Aestivation in Nature: Physiological Strategies and Evolutionary Adaptations in Hypometabolic States
Abstract
:1. Introduction of Animal Aestivation
1.1. Research Background
1.1.1. Several Typical Hypometabolism Regulations
1.1.2. Physiological and Behavioral Characteristics of Aestivation
1.1.3. Comparison of Aestivation in Ectotherms and Endotherms
1.2. Main Elements of Animal Aestivation Research
1.2.1. Antioxidant Defense
1.2.2. Nitrogen Metabolism and Ammonia Detoxification
1.2.3. Lipid Reserve and Lipid Metabolism
1.2.4. Muscle Disuse Atrophy and Muscle Protection
1.2.5. Antibacterial Immune Protection
1.2.6. Visceral Degeneration and Regeneration
1.2.7. Epigenetic Regulation during Aestivation
DNA Methylation
Post-Translational Modifications of Histones
Changes in miRNA Expression
Isolated Preservation of mRNA Transcripts
1.2.8. The Regulatory Network of Aestivation
2. Characteristics and Research Progress of Vertebrate Aestivation
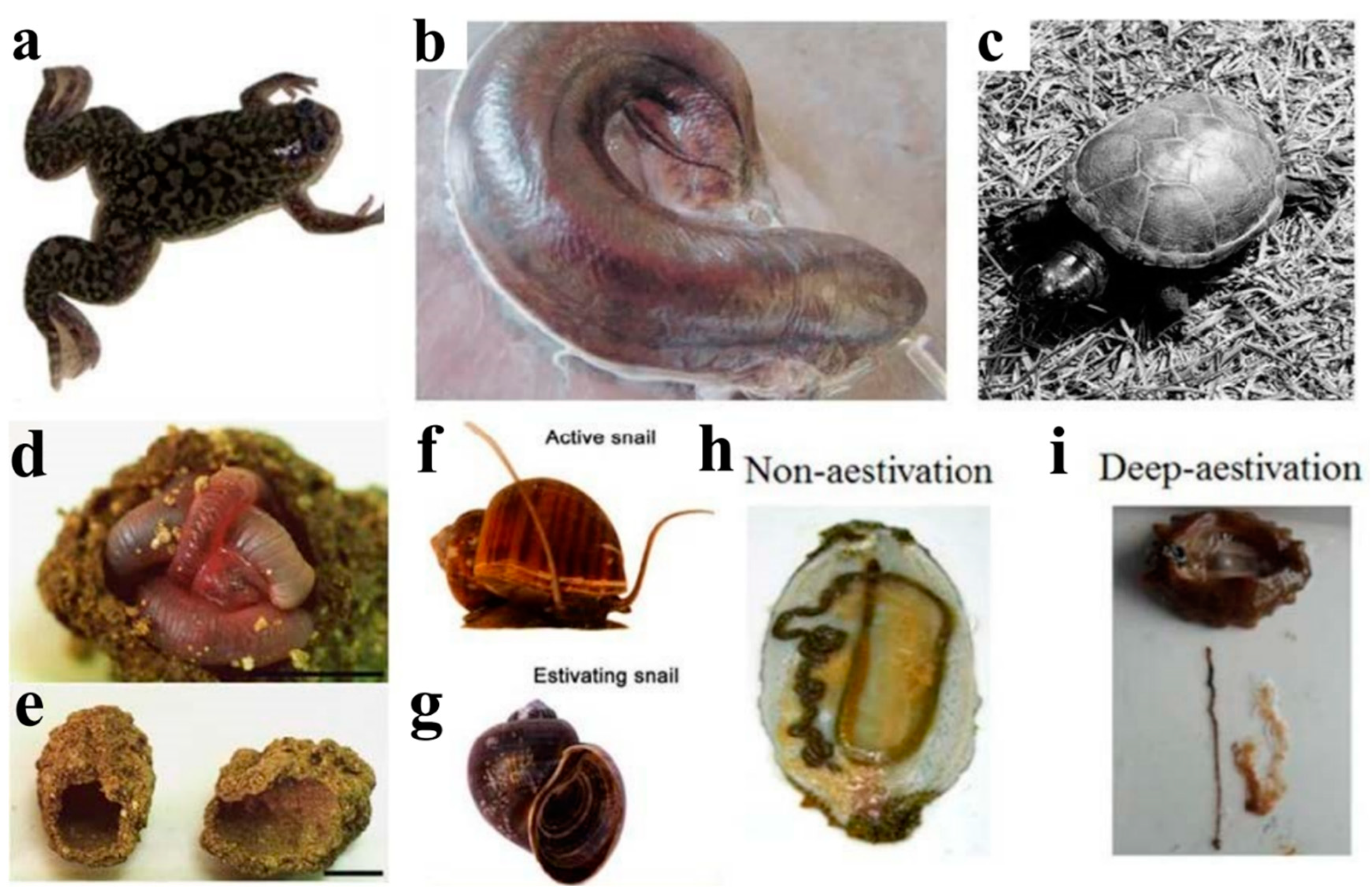
2.1. Amphibians—African Clawed Frog (Xenopus laevis)
2.2. Fish—African lungfish (P. annectens)
2.3. Reptiles—Turtles and Crocodiles
3. Characteristics and Research Progress of Invertebrate Aestivation
3.1. Annelids—Earthworms
3.2. Molluscs
3.2.1. Freshwater Snails (Pomacea canaliculate)
3.2.2. Land Snails (Theba pisana)
3.3. Echinoderms: Sea Cucumber (Apostichopus japonicus)
4. Conclusions
Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| Dormancy | The state of not being active or growing but being able to become active later. |
| Cryptobiosis | This state of “suspended animation” has been observed in a variety of invertebrate animals and plants during extreme environmental conditions. It was first described for invertebrate animals that survived an absence of water by becoming inactive and allowing their tissues to become desiccated. |
| Aestivation | Aestivation is summer dormancy, that is, long-term torpor during the summer for the survival of hot and dry periods. |
| Hibernation | The state of greatly reduced metabolic activity and lowered body temperature adopted by certain mammals as an adaptation to adverse winter conditions. |
| Torpor | The state of lowered body temperature and metabolic activity assumed by many animals in response to adverse environmental conditions, especially cold and heat. The torpid state may last overnight, as in temperate-zone hummingbirds and some insects and reptiles; or it may last for months, as in the case of true hibernation and the winter torpor of many cold-blooded vertebrates. |
| Diapause | Spontaneous interruption of the development of certain animals, marked by a reduction of metabolic activity. It is typical of many insects and mites, a few crustaceans and snails, and perhaps certain other animal groups. |
References
- Yang, H.; Yuan, X.; Zhou, Y.; Mao, Y.; Zhang, T.; Liu, Y. Effects of body size and water temperature on food consumption and growth in the sea cucumber Apostichopus japonicus (Selenka) with special reference to aestivation. Aquacult. Res. 2005, 36, 1085–1092. [Google Scholar] [CrossRef]
- Heimroth, R.D.; Casadei, E.; Benedicenti, O.; Amemiya, C.T.; Muñoz, P.; Salinas, I. The lungfish cocoon is a living tissue with antimicrobial functions. Sci. Adv. 2021, 7, eabj0829. [Google Scholar] [CrossRef] [PubMed]
- Balinsky, J.B.; Choritz, E.L.; Coe, C.G.L.; van der Schans, G.S. Amino acid metabolism and urea synthesis in naturally aestivating Xenopus laevis. Comp. Biochem. Physiol. 1967, 22, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Hoyeck, M.P.; Hadj-Moussa, H.; Storey, K.B. Estivation-responsive microRNAs in a hypometabolic terrestrial snail. PeerJ 2019, 7, e6515. [Google Scholar] [CrossRef] [PubMed]
- Staples, J.F. Metabolic Flexibility: Hibernation, Torpor, and Estivation. Compr. Physiol. 2016, 6, 737–771. [Google Scholar] [CrossRef]
- Salway, K.D.; Tattersall, G.J.; Stuart, J.A. Rapid upregulation of heart antioxidant enzymes during arousal from estivation in the Giant African snail (Achatina fulica). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 157, 229–236. [Google Scholar] [CrossRef]
- Zhao, Z.; Kudej, R.K.; Wen, H.; Fefelova, N.; Yan, L.; Vatner, D.E.; Vatner, S.F.; Xie, L.H. Antioxidant defense and protection against cardiac arrhythmias: Lessons from a mammalian hibernator (the woodchuck). FASEB J. 2018, 32, 4229–4240. [Google Scholar] [CrossRef]
- Chainy, G.B.; Paital, B.; Dandapat, J. An Overview of Seasonal Changes in Oxidative Stress and Antioxidant Defence Parameters in Some Invertebrate and Vertebrate Species. Scientifica 2016, 2016, 6126570. [Google Scholar] [CrossRef]
- Giraud-Billoud, M.; Rivera-Ingraham, G.A.; Moreira, D.C.; Burmester, T.; Castro-Vazquez, A.; Carvajalino-Fernandez, J.M.; Dafre, A.; Niu, C.; Tremblay, N.; Paital, B.; et al. Twenty years of the ‘Preparation for Oxidative Stress’ (POS) theory: Ecophysiological advantages and molecular strategies. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 234, 36–49. [Google Scholar] [CrossRef]
- Hermes-Lima, M.; Storey, J.M.; Storey, K.B. Antioxidant defenses and metabolic depression. The hypothesis of preparation for oxidative stress in land snails. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998, 120, 437–448. [Google Scholar]
- Nowakowska, A.; Rogalska, J.; Caputa, M. Adaptability of antioxidant defence system in Helix pomatia snails: Effect of forced aestivation during early spring. J. Molluscan Stud. 2015, 82, 205–207. [Google Scholar] [CrossRef]
- Nowakowska, A.; Swiderska-Kolacz, G.; Rogalska, J.; Caputa, M. Antioxidants and oxidative stress in Helix pomatia snails during estivation. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 150, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Billoud, M.; Vega, I.A.; Tosi, M.E.; Abud, M.A.; Calderon, M.L.; Castro-Vazquez, A. Antioxidant and molecular chaperone defences during estivation and arousal in the South American apple snail Pomacea canaliculata. J. Exp. Biol. 2013, 216, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Ensminger, D.C.; Salvador-Pascual, A.; Arango, B.G.; Allen, K.N.; Vazquez-Medina, J.P. Fasting ameliorates oxidative stress: A review of physiological strategies across life history events in wild vertebrates. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021, 256, 110929. [Google Scholar] [CrossRef] [PubMed]
- Ip, Y.K.; Peh, B.K.; Tam, W.L.; Wong, W.P.; Chew, S.F. Effects of intra-peritoneal injection with NH4Cl, urea, or NH4Cl+urea on nitrogen excretion and metabolism in the African lungfish Protopterus dolloi. J. Exp. Zool. A Comp. Exp. Biol. 2005, 303, 272–282. [Google Scholar] [CrossRef]
- Loong, A.M.; Ang, S.F.; Wong, W.P.; Portner, H.O.; Bock, C.; Wittig, R.; Bridges, C.R.; Chew, S.F.; Ip, Y.K. Effects of hypoxia on the energy status and nitrogen metabolism of African lungfish during aestivation in a mucus cocoon. J. Comp. Physiol. B 2008, 178, 853–865. [Google Scholar] [CrossRef]
- Hiong, K.C.; Loong, A.M.; Chew, S.F.; Ip, Y.K. Increases in urea synthesis and the ornithine-urea cycle capacity in the giant African snail, Achatina fulica, during fasting or aestivation, or after the injection with ammonium chloride. J. Exp. Zool. A Comp. Exp. Biol. 2005, 303, 1040–1053. [Google Scholar] [CrossRef]
- Chng, Y.R.; Ong, J.L.; Ching, B.; Chen, X.L.; Hiong, K.C.; Wong, W.P.; Chew, S.F.; Lam, S.H.; Ip, Y.K. Aestivation Induces Changes in the mRNA Expression Levels and Protein Abundance of Two Isoforms of Urea Transporters in the Gills of the African Lungfish, Protopterus annectens. Front. Physiol. 2017, 8, 71. [Google Scholar] [CrossRef]
- Ip, Y.K.; Chew, S.F. Nitrogen metabolism and excretion during aestivation. Prog. Mol. Subcell. Biol. 2010, 49, 63–94. [Google Scholar] [CrossRef]
- Ip, Y.K.; Yeo, P.J.; Loong, A.M.; Hiong, K.C.; Wong, W.P.; Chew, S.F. The interplay of increased urea synthesis and reduced ammonia production in the African lungfish Protopterus aethiopicus during 46 days of aestivation in a mucus cocoon. J. Exp. Zool. A Comp. Exp. Biol. 2005, 303, 1054–1065. [Google Scholar] [CrossRef]
- Kovarik, J.J.; Morisawa, N.; Wild, J.; Marton, A.; Takase-Minegishi, K.; Minegishi, S.; Daub, S.; Sands, J.M.; Klein, J.D.; Bailey, J.L.; et al. Adaptive physiological water conservation explains hypertension and muscle catabolism in experimental chronic renal failure. Acta Physiol. 2021, 232, e13629. [Google Scholar] [CrossRef]
- Secor, S.M.; Carey, H.V. Integrative Physiology of Fasting. Compr. Physiol. 2016, 6, 773–825. [Google Scholar] [CrossRef] [PubMed]
- Vanbeurden, E.K. Energy-metabolism of dormant australian water-holding frogs (Cyclorana platycephalus). Copeia 1980, 1980, 787–799. [Google Scholar] [CrossRef]
- Fishman, A.P.; Galante, R.G.; Winokur, A.; Pack, A.I. Estivation in the African Lungfish. Proc. Am. Philos. Soc. 1992, 136, 61–72. [Google Scholar]
- Sun, J.; Mu, H.; Zhang, H.; Chandramouli, K.H.; Qian, P.Y.; Wong, C.K.; Qiu, J.W. Understanding the regulation of estivation in a freshwater snail through iTRAQ-based comparative proteomics. J. Proteome Res. 2013, 12, 5271–5280. [Google Scholar] [CrossRef]
- Olsen, L.; Thum, E.; Rohner, N. Lipid metabolism in adaptation to extreme nutritional challenges. Dev. Cell 2021, 56, 1417–1429. [Google Scholar] [CrossRef]
- Houten, S.M.; Denis, S.; Argmann, C.A.; Jia, Y.Z.; Ferdinandusse, S.; Reddy, J.K.; Wanders, R.J.A. Peroxisomal L-bifunctional enzyme (Ehhadh) is essential for the production of medium-chain dicarboxylic acids. J. Lipid Res. 2012, 53, 1296–1303. [Google Scholar] [CrossRef]
- Young, K.M.; Cramp, R.L.; Franklin, C.E. Each to their own: Skeletal muscles of different function use different biochemical strategies during aestivation at high temperature. J. Exp. Biol. 2013, 216, 1012–1024. [Google Scholar] [CrossRef]
- Mantle, B.L.; Hudson, N.J.; Harper, G.S.; Cramp, R.L.; Franklin, C.E. Skeletal muscle atrophy occurs slowly and selectively during prolonged aestivation in Cyclorana alboguttata (Gunther 1867). J. Exp. Biol. 2009, 212, 3664–3672. [Google Scholar] [CrossRef]
- Reilly, B.D.; Hickey, A.J.; Cramp, R.L.; Franklin, C.E. Decreased hydrogen peroxide production and mitochondrial respiration in skeletal muscle but not cardiac muscle of the green-striped burrowing frog, a natural model of muscle disuse. J. Exp. Biol. 2014, 217, 1087–1093. [Google Scholar] [CrossRef]
- Hudson, N.J.; Lehnert, S.A.; Ingham, A.B.; Symonds, B.; Franklin, C.E.; Harper, G.S. Lessons from an estivating frog: Sparing muscle protein despite starvation and disuse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R836–R843. [Google Scholar] [CrossRef] [PubMed]
- Young, K.M.; Cramp, R.L.; White, C.R.; Franklin, C.E. Influence of elevated temperature on metabolism during aestivation: Implications for muscle disuse atrophy. J. Exp. Biol. 2011, 214, 3782–3789. [Google Scholar] [CrossRef] [PubMed]
- James, R.S. Effects of aestivation on skeletal muscle performance. Prog. Mol. Subcell. Biol. 2010, 49, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Luu, B.E.; Zhang, Y.; Storey, K.B. The regulation of Akt and FoxO transcription factors during dehydration in the African clawed frog (Xenopus laevis). Cell Stress. Chaperones 2020, 25, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.L.; Chng, Y.R.; Ching, B.; Chen, X.L.; Hiong, K.C.; Wong, W.P.; Chew, S.F.; Ip, Y.K. Molecular characterization of myostatin from the skeletal muscle of the African lungfish, Protopterus annectens, and changes in its mRNA and protein expression levels during three phases of aestivation. J. Comp. Physiol. B 2017, 187, 575–589. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, Y.; Ru, X.; Zhang, M.; Cao, X.; Yang, H. Differences in immune function and metabolites between aestivating and non-aestivating Apostichopus japonicus. Aquaculture 2016, 459, 36–42. [Google Scholar] [CrossRef]
- Ganeshan, K.; Nikkanen, J.; Man, K.; Leong, Y.A.; Sogawa, Y.; Maschek, J.A.; Van Ry, T.; Chagwedera, D.N.; Cox, J.E.; Chawla, A. Energetic Trade-Offs and Hypometabolic States Promote Disease Tolerance. Cell 2019, 177, 399–413.e12. [Google Scholar] [CrossRef]
- Heimroth, R.D.; Casadei, E.; Salinas, I. Effects of Experimental Terrestrialization on the Skin Mucus Proteome of African Lungfish (Protopterus dolloi). Front. Immunol. 2018, 9, 1259. [Google Scholar] [CrossRef]
- Delgado-Rizo, V.; Martinez-Guzman, M.A.; Iniguez-Gutierrez, L.; Garcia-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil extracellular Traps and its implications in inflammation: An Overview. Front. Immunol. 2017, 8, 20. [Google Scholar] [CrossRef]
- Bhunia, A.S.; Mukherjee, S.; Bhunia, N.S.; Ray, M.; Ray, S. Immunological resilience of a freshwater Indian mollusc during aestivation and starvation. Aquacult. Rep. 2016, 3, 1–11. [Google Scholar] [CrossRef]
- Bouma, H.R.; Henning, R.H.; Kroese, F.G.; Carey, H.V. Hibernation is associated with depression of T-cell independent humoral immune responses in the 13-lined ground squirrel. Dev. Comp. Immunol. 2013, 39, 154–160. [Google Scholar] [CrossRef]
- Field, K.A.; Sewall, B.J.; Prokkola, J.M.; Turner, G.G.; Gagnon, M.F.; Lilley, T.M.; Paul White, J.; Johnson, J.S.; Hauer, C.L.; Reeder, D.M. Effect of torpor on host transcriptomic responses to a fungal pathogen in hibernating bats. Mol. Ecol. 2018, 27, 3727–3743. [Google Scholar] [CrossRef]
- Cramp, R.L.; Franklin, C.E. Arousal and re-feeding rapidly restores digestive tract morphology following aestivation in green-striped burrowing frogs. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 142, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E.; Secor, S.M. Physiological Responses to Fasting and Estivation for the Three-Toed Amphiuma (Amphiuma tridactylum). Physiol. Biochem. Zool. 2017, 90, 240–256. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Yu, Q.; Zhang, J.; Lv, Z.; Fu, W.; Wang, T. Cell loss by apoptosis is involved in the intestinal degeneration that occurs during aestivation in the sea cucumber Apostichopus japonicus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2018, 216, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Secor, S.M.; Taylor, J.R.; Grosell, M. Selected regulation of gastrointestinal acid-base secretion and tissue metabolism for the diamondback water snake and Burmese python. J. Exp. Biol. 2012, 215, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Gavira, R.S.B.; Sartori, M.R.; Gontero-Fourcade, M.N.; Gomes, B.F.; Abe, A.S.; Andrade, D.V. The consequences of seasonal fasting during the dormancy of tegu lizards (Salvator merianae) on their postprandial metabolic response. J. Exp. Biol. 2018, 221, jeb176156. [Google Scholar] [CrossRef]
- Cramp, R.L.; Kayes, S.M.; Meyer, E.A.; Franklin, C.E. Ups and downs of intestinal function with prolonged fasting during aestivation in the burrowing frog, Cyclorana alboguttata. J. Exp. Biol. 2009, 212, 3656–3663. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Chen, M.; Storey, K.B.; Wang, T. A potential antiapoptotic regulation: The interaction of heat shock protein 70 and apoptosis-inducing factor mitochondrial 1 during heat stress and aestivation in sea cucumber. J. Exp. Zool. A Ecol. Integr. Physiol. 2018, 329, 103–111. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Xun, X.; Wang, J.; Bao, L.; Thimmappa, R.; Ding, J.; Jiang, J.; Zhang, L.; Li, T.; et al. Sea cucumber genome provides insights into saponin biosynthesis and aestivation regulation. Cell Discov. 2018, 4, 29. [Google Scholar] [CrossRef]
- Storey, K.B. Regulation of hypometabolism: Insights into epigenetic controls. J. Exp. Biol. 2015, 218, 150–159. [Google Scholar] [CrossRef]
- Hudson, N.J.; Lonhienne, T.G.; Franklin, C.E.; Harper, G.S.; Lehnert, S.A. Epigenetic silencers are enriched in dormant desert frog muscle. J. Comp. Physiol. B 2008, 178, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, Y.; Sun, L.; Chen, M. Genome-Wide DNA Methylation Signatures of Sea Cucumber Apostichopus japonicus during Environmental Induced Aestivation. Genes 2020, 11, 1020. [Google Scholar] [CrossRef] [PubMed]
- Storey, K.B.; Storey, J.M. Metabolic regulation and gene expression during aestivation. Prog. Mol. Subcell. Biol. 2010, 49, 25–45. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, A.; Storey, K.B. Comparative phosphoproteomic analysis of intestinal phosphorylated proteins in active versus aestivating sea cucumbers. J. Proteom. 2016, 135, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.A. Noncoding RNA Regulation of Dormant States in Evolutionarily Diverse Animals. Biol. Bull. 2019, 237, 192–209. [Google Scholar] [CrossRef]
- Wang, S.; Chen, M.; Yin, Y.; Storey, K.B. MiR-200-3p Is Potentially Involved in Cell Cycle Arrest by Regulating Cyclin A during Aestivation in Apostichopus japonicus. Cells 2019, 8, 843. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, J.; Zhang, X.; Jin, S.; Li, F.; Xiang, J. Clustering genomic organization of sea cucumber miRNAs impacts their evolution and expression. Genomics 2021, 113, 3544–3555. [Google Scholar] [CrossRef]
- Chew, S.F.; Ip, Y.K. Excretory nitrogen metabolism and defence against ammonia toxicity in air-breathing fishes. J. Fish. Biol. 2014, 84, 603–638. [Google Scholar] [CrossRef]
- Reilly, B.D.; Franklin, C.E. Prevention of muscle wasting and osteoporosis: The value of examining novel animal models. J. Exp. Biol. 2016, 219, 2582–2595. [Google Scholar] [CrossRef]
- Romspert, A.P. Osmoregulation of african clawed frog. Xenopus laevis, in hypersaline media. Comp. Biochem. Physiol. A Physiol. 1976, 54, 207–210. [Google Scholar] [CrossRef]
- Hillman, S.S. Roles of oxygen delivery and electrolyte levels in dehydrational death of Xenopus laevis. J. Comp. Physiol. 1978, 128, 169–175. [Google Scholar] [CrossRef]
- Cramp, R.L.; Franklin, C.E.; Meyer, E.A. The impact of prolonged fasting during aestivation on the structure of the small intestine in the green-striped burrowing frog, Cyclorana alboguttata. Acta Zool. 2005, 86, 13–24. [Google Scholar] [CrossRef]
- Hudson, N.J.; Franklin, C.E. Effect of aestivation on muscle characteristics and locomotor performance in the Green-striped burrowing frog, Cyclorana alboguttata. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2002, 172, 177–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Luu, B.E.; Storey, K.B. FoxO4 activity is regulated by phosphorylation and the cellular environment during dehydration in the African clawed frog, Xenopus laevis. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1721–1728. [Google Scholar] [CrossRef]
- Wu, C.W.; Tessier, S.N.; Storey, K.B. Regulation of the insulin-Akt signaling pathway and glycolysis during dehydration stress in the African clawed frog Xenopus laevis. Biochem. Cell Biol. 2017, 95, 663–671. [Google Scholar] [CrossRef]
- Wu, C.W.; Tessier, S.N.; Storey, K.B. Dehydration stress alters the mitogen-activated-protein kinase signaling and chaperone stress response in Xenopus laevis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2020, 246–247, 110461. [Google Scholar] [CrossRef]
- Katzenback, B.A.; Dawson, N.J.; Storey, K.B. Purification and characterization of a urea sensitive lactate dehydrogenase from the liver of the African clawed frog, Xenopus laevis. J. Comp. Physiol. B 2014, 184, 601–611. [Google Scholar] [CrossRef]
- Dawson, N.J.; Biggar, Y.; Malik, A.I.; Storey, K.B. Increased transcript levels and kinetic function of pyruvate kinase during severe dehydration in aestivating African clawed frogs, Xenopus laevis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2018, 224, 245–252. [Google Scholar] [CrossRef]
- Biggar, Y.; Ingelson-Filpula, W.A.; Storey, K.B. Pro- and anti-apoptotic microRNAs are differentially regulated during estivation in Xenopus laevis. Gene 2022, 819, 146236. [Google Scholar] [CrossRef]
- Tamaoki, K.; Okada, R.; Ishihara, A.; Shiojiri, N.; Mochizuki, K.; Goda, T.; Yamauchi, K. Morphological, biochemical, transcriptional and epigenetic responses to fasting and refeeding in intestine of Xenopus laevis. Cell Biosci. 2016, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Joss, J.M. Lungfish evolution and development. Gen. Comp. Endocrinol. 2006, 148, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Arthington, A.H. Australian lungfish, Neoceratodus forsteri, threatened by a new dam. Environ. Biol. Fishes 2008, 84, 211–221. [Google Scholar] [CrossRef]
- da Silva, G.S.; Giusti, H.; Sanchez, A.P.; do Carmo, J.M.; Glass, M.L. Aestivation in the South American lungfish, Lepidosiren paradoxa: Effects on cardiovascular function, blood gases, osmolality and leptin levels. Respir. Physiol. Neurobiol. 2008, 164, 380–385. [Google Scholar] [CrossRef]
- Frick, N.T.; Bystriansky, J.S.; Ip, Y.K.; Chew, S.F.; Ballantyne, J.S. Carbohydrate and amino acid metabolism in fasting and aestivating African lungfish (Protopterus dolloi). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 151, 85–92. [Google Scholar] [CrossRef]
- Chew, S.F.; Chan, N.K.; Loong, A.M.; Hiong, K.C.; Tam, W.L.; Ip, Y.K. Nitrogen metabolism in the African lungfish (Protopterus dolloi) aestivating in a mucus cocoon on land. J. Exp. Biol. 2004, 207, 777–786. [Google Scholar] [CrossRef]
- Ip, Y.K.; Peh, B.K.; Tam, W.L.; Lee, S.L.; Chew, S.F. Changes in salinity and ionic compositions can act as environmental signals to induce a reduction in ammonia production in the African lungfish Protopterus dolloi. J. Exp. Zool. A Comp. Exp. Biol. 2005, 303, 456–463. [Google Scholar] [CrossRef]
- Perry, S.F.; Euverman, R.; Wang, T.; Loong, A.M.; Chew, S.F.; Ip, Y.K.; Gilmour, K.M. Control of breathing in African lungfish (Protopterus dolloi): A comparison of aquatic and cocooned (terrestrialized) animals. Respir. Physiol. Neurobiol. 2008, 160, 8–17. [Google Scholar] [CrossRef]
- Staples, J.F.; Kajimura, M.; Wood, C.M.; Patel, M.; Ip, Y.K.; McClelland, G.B. Enzymatic and mitochondrial responses to 5 months of aerial exposure in the slender lungfish Protopterus dolloi. J. Fish. Biol. 2008, 73, 608–622. [Google Scholar] [CrossRef]
- Fishman, A.P.; Pack, A.I.; Delaney, R.G.; Galante, R.J. Estivation in Protopterus. J. Morphol. 1986, 190, 237–248. [Google Scholar] [CrossRef]
- Amelio, D.; Garofalo, F.; Wong, W.P.; Chew, S.F.; Ip, Y.K.; Cerra, M.C.; Tota, B. Nitric oxide synthase-dependent “on/off” switch and apoptosis in freshwater and aestivating lungfish, Protopterus annectens: Skeletal muscle versus cardiac muscle. Nitric Oxide 2013, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Amelio, D.; Garofalo, F. The NOS/NO system in an example of extreme adaptation: The African lungfish. J. Therm. Biol. 2020, 90, 102594. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, F.; Amelio, D.; Icardo, J.M.; Chew, S.F.; Tota, B.; Cerra, M.C.; Ip, Y.K. Signal molecule changes in the gills and lungs of the African lungfish Protopterus annectens, during the maintenance and arousal phases of aestivation. Nitric Oxide 2015, 44, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Loong, A.M.; Pang, C.Y.; Hiong, K.C.; Wong, W.P.; Chew, S.F.; Ip, Y.K. Increased urea synthesis and/or suppressed ammonia production in the African lungfish, Protopterus annectens, during aestivation in air or mud. J. Comp. Physiol. B 2008, 178, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Loong, A.M.; Hiong, K.C.; Wong, W.P.; Chew, S.F.; Ip, Y.K. Differential gene expression in the liver of the African lungfish, Protopterus annectens, after 6 days of estivation in air. J. Comp. Physiol. B 2012, 182, 231–245. [Google Scholar] [CrossRef]
- Hiong, K.C.; Ip, Y.K.; Wong, W.P.; Chew, S.F. Differential gene expression in the liver of the African lungfish, Protopterus annectens, after 6 months of aestivation in air or 1 day of arousal from 6 months of aestivation. PLoS ONE 2015, 10, e0121224. [Google Scholar] [CrossRef]
- Chng, Y.R.; Ong, J.L.; Ching, B.; Chen, X.L.; Wong, W.P.; Chew, S.F.; Ip, Y.K. Molecular characterization of argininosuccinate synthase and argininosuccinate lyase from the liver of the African lungfish Protopterus annectens, and their mRNA expression levels in the liver, kidney, brain and skeletal muscle during aestivation. J. Comp. Physiol. B 2014, 184, 835–853. [Google Scholar] [CrossRef]
- Ong, J.L.; Woo, J.M.; Hiong, K.C.; Ching, B.; Wong, W.P.; Chew, S.F.; Ip, Y.K. Molecular characterization of betaine-homocysteine methyltransferase 1 from the liver, and effects of aestivation on its expressions and homocysteine concentrations in the liver, kidney and muscle, of the African lungfish, Protopterus annectens. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2015, 183, 30–41. [Google Scholar] [CrossRef]
- Konno, N.; Hyodo, S.; Yamaguchi, Y.; Kaiya, H.; Miyazato, M.; Matsuda, K.; Uchiyama, M. African lungfish, Protopterus annectens, possess an arginine vasotocin receptor homologous to the tetrapod V2-type receptor. J. Exp. Biol. 2009, 212, 2183–2193. [Google Scholar] [CrossRef]
- Uchiyama, M.; Konno, N.; Shibuya, S.; Nogami, S. Cloning and expression of the epithelial sodium channel and its role in osmoregulation of aquatic and estivating African lungfish Protopterus annectens. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 183, 1–8. [Google Scholar] [CrossRef]
- Hiong, K.C.; Tan, X.R.; Boo, M.V.; Wong, W.P.; Chew, S.F.; Ip, Y.K. Aestivation induces changes in transcription and translation of coagulation factor II and fibrinogen gamma chain in the liver of the African lungfish Protopterus annectens. J. Exp. Biol. 2015, 218, 3717–3728. [Google Scholar] [CrossRef] [PubMed]
- Kennett, R.; Christi, K. Metabolic Depression in Estivating Long-Neck Turtles (Chelodina rugosa). Physiol. Zool. 1994, 67, 1087–1102. [Google Scholar] [CrossRef]
- Roe, J.H.; Georges, A.; Green, B. Energy and water flux during terrestrial estivation and overland movement in a freshwater turtle. Physiol. Biochem. Zool. 2008, 81, 570–583. [Google Scholar] [CrossRef]
- Roe, J.H.; Georges, A. Heterogeneous wetland complexes, buffer zones, and travel corridors: Landscape management for freshwater reptiles. Biol. Conserv. 2007, 135, 67–76. [Google Scholar] [CrossRef]
- Attum, O.; Kramer, A.; El Din, S.M.B. Thermal utility of desert vegetation for the Egyptian tortoise and its conservation implications. J. Arid. Environ. 2013, 96, 73–79. [Google Scholar] [CrossRef]
- Attum, O.; Eason, P.; Cobbs, G.; El Din, S.M.B. Response of a desert lizard community to habitat degradation: Do ideas about habitat specialists/generalists hold? Biol. Conserv. 2006, 133, 52–62. [Google Scholar] [CrossRef]
- Attum, O.; El Din, M.B.; El Din, S.B.; Habinan, S. Egyptian tortoise conservation: A community-based, field research program developed from a study on a captive population. Zoo. Biol. 2007, 26, 397–406. [Google Scholar] [CrossRef]
- Arnall, S.G.; Mitchell, N.J.; Kuchling, G.; Durell, B.; Kooijman, S.A.L.M.; Kearney, M.R. Life in the slow lane? A dynamic energy budget model for the western swamp turtle, Pseudemydura umbrina. J. Sea Res. 2019, 143, 89–99. [Google Scholar] [CrossRef]
- Tuma, M.W. Range, Habitat Use, and Seasonal Activity of the Yellow Mud Turtle (Kinosternon flavescens) in Northwestern Illinois: Implications for Site-Specific Conservation and Management. Chelonian Conserv. Biol. 2006, 5, 108–120. [Google Scholar] [CrossRef]
- Taplin, L.E. Osmoregulation in Crocodilians. Biol. Rev. 1988, 63, 333–377. [Google Scholar] [CrossRef]
- Christian, K.; Green, B.; Kennett, R. Some physiological consequences of estivation by freshwater crocodiles, Crocodylus johnstoni. J. Herpetol. 1996, 30, 1–9. [Google Scholar] [CrossRef]
- Cosín, D.J.D.; Ruiz, M.P.; Ramajo, M.; Gutiérrez, M. Is the aestivation of the earthworm Hormogaster elisae a paradiapause. Invertebr. Biol. 2006, 125, 250–255. [Google Scholar] [CrossRef]
- Phillipson, J. Earthworms: Their Ecology and Relationships with Soils and Land Use. K. E. Lee. Q. Rev. Biol. 1986, 61, 115–116. [Google Scholar] [CrossRef]
- Kretzschmar, A.; Bruchou, C. Weight response to the soil-water potential of the earthworm aporrectodea-longa. Biol. Fertil. Soils 1991, 12, 209–212. [Google Scholar] [CrossRef]
- McDaniel, J.P.; Stromberger, M.E.; Barbarick, K.A.; Cranshaw, W. Survival of Aporrectodea caliginosa and its effects on nutrient availability in biosolids amended soil. Appl. Soil. Ecol. 2013, 71, 1–6. [Google Scholar] [CrossRef]
- Jimenez, J.J.; Brown, G.G.; Decaens, T.; Feijoo, A.; Lavelle, P. Differences in the timing of diapause and patterns of aestivation in tropical earthworms. Pedobiologia 2000, 44, 677–694. [Google Scholar]
- Eggleton, P.; Inward, K.; Smith, J.; Jones, D.T.; Sherlock, E. A six year study of earthworm (Lumbricidae) populations in pasture woodland in southern England shows their responses to soil temperature and soil moisture. Soil. Biol. Biochem. 2009, 41, 1857–1865. [Google Scholar] [CrossRef]
- Holmstrup, M.; Loeschcke, V. Genetic variation in desiccation tolerance of Dendrobaena octaedra cocoons originating from different climatic regions. Soil. Biol. Biochem. 2003, 35, 119–124. [Google Scholar] [CrossRef]
- Petersen, C.R.; Holmstrup, M.; Malmendal, A.; Bayley, M.; Overgaard, J. Slow desiccation improves dehydration tolerance and accumulation of compatible osmolytes in earthworm cocoons (Dendrobaena octaedra Savigny). J. Exp. Biol. 2008, 211, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Bayley, M.; Overgaard, J.; Hoj, A.S.; Malmendal, A.; Nielsen, N.C.; Holmstrup, M.; Wang, T. Metabolic changes during estivation in the common earthworm Aporrectodea caliginosa. Physiol. Biochem. Zool. 2010, 83, 541–550. [Google Scholar] [CrossRef]
- Rundgren, S. Vertical Distribution of Lumbricids in Southern Sweden. Oikos 1975, 26, 299–306. [Google Scholar] [CrossRef]
- Holmstrup, M. Sensitivity of life history parameters in the earthworm Aporrectodea caliginosa to small changes in soil water potential. Soil. Biol. Biochem. 2001, 33, 1217–1223. [Google Scholar] [CrossRef]
- Valle, J.V.; Moro, R.P.; Garvin, M.H.; Trigo, D.; Cosin, N.J.D. Annual dynamics of the earthworm Hormogaster elisae (Oligochaeta, Hormogastridae) in central Spain. Soil. Biol. Biochem. 1997, 29, 309–312. [Google Scholar] [CrossRef]
- Valle, J.V.; Garvin, M.H.; Trigo, D.; Martinez, F.; Belinchon, C.; Cosin, D.J.D. Vertical distribution of Hormogaster elisae (Oligochaeta, Hormogastridae) in soil at El Molar (Central Spain). Pedobiologia 1999, 43, 859–865. [Google Scholar]
- Holmstrup, M.; Slotsbo, S.; Henriksen, P.G.; Bayley, M. Earthworms accumulate alanine in response to drought. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 199, 8–13. [Google Scholar] [CrossRef]
- Smolinski, M.B.; Varma, A.; Green, S.R.; Storey, K.B. Purification and Regulation of Pyruvate Kinase from the Foot Muscle of the Anoxia and Freeze Tolerant Marine Snail, Littorina littorea. Protein J. 2020, 39, 531–541. [Google Scholar] [CrossRef]
- Eberlee, J.C.; Storey, J.M.; Storey, K.B. Anaerobiosis, recovery from anoxia, and the role of strombine and alanopine in the oyster Crassostrea virginica. Can. J. Zool. -Rev. Can. Zool. 1983, 61, 2682–2687. [Google Scholar] [CrossRef]
- Tilikj, N.; Novo, M. How to resist soil desiccation: Transcriptional changes in a Mediterranean earthworm during aestivation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2022, 264, 111112. [Google Scholar] [CrossRef]
- Rawlings, T.A.; Hayes, K.A.; Cowie, R.H.; Collins, T.M. The identity, distribution, and impacts of non-native apple snails in the continental United States. BMC Evol. Biol. 2007, 7, 14. [Google Scholar] [CrossRef]
- Hayes, K.A.; Cowie, R.H.; Thiengo, S.C.; Strong, E.E. Comparing apples with apples: Clarifying the identities of two highly invasive Neotropical Ampullariidae (Caenogastropoda). Zool. J. Linn. Soc. 2012, 166, 723–753. [Google Scholar] [CrossRef]
- Hayes, K.A.; Joshi, R.C.; Thiengo, S.C.; Cowie, R.H. Out of South America: Multiple origins of non-native apple snails in Asia. Divers. Distrib. 2008, 14, 701–712. [Google Scholar] [CrossRef]
- Hermes-Lima, M.; Ramos-Vasconcelos, G.R.; Cardoso, L.A.; Orr, A.L.; Rivera, P.M.; Drew, K.L. Animal adaptability to oxidative stress: Gastropod estivation and mammalian hibernation. In Life in the Cold: Evolution, Mechanisms, Adaptation, and Application (Twelfth International Hibernation Symposium); Institute of Arctic Biology, University of Alaska Fairbanks: Fairbanks, AK, USA, 2004; pp. 585–593. [Google Scholar]
- Storey, K.B.; Storey, J.M. Aestivation: Signaling and hypometabolism. J. Exp. Biol. 2012, 215, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Billoud, M.; Abud, M.A.; Cueto, J.A.; Vega, I.A.; Castro-Vazquez, A. Uric acid deposits and estivation in the invasive apple-snail, Pomacea canaliculata. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 506–512. [Google Scholar] [CrossRef]
- Giraud-Billoud, M.; Castro-Vazquez, A. Tolerance to hypometabolism and arousal induced by hibernation in the apple snail Pomacea canaliculata (Caenogastropoda, Ampullariidae). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2018, 224, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Billoud, M.; Campoy-Diaz, A.D.; Dellagnola, F.A.; Rodriguez, C.; Vega, I.A. Antioxidant Responses Induced by Short-Term Activity-Estivation-Arousal Cycle in Pomacea canaliculata. Front. Physiol. 2022, 13, 805168. [Google Scholar] [CrossRef] [PubMed]
- Riddle, W.A. Physiological ecology of land snails and slugs. Mollusca 1983, 431–461. [Google Scholar] [CrossRef]
- Dittbrenner, N.; Lazzara, R.; Kohler, H.R.; Mazzia, C.; Capowiez, Y.; Triebskorn, R. Heat tolerance in Mediterranean land snails: Histopathology after exposure to different temperature regimes. J. Molluscan Stud. 2009, 75, 9–18. [Google Scholar] [CrossRef]
- Hand, S.C.; Hardewig, I. Downregulation of cellular metabolism during environmental stress: Mechanisms and implications. Annu. Rev. Physiol. 1996, 58, 539–563. [Google Scholar] [CrossRef]
- Schweizer, M.; Triebskorn, R.; Kohler, H.R. Snails in the sun: Strategies of terrestrial gastropods to cope with hot and dry conditions. Ecol. Evol. 2019, 9, 12940–12960. [Google Scholar] [CrossRef]
- Li, D.M.; Graham, L.D. Epiphragmin, the major protein of epiphragm mucus from the vineyard snail, Cernuella virgata. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 148, 192–200. [Google Scholar] [CrossRef]
- Mizrahi, T.; Goldenberg, S.; Heller, J.; Arad, Z. Natural variation in resistance to desiccation and heat shock protein expression in the land snail Theba pisana along a climatic gradient. Physiol. Biochem. Zool. 2015, 88, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Adamson, K.J.; Wang, T.; Rotgans, B.A.; Kuballa, A.V.; Storey, K.B.; Cummins, S.F. Differential peptide expression in the central nervous system of the land snail Theba pisana, between active and aestivated. Peptides 2016, 80, 61–71. [Google Scholar] [CrossRef]
- Adamson, K.J.; Wang, T.; Rotgans, B.A.; Kruangkum, T.; Kuballa, A.V.; Storey, K.B.; Cummins, S.F. Genes and associated peptides involved with aestivation in a land snail. Gen. Comp. Endocrinol. 2017, 246, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, Y.; Zhang, T.; Yuan, X.; Li, X.; Liu, Y.; Zhang, F. Metabolic characteristics of sea cucumber Apostichopus japonicus (Selenka) during aestivation. J. Exp. Mar. Biol. Ecol. 2006, 330, 505–510. [Google Scholar] [CrossRef]
- Xiang, X.; Chen, M.; Wu, C.; Zhu, A.; Yang, J.; Lv, Z.; Wang, T. Glycolytic regulation in aestivation of the sea cucumber Apostichopus japonicus: Evidence from metabolite quantification and rate-limiting enzyme analyses. Mar. Biol. 2016, 163, 167. [Google Scholar] [CrossRef]
- Xu, Q.; Gao, F.; Xu, Q.; Yang, H. Analysis of fatty acid composition of sea cucumber Apostichopus japonicus using multivariate statistics. Chin. J. Oceanol. Limnol. 2014, 32, 1314–1319. [Google Scholar] [CrossRef]
- Wang, F.Y.; Yang, Y.H.S.; Wang, X.Y.; Kun, X.; Fei, G. Antioxidant enzymes in sea cucumber Apostichopus japonicus (Selenka) during aestivation. J. Mar. Biol. Assoc. UK 2011, 91, 209–214. [Google Scholar] [CrossRef]
- Ji, T.; Dong, Y.; Dong, S. Growth and physiological responses in the sea cucumber, Apostichopus japonicus Selenka: Aestivation and temperature. Aquaculture 2008, 283, 180–187. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, M.; Wang, T.; Sun, L.; Xu, D.; Yang, H. Selection of reference genes for qRT-PCR analysis of gene expression in sea cucumber Apostichopus japonicus during aestivation. Chin. J. Oceanol. Limnol. 2014, 32, 1248–1256. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, H.; Storey, K.B.; Chen, M. Differential gene expression in the respiratory tree of the sea cucumber Apostichopus japonicus during aestivation. Mar. Genom. 2014, 18 Pt B, 173–183. [Google Scholar] [CrossRef]
- Chen, M.; Li, X.; Zhu, A.; Storey, K.B.; Sun, L.; Gao, T.; Wang, T. Understanding mechanism of sea cucumber Apostichopus japonicus aestivation: Insights from TMT-based proteomic study. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2016, 19, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yuan, Z.; Yu, S.; Yang, Y.; Li, Y.; He, C. Genome-wide identification of HSP70/110 genes in sea cucumber Apostichopus japonicus and comparative analysis of their involvement in aestivation. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2018, 28, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yuan, Z.; Ma, Z.; Li, Z.; Yu, S.; Li, Y.; He, C. Genome-wide comparative analysis of the SHSP, HSP60/10 and HSP90 genes reveals differential heat stress responses in estivation of the sea cucumber Apostichopus japonicus. Aquacult. Res. 2019, 50, 1117–1130. [Google Scholar] [CrossRef]
- Gao, L.; Yuan, Z.; Li, Y.; Ma, Z. Genome-wide comparative analysis of DNAJ genes and their co-expression patterns with HSP70s in aestivation of the sea cucumber Apostichopus japonicus. Funct. Integr. Genom. 2022, 22, 317–330. [Google Scholar] [CrossRef]
- Zhu, A.; Chen, M.; Zhang, X.; Storey, K.B. Gene structure, expression, and DNA methylation characteristics of sea cucumber cyclin B gene during aestivation. Gene 2016, 594, 82–88. [Google Scholar] [CrossRef]
| Strategy | Pattern Diagram | Ref |
|---|---|---|
| 1. Anti-oxidant defense |  | [9] |
| 2. Nitrogen metabolism and ammonia detoxification |  | [59] |
| 3. Lipid reserves and lipid metabolism |  | [26] |
| 4. Muscle disuse atrophy and muscle protection |  | [60] |
| 5. Antibacterial immune protection |  | [2] |
| 6. Visceral degeneration and regeneration. |  | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Storey, K.B.; Yang, H.; Sun, L. Aestivation in Nature: Physiological Strategies and Evolutionary Adaptations in Hypometabolic States. Int. J. Mol. Sci. 2023, 24, 14093. https://doi.org/10.3390/ijms241814093
Jiang C, Storey KB, Yang H, Sun L. Aestivation in Nature: Physiological Strategies and Evolutionary Adaptations in Hypometabolic States. International Journal of Molecular Sciences. 2023; 24(18):14093. https://doi.org/10.3390/ijms241814093
Chicago/Turabian StyleJiang, Chunxi, Kenneth B. Storey, Hongsheng Yang, and Lina Sun. 2023. "Aestivation in Nature: Physiological Strategies and Evolutionary Adaptations in Hypometabolic States" International Journal of Molecular Sciences 24, no. 18: 14093. https://doi.org/10.3390/ijms241814093






