis of carotenoid cleavage oxygenase genes and their responses to various phytohormones and abiotic stresses in apple (Malus domestica). Plant Physiol. Biochem. 2018, 123, 81–93. [Google Scholar] [CrossRef]
- Schmidt, H.; Kurtzer, R.; Eisenreich, W.; Schwab, W. The Carotenase AtCCD1 from Arabidopsis thaliana Is a Dioxygenase. J. Biol. Chem. 2006, 281, 9845–9851. [Google Scholar] [CrossRef]
- Ilg, A.; Bruno, M.; Beyer, P.; Al-Babili, S. Tomato carotenoid cleavage dioxygenases 1A and 1B: Relaxed double bond specificity leads to a plenitude of dialdehydes, mono-apocarotenoids and isoprenoid volatiles. FEBS Open Bio 2014, 4, 584–593. [Google Scholar] [CrossRef]
- Ibdah, M.; Azulay, Y.; Portnoy, V.; Wasserman, B.; Bar, E.; Meir, A.; Burger, Y.; Hirschberg, J.; Schaffer, A.A.; Katzir, N. Functional characterization of CmCCD1, a carotenoid cleavage dioxygenase from melon. Phytochemistry 2006, 67, 1579–1589. [Google Scholar] [CrossRef]
- Simkin, A.J.; Underwood, B.A.; Auldridge, M.; Loucas, H.M.; Shibuya, K.; Schmelz, E.; Clark, D.G.; Klee, H.J. Circadian Regulation of the PhCCD1 Carotenoid Cleavage Dioxygenase Controls Emission of β-Ionone, a Fragrance Volatile of Petunia Flowers. Plant Physiol. 2004, 136, 3504–3514. [Google Scholar] [CrossRef] [PubMed]
- Yahyaa, M.; Berim, A.; Isaacson, T.; Marzouk, S.; Bar, E.; Davidovich-Rikanati, R.; Lewinsohn, E.; Ibdah, M. Isolation and Functional Characterization of Carotenoid Cleavage Dioxygenase-1 from Laurus nobilis L. (Bay Laurel) Fruits. J. Agric. Food Chem. 2015, 63, 8275–8282. [Google Scholar] [CrossRef]
- García-Limones, C.; Schnäbele, K.; Blanco-Portales, R.; Luz Bellido, M.; Caballero, J.L.; Schwab, W.; Muñoz-Blanco, J. Functional Characterization of FaCCD1: A Carotenoid Cleavage Dioxygenase from Strawberry Involved in Lutein Degradation during Fruit Ripening. J. Agric. Food Chem. 2008, 56, 9277–9285. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Ji, J.; Wang, G.; Jin, C.; Guan, C.; Wu, G. Molecular cloning and characterization of a novel carotenoid cleavage dioxygenase 1 from Lycium chinense. Biotechnol. Appl. Biochem. 2015, 62, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Ohmiya, A.; Kishimoto, S.; Aida, R.; Yoshioka, S.; Sumitomo, K. Carotenoid Cleavage Dioxygenase (CmCCD4a) Contributes to White Color Formation in Chrysanthemum Petals. Plant Physiol. 2006, 142, 1193–1201. [Google Scholar] [CrossRef]
- Bruno, M.; Beyer, P.; Al-Babili, S. The potato carotenoid cleavage dioxygenase 4 catalyzes a single cleavage of β-ionone ring-containing carotenes and non-epoxidated xanthophylls. Arch. Biochem. Biophys. 2015, 572, 126–133. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, C.; Wang, Y.; Yao, X.; Wang, F.; Wu, J.; King, G.J.; Liu, K. Disruption of a CAROTENOID CLEAVAGE DIOXYGENASE 4 gene converts flower colour from white to yellow in Brassica species. New Phytol. 2015, 206, 1513–1526. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Matsuta, A.; Matsutani, K.; Yamawaki, K.; Yahata, M.; Wahyudi, A.; Motohashi, R.; Kato, M. Enzymatic Formation of β-Citraurin from β-Cryptoxanthin and Zeaxanthin by Carotenoid Cleavage Dioxygenase 4 in the Flavedo of Citrus Fruit. Plant Physiol. 2013, 163, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Hu, L.; Zhu, L.; Chen, Y.; Qiu, C.; Fan, R.; Ma, X.; Cao, Z.; Chen, J.; Shi, J.; et al. Genome-Wide Identification and Expression Analysis of CCO Gene Family in Liriodendron chinense. Plants 2023, 12, 1975. [Google Scholar] [CrossRef]
- Baba, S.A.; Jain, D.; Abbas, N.; Ashraf, N. Overexpression of Crocus carotenoid cleavage dioxygenase, CsCCD4b, in Arabidopsis imparts tolerance to dehydration, salt and oxidative stresses by modulating ROS machinery. J. Plant Physiol. 2015, 189, 114–125. [Google Scholar] [CrossRef]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The Path from β-Carotene to Carlactone, a Strigolactone-Like Plant Hormone. Science 2012, 335, 1348–1351. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Novero, M.; Charnikhova, T.; Ferrandino, A.; Schubert, A.; Ruyter-Spira, C.; Bonfante, P.; Lovisolo, C.; Bouwmeester, H.J.; Cardinale, F. CAROTENOID CLEAVAGE DIOXYGENASE 7 modulates plant growth, reproduction, senescence, and determinate nodulation in the model legume Lotus japonicus. J. Exp. Bot. 2013, 64, 1967–1981. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, D.; Lin, J.; Zhu, T.; Liu, N.; Yang, X.; Ma, J.; Sui, S. Carotenoid Cleavage Dioxygenase Genes of Chimonanthus praecox, CpCCD7 and CpCCD8, Regulate Shoot Branching in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 8750. [Google Scholar] [CrossRef]
- Pan, X.; Zheng, H.; Zhao, J.; Xu, Y.; Li, X. ZmCCD7/ZpCCD7 encodes a carotenoid cleavage dioxygenase mediating shoot branching. Planta 2016, 243, 1407–1418. [Google Scholar] [CrossRef]
- Wei, H.; Movahedi, A.; Liu, G.; Li, Y.; Liu, S.; Yu, C.; Chen, Y.; Zhong, F.; Zhang, J. Comprehensive Analysis of Carotenoid Cleavage Dioxygenases Gene Family and Its Expression in Response to Abiotic Stress in Poplar. Int. J. Mol. Sci. 2022, 23, 1418. [Google Scholar] [CrossRef]
- Qin, X.; Zeevaart, J.A.D. Overexpression of a 9-cis-Epoxycarotenoid Dioxygenase Gene in Nicotiana plumbaginifolia Increases Abscisic Acid and Phaseic Acid Levels and Enhances Drought Tolerance. Plant Physiol. 2002, 128, 544–551. [Google Scholar] [CrossRef]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001, 27, 325–333. [Google Scholar] [CrossRef]
- Fujita, Y.; Fujita, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 2011, 124, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, Y.; Liu, Y.; Zhang, F.; Wang, Z.; Wang, H.; Wang, F.; Li, D.; Mao, D.; Luan, S.; et al. 9-cis-Epoxycarotenoid Dioxygenase 3 Regulates Plant Growth and Enhances Multi-Abiotic Stress Tolerance in Rice. Front. Plant Sci. 2018, 9, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Jiang, Z.; Tang, L.; Zhang, S.; Li, X.; Wang, H.; Liu, C.; Chi, J.; Zhang, X.; Zhang, J. Genome-wide characterization of carotenoid oxygenase gene family in three cotton species and functional identification of GaNCED3 in drought and salt stress. J. Appl. Genet. 2021, 62, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Changan, S.S.; Ali, K.; Kumar, V.; Garg, N.K.; Tyagi, A. Abscisic acid biosynthesis under water stress: Anomalous behavior of the 9-cis-epoxycarotenoid dioxygenase1 (NCED1) gene in rice. Biol. Plant. 2018, 62, 663–670. [Google Scholar] [CrossRef]
- Hu, F.; Huang, L.; Bao, Y.; Qin, S.; Min, N.; Lyu, J.; Zhang, S.; Huang, G.; Zhang, J.; Wang, W.; et al. An ABA Synthesis Enzyme Allele OsNCED2 Promotes the Aerobic Adaption in Upland Rice; Cold Spring Harbor Laboratory Press: New York, NY, USA; Cold Spring Harbor: Cold Spring Harbor, NY, USA, 2020. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, Y.; Xie, N.; Guo, Y.; Zhang, F.; Xiang, Z.; Wang, R.; Wang, F.; Gao, Q.; Tian, L.; et al. OsNCED5, a 9-cis-epoxycarotenoid dioxygenase gene, regulates salt and water stress tolerance and leaf senescence in rice. Plant Sci. 2019, 287, 110188. [Google Scholar] [CrossRef] [PubMed]
- Xian, L.; Sun, P.; Hu, S.; Wu, J.; Liu, J. Molecular cloning and characterization of CrNCED1, a gene encoding 9-cis-epoxycarotenoid dioxygenase in Citrus reshni, with functions in tolerance to multiple abiotic stresses. Planta 2014, 239, 61–77. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Z. Cloning of a 9-cis-epoxycarotenoid dioxygenase gene (SgNCED1) from Stylosanthes guianensis and its expression in response to abiotic stresses. Plant Cell Rep. 2007, 26, 1383–1390. [Google Scholar] [CrossRef]
- Ahrazem, O.; Rubio-Moraga, A.; Trapero, A.; Gomez-Gomez, L. Developmental and stress regulation of gene expression for a 9-cis-epoxycarotenoid dioxygenase, CstNCED, isolated from Crocus sativus stigmas. J. Exp. Bot. 2012, 63, 681–694. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, Y.; Yang, C.; Li, J.; Rui, X.; Ding, F.; Hu, F.; Wang, X.; Ma, W.; Zhou, K. Genome-wide identification and expression analysis of carotenoid cleavage oxygenase genes in Litchi (Litchi chinensis Sonn.). BMC Plant Biol. 2022, 22, 394–412. [Google Scholar] [CrossRef]
- Cheng, D.; Wang, Z.; Li, S.; Zhao, J.; Wei, C.; Zhang, Y. Genome-Wide Identification of CCD Gene Family in Six Cucurbitaceae Species and Its Expression Profiles in Melon. Genes 2022, 13, 262. [Google Scholar] [CrossRef]
- Phadungsawat, B.; Watanabe, K.; Mizuno, S.; Kanekatsu, M.; Suzuki, S. Expression of CCD4 gene involved in carotenoid degradation in yellow-flowered Petunia × hybrida. Sci. Hortic. 2020, 261, 108916. [Google Scholar] [CrossRef]
- Auldridge, M.E.; Block, A.; Vogel, J.T.; Dabney-Smith, C.; Mila, I.; Bouzayen, M.; Magallanes-Lundback, M.; DellaPenna, D.; McCarty, D.R.; Klee, H.J. Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J. 2006, 45, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, Q.; Li, P.; Zhang, S.; Liu, C.; Jin, J.; Cao, P.; Yang, Y. Carotenoid Cleavage Dioxygenases: Identification, Expression, and Evolutionary Analysis of This Gene Family in Tobacco. Int. J. Mol. Sci. 2019, 20, 5796. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jia, L.; Duan, M.; Chen, X.; Qiao, C.; Ma, J.; Zhang, C.; Jing, F.; Zhang, S.; Yang, B.; et al. Genome-wide identification and expression profiling of the carotenoid cleavage dioxygenase (CCD) gene family in Brassica napus L. PLoS ONE 2020, 15, e238179. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, G.; Gu, T.; Ding, J.; Li, Y. Bioinformatic and expression analyses on carotenoid dioxygenase genes in fruit development and abiotic stress responses in Fragaria vesca. Mol. Genet. Genom. 2017, 292, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Daruwalla, A.; Kiser, P.D. Structural and Mechanistic Aspects of Carotenoid Cleavage Dioxygenases (CCDs). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158590. [Google Scholar] [CrossRef]
- Pei, X.; Wang, X.; Fu, G.; Chen, B.; Nazir, M.F.; Pan, Z.; He, S.; Du, X. Identification and functional analysis of 9-cis-epoxy carotenoid dioxygenase (NCED) homologs in G. hirsutum. Int. J. Biol. Macromol. 2021, 182, 298–310. [Google Scholar] [CrossRef]
- Bruno, M.; Vermathen, M.; Alder, A.; Wüst, F.; Schaub, P.; Steen, R.; Beyer, P.; Ghisla, S.; Al Babili, S. Insights into the formation of carlactone from in-depth analysis of the CCD8-catalyzed reactions. FEBS Lett. 2017, 591, 792–800. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, X.; Quan, S.; Zhang, M.; Kang, C.; Guo, C.; Zhang, Z.; Niu, J. Genome-Wide Identification and Expression Analysis of NCED Gene Family in Pear and Its Response to Exogenous Gibberellin and Paclobutrazol. Int. J. Mol. Sci. 2023, 24, 7566. [Google Scholar] [CrossRef]
- Paterson, A.H.; Bowers, J.E.; Chapman, B.A. Ancient polyploidization predating divergence of the cereals, and its consequences for comparative genomics. Proc. Natl. Acad. Sci. USA 2004, 101, 9903–9908. [Google Scholar] [CrossRef]
- Sun, Z.; Hans, J.; Walter, M.H.; Matusova, R.; Beekwilder, J.; Verstappen, F.W.A.; Ming, Z.; van Echtelt, E.; Strack, D.; Bisseling, T.; et al. Cloning and characterisation of a maize carotenoid cleavage dioxygenase (ZmCCD1) and its involvement in the biosynthesis of apocarotenoids with various roles in mutualistic and parasitic interactions. Planta 2008, 228, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Ilg, A.; Beyer, P.; Al-Babili, S. Characterization of the rice carotenoid cleavage dioxygenase 1 reveals a novel route for geranial biosynthesis. FEBS J. 2009, 276, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Oda-Yamamizo, C.; Sage-Ono, K.; Ohmiya, A.; Ono, M. Alteration of flower colour in Ipomoea nil through CRISPR/Cas9-mediated mutagenesis of carotenoid cleavage dioxygenase 4. Transgenic Res. 2018, 27, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Lim, S.; Kim, J.K.; Jung, E.S.; John, K.M.M.; You, M.; Ahn, S.; Lee, C.H.; Ha, S. In planta cleavage of carotenoids by Arabidopsis carotenoid cleavage dioxygenase 4 in transgenic rice plants. Plant Biotechnol. Rep. 2016, 10, 291–300. [Google Scholar] [CrossRef]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef]
- Booker, J.; Auldridge, M.; Wills, S.; McCarty, D.; Klee, H.; Leyser, O. MAX3/CCD7 Is a Carotenoid Cleavage Dioxygenase Required for the Synthesis of a Novel Plant Signaling Molecule. Curr. Biol. 2004, 14, 1232–1238. [Google Scholar] [CrossRef]
- Simkin, A.J.; Schwartz, S.H.; Auldridge, M.; Taylor, M.G.; Klee, H.J. The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles β-ionone, pseudoionone, and geranylacetone. Plant J. 2004, 40, 882–892. [Google Scholar] [CrossRef]
- Yoshioka, S.; Aida, R.; Yamamizo, C.; Shibata, M.; Ohmiya, A. The carotenoid cleavage dioxygenase 4 (CmCCD4a) gene family encodes a key regulator of petal color mutation in chrysanthemum. Euphytica 2012, 184, 377–387. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Zhang, R.; Shi, D.; Lin, N.; Guo, P.; Wang, Y.; Shang, F.; Liu, Y. Transcriptome and carotenoids profiling of flowers in different Osmanthus fragrans cultivars provide insight into transcriptional control network of carotenoid-related genes expression. Sci. Hortic. 2022, 303, 111201. [Google Scholar] [CrossRef]
- Ureshino, K.; Nakayama, M.; Miyajima, I. Contribution made by the carotenoid cleavage dioxygenase 4 gene to yellow colour fade in azalea petals. Euphytica 2016, 207, 401–417. [Google Scholar] [CrossRef]
- Hai, N.T.L.; Masuda, J.; Miyajima, I.; Thien, N.Q.; Mojtahedi, N.; Hiramatsu, M.; Kim, J.; Okubo, H. Involvement of Carotenoid Cleavage Dioxygenase 4 Gene in Tepal Color Change in Lilium brownii var. colchesteri. J. Jpn. Soc. Hortic. Sci. 2012, 81, 366–373. [Google Scholar] [CrossRef]
- Auldridge, M.E.; McCarty, D.R.; Klee, H.J. Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr. Opin. Plant Biol. 2006, 9, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Chernys, J.T.; Zeevaart, J.A.D. Characterization of the 9-Cis-Epoxycarotenoid Dioxygenase Gene Family and the Regulation of Abscisic Acid Biosynthesis in Avocado1. Plant Physiol. 2000, 124, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; North, H.; Frey, A.; Sotta, B.; Seo, M.; Okamoto, M.; Nambara, E.; Marion-Poll, A. Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J. 2006, 45, 309–319. [Google Scholar] [CrossRef]
- Næsted, H.; Holm, A.; Jenkins, T.; Nielsen, H.B.; Harris, C.A.; Beale, M.H.; Andersen, M.; Mant, A.; Scheller, H.; Camara, B.; et al. Arabidopsis VARIEGATED 3 encodes a chloroplast-targeted, zinc-finger protein required for chloroplast and palisade cell development. J. Cell Sci. 2004, 117, 4807–4818. [Google Scholar] [CrossRef]
- Watanabe, M.; Mochida, K.; Kato, T.; Tabata, S.; Yoshimoto, N.; Noji, M.; Saito, K. Comparative Genomics and Reverse Genetics Analysis Reveal Indispensable Functions of the Serine Acetyltransferase Gene Family in Arabidopsis. Plant Cell 2008, 20, 2484–2496. [Google Scholar] [CrossRef]
- Freeman, J.L.; Salt, D.E. The metal tolerance profile of Thlaspi goesingense is mimicked in Arabidopsis thaliana heterologously expressing serine acetyl-transferase. BMC Plant Biol. 2007, 7, 63. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Sun, L.; Zhao, F.; Huang, B.; Yang, W.; Tao, Y.; Wang, J.; Yuan, Z.; Fan, G.; et al. The genome of Prunus mume. Nat. Commun. 2012, 3, 1318. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Geourjon, C.; Deleage, G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput. Appl. Biosci. 1995, 11, 681–684. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yuan, C.; Cong, T.; Wang, J.; Zhang, Q. Genome-wide identification of three-amino-acid-loop-extension gene family and their expression profile under hormone and abiotic stress treatments during stem development of Prunus mume. Front. Plant Sci. 2022, 13, 1006360–1006381. [Google Scholar] [CrossRef] [PubMed]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Yang, S.; Kim, E.; Ko, Y.; Hwang, S.; Shin, J.; Shim, J.E.; Shim, H.; Kim, H.; Kim, C.; et al. AraNet v2: An improved database of co-functional gene networks for the study of Arabidopsis thaliana and 27 other nonmodel plant species. Nucleic Acids Res. 2015, 43, D996–D1002. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, A.; Bao, F.; Cheng, W.; Cheng, T.; Zhang, Q. Phylogeny of PmCCD Gene Family and Expression Analysis of Flower Coloration and Stress Response in Prunus mume. Int. J. Mol. Sci. 2023, 24, 13950. https://doi.org/10.3390/ijms241813950
Ding A, Bao F, Cheng W, Cheng T, Zhang Q. Phylogeny of PmCCD Gene Family and Expression Analysis of Flower Coloration and Stress Response in Prunus mume. International Journal of Molecular Sciences. 2023; 24(18):13950. https://doi.org/10.3390/ijms241813950
Chicago/Turabian StyleDing, Aiqin, Fei Bao, Wenhui Cheng, Tangren Cheng, and Qixiang Zhang. 2023. "Phylogeny of PmCCD Gene Family and Expression Analysis of Flower Coloration and Stress Response in Prunus mume" International Journal of Molecular Sciences 24, no. 18: 13950. https://doi.org/10.3390/ijms241813950
APA StyleDing, A., Bao, F., Cheng, W., Cheng, T., & Zhang, Q. (2023). Phylogeny of PmCCD Gene Family and Expression Analysis of Flower Coloration and Stress Response in Prunus mume. International Journal of Molecular Sciences, 24(18), 13950. https://doi.org/10.3390/ijms241813950





