Heterologous Interactions with Galectins and Chemokines and Their Functional Consequences
Abstract
1. Introduction
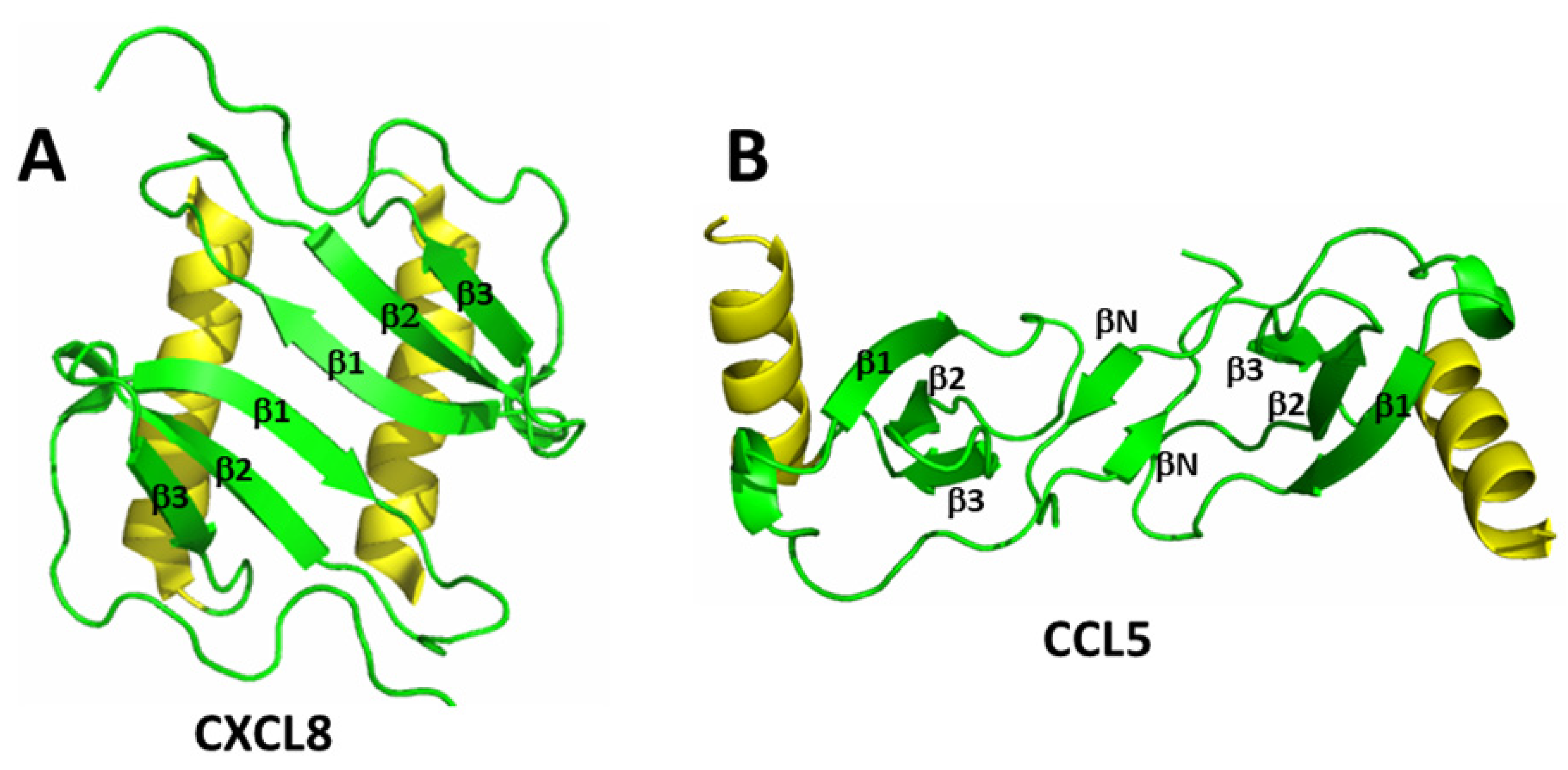
2. Chemokines
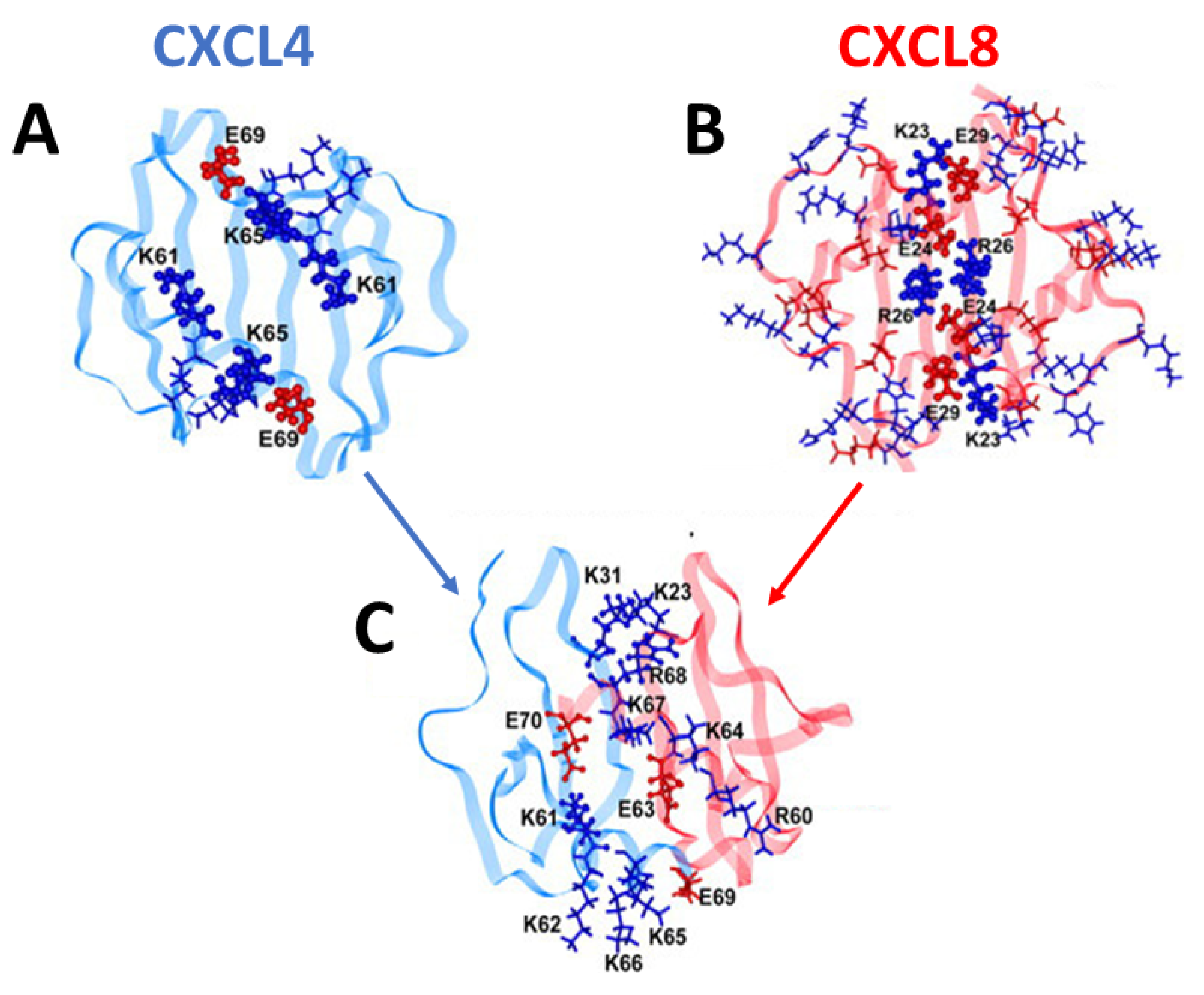
2.1. Chemokine Heterodimers
2.2. Functional Considerations
3. Galectins
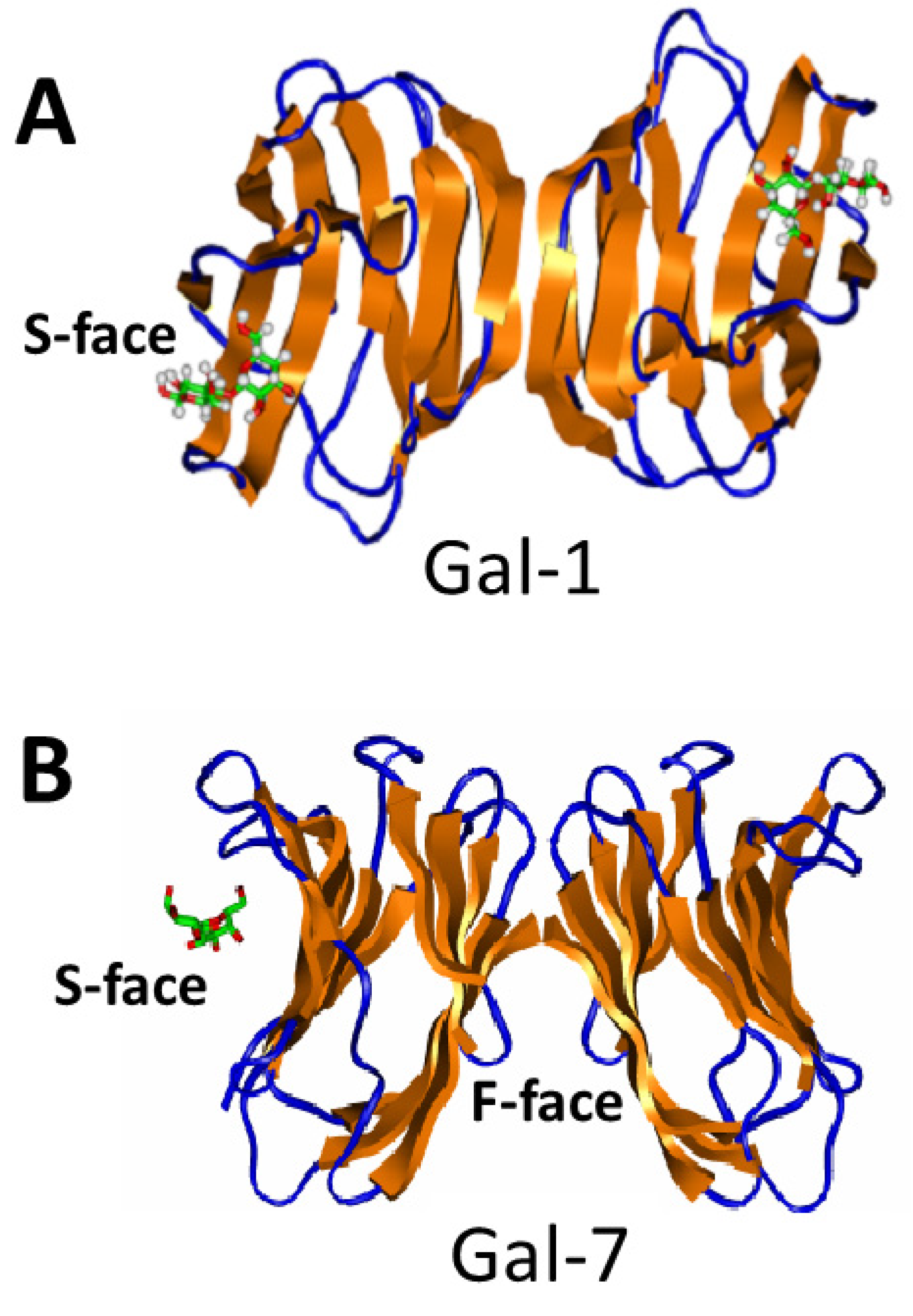
3.1. Galectin-Galectin Heterodimers
3.2. Functional Considerations
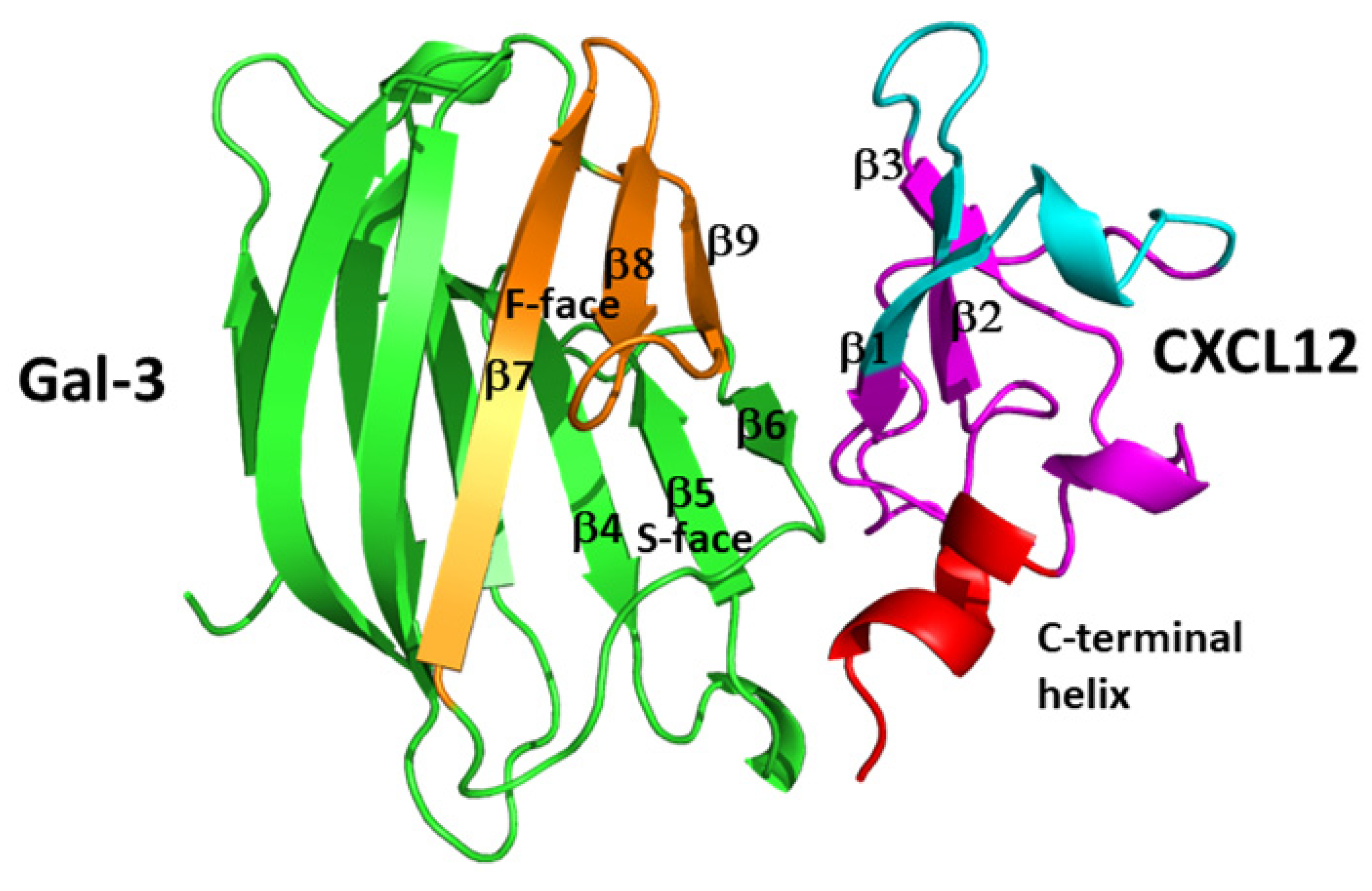
4. Chemokine–Galectin Heterodimers
5. Conclusions
Funding
Conflicts of Interest
References
- Guan, E.; Wang, J.; Norcross, M.A. Identification of Human Macrophage Inflammatory Proteins 1α and 1β as a Native Secreted Heterodimer. J. Biol. Chem. 2001, 276, 12404–12409. [Google Scholar] [CrossRef] [PubMed]
- Dudek, A.Z.; Nesmelova, I.; Mayo, K.H.; Verfaillie, C.M.; Pitchford, E.; Slungaard, A. Platelet Factor 4 Promotes Adhesion of Hematopoietic Progenitor Cells and Binds IL-8: Novel Mechanisms for Modulation of Hematopoiesis. Blood 2003, 101, 4687–4694. [Google Scholar] [CrossRef] [PubMed]
- Nesmelova, I.; Sham, Y.; Dudek, A.Z.; van Eijk, L.I.; Wu, G.; Slungaard, A.; Mortari, F.; Griffioen, A.W.; Mayo, K.H. Platelet Factor 4 and Interleukin-8 CXC Chemokine Heterodimer Formation Modulates Function at the Quaternary Structural Level. J. Biol. Chem. 2005, 280, 4948–4958. [Google Scholar] [CrossRef]
- Nesmelova, I.V.; Sham, Y.; Gao, J.; Mayo, K.H. CXC-chemokines associate with CC-chemokines to form mixed heterodimers: RANTES and PF4 monomers associate as CC-type heterodimers. J. Biol. Chem. 2008, 283, 24155–24166. [Google Scholar] [CrossRef]
- Koenen, R.; von Hundelhausen, P.; Nesmelova, I.V.; Zernecke, A.; Liehn, E.A.; Sarabi, A.; Kramp, B.K.; Piccinini, A.; Paludan, S.R.; Kowalska, M.A.; et al. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat. Med. 2009, 15, 97–103. [Google Scholar] [CrossRef] [PubMed]
- von Hundelshausen, P.; Agten, S.; Eckardt, V.; Schmitt, M.; Blanchet, X.; Neideck, C.; Ippel, H.; Bidzhekov, K.; Wichapong, K.; Faussner, A.; et al. Chemokine interactome mapping enables tailored intervention in acute and chronic inflammation. Sci. Transl. Med. 2017, 9, 384–392. [Google Scholar] [CrossRef]
- Miller, M.C.; Ludwig, A.K.; Wichapong, K.; Kaltner, H.; Kopitz, J.; Gabius, H.-J.; Mayo, K.H. Adhesion/growth-regulatory galectins tested in combination: Evidence for synergistic functional cooperation and subunit swapping to form heterodimers. Biochem. J. 2018, 475, 1003–1018. [Google Scholar] [CrossRef]
- Dings, R.P.M.; Kumar, N.; Mikkelson, S.; Gabius, H.-J.; Mayo, K.H. Stimulating cellular galectins networks by mixing galectins in vitro reveals synergistic activity. Biochem. Biophys. Rep. 2021, 28, 101–116. [Google Scholar]
- Eckardt, V.; Miller, M.C.; Blanchet, X.; Duan, R.; Leberzammer, J.; Duchene, J.; Soehnlein, O.; Megens, R.T.; Ludwig, A.K.; Dregni, A.; et al. Chemokines and galectins form heterodimers to modulate inflammation. EMBO Rep. 2020, 21, e47852. [Google Scholar] [CrossRef]
- Baggiolini, M. Chemokines and leukocyte traffic. Nature 1998, 392, 565–568. [Google Scholar] [CrossRef]
- Mackay, C.R. Chemokines: Immunology’s high impact factors. Nat. Immunol. 2001, 2, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Youn, B.S.; Mantel, C.; Broxmeyer, H.E. Chemokines, chemokine receptors and hematopoiesis. Immunol. Rev. 2000, 177, 150–174. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.E.; Polverini, P.J.; Kunkel, S.L.; Harlow, L.A.; DiPietro, L.A.; Elner, V.M.; Elner, S.G.; Strieter, R.M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258, 1798–1801. [Google Scholar] [CrossRef] [PubMed]
- Belperio, J.A.; Keane, M.P.; Arenberg, D.A.; Addison, C.L.; Ehlert, J.E.; Burdick, M.D.; Strieter, R.M. CXC Chemokines in Angiogenesis. J. Leukoc. Biol. 2000, 68, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zlotnik, A.; Yoshie, O. Chemokines: A new classification system and their role in immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Clore, G.M.; Gronenborn, A.M. Three-dimensional structures of α- and β-chemokines. FASEB J. 1995, 9, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.A.; Buelow, B.J.; Nevins, A.M.; Jones, S.E.; Peterson, F.C.; Gundry, R.L.; Grayson, M.H.; Volkman, B.F. Structure-function analysis of CCL28 in the development of post-viral asthma. J. Biol. Chem. 2015, 290, 4528–4536. [Google Scholar] [CrossRef]
- Yang, Y.; Mayo, K.H.; Daly, T.; Barry, J.K.; La Rosa, G.J. Subunit Association and Structural Analysis of Platelet Basic Protein and Related Proteins Investigated by 1H-NMR Spectroscopy and Circular Dichroism. J. Biol. Chem. 1994, 269, 20110–20118. [Google Scholar] [CrossRef]
- Clark-Lewis, I.; Kim, K.S.; Rajarathnam, K.; Gong, J.H.; Dewald, B.; Moser, B.; Baggiolini, M.; Sykes, B.D. Structure-activity relationships of chemokines. J. Leukoc. Biol. 1995, 57, 703–711. [Google Scholar] [CrossRef]
- Wang, X.; Watson, C.; Sharp, J.S.; Handel, T.M.; Prestegard, J.H. Oligomeric structure of the chemokine CCL5/RANTES from NMR, MS, and SAXS data. Structure 2011, 19, 1138–1148. [Google Scholar] [CrossRef]
- Jansma, A.L.; Kirkpatrick, J.P.; Hsu, A.R.; Handel, T.M.; Nietlispach, D. NMR analysis of the structure, dynamics, and unique oligomerization properties of the chemokine CCL27. J. Biol. Chem. 2010, 285, 14424–14437. [Google Scholar] [CrossRef] [PubMed]
- Clore, G.M.; Appella, E.; Yamada, M.; Matsushima, K.; Gronenborn, A.M. Three dimensional structure of interleukin 8 in solution. Biochemistry 1990, 29, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.G.; Triandafillou, C.G.; Huang, T.Y.; Zulueta, M.M.; Banerjee, S.; Dinner, A.R.; Hung, S.C.; Tang, W.J. Crystal structure of CC chemokine 5 (CCL5). Proc. Natl. Acad. Sci. USA 2016, 113, 5000–5005. [Google Scholar] [CrossRef] [PubMed]
- Skelton, N.J.; Aspiras, F.; Ogez, J.; Schall, T.J. Proton NMR assignments and solution conformation of RANTES, a chemokine of the CC type. Biochemistry 1995, 34, 5329–5342. [Google Scholar] [CrossRef]
- Mayo, K.H.; Roongta, V.; Barker, S.; Milius, R.; Ilyina, E.; Quinlan, C.; La Rosa, G.; Daly, T. NMR Solution Structure of the 32 kD Tetrameric Platelet Factor-4 ELR-Motif N-terminal Chimer: A Symmetric Tetramer. Biochemistry 1995, 34, 11399–11409. [Google Scholar] [CrossRef]
- Swaminathan, G.J.; Holloway, D.E.; Colvin, R.A.; Campanella, K.; Papageorgiou, A.C.; Luster, A.D.; Acharya, K.R. Crystal Structures of Oligomeric Forms of the IP-10/CXCL10 Chemokine. Structure 2003, 11, 521–532. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Bancroft, D.P.; Lai, C.K.; Maione, T.E. Crystal structure of recombinant human platelet factor 4. Biochemistry 1994, 33, 8361–8366. [Google Scholar] [CrossRef]
- Paoletti, S.; Petkovic, V.; Sebastiani, S.; Danelon, M.G.; Uguccioni, M.; Gerber, B.O. A rich chemokine environment strongly enhances leukocyte migration and activities. Blood 2005, 105, 3405–3412. [Google Scholar] [CrossRef]
- Campanella, G.S.V.; Grimm, J.; Manice, L.A.; Colvin, R.A.; Medoff, B.D.; Woitkiewicz, G.R.; Weissleder, R.; Luster, A.D. Oligomerization of CXCL10 Is Necessary for Endothelial Cell Presentation and In Vivo Activity. J. Immunol. 2006, 177, 6991–6998. [Google Scholar] [CrossRef]
- Rek, A.; Brandner, B.; Geretti, E.; Kungl, A.J. A biophysical insight into the RANTES–glycosaminoglycan interaction. Biochim. Biophys. Acta 2009, 1794, 577–582. [Google Scholar] [CrossRef]
- Ren, M.; Guo, Q.; Guo, L.; Lenz, M.; Qian, F.; Koenen, R.R.; Xu, H.; Schilling, A.B.; Weber, C.; Ye, R.D.; et al. Polymerization of MIP-1 chemokine (CCL3 and CCL4) and clearance of MIP1 by insulin-degrading enzyme. EMBO J. 2010, 29, 3952–3966. [Google Scholar] [CrossRef] [PubMed]
- Mayo, K.H.; Chen, M.-J. Human Platelet Factor 4 Monomer-Dimer-Tetramer Equilibria Investigated by NMR Spectroscopy. Biochemistry 1989, 28, 9469–9478. [Google Scholar] [CrossRef]
- Chen, M.J.; Mayo, K.H. Human Platelet Factor 4 Subunit Association-Dissociation Thermodynamics and Kinetics. Biochemistry 1991, 30, 6402–6411. [Google Scholar] [CrossRef]
- Mayo, K.H. Low Affinity Platelet Factor 4 1H-NMR Derived Aggregate Equilibria Indicate Physiological Preference for Monomers over Dimers and Tetramers. Biochemistry 1991, 30, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Young, H.; Roongta, V.; Daly, T.J.; Mayo, K.H. NMR Structure and Dynamics of Monomeric Neutrophil Activating Peptide-2. Biochem. J. 1999, 338, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mayo, K.H. Alcohol-Induced Protein Folding Transitions in Platelet Factor- 4: The O-State. Biochemistry 1993, 32, 8661–8671. [Google Scholar] [CrossRef]
- Yang, Y.; Barker, S.; Chen, M.-J.; Mayo, K.H. Effect of Low Molecular Weight Aliphatic Alcohols and Related Compounds on Platelet Factor-4 Subunit Association. J. Biol. Chem. 1993, 268, 9223–9229. [Google Scholar] [CrossRef]
- Veldkamp, C.T.; Ziarek, J.J.; Su, J.; Basnet, H.; Lennertz, R.; Weiner, J.J.; Peterson, F.C.; Baker, J.E.; Volkman, B.F. Monomeric structure of the cardio-protective chemokine SDF-1/CXCL12. Protein Sci. 2009, 18, 1359–1369. [Google Scholar] [CrossRef]
- Veldkamp, C.T.; Peterson, F.C.; Pelzek, A.J.; Volkman, B.F. The monomer-dimer equilibrium of stromal cell-derived factor-1 (CXCL12) is altered by pH, phosphate, sulfate, and heparin. Protein Sci. 2005, 14, 1071–1081. [Google Scholar] [CrossRef]
- Crump, M.P.; Rajarathnam, K.; Kim, K.-S.; Clark-Lewis, I.; Sykes, B.D. Solution Structure of Eotaxin, a Chemokine That Selectively Recruits Eosinophils in Allergic Inflammation. J. Biol. Chem. 1998, 273, 22471–22479. [Google Scholar] [CrossRef]
- Ellyard, J.I.; Simson, L.; Bezos, A.; Johnston, K.; Freeman, C.; Parish, C.R. Eotaxin Selectively Binds Heparin. J. Biol. Chem. 2007, 282, 15238–15247. [Google Scholar] [CrossRef]
- Proudfoot, A.E. The BBXB Motif of RANTES Is the Principal Site for Heparin Binding and Controls Receptor Selectivity. J. Biol. Chem. 2001, 276, 10620–10626. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.J.; Oh, Y.I.; Chang, S.-K.; Hsieh-Wilson, L.C. Tunable Heparan Sulfate Mimetics for Modulating Chemokine Activity. J. Am. Chem. Soc. 2013, 135, 10898–10901. [Google Scholar] [CrossRef] [PubMed]
- Salanga, C.L.; Handel, T.M. Chemokine oligomerization and interactions with receptors and glycosaminoglycans: The role of structural dynamics in function. Exp. Cell Res. 2011, 317, 590–601. [Google Scholar] [CrossRef]
- Proudfoot, A.E. Chemokines and Glycosaminoglycans. Front. Immunol. 2015, 6, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.P.; Johnson, Z.; Borlat, F.; Zwahlen, C.; Kungl, A.; Roulin, K.; Harrenga, A.; Wells, T.N.; Proudfoot, A.E. The X-ray Structure of RANTES Heparin-Derived Disaccharides Allows the Rational Design of Chemokine Inhibitors. Structure 2004, 12, 2081–2093. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Andaya, A.; Bleiholder, C.; Leary, J.A. Differentiation of CC vs. CXC Chemokine Dimers with GAG Octasaccharide Binding Partners: An Ion Mobility Mass Spectrometry Approach. J. Am. Chem. Soc. 2013, 135, 4325–4332. [Google Scholar] [CrossRef]
- Mayo, K.H.; Ilyina, E.; Roongta, V.; Dundas, M.; Joseph, J.; Lai, C.K.; Maione, T.; Daly, T. Heparin Binding to Platelet Factor-4. An NMR and Site-Directed Mutagenesis Study: Arginine Residues Crucial for Binding. Biochem. J. 1995, 312, 357–365. [Google Scholar] [CrossRef]
- Dyer, D.P.; Salanga, C.L.; Volkman, B.F.; Kawamura, T.; Handel, T.M. The dependence of chemokine-glycosaminoglycan interactions on chemokine oligomerization. Glycobiology 2016, 26, 312–326. [Google Scholar] [CrossRef]
- Hoogewerf, A.J.; Kuschert, G.S.; Proudfoot, A.E.; Borlat, F.; Clark-Lewis, I.; Power, C.A.; Wells, T.N. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry 1997, 36, 13570–13578. [Google Scholar] [CrossRef]
- Proudfoot, A.E.; Handel, T.M.; Johnson, Z.; Lau, E.K.; Li Wang, P.; Clark-Lewis, I.; Borlat, F.; Wells, T.N.; Kosco-Vilbois, M.H. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc. Natl. Acad. Sci. USA 2003, 100, 1885–1890. [Google Scholar] [CrossRef] [PubMed]
- Crown, S.E.; Yu, Y.; Sweeney, M.D.; Leary, J.A.; Handel, T.M. Heterodimerization of CCR2 chemokines and regulation by glycosaminoglycan binding. J. Biol. Chem. 2006, 281, 25438–25446. [Google Scholar] [CrossRef] [PubMed]
- Verkaar, F.; van Offenbeek, J.; van der Lee, M.M.C.; van Lith, L.H.C.J.; Watts, A.O.; Rops, A.L.W.M.M.; Aguilar, D.C.; Ziarek, J.J.; van der Vlag, J.; Handel, T.M.; et al. Chemokine cooperativity is caused by competitive glycosaminoglycan binding. J. Immunol. 2014, 192, 3908–3914. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, D.V.; Young, H.; Linhardt, R.J.; Mayo, K.H. Heparin Dodecasaccharide Binding to Platelet Factor-4 and Growth-related Protein-α: Induction of a Partially Folded State and Implications for Heparin-Induced Thromnocytopenia. J. Biol. Chem. 1999, 274, 25317–25329. [Google Scholar] [CrossRef]
- Brandhofer, M.; Hoffmann, A.; Blanchet, X.; Siminkovitch, E.; Rohlfing, A.-K.; El Bounkari, O.; Nestele, J.A.; Bild, A.; Kontos, C.; Hille, K.; et al. Heterocomplexes between the Atypical Chemokine MIF and the CXC-Motif Chemokine CXCL4L1 Regulate Inflammation and Thrombus Formation. Cell. Mol. Life Sci. 2022, 79, 512. [Google Scholar] [CrossRef] [PubMed]
- Jansma, A.; Handel, T.M.; Hamel, D.J. Chapter 2. Homo- and hetero-oligomerization of chemokines. Methods Enzymol. 2009, 461, 31–50. [Google Scholar]
- Koenen, R.R.; Weber, C. Therapeutic targeting of chemokine interactions in atherosclerosis. Nat. Rev. Drug Discov. 2010, 9, 141–153. [Google Scholar] [CrossRef]
- Raman, D.; Sobolik-Delmaire, T.; Richmond, A. Chemokines in health and disease. Exp. Cell Res. 2011, 317, 575–589. [Google Scholar] [CrossRef]
- Viola, A.; Luster, A.D. Chemokines and Their Receptors: Drug Targets in Immunity and Inflammation. Ann. Rev. Pharmacol. Toxicol. 2008, 48, 171–197. [Google Scholar] [CrossRef]
- Allen, S.J.; Crown, S.E.; Handel, T.M. Chemokine: Receptor structure, interactions, and antagonism. Annu. Rev. Immunol. 2007, 25, 787–820. [Google Scholar] [CrossRef]
- Park, S.H.; Das, B.B.; Casagrande, F.; Tian, Y.; Nothnagel, H.J.; Chu, M.; Kiefer, H.; Maier, K.; DeAngelis, A.A.; Marassi, F.M.; et al. Structure of the chemokine receptor CXCR1 in phospholipid bilayers. Nature 2012, 491, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Thelen, M.; Stein, J.V. How chemokines invite leukocytes to dance. Nat. Immun. 2008, 9, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Chien, E.Y.; Mol, C.D.; Fenalti, G.; Liu, W.; Katritch, V.; Abagyan, R.; Brooun, A.; Wells, P.; Bi, F.C.; et al. Structures of the CXCR4 Chemokine GPCR with Small-Molecule and Cyclic Peptide Antagonists. Science 2010, 330, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Hemmerich, S.; Paavola, C.; Bloom, A.; Bhakta, S.; Freedman, R.; Grunberger, D.; Krstenansky, J.; Lee, S.; McCarley, D.; Mulkins, M.; et al. Identification of residues in the monocyte chemotactic protein-1 that contact the MCP-1 receptor, CCR2. Biochemistry 1999, 38, 13013–13025. [Google Scholar] [CrossRef]
- Qin, L.; Kufareva, I.; Holden, L.G.; Wang, C.; Zheng, Y.; Zhao, C.; Fenalti, G.; Wu, H.; Han, G.W.; Cherezov, V.; et al. Crystal structure of the chemokine receptor CXCR4 in complex with a viral chemokine. Science 2015, 347, 1117–11122. [Google Scholar] [CrossRef] [PubMed]
- Strieter, R.M.; Polverini, P.J.; Kunkel, S.L.; Arenberg, D.A.; Burdick, M.D.; Kasper, J.; Dzuiba, J.; VanDamme, J.; Walz, A.; Marriott, D. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J. Biol. Chem. 1995, 270, 27348–27357. [Google Scholar] [CrossRef] [PubMed]
- Blanpain, C.; Doranz, B.J.; Bondue, A.; Govaerts, C.; De Leener, A. The core domain of chemokines bind CCR5 extracellular domains while their amino terminus interacts with the transmembrane helix bundle. J. Biol. Chem. 2002, 278, 5179–5187. [Google Scholar] [CrossRef]
- Gouwy, M. Synergy between proinflammatory ligands of G protein-coupled receptors in neutrophil activation and migration. J. Leukoc. Biol. 2004, 76, 185–194. [Google Scholar] [CrossRef]
- Gouwy, M.; Struyf, S.; Noppen, S.; Schutyser, E.; Springael, J.-Y.; Parmentier, M.; Proost, P.; VanDamme, J. Synergy between Co-produced CC and CXC Chemokines in Monocyte Chemotaxis through Receptor-Mediated Events. Mol. Pharmacol. 2008, 74, 485–495. [Google Scholar] [CrossRef]
- von Hundelshausen, P.; Koenen, R.R.; Sack, M.; Mause, S.F.; Adriaens, W.; Proudfoot, A.E.; Hackeng, T.M.; Weber, C. Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood 2005, 105, 924–930. [Google Scholar] [CrossRef]
- Agten, S.M.; Koenen, R.; Ippel, H.; Eckert, V.; von Hundelshausen, P.; Mayo, K.H.; Weber, C.; Hackeng, T.M. Probing Functional Heteromeric Chemokine Protein-Protein Interactions through Conformation-assisted Oxime-Linkage. Angewante Chem. 2016, 55, 14963–14966. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.P.; Volkman, B.; Dréau, D.; Nesmelova, I.V. A new obligate CXCL4-CXCL12 heterodimer for studying chemokine heterodimer activities and mechanisms. Sci. Rep. 2022, 12, 17204. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.L.; Colombo, M.; Gonzales, J.J.; Hollander, W.; Schmid, K. The glycosaminoglycans of the human artery and their changes in atherosclerosis. J. Clin. Investig. 1976, 58, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.R.; Gallo, R.L. Glycosaminoglycans and their proteoglycans: Host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006, 20, 9–22. [Google Scholar] [CrossRef]
- Handel, T.M.; Johnson, Z.; Crown, S.E.; Lau, E.K.; Proodfoot, A.E. Regulation of protein function by glycosaminoglycans—As exemplified by chemokines. Ann. Rev. Biochem. 2005, 74, 385–410. [Google Scholar] [CrossRef]
- Gouwy, M.; Struyf, S.; Berghmans, N.; Vanormelingen, C.; Schols, D.; Van Damme, J. CXCR4 and CCR5 ligands cooperate in monocyte and lymphocyte migration and in inhibition of dual-tropic (R5/X4) HIV-1 infection. Eur. J. Immunol. 2011, 41, 963–973. [Google Scholar] [CrossRef]
- Gouwy, M.; Schiraldi, M.; Struyf, S.; Van Damme, J.; Uguccioni, M. Possible mechanisms involved in chemokine synergy fine tuning the inflammatory response. Immunol. Lett. 2012, 145, 10–14. [Google Scholar] [CrossRef]
- Gouwy, M.; Struyf, S.; Leutenez, L.; Pörtner, N.; Sozzani, S.; Van Damme, J. Chemokines and other GPCR ligands synergize in receptor-mediated migration of monocyte-derived immature and mature dendritic cells. Immunobiology 2014, 219, 218–229. [Google Scholar] [CrossRef]
- Mortier, A.; Van Damme, J.; Proost, P. Overview of the mechanisms regulating chemokine activity and availability. Immunol. Lett. 2012, 145, 2–9. [Google Scholar] [CrossRef]
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.; Cummings, R.D.; Drickamer, K.; Feizi, T.; Gitt, M.A.; Hirabayashi, J.; Hughes, C.; Kasai, K. Galectins: A family of animal β-galactoside-binding lectins. Cell 1994, 76, 597–598. [Google Scholar] [CrossRef]
- Barondes, S.H. Galectins: A personal overview. Trends Glycosci. Glycotechnol. 1998, 9, 1–7. [Google Scholar] [CrossRef]
- Cooper, D.N.; Barondes, S.H. God must love galectins; he made so many of them. Glycobiology 1990, 9, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.N. Galectinomics: Finding themes in complexity. Biochim. Biophys. Acta 2002, 1572, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.; Iqbal, A.J.; Gittens, B.R.; Cervone, C.; Perretti, M. The effect of galectins on leukocyte trafficking in inflammation: Sweet or sour? Ann. N.Y. Acad. Sci. 2012, 1253, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Camby, I.; Le Mercier, M.; Lefranc, F.; Kiss, R. Galectin-1: A small protein with major functions. Glycobiology 2006, 16, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Teichberg, V.I.; Silman, I.; Beitsch, D.D.; Resheff, G. A β-D-galactoside binding protein from electric organ tissue of Electrophorus electricus. Proc. Natl. Acad. Sci. USA 1975, 72, 1383–1387. [Google Scholar] [CrossRef]
- Hirabayashi, J.; Kasai, K. The family of metazoan metal-independent β-galactoside-binding lectins: Structure, function and molecular evolution. Glycobiology 1993, 3, 297–304. [Google Scholar] [CrossRef]
- Kasai, K.; Hirabayashi, J. Galectins: A family of animal lectins that decipher glycocodes. J. Biochem. 1996, 119, 1–8. [Google Scholar] [CrossRef]
- Ippel, H.; Miller, M.C.; Vértesy, S.; Zheng, Y.; Cañada, F.J.; Suylen, D.; Umemoto, K.; Romano, C.; Hackeng, T.; Tai, G.; et al. Intra- and intermolecular interactions of human galectin-3: Assessment by full-assignment-based NMR. Glycobiology 2016, 26, 888–903. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, X.; Cheng, H.; Miller, M.C.; He, Z.; Gu, H.; Zhang, Z.; Raz, A.; Mayo, K.H.; Tai, G.; et al. Galectin-3 N-terminal tail prolines modulate cell activity and glycan-mediated oligomerization/phase separation. Proc. Natl. Acad. Sci. USA 2021, 118, e2021074118. [Google Scholar] [CrossRef]
- Si, Y.; Yao, Y.; Ayala, G.; Li, X.; Han, Q.; Zhang, W.; Tai, G.; Mayo, K.H.; Zhou, Y.; Su, J. Human galectin-16 has a pseudo ligand binding site and plays a role in regulating c-Rel-mediated lymphocyte activity. Biochim. Biophys. Acta 2021, 1865, e129755. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Li, Y.; Yang, T.; Li, X.; Ayala, G.J.; Mayo, K.H.; Tai, G.; Su, J.; Zhou, Y. Structure-function studies of galectin-14, an important effector molecule in embryology. FEBS J. 2021, 288, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, Y.; Liu, T.; Gu, K.; Han, Q.; Zhang, W.; Ayala, G.J.; Liu, Y.; Na, H.; Yu, J.; et al. Actin binding to galectin-13/placental protein-13 occurs independently of the galectin canonical ligand binding site. Glycobiology 2021, 31, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, T.; Horie, H. Structural and functional studies of galectin-1: A novel axonal regeneration-promoting activity for oxidized galectin-1. Curr. Drug Targets 2005, 6, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Kasai, K. Effect of amino acid substitution by sited-directed mutagenesis on the carbohydrate recognition and stability of human 14-kDa β-galactoside-binding lectin. J. Biol. Chem. 1991, 266, 23648–23653. [Google Scholar] [CrossRef]
- Tracey, B.M.; Feizi, T.; Abbott, W.M.; Carruthers, R.A.; Green, B.N.; Lawson, A.M. Subunit molecular mass assignment of 14,654 Da to the soluble β-galactoside-binding lectin from bovine heart muscle and demonstration of intramolecular disulfide bonding associated with oxidative inactivation. J. Biol. Chem. 1992, 267, 10342–10347. [Google Scholar] [CrossRef]
- Nesmelova, I.V.; Ermakova, E.; Daragan, V.A.; Pang, M.; Menéndez, M.; Lagartera, L.; Solís, D.; Baum, L.G.; Mayo, K.H. Lactose binding to galectin-1 modulates structural dynamics, increases conformational entropy, and occurs with apparent negative cooperativity. J. Mol. Biol. 2010, 397, 1209–1230. [Google Scholar] [CrossRef]
- Ermakova, E.; Miller, M.C.; Nesmelova, I.V.; Lopez-Merino, L.; Berbís, M.A.; Nesmelov, Y.; Lagartera, L.; Daragan, V.A.; André, S.; Cañada, F.J.; et al. Lactose Binding to Human Galectin-7 (p53-induced gene 1) Induces Long-range Effects through the Protein Resulting in Increased Dimer Stability and Evidence for Positive Cooperativity. Glycobiology 2013, 23, 508–523. [Google Scholar] [CrossRef]
- Miller, M.C.; Nesmelova, I.V.; Daragan, V.A.; Ippel, H.; Michalak, M.; Dregni, A.; Kaltner, H.; Kopitz, J.; Gabius, H.-J.; Mayo, K.H. Identification of Pro4 prolyl peptide bond isomerization in human galectin-7 and its impact on structural dynamics and glycan affinity. Biochem. J. 2020, 477, 3147–3165. [Google Scholar] [CrossRef]
- Bourne, Y.; Bolgiano, B.; Liao, D.I.; Strecker, G.; Cantau, P.; Herzberg, O.; Feizi, T.; Cambillau, C. Crosslinking of mammalian lectin (galectin-1) by complex biantennary saccharides. Nat. Struct. Biol. 1994, 1, 863–870. [Google Scholar] [CrossRef]
- Nagae, M.; Nishi, N.; Murata, T.; Usui, T.; Nakamura, T.; Wakatsuki, S.; Kato, R. Crystal structure of the galectin-9 N-terminal carbohydrate recognition domain from Mus musculus reveals the basic mechanism of carbohydrate recognition. J. Biol. Chem. 2006, 281, 35884–35893. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Alves, I.; Gaifem, J.; Rabinovich, G.A. Immune regulatory networks coordinated by glycans and glycan-binding proteins in autoimmunity and infection. Cell. Molecul. Immun. 2023. [Google Scholar] [CrossRef]
- Miller, M.C.; Ribeiro, J.P.; Roldós, V.; Martín-Santamaría, S.; Cañada, F.J.; Nesmelova, I.A.; André, S.; Pang, M.; Klyosov, A.A.; Baum, L.G.; et al. Structural aspects of binding of α-linked digalactosides to human galectin-1. Glycobiology 2011, 21, 1627–1641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Miller, M.C.; Xu, X.; Zheng, Y.; Song, C.; Zhang, F.; Zhou, Y.; Tai, G.; Mayo, K.H. NMR-based insight into galectin-3 binding to endothelial cell adhesion molecule CD146: Evidence for non-cannonical interactions with the lectin’s CRD b-sandwich F-face. Glycobiology 2019, 29, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Nesmelova, I.V.; Klyosov, A.; Platt, D.; Mayo, K.H. The carbohydrate binding domain on galectin-1 is more extensive for a complex glycan than for simple saccharides: Implications for galectin-glycan interactions at the cell surface. Biochem. J. 2009, 421, 211–221. [Google Scholar] [CrossRef]
- Miller, M.; Klyosov, A.; Mayo, K.H. The α-galactomannan Davanat binds galectin-1 at asite different from the conventional galectin carbohydrate binding site. Glycobiology 2009, 19, 1034–1045. [Google Scholar] [CrossRef]
- Miller, M.C.; Klyosov, A.; Mayo, K.H. Structural Features for α-galactomannan binding to galectin-1. Glycobiology 2012, 22, 543–551. [Google Scholar] [CrossRef]
- Miller, M.C.; Ippel, H.; Suylen, D.; Klyosov, A.A.; Traber, P.G.; Hackeng, T.; Mayo, K.H. Binding of Polysaccharides to Human Galectin-3 at a Non-Canonical Site in its Carbohydrate Recognition Domain. Glycobiology 2016, 26, 88–99. [Google Scholar] [CrossRef]
- Miller, M.C.; Zheng, Y.; Yan, J.; Zhou, Y.; Tai, G.; Mayo, K.H. Novel polysaccharide binding to the N-terminal tail of galection-3 is likely modulated by proline isomerization. Glycobiology 2017, 27, 1038–1051. [Google Scholar] [CrossRef]
- Zhang, T.; Miller, M.C.; Lan, Y.; Zheng, Y.; Liu, F.; Zhao, D.; Su, J.; Mayo, K.H.; Tai, G.; Zhou, Y. Macromolecular assemblies of complex polysaccharides with Galectin-3 and their synergestic effects on function. Biochem. J. 2017, 474, 3849–3868. [Google Scholar] [CrossRef]
- Zheng, Y.; Su, J.; Miller, M.C.; Zhang, T.; Mayzel, M.; Tai, G.; Mayo, K.H.; Zhou, Y. Topsy-turvy binding of negatively-charged homogalacturonan oligosaccharides to galectin-3. Glycobiology 2021, 31, 341–350. [Google Scholar] [CrossRef]
- Miller, M.C.; Cai, C.; Wichapong, K.; Bhaduri, S.; Pohl, N.L.B.; Linhardt, R.J.; Gabius, H.-J.; Mayo, K.H. Structural insight into the binding of human galectins to corneal keratan sulfate, its desulfated form and related saccharides. Sci. Rep. 2020, 10, 15708–15713. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Hashidate, T.; Arata, Y.; Nishi, N.; Nakamura, T.; Hirashima, M.; Urashima, T.; Oka, T.; Futai, M.; Muller, W.E.; et al. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim. Biophys. Acta 2002, 1572, 232–254. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Hsu, D.K.; Liu, F.T. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc. Natl. Acad. Sci. USA 1996, 93, 6737–6742. [Google Scholar] [CrossRef] [PubMed]
- Massa, S.M.; Cooper, D.N.; Leffler, H.; Barondes, S.H. L-29, an endogenous lectin, binds to glycoconjugate ligands with positive cooperativity. Biochemistry 1993, 32, 260–267. [Google Scholar] [CrossRef]
- Kuklinski, S.; Probstmeier, R. Homophilic binding properties of galectin-3: Involvement of the carbohydrate recognition domain. J. Neurochem. 1998, 70, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Gabius, H.-J.; Andre, S.; Kaltner, H.; Sabesan, S.; Roy, R.; Liu, B.; Macaluso, F.; Brewer, C.F. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 2004, 279, 10841–10847. [Google Scholar] [CrossRef] [PubMed]
- Rini, J.M. Lectin structure. Annu. Rev. Biophys. Biomol. Struct. 1995, 24, 551–577. [Google Scholar] [CrossRef]
- Gitt, M.A.; Wiser, M.F.; Leffler, H.; Herrmann, J.; Xia, Y.R.; Massa, S.M.; Cooper, D.N.; Lusis, A.J.; Barondes, S.H. Sequence and mapping of galectin-5, a β-galactoside-binding lectin, found in rat erythrocytes. J. Biol. Chem. 1995, 270, 5032–5038. [Google Scholar] [CrossRef]
- Madsen, P.; Rasmussen, H.H.; Flint, T.; Gromov, P.; Kruse, T.A.; Honore, B.; Vorum, H.; Celis, J.E. Cloning, expression, and chromosome mapping of human galectin-7. J. Biol. Chem. 1995, 270, 5823–5829. [Google Scholar] [CrossRef]
- Leonidas, D.D.; Vatzaki, E.H.; Vorum, H.; Celis, J.E.; Madsen, P.; Acharya, K.R. Structural basis for the recognition of carbohydrates by human galectin-7. Biochemistry 1998, 37, 13930–13940. [Google Scholar] [PubMed]
- Leonidas, D.D.; Elbert, B.L.; Zhou, Z.; Leffler, H.; Ackerman, S.J.; Acharya, K.R. Crystal structure of human Charcot-Leyden crystal protein, an eosinophil lysophospholipase, identifies it as a new member of the carbohydrate-binding family of galectins. Structure 1995, 3, 1379–1393. [Google Scholar] [PubMed]
- Miura, T.; Takahashi, M.; Horie, H.; Kurushima, H.; Tsuchimoto, D.; Sakumi, K.; Nakabeppu, Y. Galectin-1β, a natural monomeric form of galectin-1 lacking its six amino-terminal residues promotes axonal regeneration but not cell death. Cell Death Differ. 2004, 11, 1076–1083. [Google Scholar] [CrossRef]
- Brewer, F.C. Binding and cross-linking properties of galectins. Biochim. Biophys. Acta 2002, 1572, 255–262. [Google Scholar] [CrossRef]
- Liu, F.T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar]
- Thiemann, S.; Baum, L.G. The road less traveled: Regulation of leukocyte migration across vascular and lymphatic endothelium by galectins. J. Clin. Immunol. 2011, 31, 2–9. [Google Scholar] [PubMed]
- Thiemann, S.; Baum, L.G. Galectins and immune responses-just how do they do those things they do? Annu. Rev. Immunol. 2016, 34, 243–264. [Google Scholar] [CrossRef]
- Liu, F.T.; Yang, R.Y.; Hsu, D.K. Galectins in acute and chronic inflammation. Ann. N.Y. Acad. Sci. 2012, 1253, 80–91. [Google Scholar]
- Kaltner, H.; Toegel, S.; Caballero, G.G.; Manning, J.C.; Ledeen, R.W.; Gabius, H.-J. Galectins: Their network and roles in immunity/tumor growth control. Histochem. Cell Biol. 2017, 147, 239–256. [Google Scholar]
- Demetriou, M.; Nabi, I.R.; Dennis, J.W. Galectins as adaptors: Linking glycosylation and metabolism with extracellular cues. Trends Glycosci. Glycotechnol. 2018, 30, 167–177. [Google Scholar]
- Kasai, K. Galectins: Quadruple-faced proteins. Trends Glycosci. Glycotechnol. 2018, 30, 221–223. [Google Scholar] [CrossRef]
- de Jong, C.; Gabius, H.-J.; Baron, W. The emerging role of galectins in (re)myelination and its potential for developing new approaches to treat multiple sclerosis. Cell. Mol. Life Sci. 2020, 77, 1289–1317. [Google Scholar] [PubMed]
- Caballero, G.; Kaltner, H.; Kutzner, T.J.; Ludwig, A.K.; Manning, J.C.; Schmidt, S.; Sinowatz, F.; Gabius, H.-J. How galectins have become multifunctional proteins. Histol. Histopathol. 2020, 35, 509–539. [Google Scholar]
- Timoshenko, A.V.; Gorudko, I.V.; Maslakova, O.V.; André, S.; Kuwabara, I.; Liu, F.-T.; Kaltner, H.; Gabius, H.-J. Analysis of selected blood and immune cell responses to carbohydrate-dependent surface binding of proto- and chimera-type galectins. Mol. Cell. Biochem. 2003, 250, 139–149. [Google Scholar] [PubMed]
- Hong, M.-H.; Weng, I.C.; Liu, F.-T. Galectins as intracellular regulators of cellular responses through the detection of damaged endocytic vesicles. Trends Glycosci. Glycotechnol. 2018, 30, 179–184. [Google Scholar]
- Sato, S. Cytosolic galectins and their release and roles as carbohydrate-binding proteins in host–pathogen interaction. Trends Glycosci. Glycotechnol. 2018, 30, 199–209. [Google Scholar]
- Leffler, H. Galectins structure and function—A synopsis. Results Probl. Cell Differ. 2001, 33, 57–83. [Google Scholar]
- Neri, D.; Bicknell, R. Tumour vascular targeting. Nat. Rev. Cancer 2005, 5, 436–446. [Google Scholar]
- Perillo, N.L.; Pace, K.E.; Seilhamer, J.J.; Baum, L.G. Apoptosis of T cells mediated by galectin- 1. Nature 1995, 378, 736–739. [Google Scholar] [CrossRef]
- Cherayil, B.J.; Weiner, S.J.; Pillai, S. The Mac-2 antigen is a galactose-specific lectin that binds IgE. J. Exp. Med. 1989, 170, 1959–1972. [Google Scholar] [CrossRef]
- Gil, C.D.; La, M.; Perretti, M.; Oliani, S.M. Interaction of human neutrophils with endothelial cells regulates the expression of endogenous proteins annexin 1, galectin-1 and galectin-3. Cell Biol. Int. 2006, 30, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.; Follin, P.; Leffler, H.; Dahlgren, C. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood 1998, 91, 3430–3438. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.L.; Zhao, X.Q.; Jiang, C.; You, Y.; Chen, X.P.; Jiang, Y.Y.; Jia, X.M.; Lin, X. C-type lectin receptors dectin-3 and dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 2013, 39, 324–334. [Google Scholar] [CrossRef]
- Ostrop, J.; Lang, R. Contact, collaboration, and conflict: Signal integration of Syk-coupled C-type lectin receptors. J. Immunol. 2017, 198, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Vértesy, S.; Michalak, M.; Miller, M.C.; Schnölzer, M.; André, S.; Kopitz, J.; Mayo, K.H.; Gabius, H.-J. Structural significance of galectin design: Impairment of homodimer stability by linker insertion and partial reversion by ligand presence. Prot. Eng. Des. Sel. 2015, 28, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Moussodia, R.O.; Murzeau, C.; Sun, H.J.; Klein, M.L.; Vértesy, S.; André, S.; Roy, R.; Gabius, H.-J.; Percec, V. Dissecting molecular aspects of cell interactions using glycodendrimersomes with programmable glycan presentation and engineered human lectins. Angew. Chem. Int. Ed. 2015, 54, 4036–4040. [Google Scholar] [CrossRef] [PubMed]
- Papaspyridonos, M.; McNeill, E.; de Bono, J.P.; Smith, A.; Burnand, K.G.; Channon, K.M.; Greaves, D.R. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 433–440. [Google Scholar] [CrossRef]
- Masamune, A.; Satoh, M.; Hirabayashi, J.; Kasai, K.; Satoh, K.; Shimosegawa, T. Galectin-1 induces chemokine production and proliferation in pancreatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, 729–736. [Google Scholar] [CrossRef]
- Qian, D.; Lu, Z.; Xu, Q.; Wu, P.; Tian, L.; Zhao, L.; Cai, B.; Yin, J.; Wu, Y.; Staveley-O’Carroll, K.F.; et al. Galectin-1-driven upregulation of SDF-1 in pancreatic stellate cells promotes pancreatic cancer metastasis. Cancer Lett. 2017, 397, 43–51. [Google Scholar] [CrossRef]
- Filer, A.; Bik, M.; Parsonage, G.N.; Fitton, J.; Trebilcock, E.; Howlett, K.; Cook, M.; Raza, K.; Simmons, D.L.; Thomas, A.M.; et al. Galectin 3 induces a distinctive pattern of cytokine and chemokine production in rheumatoid synovial fibroblasts via selective signaling pathways. Arthritis Rheumatol. 2009, 60, 1604–1614. [Google Scholar] [CrossRef]
- Toegel, S.; Weinmann, D.; Andre, S.; Walzer, S.M.; Bilban, M.; Schmidt, S.; Chiari, C.; Windhager, R.; Krall, C.; Bennani-Baiti, I.M.; et al. Galectin-1 couples glycobiology to inflammation in osteoarthritis through the activation of an NF-κB-regulated gene network. J. Immunol. 2016, 196, 1910–1921. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, D.; Kenn, M.; Schmidt, S.; Schmidt, K.; Walzer, S.M.; Kubista, B.; Windhager, R.; Schreiner, W.; Toegel, S.; Gabius, H.-J. Galectin-8 induces functional disease markers in human osteoarthritis and cooperates with galectins-1 and -3. Cell. Mol. Life Sci. 2018, 75, 4187–4205. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Duckworth, C.A.; Fu, B.; Pritchard, D.M.; Rhodes, J.M.; Yu, L.G. Circulating galectins -2, -4 and -8 in cancer patients make important contributions to the increased circulation of several cytokines and chemokines that promote angiogenesis and metastasis. Br. J. Cancer 2014, 110, 741–752. [Google Scholar] [CrossRef]
- Cattaneo, V.; Tribulatti, M.V.; Carabelli, J.; Carestia, A.; Schattner, M.; Campetella, O. Galectin-8 elicits pro-inflammatory activities in the endothelium. Glycobiology 2014, 24, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Carabelli, J.; Quattrocchi, V.; D’Antuono, A.; Zamorano, P.; Tribulatti, M.V.; Campetella, O. Galectin-8 activates dendritic cells and stimulates antigen-specific immune response elicitation. J. Leukoc. Biol. 2017, 102, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Nesmelova, I.V.; Pang, M.; Baum, L.G.; Mayo, K.H. 1H, 13C, and 15N backbone and side-chain chemical shift assignments for the 29 kDa human galectin-1 protein dimer. Biomol. NMR Assign. 2008, 2, 203–205. [Google Scholar] [CrossRef]
- Ippel, H.; Miller, M.C.; Berbis, M.A.; Suylen, D.; André, S.; Hackeng, T.M.; Cañada, F.J.; Weber, C.; Gabius, H.-J.; Jiménez-Barbero, J.; et al. 1H, 13C, and 15N backbone and side-chain chemical shift assignments for the 36 proline-containing, full length 29 kDa human chimera-type galectin-3. Biomol. NMR Assign. 2015, 9, 59–63. [Google Scholar] [CrossRef]
- Crump, M.P.; Gong, J.H.; Loetscher, P.; Rajarathnam, K.; Amara, A.; Arenzana-Seisdedos, F.; Virelizier, J.L.; Baggiolini, M.; Sykes, B.D.; Clark-Lewis, I. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 1997, 16, 6996–7007. [Google Scholar] [CrossRef]
- Gozansky, E.K.; Louis, J.M.; Caffrey, M.; Clore, G.M. Mapping the binding of the N-terminal extracellular tail of the CXCR4 receptor to stromal cell-derived factor-1α. J. Mol. Biol. 2005, 345, 651–658. [Google Scholar] [CrossRef]
- Hsu, D.K.; Zuberi, R.I.; Liu, F.T. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J. Biol. Chem. 1992, 267, 14167–14174. [Google Scholar] [CrossRef]
- Herrmann, J.; Turck, C.W.; Atchison, R.E.; Huflejt, M.E.; Poulter, L.; Gitt, M.A.; Burlingame, A.L.; Barondes, S.H.; Leffler, H. Primary structure of the soluble lactose binding lectin L-29 from rat and dog and interaction of its non-collagenous proline-, glycine-tyrosine-rich sequence with bacterial and tissue collagenase. J. Biol. Chem. 1993, 268, 26704–26711. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, J.; Fridman, R.; Nangia-Makker, P.; Kleiner, D.E.; Liotta, L.A.; Stetler-Stevenson, W.G.; Raz, A. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry 1994, 33, 14109–14114. [Google Scholar] [CrossRef] [PubMed]
- Rajarathnam, K.; Desai, U.R. Structural insights into how proteoglycans determine chemokine–CXCR1/CXCR2 interactions: Progress and challenges. Front. Immunol. 2020, 11, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Talaga, M.L.; Fan, N.; Fueri, A.L.; Brown, R.K.; Bandyopadhyay, P.; Dam, T.K. Multitasking human lectin galectin-3 interacts with sulfated glycosaminoglycans and chondroitin sulfate proteoglycans. Biochemistry 2016, 55, 4541–4551. [Google Scholar] [CrossRef]
- Peranzoni, E.; Lemoine, J.; Vimeux, L.; Feuillet, V.; Barrin, S.; Kantari-Mimoun, C.; Bercovici, N.; Guerin, M.; Biton, J.; Ouakrim, H.; et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc. Natl. Acad. Sci. USA 2018, 115, 4041–4050. [Google Scholar] [CrossRef]
- Gordon-Alonso, M.; Hirsch, T.; Wildmann, C.; van der Bruggen, P. Galectin-3 captures interferon-γ in the tumor matrix reducing chemokine gradient production and T-cell tumor infiltration. Nat. Commun. 2017, 8, 793–798. [Google Scholar] [CrossRef]
- von Hundelshausen, P.; Wichapong, K.; Gabius, H.-J.; Mayo, K.H. The marriage of chemokines and galectins as functional heterodimers. Cell. Mol. Life Sci. 2021, 78, 8073–8095. [Google Scholar] [CrossRef]

| Heterodimer | Functional Effects | References |
|---|---|---|
| CXCL4–CXCL8 | Enhance EC proliferation, Baf3 cell migration | [3] |
| CCL3/4, CCL2/8 | Activate CCR5 intra-cellular signaling | [1] |
| CXCL4–CCL5 | Modulate GPCR-mediated signal transduction | [4,5,6] |
| CXCL4L1-MIF | Regulate inflammation and thrombus formation | [55] |
| Gal-7-Gal-1 or Gal-3 | Increase EC and leukocyte cell surface binding | [7] |
| Enhance hemagglutination and leukocyte apoptosis | ||
| Attenuate EC growth and migration | ||
| Gal-3-CXCL12 | Inhibit CXCR3-expressing lymphocyte migration | [9,166] |
| Enhance upregulation of CXCL9, L10, L11 | ||
| Inhibit CXCL12-induced T-cell, neutrophil migration | ||
| Gal-3-CCL26 | Inhibit CCL26-induced eosinophil migration | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayo, K.H. Heterologous Interactions with Galectins and Chemokines and Their Functional Consequences. Int. J. Mol. Sci. 2023, 24, 14083. https://doi.org/10.3390/ijms241814083
Mayo KH. Heterologous Interactions with Galectins and Chemokines and Their Functional Consequences. International Journal of Molecular Sciences. 2023; 24(18):14083. https://doi.org/10.3390/ijms241814083
Chicago/Turabian StyleMayo, Kevin H. 2023. "Heterologous Interactions with Galectins and Chemokines and Their Functional Consequences" International Journal of Molecular Sciences 24, no. 18: 14083. https://doi.org/10.3390/ijms241814083
APA StyleMayo, K. H. (2023). Heterologous Interactions with Galectins and Chemokines and Their Functional Consequences. International Journal of Molecular Sciences, 24(18), 14083. https://doi.org/10.3390/ijms241814083





