The Expression of Major Facilitator Superfamily Domain-Containing Protein2a (Mfsd2a) and Aquaporin 4 Is Altered in the Retinas of a 5xFAD Mouse Model of Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
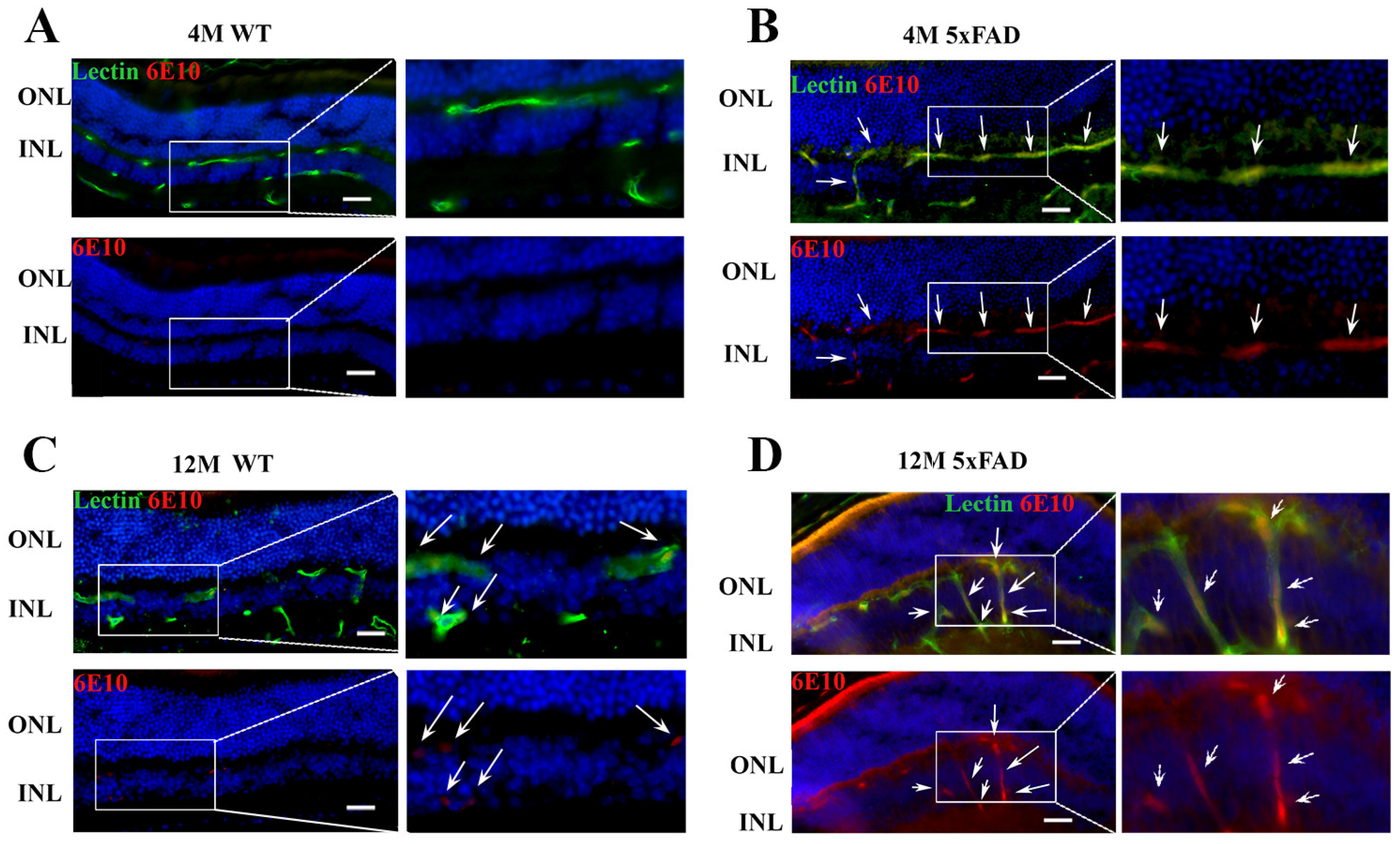
2.1. Amyloid β Accumulates in Retinal Blood Vessels in 4 M and 12 M 5xFAD and 12 M WT Mice
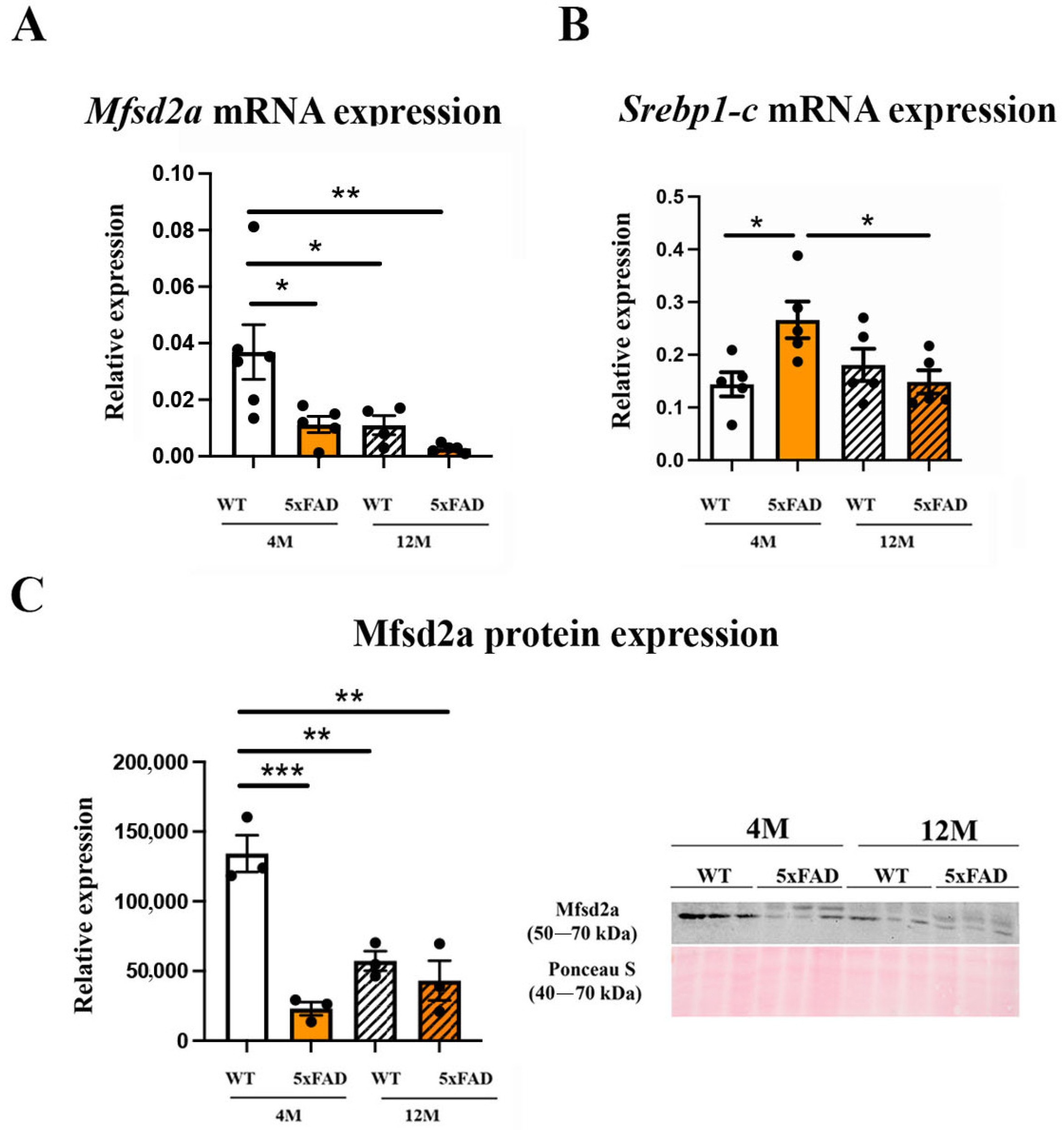
2.2. Mfsd2a mRNA and Protein Expression Are Decreased in the 4 M and 12 M 5xFAD and 12 M WT Retinas
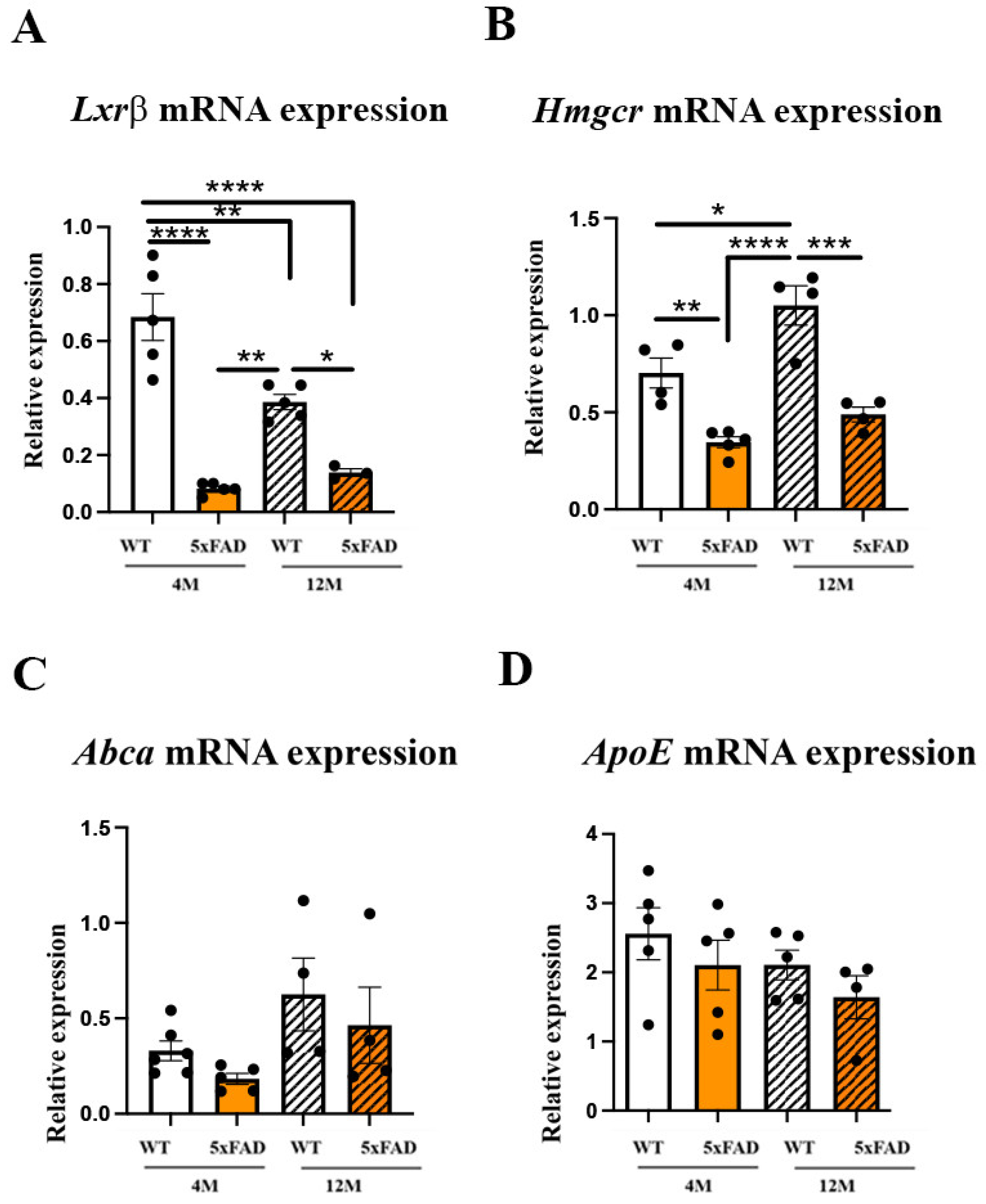
2.3. The Expression Levels of Genes Regulating Cholesterol Synthesis Are Altered in the 4 M and 12 M 5xFAD and 12 M WT Retinas, While the Expression Levels of Genes Regulating Cholesterol Transport Remain Unchanged
2.4. Aqp4 mRNA and Protein Expression Are Increased in 4 M and 12 M 5xFAD and 12 M WT Retinas
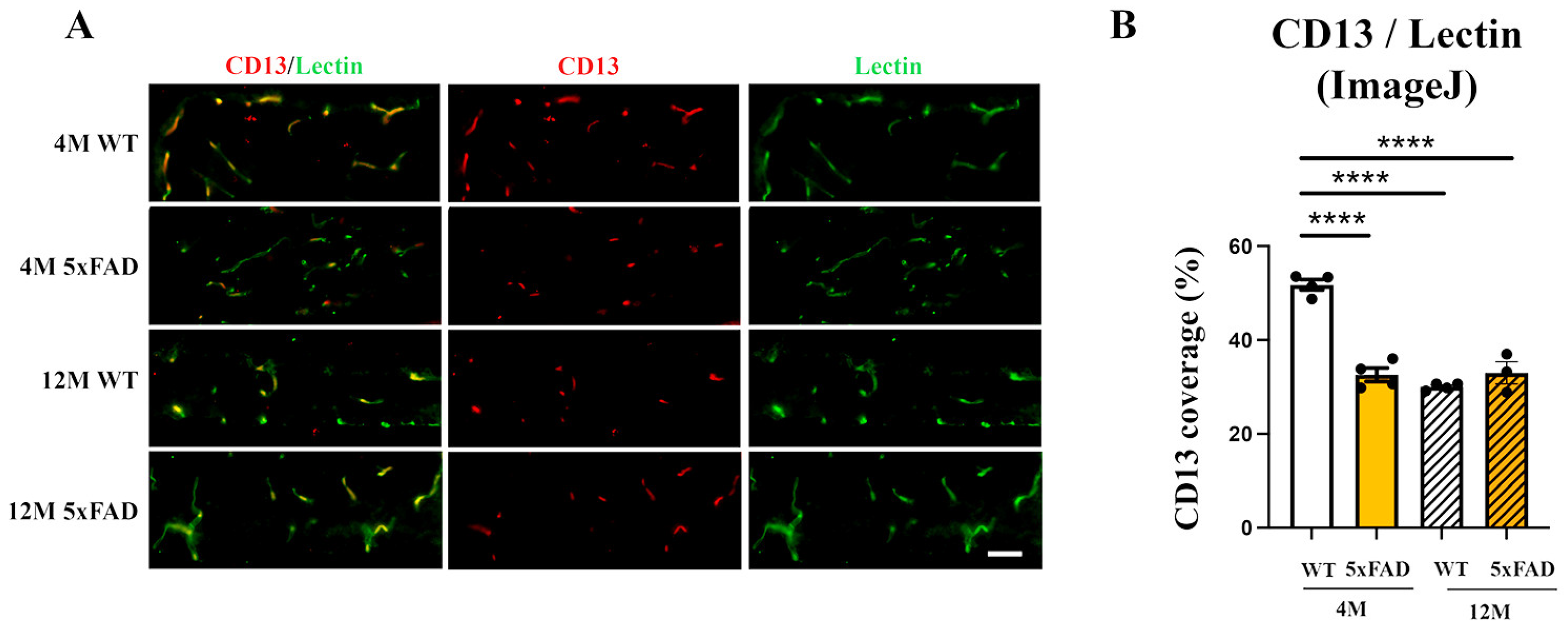
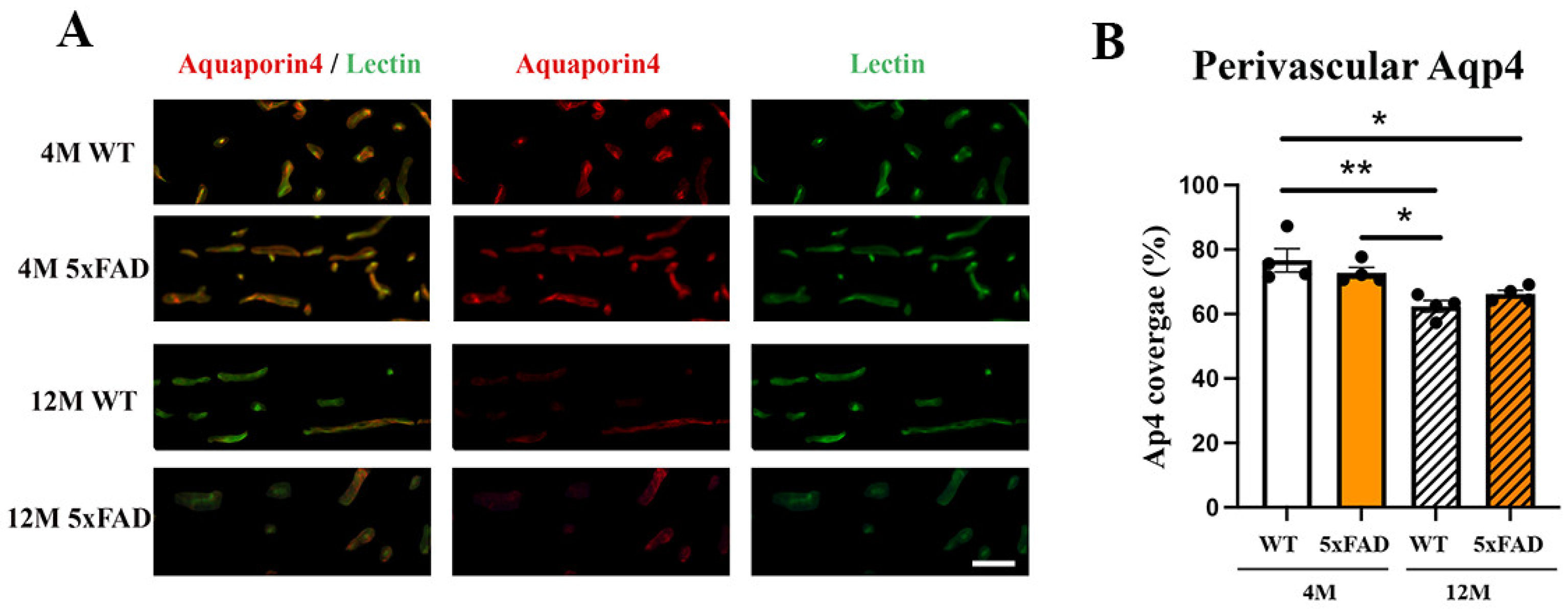
2.5. Perivascular Aqp4 Expression Is Decreased in 12 M Wild-Type and 5xFAD Retinas
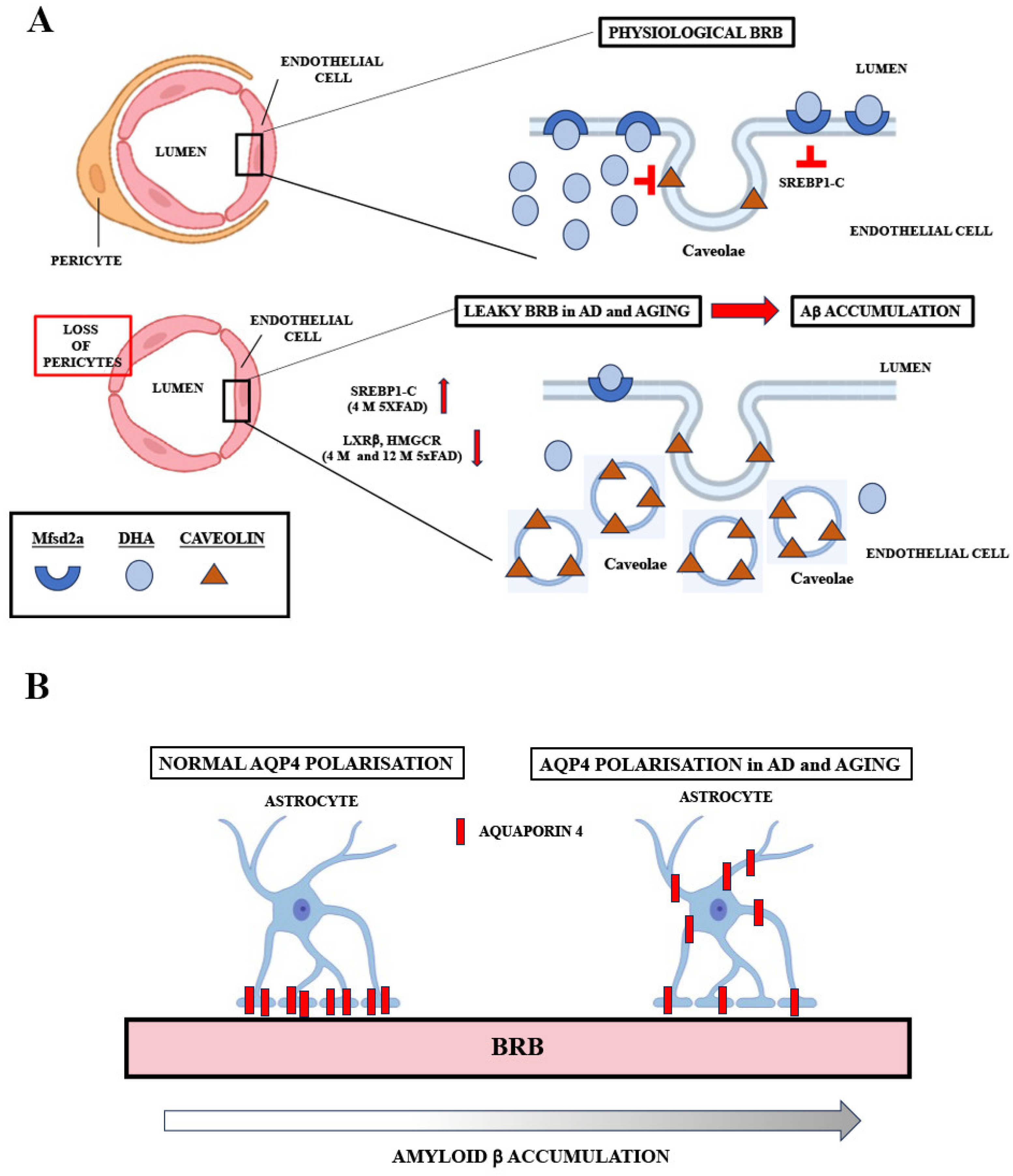
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Tissue Collection
4.3. Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR)
4.3.1. RNA Isolation, and Reverse Transcription
4.3.2. Quantitative Real-Time RT-PCR (qRT-PCR)
4.4. Western Blot Analysis
4.5. Immunohistochemistry
4.6. Quantification of Perivascular Aqp4 Expression
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breteler, M.M. Vascular risk factors for Alzheimer’s disease: An epidemiologic perspective. Neurobiol. Aging 2000, 21, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.D.; Zlokovic, B.V. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009, 118, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Koronyo, Y.; Rentsendorj, A.; Fuchs, D.T.; Sheyn, J.; Black, K.L.; Mirzaei, N.; Koronyo-Hamaoui, M. Retinal Vasculopathy in Alzheimer’s Disease. Front. Neurosci. 2021, 15, 731614. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.O.; Preston, S.D.; Subash, M.; Carare, R.O. Cerebral amyloid angiopathy in the aetiology and immunotherapy of Alzheimer disease. Alzheimer’s Res. Ther. 2009, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Zipser, B.D.; Johanson, C.E.; Gonzalez, L.; Berzin, T.M.; Tavares, R.; Hulette, C.M.; Vitek, M.P.; Hovanesian, V.; Stopa, E.G. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol. Aging 2007, 28, 977–986. [Google Scholar] [CrossRef] [PubMed]
- van de Haar, H.J.; Jansen, J.F.A.; van Osch, M.J.P.; van Buchem, M.A.; Muller, M.; Wong, S.M.; Hofman, P.A.M.; Burgmans, S.; Verhey, F.R.J.; Backes, W.H. Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging. Neurobiol. Aging 2016, 45, 190–196. [Google Scholar] [CrossRef]
- Marchesi, V.T. Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: Implications for early detection and therapy. FASEB J. 2011, 25, 5–13. [Google Scholar] [CrossRef]
- Carare, R.O.; Hawkes, C.A.; Jeffrey, M.; Kalaria, R.N.; Weller, R.O. Review: Cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy. Neuropathol. Appl. Neurobiol. 2013, 39, 593–611. [Google Scholar] [CrossRef]
- Deane, R.; Bell, R.D.; Sagare, A.; Zlokovic, B.V. Clearance of amyloid-beta peptide across the blood-brain barrier: Implication for therapies in Alzheimer’s disease. CNS Neurol. Dis. Drug Targets 2009, 8, 16–30. [Google Scholar] [CrossRef]
- Zlokovic, B.V.; Deane, R.; Sagare, A.P.; Bell, R.D.; Winkler, E.A. Low-density lipoprotein receptor-related protein-1: A serial clearance homeostatic mechanism controlling Alzheimer’s amyloid beta-peptide elimination from the brain. J. Neurochem. 2010, 115, 1077–1089. [Google Scholar] [CrossRef]
- Schultz, N.; Brannstrom, K.; Byman, E.; Moussaud, S.; Nielsen, H.M.; Netherlands Brain Bank; Olofsson, A.; Wennstrom, M. Amyloid-beta 1–40 is associated with alterations in NG2+ pericyte population ex vivo and in vitro. Aging Cell 2018, 17, e12728. [Google Scholar] [CrossRef]
- Kook, S.Y.; Hong, H.S.; Moon, M.; Ha, C.M.; Chang, S.; Jung, I.M. Abeta1–42-RAGE interaction disrupts tight junctions of the blood–brain barrier via Ca2+-calcineurin signaling. J. Neurosci. 2012, 32, 8845–8854. [Google Scholar] [CrossRef]
- Hunter, J.M.; Kwan, J.; Ahmadi, M.M.; Maarouf, C.L.; Kokjohn, T.A.; Belden, C.; Sabbagh, M.N.; Beach, T.G.; Roher, A.E. Morphological and pathological evolution of the brain microcirculation in aging and Alzheimer’s disease. PLoS ONE 2012, 7, e36893. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Sagare, A.P.; Ma, Q.; Halliday, M.R.; Kong, P.; Kisler, K.; Winkler, E.A.; Ramanathan, A.; Kanekiyo, T.; Bu, G.; et al. Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat. Neurosci. 2015, 18, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.R.; Thore, C.R. Review: Cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol. Appl. Neurobiol. 2011, 37, 56–74. [Google Scholar] [CrossRef]
- Shi, H.; Koronyo, Y.; Rentsendorj, A.; Regis, G.C.; Sheyn, J.; Fuchs, D.T.; Kramerov, A.A.; Ljubimov, A.V.; Dumitrascu, O.M.; Rodriguez, A.R.; et al. Identification of early pericyte loss and vascular amyloidosis in Alzheimer’s disease retina. Acta Neuropathol. 2020, 139, 813–836. [Google Scholar] [CrossRef]
- Koronyo, Y.; Biggs, D.; Barron, E.; Boyer, D.S.; Pearlman, J.A.; Au, W.J.; Kile, S.J.; Blanco, A.; Fuchs, D.-T.; Ashfaq, A.; et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight 2017, 2, e93621. [Google Scholar] [CrossRef]
- Shi, H.; Koronyo, Y.; Fuchs, D.-T.; Sheyn, J.; Jallow, O.; Mandalia, K.; Graham, S.L.; Gupta, V.K.; Mirzaei, M.; Kramerov, A.A.; et al. Retinal arterial Aβ40 deposition is linked with tight junction loss and cerebral amyloid angiopathy in MCI and AD patients. Alzheimer’s Dement. 2023, in press. [Google Scholar] [CrossRef]
- Zhang, M.; Zhong, L.; Han, X.; Xiong, G.; Xu, D.; Zhang, S.; Cheng, H.; Chiu, K.; Xu, Y. Brain and Retinal Abnormalities in the 5xFAD Mouse Model of Alzheimer’s Disease at Early Stages. Front. Neurosci. 2021, 15, 681831. [Google Scholar] [CrossRef]
- Shi, H.; Koronyo, Y.; Fuchs, D.T.; Sheyn, J.; Wawrowsky, K.; Lahiri, S.; Black, K.L.; Koronyo-Hamaoui, M. Retinal capillary degeneration and blood-retinal barrier disruption in murine models of Alzheimer’s disease. Acta Neuropathol. Commun. 2020, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Andreone, B.J.; Chow, B.W.; Tata, A.; Lacoste, B.; Ben-Zvi, A.; Bullock, K.; Deik, A.A.; Ginty, D.D.; Clish, C.B.; Gu, C. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 2017, 94, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.W.; Gu, C. Gradual Suppression of Transcytosis Governs Functional Blood-Retinal Barrier Formation. Neuron 2017, 93, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef]
- Yang, Y.-R.; Xiong, X.-Y.; Liu, J.; Wu, L.-R.; Zhong, Q.; Zhou, K.; Meng, Z.-Y.; Liu, L.; Wang, F.-X.; Gong, Q.-W.; et al. Mfsd2a (Major Facilitator Superfamily Domain Containing 2a) Attenuates Intracerebral Hemorrhage-Induced Blood-Brain Barrier Disruption by Inhibiting Vesicular Transcytosis. J. Am. Heart Assoc. 2017, 6, e005811. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Wang, H.-L.; Li, P.-C.; Hong, C.-D.; Chen, A.-Q.; Qiu, Y.-M.; Zeng, A.-P.; Zhou, Y.-F.; Hu, B.; Li, Y.-N. Mfsd2a overexpression alleviates vascular dysfunction in diabetic retinopathy. Pharm. Res. 2021, 171, 105755. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.H.; Huang, S.; Fu, Z.; Tomita, Y.; Britton, W.R.; Cho, S.S.; Chen, C.T.; Sun, Y.; Ma, J.X.; et al. Wnt signaling activates MFSD2A to suppress vascular endothelial transcytosis and maintain blood-retinal barrier. Sci. Adv. 2020, 6, eaba7457. [Google Scholar] [CrossRef]
- Qu, C.; Song, H.; Shen, J.; Xu, L.; Li, Y.; Qu, C.; Li, T.; Zhang, J. Mfsd2a Reverses Spatial Learning and Memory Impairment Caused by Chronic Cerebral Hypoperfusion via Protection of the Blood–Brain Barrier. Front. Neurosci. 2020, 14, 461. [Google Scholar] [CrossRef]
- Iwao, T.; Takata, F.; Matsumoto, J.; Aridome, H.; Yasunaga, M.; Yokoya, M.; Kataoka, Y.; Dohgu, S. Aging decreases docosahexaenoic acid transport across the blood-brain barrier in C57BL/6J mice. PLoS ONE 2023, 18, e0281946. [Google Scholar] [CrossRef]
- Jessen, N.A.; Munk, A.S.; Lundgaard, I.; Nedergaard, M. The glymphatic system: A beginner’s guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. Paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lou, N.; Eberhardt, A.; Yang, Y.; Kusk, P.; Xu, Q.; Forstera, B.; Peng, S.; Shi, M.; Ladron-de-Guevara, A.; et al. An ocular glymphatic clearance system removes β-amyloid from the rodent eye. Sci. Transl. Med. 2020, 12, eaaw3210. [Google Scholar] [CrossRef]
- Kitchen, P.; Salman, M.M.; Halsey, A.M.; Clarke-Bland, C.; MacDonald, J.A.; Ishida, H.; Vogel, H.J.; Almutri, S.; Logan, A.; Kreida, S.; et al. Targeting aquaporin-4 subcellular localization to treat central nervous system edema. Cell 2020, 181, 784–799.e19. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, D.M.; Simon, M.; Haswell, J.D.; D’Abreo, D.; Murchison, C.; Quinn, J.F.; Grafe, M.R.; Woltjer, R.L.; Kaye, J.; Iliff, J.J. Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol. 2017, 74, 91. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, A.; Yamamoto, T.; Shimizu, K.; Yoshikazu, U.; Nishizawa, M.; Takahashi, H.; Kakita, A. Characteristics of aquaporin expression surrounding senile plaques and cerebral amyloid angiopathy in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2012, 71, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Moftakhar, P.; Lynch, M.D.; Pomakian, J.L.; Vinters, H.V. Aquaporin expression in the brains of patients with or without cerebral amyloid angiopathy. J. Neuropathol. Exp. Neurol. 2010, 69, 1201–1209. [Google Scholar] [CrossRef]
- Yang, J.; Lunde, L.K.; Nuntagij, P.; Oguchi, T.; Camassa, L.M.A.; Nilsson, L.N.G.; Xu, Y.; Amiry-Moghaddam, M.; Ottersen, O.P.; Torp, R. Loss of astrocyte polarisation in the Tg-ArcSwe mouse model of Alzheimers disease. J. Alzheimer’s Dis. 2011, 27, 711–722. [Google Scholar] [CrossRef]
- Ishida, K.; Misawa, K.; Nishimura, H.; Hirata, T.; Yamamoto, M.; Ota, N. 5-Caffeoylquinic Acid Ameliorates Cognitive Decline and Reduces Aβ Deposition by Modulating Aβ Clearance Pathways in APP/PS2 Transgenic Mice. Nutrients 2020, 12, 494. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal β-Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice with Five Familial Alzheimer’s Disease Mutations: Potential Factors in Amyloid Plaque Formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Giannoni, P.; Arango-Lievano, M.; Neves, I.D.; Rousset, M.C.; Baranger, K.; Rivera, S.; Jeanneteau, F.; Claeysen, S.; Marchi, N. Cerebrovascular pathology during the progression of experimental Alzheimer’s disease. Neurobiol. Dis. 2016, 88, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, R.; Chow, K.M.; Derafshi, Z.; Fautsch, M.P.; Hetling, J.R.; Rodgers, D.W.; Hersh, L.B.; Pepperberg, D.R. Reduction of amyloid-beta levels in mouse eye tissues by intra-vitreally delivered neprilysin. Exp. Eye Res. 2015, 138, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Matei, N.; Leahy, S.; Blair, N.P.; Burford, J.; Rahimi, M.; Shahidi, M. Retinal Vascular Physiology Biomarkers in a 5XFAD Mouse Model of Alzheimer’s Disease. Cells 2022, 11, 2413. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Alldred, M.J.; Ginsberg, S.D.; Ohno, M. Sex- and brain region-specific acceleration of β-amyloidogenesis following behavioral stress in a mouse model ofAlzheimer’s disease. Mol. Brain 2010, 3, 34. [Google Scholar] [CrossRef]
- Walker, K.A.; Chen, J.; Shi, L.; Yang, Y.; Fornage, M.; Zhou, L.; Schlosser, P.; Surapaneni, A.; Grams, M.E.; Duggan, M.R.; et al. Proteomics analysis of plasma from middle-aged adults identifies protein markers of dementia risk in later life. Sci. Transl. Med. 2023, 15, eadf5681. [Google Scholar] [CrossRef]
- Zhao, Z.; Zlokovic, B.V. Blood-brain barrier: A dual life of MFSD2A? Neuron 2014, 82, 728–730. [Google Scholar] [CrossRef]
- Lobanova, E.S.; Schuhmann, K.; Finkelstein, S.; Lewis, T.R.; Cady, M.A.; Hao, Y.; Keuthan, C.; Ash, J.D.; Burns, M.E.; Shevchenko, A.; et al. Disrupted Blood-Retina Lysophosphatidylcholine Transport Impairs Photoreceptor Health But Not Visual Signal Transduction. J. Neurosci. 2019, 39, 9689–9701. [Google Scholar] [CrossRef]
- Li, P.; Wu, Y.; Hamlett, E.D.; Goodwin, A.J.; Halushka, P.V.; Carroll, S.L.; Liu, M.; Fan, H. Suppression of Fli-1 protects against pericyte loss and cognitive deficits in Alzheimer’s disease. Mol. Ther. 2022, 30, 1451–1464. [Google Scholar] [CrossRef]
- Kalaany, N.Y.; Mangelsdorf, D.J. LXRs and FXR: The yin and yang of cholesterol and fat metabolism. Ann. Rev. Physiol. 2006, 68, 159–191. [Google Scholar] [CrossRef]
- Siperstein, M.D.; Fagan, V.M. Feedback control of mevalonate synthesis by dietary cholesterol. J. Biol. Chem. 1966, 241, 602–609. [Google Scholar] [CrossRef]
- Mauch, D.H.; Nagler, K.; Schumacher, S.; Goritz, C.; Muller, E.C.; Otto, A.; Pfrieger, F.W. CNS synaptogenesis promoted by glia-derived cholesterol. Science 2001, 294, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A.; et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Rasool, S.; Yang, Z.; Glabe, C.G.; Schreiber, S.S.; Ge, J.; Tan, Z. Amyloid-peptide vaccinations reduce {beta}-amyloid plaques but exacerbate vascular deposition and inflammation in the retina of Alzheimer’s transgenic mice. Am. J. Pathol. 2009, 175, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Son, T.; Klatt, D.; Yao, X. Concurrent OCT and OCT angiography of retinal neurovascular degeneration in the 5XFAD Alzheimer’s disease mice. Neurophotonics 2021, 8, 035002. [Google Scholar] [CrossRef]
- Lim, J.K.H.; Li, Q.X.; He, Z.; Vingrys, A.J.; Chinnery, H.R.; Mullen, J.; Bui, B.V.; Nguyen, C.T.O. Retinal Functional and Structural Changes in the 5xFAD Mouse Model of Alzheimer’s Disease. Front. Neurosci. 2020, 14, 862. [Google Scholar] [CrossRef]
- Zhukov, O.; He, C.; Soylu-Kucharz, R.; Cai, C.; Lauritzen, A.D.; Aldana, B.I.; Björkqvist, M.; Lauritzen, M.; Kucharz, K. Preserved blood-brain barrier and neurovascular coupling in female 5xFAD model of Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1089005. [Google Scholar] [CrossRef]
- Chan, J.P.; Wong, B.H.; Chin, C.F.; Galam, D.L.A.; Foo, J.C.; Wong, L.C.; Ghosh, S.; Wenk, M.R.; Gassiot, A.C.; Silver, D.L. The lysolipid transporter Mfsd2a regulates lipogenesis in the developing brain. PLoS Biol. 2018, 16, e2006443. [Google Scholar] [CrossRef]
- Boergesen, M.; Pedersen, T.A.; Gross, B.; van Heeringen, S.J.; Hagenbeek, D.; Bindesboll, C.; Caron, S.; Lalloyer, F.; Steffensen, K.R.; Nebb, H.I.; et al. Genome-wide profiling of liver X receptor, retinoid X receptor, and peroxisome proliferator-activated receptor alpha in mouse liver reveals extensive sharing of binding sites. Mol. Cell. Biol. 2012, 32, 852–867. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef]
- Chen, W.; Jump, D.B.; Esselman, W.J.; Busik, J.V. Inhibition of cytokine signaling in human retinal endothelial cells through modification of caveolae/lipid rafts by docosahexaenoic acid. Investig. Ophthalmol. Vis. Sci. 2007, 48, 18–26. [Google Scholar] [CrossRef]
- Simon, M.; Wang, M.X.; Ismail, O.; Braun, M.; Schindler, A.G.; Reemmer, J.; Wang, Z.; Haveliwala, M.A.; O’Boyle, R.P.; Han, W.Y.; et al. Loss of perivascular aquaporin-4 localization impairs glymphatic exchange and promotes amyloid β plaque formation in mice. Alzheimer’s Res. Ther. 2022, 14, 59. [Google Scholar] [CrossRef]
- Katoozi, S.; Rao, S.B.; Skauli, N.; Froehner, S.C.; Ottersen, O.P.; Adams, M.E.; Amiry-Moghaddam, M. Functional specialization of retinal Müller cell endfeet depends on an interplay between two syntrophin isoforms. Mol. Brain 2020, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Duan, T.; Verkman, A.S. Aquaporin-4 reduces neuropathology in a mouse model of Alzheimer’s disease by remodeling peri-plaque astrocyte structure. Acta Neuropathol. Commun. 2019, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Ikegawa, N.; Yoshida, K.; Muramatsu, K.; Hattori, S.; Kawai, K.; Murakami, M.; Tanaka, T.; Goda, W.; Goto, M.; et al. Behavioral and electrophysiological evidence for a neuroprotective role of aquaporin-4 in the 5xFAD transgenic mice model. Acta Neuropathol. Commun. 2020, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Rosu, G.C.; Catalin, B.; Balseanu, T.A.; Laurentiu, M.; Claudiu, M.; Kumar-Singh, S.; Daniel, P. Inhibition of Aquaporin 4 Decreases Amyloid Aβ40 Drainage Around Cerebral Vessels. Mol. Neurobiol. 2020, 57, 4720–4734. [Google Scholar] [CrossRef]
- Wang, J.Z.; Xiao, N.; Zhang, Y.Z.; Zhao, C.X.; Guo, X.H.; Lu, L.M. Mfsd2a-based pharmacological strategies for drug delivery across the blood-brain barrier. Pharmacol. Res. 2016, 104, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, D.; Shen, Q.; Gao, L.; Zhuang, P.; Zhang, Y.; Guo, H. Storax Inhibits Caveolae-Mediated Transcytosis at Blood-Brain Barrier After Ischemic Stroke in Rats. Front. Pharmacol. 2022, 13, 876235. [Google Scholar] [CrossRef]
- Sugasini, D.; Park, J.C.; McAnany, J.J.; Kim, T.H.; Ma, G.; Yao, X.; Antharavally, B.; Oroskar, A.; Oroskar, A.A.; Layden, B.T.; et al. Improvement of retinal function in Alzheimer disease-associated retinopathy by dietary lysophosphatidylcholine-EPA/DHA. Sci. Rep. 2023, 13, 9179. [Google Scholar] [CrossRef]
- Macura Jovanovic, I.; Djuricic, I.; Major, T.; Milanovic, D.; Brkic, B.; Sobajic, S.; Kanazir, S.; Ivkovic, S. The high-dose fish oil supplementation increased Mfsd2a expression without altering DHA levels in the healthy retina. J. Funct. Foods 2022, 99, 105302. [Google Scholar] [CrossRef]
- Xu, Z.; Xiao, N.; Chen, Y.; Huang, H.; Marshall, C.; Gao, J.; Cai, Z.; Wu, T.; HU, G.; Xiao, M. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol. Neurodegener. 2015, 10, 58. [Google Scholar] [CrossRef]
- Ren, H.; Luo, C.; Feng, Y.; Yao, X.; Shi, Z.; Liang, F.; Kang, J.X.; Wan, J.B.; Pei, Z.; Su, H. Omega-3 polyunsaturated fatty acids promote amyloid-β clearance from the brain through mediating the function of the glymphatic system. FASEB J. 2017, 31, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Lianoglou, S.; Sandmann, T.; Calvert, M.; Suh, J.H.; Thomsen, E.; Dugas, J.; Pizzo, M.E.; DeVos, S.L.; Earr, T.K.; et al. Novel App knock-in mouse model shows key features of amyloid pathology and reveals profound metabolic dysregulation of microglia. Mol. Neurodegener. 2022, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Method 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Okawa, H.; Sampath, A.P.; Laughlin, S.B.; Fain, G.L. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr. Biol. 2008, 18, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Smedowski, A.; Liu, X.; Podracka, L.; Akhtar, S.; Trzeciecka, A.; Pietrucha-Dutczak, M.; Lewin-Kowalik, J.; Urtti, A.; Ruponen, M.; Kaarniranta, K.; et al. Increased intraocular pressure alters the cellular distribution of HuR protein in retinal ganglion cells—A possible sign of endogenous neuroprotection failure. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 296–306. [Google Scholar] [CrossRef]
- Wang, L.; Mao, X. Role of Retinal Amyloid-β in Neurodegenerative Diseases: Overlapping Mechanisms and Emerging Clinical Applications. Int. J. Mol. Sci. 2021, 22, 2360. [Google Scholar] [CrossRef]

| Gene | Orientation | Sequence |
|---|---|---|
| HMGCR | F (5′-3′) | TTG GTC CTT GTT CAC GCT CAT |
| R (3′-5′) | TTC GCC AGA CCC AAG GAA AC | |
| SREBP1-C | F (5′-3′) | ACG GAG CCA TGG ATT GCA |
| R (3′-5′) | AAG TCA CTG TCT TGG TTG TTGATGA | |
| LXRBETA | F (5′-3′) | AGC GTC CAT TCA GAG CAA GTG |
| R (3′-5′) | CAC TCG TGG ACA TCC CAG ATC T | |
| ABCA | F (5′-3′) | AGG CCG CAC CAT TAT TTT GTC |
| R (3′-5′) | GGC AAT TCT GTC CCC AAG GAT | |
| APOE | F (5′-3′) | GGC CCA GGA GGA GAA TCA ATGA G |
| R (3′-5′) | CCT GGC TGG ATA TGG ATG TTG | |
| MFSD2A | F (5′-3′) | AGA AGC AGC AAC TGT CCA TTT |
| R (3′-5′) | CTC GGC CCA CAA AAA GGA TAA T | |
| HPRT | F (5′-3′) | CTC ATG GAC TGA TTA TGG ACA GGA C |
| R (3′-5′) | GCA GGT CAG CAA AGA ACT TAT AGC C | |
| AQP4 | F (5′-3′) | AGC AAT TGG ATT TTC CGT TG |
| R (3′-5′) | TGA GCT CCA CAT CAG GAC AG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanovic Macura, I.; Zivanovic, A.; Perovic, M.; Ciric, J.; Major, T.; Kanazir, S.; Ivkovic, S. The Expression of Major Facilitator Superfamily Domain-Containing Protein2a (Mfsd2a) and Aquaporin 4 Is Altered in the Retinas of a 5xFAD Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 14092. https://doi.org/10.3390/ijms241814092
Jovanovic Macura I, Zivanovic A, Perovic M, Ciric J, Major T, Kanazir S, Ivkovic S. The Expression of Major Facilitator Superfamily Domain-Containing Protein2a (Mfsd2a) and Aquaporin 4 Is Altered in the Retinas of a 5xFAD Mouse Model of Alzheimer’s Disease. International Journal of Molecular Sciences. 2023; 24(18):14092. https://doi.org/10.3390/ijms241814092
Chicago/Turabian StyleJovanovic Macura, Irena, Ana Zivanovic, Milka Perovic, Jelena Ciric, Tamara Major, Selma Kanazir, and Sanja Ivkovic. 2023. "The Expression of Major Facilitator Superfamily Domain-Containing Protein2a (Mfsd2a) and Aquaporin 4 Is Altered in the Retinas of a 5xFAD Mouse Model of Alzheimer’s Disease" International Journal of Molecular Sciences 24, no. 18: 14092. https://doi.org/10.3390/ijms241814092
APA StyleJovanovic Macura, I., Zivanovic, A., Perovic, M., Ciric, J., Major, T., Kanazir, S., & Ivkovic, S. (2023). The Expression of Major Facilitator Superfamily Domain-Containing Protein2a (Mfsd2a) and Aquaporin 4 Is Altered in the Retinas of a 5xFAD Mouse Model of Alzheimer’s Disease. International Journal of Molecular Sciences, 24(18), 14092. https://doi.org/10.3390/ijms241814092






