Crosstalk between Platelets and SARS-CoV-2: Implications in Thrombo-Inflammatory Complications in COVID-19
Abstract
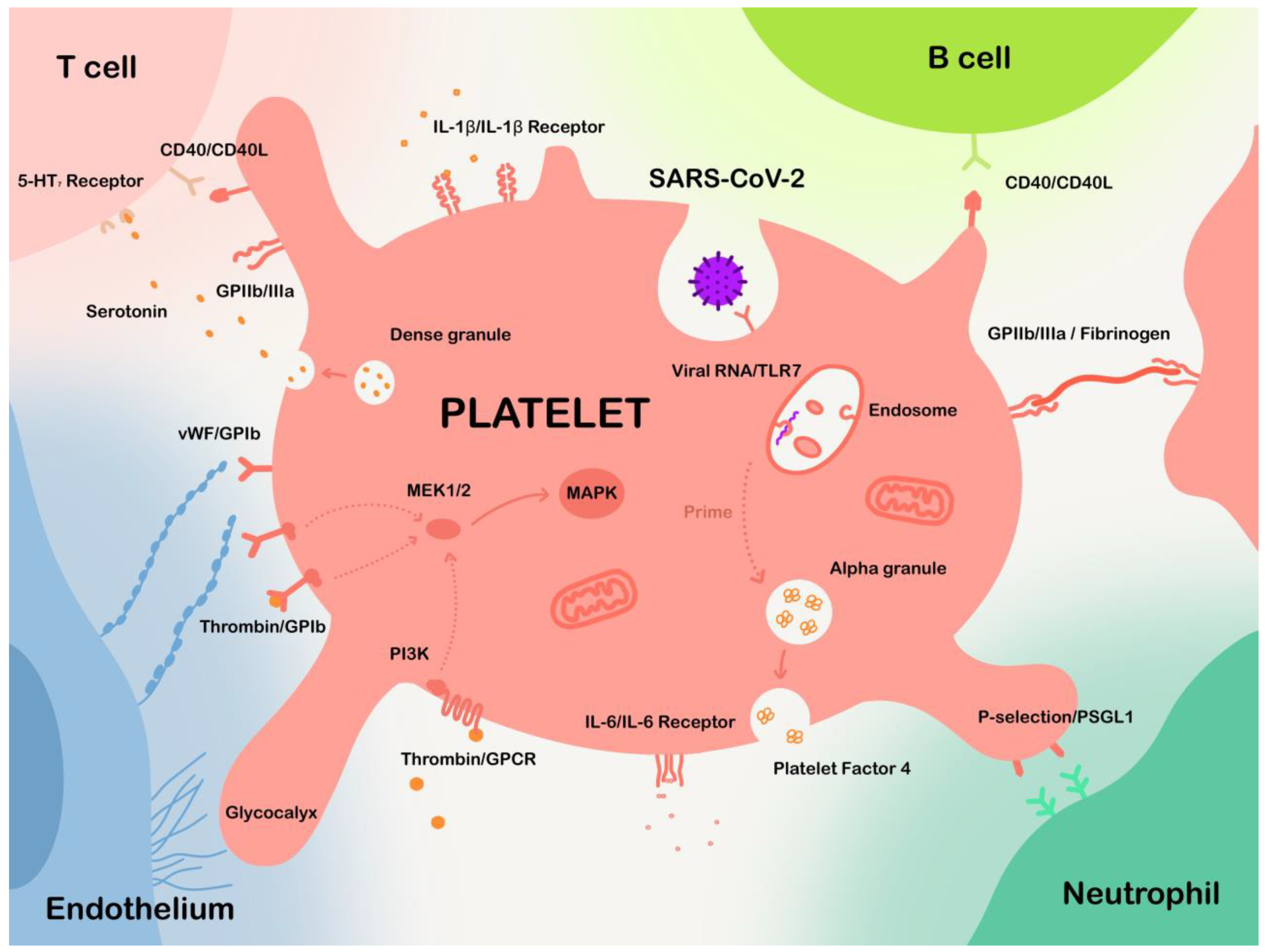
:1. Introduction
2. Roles of Platelets in Physiology and Pathobiology
2.1. Platelets in Hemostasis and Thrombosis
2.2. Platelets in Innate and Adaptive Immunity
2.3. Platelets in Microbial Infections
3. Thrombotic Events and Platelet Alteration in COVID-19
3.1. Thrombotic Events in COVID-19
3.2. Platelet Count and Volume Change in COVID-19 Patients
3.3. Platelet Activation in COVID-19
3.3.1. Platelets Are Sensitized in COVID-19 Patients
3.3.2. Mechanisms of Platelet Activation in COVID-19
Inflammation-Induced Platelet Activation
Endothelial Dysfunction Influences Platelet Activation and Aggregation
SARS-CoV-2 May Invade Platelets to Affect Their Function
3.3.3. Platelet Transcriptome Alteration
3.3.4. Signaling Effects on Platelet Activation
4. Potential Antiviral Effects of Platelets on SARS-CoV-2
4.1. Internalizing SARS-CoV-2
4.2. Releasing Bioactive Molecules to Interfere with SARS-CoV-2 Infection
4.3. Modulating Innate Immune Cells
4.4. Enhancing T-Lymphocyte and B-Lymphocyte Function
5. Potential Platelet-Targeted Treatment of COVID-19
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-converting enzyme 2 |
| ADAMTS13 | Enzyme A disintegrin and metalloprotease with thrombospondin motifs-13 |
| CMV | Cytomegalovirus |
| COVID-19 | Coronavirus disease 2019 |
| cPLA2 | Cytosolic phospholipase A2 |
| DVT | Deep vein thrombosis |
| HSV-1 | Herpes simplex virus type 1 |
| ICU | Intensive care unit |
| IL | Interleukin |
| MAPK | Mitogen-activated protein kinase |
| MERS-CoV | Middle East respiratory syndrome coronavirus |
| NET | Neutrophil extracellular trap |
| NK cell | Natural killer cell |
| PF4 | Platelet factor 4 |
| PI3K | Phosphoinositide 3-kinase |
| PRR | Pathogen-recognition receptor |
| PSGL-1 | P-selectin glycoprotein ligand 1 |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor alpha |
| VEGF | Vascular endothelial growth factor |
| vWF | von Willebrand factor |
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological Findings of COVID-19 Associated with Acute Respiratory Distress Syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Cao, X. COVID-19: Immunopathology and Its Implications for Therapy. Nat. Rev. Immunol. 2020, 20, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Toussi, S.S.; Hammond, J.L.; Gerstenberger, B.S.; Anderson, A.S. Therapeutics for COVID-19. Nat. Microbiol. 2023, 8, 771–786. [Google Scholar] [CrossRef]
- Sette, A.; Sidney, J.; Crotty, S. T Cell Responses to SARS-CoV-2. Annu. Rev. Immunol. 2023, 41, 343–373. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.B.; June, C.H. Cytokine Release Syndrome in Severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef]
- Schett, G.; Sticherling, M.; Neurath, M.F. COVID-19: Risk for Cytokine Targeting in Chronic Inflammatory Diseases? Nat. Rev. Immunol. 2020, 20, 271–272. [Google Scholar] [CrossRef]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective Treatment of Severe COVID-19 Patients with Tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Dela Cruz, C.S.; Wang, Y.; Wu, C.; Xiao, Y.; et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin. Infect. Dis. 2020, 71, 778–785. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef] [PubMed]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N. Engl. J. Med. 2021, 384, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Adams, A.C.; Hufford, M.M.; de la Torre, I.; Winthrop, K.; Gottlieb, R.L. Neutralizing Monoclonal Antibodies for Treatment of COVID-19. Nat. Rev. Immunol. 2021, 21, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, P.J.M.; Caniels, T.G.; van der Straten, K.; Snitselaar, J.L.; Aldon, Y.; Bangaru, S.; Torres, J.L.; Okba, N.M.A.; Claireaux, M.; Kerster, G.; et al. Potent Neutralizing Antibodies from COVID-19 Patients Define Multiple Targets of Vulnerability. Science 2020, 369, 643–650. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Ellebedy, A.H. The Germinal Centre B Cell Response to SARS-CoV-2. Nat. Rev. Immunol. 2022, 22, 7–18. [Google Scholar] [CrossRef]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef]
- Chandran, A.; Rosenheim, J.; Nageswaran, G.; Swadling, L.; Pollara, G.; Gupta, R.K.; Burton, A.R.; Guerra-Assunção, J.A.; Woolston, A.; Ronel, T.; et al. Rapid Synchronous Type 1 IFN and Virus-Specific T Cell Responses Characterize First Wave Non-Severe SARS-CoV-2 Infections. Cell Rep. Med. 2022, 3, 100557. [Google Scholar] [CrossRef]
- Szabo, P.A.; Dogra, P.; Gray, J.I.; Wells, S.B.; Connors, T.J.; Weisberg, S.P.; Krupska, I.; Matsumoto, R.; Poon, M.M.L.; Idzikowski, E.; et al. Longitudinal Profiling of Respiratory and Systemic Immune Responses Reveals Myeloid Cell-Driven Lung Inflammation in Severe COVID-19. Immunity 2021, 54, 797–814.e6. [Google Scholar] [CrossRef]
- Melms, J.C.; Biermann, J.; Huang, H.; Wang, Y.; Nair, A.; Tagore, S.; Katsyv, I.; Rendeiro, A.F.; Amin, A.D.; Schapiro, D.; et al. A Molecular Single-Cell Lung Atlas of Lethal COVID-19. Nature 2021, 595, 114–119. [Google Scholar] [CrossRef]
- Kaneko, N.; Kuo, H.-H.; Boucau, J.; Farmer, J.R.; Allard-Chamard, H.; Mahajan, V.S.; Piechocka-Trocha, A.; Lefteri, K.; Osborn, M.; Bals, J.; et al. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell 2020, 183, 143–157.e13. [Google Scholar] [CrossRef]
- Schurink, B.; Roos, E.; Radonic, T.; Barbe, E.; Bouman, C.S.C.; De Boer, H.H.; De Bree, G.J.; Bulle, E.B.; Aronica, E.M.; Florquin, S.; et al. Viral Presence and Immunopathology in Patients with Lethal COVID-19: A Prospective Autopsy Cohort Study. Lancet Microbe 2020, 1, e290–e299. [Google Scholar] [CrossRef]
- Masso-Silva, J.A.; Moshensky, A.; Lam, M.T.Y.; Odish, M.F.; Patel, A.; Xu, L.; Hansen, E.; Trescott, S.; Nguyen, C.; Kim, R.; et al. Increased Peripheral Blood Neutrophil Activation Phenotypes and Neutrophil Extracellular Trap Formation in Critically Ill Coronavirus Disease 2019 (COVID-19) Patients: A Case Series and Review of the Literature. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 74, 479–489. [Google Scholar] [CrossRef]
- Radermecker, C.; Detrembleur, N.; Guiot, J.; Cavalier, E.; Henket, M.; d’Emal, C.; Vanwinge, C.; Cataldo, D.; Oury, C.; Delvenne, P.; et al. Neutrophil Extracellular Traps Infiltrate the Lung Airway, Interstitial, and Vascular Compartments in Severe COVID-19. J. Exp. Med. 2020, 217, e20201012. [Google Scholar] [CrossRef]
- Lipsitch, M.; Grad, Y.H.; Sette, A.; Crotty, S. Cross-Reactive Memory T Cells and Herd Immunity to SARS-CoV-2. Nat. Rev. Immunol. 2020, 20, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Tarke, A.; Sidney, J.; Kidd, C.K.; Dan, J.M.; Ramirez, S.I.; Yu, E.D.; Mateus, J.; da Silva Antunes, R.; Moore, E.; Rubiro, P.; et al. Comprehensive Analysis of T Cell Immunodominance and Immunoprevalence of SARS-CoV-2 Epitopes in COVID-19 Cases. Cell Rep. Med. 2021, 2, 100204. [Google Scholar] [CrossRef] [PubMed]
- Bowles, L.; Platton, S.; Yartey, N.; Dave, M.; Lee, K.; Hart, D.P.; MacDonald, V.; Green, L.; Sivapalaratnam, S.; Pasi, K.J.; et al. Lupus Anticoagulant and Abnormal Coagulation Tests in Patients with Covid-19. N. Engl. J. Med. 2020, 383, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of Thrombotic Complications in Critically Ill ICU Patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of Venous Thromboembolism in Patients with Severe Novel Coronavirus Pneumonia. J. Thromb. Haemost. 2020, 18, 1421–1424. [Google Scholar] [CrossRef]
- Luo, W.-R.; Yu, H.; Gou, J.-Z.; Li, X.-X.; Sun, Y.; Li, J.-X.; He, J.-X.; Liu, L. Histopathologic Findings in the Explant Lungs of a Patient With COVID-19 Treated with Bilateral Orthotopic Lung Transplant. Transplantation 2020, 104, e329–e331. [Google Scholar] [CrossRef]
- Jiang, S.-Q.; Huang, Q.-F.; Xie, W.-M.; Lv, C.; Quan, X.-Q. The Association between Severe COVID-19 and Low Platelet Count: Evidence from 31 Observational Studies Involving 7613 Participants. Br. J. Haematol. 2020, 190, e29–e33. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M.; Henry, B.M. Thrombocytopenia Is Associated with Severe Coronavirus Disease 2019 (COVID-19) Infections: A Meta-Analysis. Clin. Chim. Acta 2020, 506, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, J.; Ni, H. Crosstalk Between Platelets and Microbial Pathogens. Front. Immunol. 2020, 11, 1962. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Andrews, M.; Yang, Y.; Lang, S.; Jin, J.W.; Cameron-Vendrig, A.; Zhu, G.; Reheman, A.; Ni, H. Platelets in Thrombosis and Hemostasis: Old Topic with New Mechanisms. Cardiovasc. Hematol. Disord. Drug Targets 2012, 12, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.R.; Zhang, D.; Oswald, B.E.; Carrim, N.; Wang, X.; Hou, Y.; Zhang, Q.; Lavalle, C.; McKeown, T.; Marshall, A.H.; et al. Platelets Are Versatile Cells: New Discoveries in Hemostasis, Thrombosis, Immune Responses, Tumor Metastasis and Beyond. Crit. Rev. Clin. Lab. Sci. 2016, 53, 409–430. [Google Scholar] [CrossRef]
- Xu, X.R.; Carrim, N.; Neves, M.A.D.; McKeown, T.; Stratton, T.W.; Coelho, R.M.P.; Lei, X.; Chen, P.; Xu, J.; Dai, X.; et al. Platelets and Platelet Adhesion Molecules: Novel Mechanisms of Thrombosis and Anti-Thrombotic Therapies. Thromb. J. 2016, 14, 29. [Google Scholar] [CrossRef]
- Elzey, B.D.; Schmidt, N.W.; Crist, S.A.; Kresowik, T.P.; Harty, J.T.; Nieswandt, B.; Ratliff, T.L. Platelet-Derived CD154 Enables T-Cell Priming and Protection against Listeria Monocytogenes Challenge. Blood 2008, 111, 3684–3691. [Google Scholar] [CrossRef]
- Shih, A.H.; Dai, C.; Hu, X.; Rosenblum, M.K.; Koutcher, J.A.; Holland, E.C. Dose-Dependent Effects of Platelet-Derived Growth Factor-B on Glial Tumorigenesis. Cancer Res. 2004, 64, 4783–4789. [Google Scholar] [CrossRef]
- Loo, J.; Spittle, D.A.; Newnham, M. COVID-19, Immunothrombosis and Venous Thromboembolism: Biological Mechanisms. Thorax 2021, 76, 412–420. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Banerjee, M. Immune Thrombocytopenia Secondary to COVID-19: A Systematic Review. SN Compr. Clin. Med. 2020, 2, 2048–2058. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Wang, X.; Yang, L.; Li, H.; Wang, Y.; Liu, M.; Zhao, X.; Xie, Y.; Yang, Y.; et al. SARS-CoV-2 Binds Platelet ACE2 to Enhance Thrombosis in COVID-19. J. Hematol. Oncol. 2020, 13, 120. [Google Scholar] [CrossRef]
- Wang, Y.; Gallant, R.C.; Ni, H. Extracellular Matrix Proteins in the Regulation of Thrombus Formation. Curr. Opin. Hematol. 2016, 23, 280–287. [Google Scholar] [CrossRef]
- Ruggeri, Z.M. Mechanisms Initiating Platelet Thrombus Formation. Thromb. Haemost. 1997, 78, 611–616. [Google Scholar] [CrossRef]
- Ni, H.; Denis, C.V.; Subbarao, S.; Degen, J.L.; Sato, T.N.; Hynes, R.O.; Wagner, D.D. Persistence of Platelet Thrombus Formation in Arterioles of Mice Lacking Both von Willebrand Factor and Fibrinogen. J. Clin. Investig. 2000, 106, 385–392. [Google Scholar] [CrossRef]
- Reheman, A.; Yang, H.; Zhu, G.; Jin, W.; He, F.; Spring, C.M.; Bai, X.; Gross, P.L.; Freedman, J.; Ni, H. Plasma Fibronectin Depletion Enhances Platelet Aggregation and Thrombus Formation in Mice Lacking Fibrinogen and von Willebrand Factor. Blood 2009, 113, 1809–1817. [Google Scholar] [CrossRef]
- Dunne, E.; Spring, C.M.; Reheman, A.; Jin, W.; Berndt, M.C.; Newman, D.K.; Newman, P.J.; Ni, H.; Kenny, D. Cadherin 6 Has a Functional Role in Platelet Aggregation and Thrombus Formation. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1724–1731. [Google Scholar] [CrossRef]
- Monroe, D.M.; Hoffman, M.; Roberts, H.R. Platelets and Thrombin Generation. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1381–1389. [Google Scholar] [CrossRef]
- Shih, A.H.; Holland, E.C. Platelet-Derived Growth Factor (PDGF) and Glial Tumorigenesis. Cancer Lett. 2006, 232, 139–147. [Google Scholar] [CrossRef]
- Silvis, S.M.; De Sousa, D.A.; Ferro, J.M.; Coutinho, J.M. Cerebral Venous Thrombosis. Nat. Rev. Neurol. 2017, 13, 555–565. [Google Scholar] [CrossRef]
- Grover, S.P.; Mackman, N. Intrinsic Pathway of Coagulation and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 331–338. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.C. Mechanisms of Thrombus Formation. N. Engl. J. Med. 2008, 359, 938–949. [Google Scholar] [CrossRef]
- Senzel, L.; Chang, C. Platelet Phagocytosis by Neutrophils. Blood 2013, 122, 1543. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.; Kerrigan, S.W.; Watson, S.P. Platelets and the Innate Immune System: Mechanisms of Bacterial-induced Platelet Activation. J. Thromb. Haemost. 2011, 9, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R. Platelets: At the Nexus of Antimicrobial Defence. Nat. Rev. Microbiol. 2014, 12, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Rondina, M.T. The Era of Thromboinflammation: Platelets Are Dynamic Sensors and Effector Cells During Infectious Diseases. Front. Immunol. 2019, 10, 2204. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, S.; Hu, L.; Zhai, L.; Xue, R.; Ye, J.; Chen, L.; Cheng, G.; Mruk, J.; Kunapuli, S.P.; et al. Nucleotide-Binding Oligomerization Domain 2 Receptor Is Expressed in Platelets and Enhances Platelet Activation and Thrombosis. Circulation 2015, 131, 1160–1170. [Google Scholar] [CrossRef]
- Aslam, R.; Speck, E.R.; Kim, M.; Crow, A.R.; Bang, K.W.A.; Nestel, F.P.; Ni, H.; Lazarus, A.H.; Freedman, J.; Semple, J.W. Platelet Toll-like Receptor Expression Modulates Lipopolysaccharide-Induced Thrombocytopenia and Tumor Necrosis Factor-α Production in vivo. Blood 2006, 107, 637–641. [Google Scholar] [CrossRef]
- Clemetson, K.J.; Clemetson, J.M.; Proudfoot, A.E.; Power, C.A.; Baggiolini, M.; Wells, T.N. Functional Expression of CCR1, CCR3, CCR4, and CXCR4 Chemokine Receptors on Human Platelets. Blood 2000, 96, 4046–4054. [Google Scholar] [CrossRef]
- Semple, J.W.; Italiano, J.E.; Freedman, J. Platelets and the Immune Continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef]
- Elzey, B.D.; Ratliff, T.L.; Sowa, J.M.; Crist, S.A. Platelet CD40L at the Interface of Adaptive Immunity. Thromb. Res. 2011, 127, 180–183. [Google Scholar] [CrossRef]
- Rachidi, S.; Metelli, A.; Riesenberg, B.; Wu, B.X.; Nelson, M.H.; Wallace, C.; Paulos, C.M.; Rubinstein, M.P.; Garrett-Mayer, E.; Hennig, M.; et al. Platelets Subvert T Cell Immunity against Cancer via GARP-TGFβ Axis. Sci. Immunol. 2017, 2, eaai7911. [Google Scholar] [CrossRef]
- Bao, W.; Bussel, J.B.; Heck, S.; He, W.; Karpoff, M.; Boulad, N.; Yazdanbakhsh, K. Improved Regulatory T-Cell Activity in Patients with Chronic Immune Thrombocytopenia Treated with Thrombopoietic Agents. Blood 2010, 116, 4639–4645. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, A.; Rescigno, M. The Biology of Intestinal Immunoglobulin A Responses. Immunity 2008, 28, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, M.; Ahmadi, J.; Hosseini, E. Platelet-Leukocyte Crosstalk in COVID-19: How Might the Reciprocal Links between Thrombotic Events and Inflammatory State Affect Treatment Strategies and Disease Prognosis? Thromb. Res. 2022, 213, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, M.; Hosseini, E. Platelet-Leukocyte Crosstalk: Linking Proinflammatory Responses to Procoagulant State. Thromb. Res. 2013, 131, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, M.; Kaplan, Z.S.; Alwis, I.; Schoenwaelder, S.M.; Ashworth, K.J.; Westein, E.; Hosseini, E.; Salem, H.H.; Slattery, R.; McColl, S.R.; et al. The CXCR1/2 Ligand NAP-2 Promotes Directed Intravascular Leukocyte Migration through Platelet Thrombi. Blood 2013, 121, 4555–4566. [Google Scholar] [CrossRef]
- Canzano, P.; Brambilla, M.; Porro, B.; Cosentino, N.; Tortorici, E.; Vicini, S.; Poggio, P.; Cascella, A.; Pengo, M.F.; Veglia, F.; et al. Platelet and Endothelial Activation as Potential Mechanisms Behind the Thrombotic Complications of COVID-19 Patients. JACC Basic Transl. Sci. 2021, 6, 202–218. [Google Scholar] [CrossRef]
- McMorran, B.J.; Marshall, V.M.; De Graaf, C.; Drysdale, K.E.; Shabbar, M.; Smyth, G.K.; Corbin, J.E.; Alexander, W.S.; Foote, S.J. Platelets Kill Intraerythrocytic Malarial Parasites and Mediate Survival to Infection. Science 2009, 323, 797–800. [Google Scholar] [CrossRef]
- Qu, M.; Liu, Q.; Zhao, H.-G.; Peng, J.; Ni, H.; Hou, M.; Jansen, A.J.G. Low Platelet Count as Risk Factor for Infections in Patients with Primary Immune Thrombocytopenia: A Retrospective Evaluation. Ann. Hematol. 2018, 97, 1701–1706. [Google Scholar] [CrossRef]
- Gaertner, F.; Massberg, S. Patrolling the Vascular Borders: Platelets in Immunity to Infection and Cancer. Nat. Rev. Immunol. 2019, 19, 747–760. [Google Scholar] [CrossRef]
- Verschoor, A.; Neuenhahn, M.; Navarini, A.A.; Graef, P.; Plaumann, A.; Seidlmeier, A.; Nieswandt, B.; Massberg, S.; Zinkernagel, R.M.; Hengartner, H.; et al. A Platelet-Mediated System for Shuttling Blood-Borne Bacteria to CD8α+ Dendritic Cells Depends on Glycoprotein GPIb and Complement C3. Nat. Immunol. 2011, 12, 1194–1201. [Google Scholar] [CrossRef]
- Fitzgerald, J.R.; Foster, T.J.; Cox, D. The Interaction of Bacterial Pathogens with Platelets. Nat. Rev. Microbiol. 2006, 4, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.R.; Loughman, A.; Keane, F.; Brennan, M.; Knobel, M.; Higgins, J.; Visai, L.; Speziale, P.; Cox, D.; Foster, T.J. Fibronectin-Binding Proteins of Staphylococcus Aureus Mediate Activation of Human Platelets via Fibrinogen and Fibronectin Bridges to Integrin GPIIb/IIIa and IgG Binding to the FcγRIIa Receptor. Mol. Microbiol. 2006, 59, 212–230. [Google Scholar] [CrossRef] [PubMed]
- Loughman, A.; Fitzgerald, J.R.; Brennan, M.P.; Higgins, J.; Downer, R.; Cox, D.; Foster, T.J. Roles for Fibrinogen, Immunoglobulin and Complement in Platelet Activation Promoted by Staphylococcus Aureus Clumping Factor A: Platelet Activation by Staphylococcus Aureus ClfA. Mol. Microbiol. 2005, 57, 804–818. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.; Flaumenhaft, R. Platelet α–Granules: Basic Biology and Clinical Correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef]
- Tang, Y.-Q.; Yeaman, M.R.; Selsted, M.E. Antimicrobial Peptides from Human Platelets. Infect. Immun. 2002, 70, 6524–6533. [Google Scholar] [CrossRef]
- Yeaman, M.R. Platelets in Defense against Bacterial Pathogens. Cell. Mol. Life Sci. 2010, 67, 525–544. [Google Scholar] [CrossRef] [PubMed]
- McMorran, B.J.; Wieczorski, L.; Drysdale, K.E.; Chan, J.-A.; Huang, H.M.; Smith, C.; Mitiku, C.; Beeson, J.G.; Burgio, G.; Foote, S.J. Platelet Factor 4 and Duffy Antigen Required for Platelet Killing of Plasmodium Falciparum. Science 2012, 338, 1348–1351. [Google Scholar] [CrossRef]
- Assinger, A.; Kral, J.B.; Yaiw, K.C.; Schrottmaier, W.C.; Kurzejamska, E.; Wang, Y.; Mohammad, A.-A.; Religa, P.; Rahbar, A.; Schabbauer, G.; et al. Human Cytomegalovirus–Platelet Interaction Triggers Toll-Like Receptor 2–Dependent Proinflammatory and Proangiogenic Responses. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 801–809. [Google Scholar] [CrossRef]
- Forghani, B.; Schmidt, N.J. Association of Herpes Simplex Virus with Platelets of Experimentally Infected Mice. Arch. Virol. 1983, 76, 269–274. [Google Scholar] [CrossRef]
- Sottnek, H.M.; Campbell, W.G.; Cassel, W.A. The Pathogenesis of Vaccinia Virus Toxicity. II. An Electron Microscopic Study. Lab. Investig. J. Tech. Methods Pathol. 1975, 33, 522–532. [Google Scholar]
- Visser, M.R.; Tracy, P.B.; Vercellotti, G.M.; Goodman, J.L.; White, J.G.; Jacob, H.S. Enhanced Thrombin Generation and Platelet Binding on Herpes Simplex Virus-Infected Endothelium. Proc. Natl. Acad. Sci. USA 1988, 85, 8227–8230. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M.; Vitseva, O.; MacKay, C.R.; Beaulieu, L.M.; Benjamin, E.J.; Mick, E.; Kurt-Jones, E.A.; Ravid, K.; Freedman, J.E. Platelet-TLR7 Mediates Host Survival and Platelet Count during Viral Infection in the Absence of Platelet-Dependent Thrombosis. Blood 2014, 124, 791–802. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.J.; Campos-de-Magalhães, M.; Brandão-Mello, C.E.; de Oliveira, R.V.; do Espirito-Santo, M.P.; Yoshida, C.F.T.; Lampe, E. Detection of Hepatitis C Virus in Platelets: Evaluating Its Relationship to Antiviral Therapy Outcome. Hepato Gastroenterol. 2009, 56, 429–436. [Google Scholar]
- Negrotto, S.; Jaquenod De Giusti, C.; Rivadeneyra, L.; Ure, A.E.; Mena, H.A.; Schattner, M.; Gomez, R.M. Platelets Interact with Coxsackieviruses B and Have a Critical Role in the Pathogenesis of Virus-induced Myocarditis. J. Thromb. Haemost. 2015, 13, 271–282. [Google Scholar] [CrossRef]
- Danon, D.; Jerushalmy, Z.; De Vries, A. Incorporation of Influenza Virus in Human Blood Platelets in Vitro. Electron Microscopical Observation. Virology 1959, 9, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Onlamoon, N.; Noisakran, S.; Hsiao, H.-M.; Duncan, A.; Villinger, F.; Ansari, A.A.; Perng, G.C. Dengue Virus–Induced Hemorrhage in a Nonhuman Primate Model. Blood 2010, 115, 1823–1834. [Google Scholar] [CrossRef]
- Barrett, T.J.; Schlegel, M.; Zhou, F.; Gorenchtein, M.; Bolstorff, J.; Moore, K.J.; Fisher, E.A.; Berger, J.S. Platelet Regulation of Myeloid Suppressor of Cytokine Signaling 3 Accelerates Atherosclerosis. Sci. Transl. Med. 2019, 11, eaax0481. [Google Scholar] [CrossRef]
- Rolfes, V.; Ribeiro, L.S.; Hawwari, I.; Böttcher, L.; Rosero, N.; Maasewerd, S.; Santos, M.L.S.; Próchnicki, T.; Silva, C.M.D.S.; Wanderley, C.W.D.S.; et al. Platelets Fuel the Inflammasome Activation of Innate Immune Cells. Cell Rep. 2020, 31, 107615. [Google Scholar] [CrossRef]
- Taus, F.; Salvagno, G.; Canè, S.; Fava, C.; Mazzaferri, F.; Carrara, E.; Petrova, V.; Barouni, R.M.; Dima, F.; Dalbeni, A.; et al. Platelets Promote Thromboinflammation in SARS-CoV-2 Pneumonia. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2975–2989. [Google Scholar] [CrossRef]
- Demelo-Rodríguez, P.; Cervilla-Muñoz, E.; Ordieres-Ortega, L.; Parra-Virto, A.; Toledano-Macías, M.; Toledo-Samaniego, N.; García-García, A.; García-Fernández-Bravo, I.; Ji, Z.; de-Miguel-Diez, J.; et al. Incidence of Asymptomatic Deep Vein Thrombosis in Patients with COVID-19 Pneumonia and Elevated D-Dimer Levels. Thromb. Res. 2020, 192, 23–26. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Karp Leaf, R.S.; Dzik, W.H.; Carlson, J.C.T.; Fogerty, A.E.; Waheed, A.; Goodarzi, K.; Bendapudi, P.K.; Bornikova, L.; Gupta, S.; et al. COVID-19 and Coagulation: Bleeding and Thrombotic Manifestations of SARS-CoV-2 Infection. Blood 2020, 136, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tritschler, T.; Kahn, S.R.; Rodger, M.A. Venous Thromboembolism. Lancet 2021, 398, 64–77. [Google Scholar] [CrossRef]
- Suh, Y.J.; Hong, H.; Ohana, M.; Bompard, F.; Revel, M.-P.; Valle, C.; Gervaise, A.; Poissy, J.; Susen, S.; Hékimian, G.; et al. Pulmonary Embolism and Deep Vein Thrombosis in COVID-19: A Systematic Review and Meta-Analysis. Radiology 2021, 298, E70–E80. [Google Scholar] [CrossRef] [PubMed]
- Hariri, L.P.; North, C.M.; Shih, A.R.; Israel, R.A.; Maley, J.H.; Villalba, J.A.; Vinarsky, V.; Rubin, J.; Okin, D.A.; Sclafani, A.; et al. Lung Histopathology in Coronavirus Disease 2019 as Compared with Severe Acute Respiratory Sydrome and H1N1 Influenza: A Systematic Review. CHEST 2021, 159, 73–84. [Google Scholar] [CrossRef]
- Nahum, J.; Morichau-Beauchant, T.; Daviaud, F.; Echegut, P.; Fichet, J.; Maillet, J.-M.; Thierry, S. Venous Thrombosis Among Critically Ill Patients with Coronavirus Disease 2019 (COVID-19). JAMA Netw. Open 2020, 3, e2010478. [Google Scholar] [CrossRef]
- Wichmann, D.; Sperhake, J.-P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann. Intern. Med. 2020, 173, 268–277. [Google Scholar] [CrossRef]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High Risk of Thrombosis in Patients with Severe SARS-CoV-2 Infection: A Multicenter Prospective Cohort Study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Poissy, J.; Goutay, J.; Caplan, M.; Parmentier, E.; Duburcq, T.; Lassalle, F.; Jeanpierre, E.; Rauch, A.; Labreuche, J.; Susen, S.; et al. Pulmonary Embolism in Patients With COVID-19: Awareness of an Increased Prevalence. Circulation 2020, 142, 184–186. [Google Scholar] [CrossRef]
- Yang, M.; Hon, E.K.L.; Li, K.; Fok, T.-F.; Li, C. The Effect of SARS Coronavirus on Blood System: Its Clinical Findings and the Pathophysiologic Hypothesis. J. Exp. Hematol. 2003, 11, 217–221. [Google Scholar]
- Wong, R.S.M.; Wu, A.; To, K.F.; Lee, N.; Lam, C.W.K.; Wong, C.K.; Chan, P.K.S.; Ng, M.H.L.; Yu, L.M.; Hui, D.S.; et al. Haematological Manifestations in Patients with Severe Acute Respiratory Syndrome: Retrospective Analysis. BMJ 2003, 326, 1358–1362. [Google Scholar] [CrossRef] [PubMed]
- Assiri, A.; Al-Tawfiq, J.A.; Al-Rabeeah, A.A.; Al-Rabiah, F.A.; Al-Hajjar, S.; Al-Barrak, A.; Flemban, H.; Al-Nassir, W.N.; Balkhy, H.H.; Al-Hakeem, R.F.; et al. Epidemiological, Demographic, and Clinical Characteristics of 47 Cases of Middle East Respiratory Syndrome Coronavirus Disease from Saudi Arabia: A Descriptive Study. Lancet Infect. Dis. 2013, 13, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-M.; Na, B.-J.; Jung, Y.; Lim, H.-S.; Seo, J.-E.; Park, S.-A.; Cho, Y.-S.; Song, E.-H.; Seo, J.-Y.; Kim, S.-R.; et al. Clinical and Laboratory Findings of Middle East Respiratory Syndrome Coronavirus Infection. Jpn. J. Infect. Dis. 2019, 72, 160–167. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, W.; Guo, Y.; Chen, L.; Zhang, L.; Zhao, S.; Long, D.; Yu, L. Association between Platelet Parameters and Mortality in Coronavirus Disease 2019: Retrospective Cohort Study. Platelets 2020, 31, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Harari, E.; Cipok, M.; Laish-Farkash, A.; Bryk, G.; Yahud, E.; Sela, Y.; Lador, N.K.; Mann, T.; Mayo, A.; et al. Immature Platelets in Patients Hospitalized with Covid-19. J. Thromb. Thrombolysis 2021, 51, 608–616. [Google Scholar] [CrossRef]
- Güçlü, E.; Kocayiğit, H.; Okan, H.D.; Erkorkmaz, U.; Yürümez, Y.; Yaylacı, S.; Koroglu, M.; Uzun, C.; Karabay, O. Effect of COVID-19 on Platelet Count and Its Indices. Rev. Assoc. Médica Bras. 2020, 66, 1122–1127. [Google Scholar] [CrossRef]
- Wool, G.D.; Miller, J.L. The Impact of COVID-19 Disease on Platelets and Coagulation. Pathobiology 2021, 88, 15–27. [Google Scholar] [CrossRef]
- McDonald, T.P.; Odell, T.T.; Gosslee, D.G. Platelet Size in Relation to Platelet Age. Proc. Soc. Exp. Biol. Med. 1964, 115, 684–689. [Google Scholar] [CrossRef]
- Ksiazek, T.G.; Erdman, D.; Goldsmith, C.S.; Zaki, S.R.; Peret, T.; Emery, S.; Tong, S.; Urbani, C.; Comer, J.A.; Lim, W.; et al. A Novel Coronavirus Associated with Severe Acute Respiratory Syndrome. N. Engl. J. Med. 2003, 348, 1953–1966. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Pasqualini, R.; Arap, W. Aminopeptidase N Inhibitors and SARS. Lancet 2003, 361, 1558. [Google Scholar] [CrossRef]
- Yang, M.; Li, K.; Chui, C.M.Y.; Yuen, P.M.P.; Chan, P.K.; Chuen, C.K.Y.; Li, C.K.; Fok, A.F. Expression of Interleukin (IL) 1 Type I and Type II Receptors in Megakaryocytic Cells and Enhancing Effects of IL-1β on Megakaryocytopoiesis and NF-E2 Expression. Br. J. Haematol. 2000, 111, 371–380. [Google Scholar] [CrossRef]
- Lefrançais, E.; Ortiz-Muñoz, G.; Caudrillier, A.; Mallavia, B.; Liu, F.; Sayah, D.M.; Thornton, E.E.; Headley, M.B.; David, T.; Coughlin, S.R.; et al. The Lung Is a Site of Platelet Biogenesis and a Reservoir for Haematopoietic Progenitors. Nature 2017, 544, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhou, Q.; Xu, J. Mechanism of Thrombocytopenia in COVID-19 Patients. Ann. Hematol. 2020, 99, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.D.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef] [PubMed]
- Beasley, R.; Raymond, N.; Hill, S.; Nowitz, M.; Hughes, R. EThrombosis: The 21st Century Variant of Venous Thromboembolism Associated with Immobility. Eur. Respir. J. 2003, 21, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chu, H.; Li, C.; Wong, B.H.-Y.; Cheng, Z.-S.; Poon, V.K.-M.; Sun, T.; Lau, C.C.-Y.; Wong, K.K.-Y.; Chan, J.Y.-W.; et al. Active Replication of Middle East Respiratory Syndrome Coronavirus and Aberrant Induction of Inflammatory Cytokines and Chemokines in Human Macrophages: Implications for Pathogenesis. J. Infect. Dis. 2014, 209, 1331–1342. [Google Scholar] [CrossRef]
- Chong, P.Y.; Chui, P.; Ling, A.E.; Franks, T.J.; Tai, D.Y.H.; Leo, Y.S.; Kaw, G.J.L.; Wansaicheong, G.; Chan, K.P.; Ean Oon, L.L.; et al. Analysis of Deaths during the Severe Acute Respiratory Syndrome (SARS) Epidemic in Singapore: Challenges in Determining a SARS Diagnosis. Arch. Pathol. Lab. Med. 2004, 128, 195–204. [Google Scholar] [CrossRef]
- Zaid, Y.; Puhm, F.; Allaeys, I.; Naya, A.; Oudghiri, M.; Khalki, L.; Limami, Y.; Zaid, N.; Sadki, K.; Ben El Haj, R.; et al. Platelets Can Associate With SARS-CoV-2 RNA and Are Hyperactivated in COVID-19. Circ. Res. 2020, 127, 1404–1418. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An Inflammatory Cytokine Signature Predicts COVID-19 Severity and Survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Connors, J.M.; Warkentin, T.E.; Thachil, J.; Levi, M. Managing Thrombosis and Cardiovascular Complications of COVID-19: Answering the Questions in COVID-19-Associated Coagulopathy. Expert Rev. Respir. Med. 2021, 15, 1003–1011. [Google Scholar] [CrossRef]
- Ishibashi, T.; Kimura, H.; Shikama, Y.; Uchida, T.; Kariyone, S.; Hirano, T.; Kishimoto, T.; Takatsuki, F.; Akiyama, Y. Interleukin-6 Is a Potent Thrombopoietic Factor In Vivo in Mice. Blood 1989, 74, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Bester, J.; Pretorius, E. Effects of IL-1β, IL-6 and IL-8 on Erythrocytes, Platelets and Clot Viscoelasticity. Sci. Rep. 2016, 6, 32188. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Bester, J.; Pretorius, E. The Inflammatory Effects of TNF-α and Complement Component 3 on Coagulation. Sci. Rep. 2018, 8, 1812. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Vargas, M.; Bonilla-Abadía, F.; Cañas, C.A. Potential Role for Tissue Factor in the Pathogenesis of Hypercoagulability Associated with in COVID-19. J. Thromb. Thrombolysis 2020, 50, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.D.; Marder, V.J. Biosynthesis of von Willebrand Protein by Human Endothelial Cells: Processing Steps and Their Intracellular Localization. J. Cell Biol. 1984, 99, 2123–2130. [Google Scholar] [CrossRef]
- Cao, W.; Niiya, M.; Zheng, X.; Shang, D.; Zheng, X.L. Inflammatory Cytokines Inhibit ADAMTS13 Synthesis in Hepatic Stellate Cells and Endothelial Cells. J. Thromb. Haemost. JTH 2008, 6, 1233–1235. [Google Scholar] [CrossRef]
- Delgadillo, L.F.; Lomakina, E.B.; Kuebel, J.; Waugh, R.E. Changes in Endothelial Glycocalyx Layer Protective Ability after Inflammatory Stimulus. Am. J. Physiol. Cell Physiol. 2021, 320, C216–C224. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial Cell Infection and Endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Antonopoulos, A.S.; Oikonomou, E.; Tsioufis, C.; Tousoulis, D. Inflammatory Mediators of Platelet Activation: Focus on Atherosclerosis and COVID-19. Int. J. Mol. Sci. 2021, 22, 11170. [Google Scholar] [CrossRef]
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox Biology of Respiratory Viral Infections. Viruses 2018, 10, 392. [Google Scholar] [CrossRef]
- Barrett, T.J.; Bilaloglu, S.; Cornwell, M.; Burgess, H.M.; Virginio, V.W.; Drenkova, K.; Ibrahim, H.; Yuriditsky, E.; Aphinyanaphongs, Y.; Lifshitz, M.; et al. Platelets Contribute to Disease Severity in COVID-19. J. Thromb. Haemost. 2021, 19, 3139–3153. [Google Scholar] [CrossRef] [PubMed]
- Manne, B.K.; Denorme, F.; Middleton, E.A.; Portier, I.; Rowley, J.W.; Stubben, C.; Petrey, A.C.; Tolley, N.D.; Guo, L.; Cody, M.; et al. Platelet Gene Expression and Function in Patients with COVID-19. Blood 2020, 136, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, W.; Zhang, Z.; Deng, Y.; Lian, J.-Q.; Du, P.; Wei, D.; Zhang, Y.; Sun, X.-X.; Gong, L.; et al. CD147-Spike Protein Is a Novel Route for SARS-CoV-2 Infection to Host Cells. Signal Transduct. Target. Ther. 2020, 5, 283. [Google Scholar] [CrossRef] [PubMed]
- Shilts, J.; Crozier, T.W.M.; Greenwood, E.J.D.; Lehner, P.J.; Wright, G.J. No Evidence for Basigin/CD147 as a Direct SARS-CoV-2 Spike Binding Receptor. Sci. Rep. 2021, 11, 413. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, J.; Zhao, S.; Gomez Castro, M.F.; Laurent-Rolle, M.; Dong, J.; Ran, X.; Damani-Yokota, P.; Tang, H.; Karakousi, T.; et al. SARS-CoV-2 Exacerbates Proinflammatory Responses in Myeloid Cells through C-Type Lectin Receptors and Tweety Family Member 2. Immunity 2021, 54, 1304–1319.e9. [Google Scholar] [CrossRef]
- Bray, P.F.; McKenzie, S.E.; Edelstein, L.C.; Nagalla, S.; Delgrosso, K.; Ertel, A.; Kupper, J.; Jing, Y.; Londin, E.; Loher, P.; et al. The Complex Transcriptional Landscape of the Anucleate Human Platelet. BMC Genom. 2013, 14, 293. [Google Scholar] [CrossRef]
- Estevez, B.; Du, X. New Concepts and Mechanisms of Platelet Activation Signaling. Physiology 2017, 32, 162–177. [Google Scholar] [CrossRef]
- Manne, B.K.; Münzer, P.; Badolia, R.; Walker-Allgaier, B.; Campbell, R.A.; Middleton, E.; Weyrich, A.S.; Kunapuli, S.P.; Borst, O.; Rondina, M.T. PDK1 Governs Thromboxane Generation and Thrombosis in Platelets by Regulating Activation of Raf1 in the MAPK Pathway. J. Thromb. Haemost. 2018, 16, 1211–1225. [Google Scholar] [CrossRef]
- Patel, P.; Naik, U.P. Platelet MAPKs—A 20+ Year History: What Do We Really Know? J. Thromb. Haemost. 2020, 18, 2087–2102. [Google Scholar] [CrossRef]
- Shankar, H.; Garcia, A.; Prabhakar, J.; Kim, S.; Kunapuli, S.P. P2Y12 Receptor-Mediated Potentiation of Thrombin-Induced Thromboxane A2 Generation in Platelets Occurs through Regulation of Erk1/2 Activation. J. Thromb. Haemost. JTH 2006, 4, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.U.; Patel, P.; Derstine, R.; Turaga, R.; Chen, X.; Golla, K.; Neeves, K.B.; Ichijo, H.; Naik, U.P. Ask1 Regulates Murine Platelet Granule Secretion, Thromboxane A2 Generation, and Thrombus Formation. Blood 2017, 129, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Zhang, L.; Zhang, M.; Yang, W.; Wang, K.; Zhang, J.; Otsu, K.; Huang, G.; Fan, X.; Liu, J. Platelet-Specific P38α Deficiency Improved Cardiac Function After Myocardial Infarction in Mice. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e185–e196. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, M.; Shirai, T.; Tamura, S.; Suzuki-Inoue, K.; Ehata, S.; Takahashi, K.; Miyazono, K.; Hayakawa, Y.; Sato, T.; Takeda, K.; et al. ASK1 Facilitates Tumor Metastasis through Phosphorylation of an ADP Receptor P2Y12 in Platelets. Cell Death Differ. 2017, 24, 2066–2076. [Google Scholar] [CrossRef]
- Caldwell, A.T.; Watkins, E.B. Chapter Seven—Recent Advances in the Development of P2Y12 Receptor Antagonists as Antiplatelet Agents. In Annual Reports in Medicinal Chemistry; Desai, M.C., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 49, pp. 87–99. [Google Scholar]
- Delaney, M.K.; Kim, K.; Estevez, B.; Xu, Z.; Stojanovic-Terpo, A.; Shen, B.; Ushio-Fukai, M.; Cho, J.; Du, X. Differential Roles of the NADPH-Oxidase 1 and 2 in Platelet Activation and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 846–854. [Google Scholar] [CrossRef]
- Nieswandt, B.; Bergmeier, W.; Eckly, A.; Schulte, V.; Ohlmann, P.; Cazenave, J.P.; Zirngibl, H.; Offermanns, S.; Gachet, C. Evidence for Cross-Talk between Glycoprotein VI and Gi-Coupled Receptors during Collagen-Induced Platelet Aggregation. Blood 2001, 97, 3829–3835. [Google Scholar] [CrossRef]
- Stefanini, L.; Roden, R.C.; Bergmeier, W. CalDAG-GEFI Is at the Nexus of Calcium-Dependent Platelet Activation. Blood 2009, 114, 2506–2514. [Google Scholar] [CrossRef]
- Estevez, B.; Stojanovic-Terpo, A.; Delaney, M.K.; O’Brien, K.A.; Berndt, M.C.; Ruan, C.; Du, X. LIM Kinase-1 Selectively Promotes Glycoprotein Ib-IX-Mediated TXA2 Synthesis, Platelet Activation, and Thrombosis. Blood 2013, 121, 4586–4594. [Google Scholar] [CrossRef]
- Sahai, A.; Bhandari, R.; Koupenova, M.; Freedman, J.; Godwin, M.; McIntyre, T.; Chung, M.; Iskandar, J.-P.; Kamran, H.; Aggarwal, A.; et al. SARS-CoV-2 Receptors Are Expressed on Human Platelets and the Effect of Aspirin on Clinical Outcomes in COVID-19 Patients. Res. Sq. 2020. preprint. [Google Scholar] [CrossRef]
- Solomon Tsegaye, T.; Gnirß, K.; Rahe-Meyer, N.; Kiene, M.; Krämer-Kühl, A.; Behrens, G.; Münch, J.; Pöhlmann, S. Platelet Activation Suppresses HIV-1 Infection of T Cells. Retrovirology 2013, 10, 48. [Google Scholar] [CrossRef]
- Parker, Z.F.; Rux, A.H.; Riblett, A.M.; Lee, F.-H.; Rauova, L.; Cines, D.B.; Poncz, M.; Sachais, B.S.; Doms, R.W. Platelet Factor 4 Inhibits and Enhances HIV-1 Infection in a Concentration-Dependent Manner by Modulating Viral Attachment. AIDS Res. Hum. Retroviruses 2016, 32, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Bye, A.P.; Hoepel, W.; Mitchell, J.L.; Jégouic, S.; Loureiro, S.; Sage, T.; Vidarsson, G.; Nouta, J.; Wuhrer, M.; De Taeye, S.; et al. Aberrant Glycosylation of Anti-SARS-CoV-2 Spike IgG Is a Prothrombotic Stimulus for Platelets. Blood 2021, 138, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Klinger, M.H.F.; Wilhelm, D.; Bubel, S.; Sticherling, M.; Schröder, J.-M.; Kühnel, W. Immunocytochemical Localization of the Chemokines RANTES and MIP-1α within Human Platelets and Their Release during Storage. Int. Arch. Allergy Immunol. 1995, 107, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.; Rex, S.; Vitseva, O.; Beaulieu, L.; Tanriverdi, K.; Chakrabarti, S.; Hayashi, C.; Genco, C.A.; Iafrati, M.; Freedman, J.E. Stimulation of Toll-Like Receptor 2 in Human Platelets Induces a Thromboinflammatory Response Through Activation of Phosphoinositide 3-Kinase. Circ. Res. 2009, 104, 346–354. [Google Scholar] [CrossRef]
- Kaplan, M.J.; Radic, M. Neutrophil Extracellular Traps: Double-Edged Swords of Innate Immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef]
- Jenne, C.N.; Kubes, P. Virus-Induced NETs—Critical Component of Host Defense or Pathogenic Mediator? PLoS Pathog. 2015, 11, e1004546. [Google Scholar] [CrossRef]
- Kraemer, B.F.; Campbell, R.A.; Schwertz, H.; Cody, M.J.; Franks, Z.; Tolley, N.D.; Kahr, W.H.A.; Lindemann, S.; Seizer, P.; Yost, C.C.; et al. Novel Anti-Bacterial Activities of β-Defensin 1 in Human Platelets: Suppression of Pathogen Growth and Signaling of Neutrophil Extracellular Trap Formation. PLoS Pathog. 2011, 7, e1002355. [Google Scholar] [CrossRef]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 Activates Neutrophil Extracellular Traps to Ensnare Bacteria in Septic Blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef]
- Langer, H.F.; Daub, K.; Braun, G.; Schönberger, T.; May, A.E.; Schaller, M.; Stein, G.M.; Stellos, K.; Bueltmann, A.; Siegel-Axel, D.; et al. Platelets Recruit Human Dendritic Cells Via Mac-1/JAM-C Interaction and Modulate Dendritic Cell Function In Vitro. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1463–1470. [Google Scholar] [CrossRef]
- Malengier-Devlies, B.; Filtjens, J.; Ahmadzadeh, K.; Boeckx, B.; Vandenhaute, J.; De Visscher, A.; Bernaerts, E.; Mitera, T.; Jacobs, C.; Vanderbeke, L.; et al. Severe COVID-19 Patients Display Hyper-Activated NK Cells and NK Cell-Platelet Aggregates. Front. Immunol. 2022, 13, 861251. [Google Scholar] [CrossRef] [PubMed]
- Elzey, B.D.; Tian, J.; Jensen, R.J.; Swanson, A.K.; Lees, J.R.; Lentz, S.R.; Stein, C.S.; Nieswandt, B.; Wang, Y.; Davidson, B.L.; et al. Platelet-Mediated Modulation of Adaptive Immunity. Immunity 2003, 19, 9–19. [Google Scholar] [CrossRef] [PubMed]
- León-Ponte, M.; Ahern, G.P.; O’Connell, P.J. Serotonin Provides an Accessory Signal to Enhance T-Cell Activation by Signaling through the 5-HT7 Receptor. Blood 2007, 109, 3139–3146. [Google Scholar] [CrossRef]
- O’Connell, P.J.; Wang, X.; Leon-Ponte, M.; Griffiths, C.; Pingle, S.C.; Ahern, G.P. A Novel Form of Immune Signaling Revealed by Transmission of the Inflammatory Mediator Serotonin between Dendritic Cells and T Cells. Blood 2006, 107, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Scherlinger, M.; Guillotin, V.; Douchet, I.; Vacher, P.; Boizard-Moracchini, A.; Guegan, J.-P.; Garreau, A.; Merillon, N.; Vermorel, A.; Ribeiro, E.; et al. Selectins Impair Regulatory T Cell Function and Contribute to Systemic Lupus Erythematosus Pathogenesis. Sci. Transl. Med. 2021, 13, eabi4994. [Google Scholar] [CrossRef]
- Cognasse, F.; Hamzeh-Cognasse, H.; Lafarge, S.; Chavarin, P.; Cogné, M.; Richard, Y.; Garraud, O. Human Platelets Can Activate Peripheral Blood B Cells and Increase Production of Immunoglobulins. Exp. Hematol. 2007, 35, 1376–1387. [Google Scholar] [CrossRef]
- Viallard, J.-F.; Solanilla, A.; Gauthier, B.; Contin, C.; Déchanet, J.; Grosset, C.; Moreau, J.-F.; Praloran, V.; Nurden, P.; Pellegrin, J.-L.; et al. Increased Soluble and Platelet-Associated CD40 Ligand in Essential Thrombocythemia and Reactive Thrombocytosis. Blood 2002, 99, 2612–2614. [Google Scholar] [CrossRef]
- Müller, K.; Chatterjee, M.; Rath, D.; Geisler, T. Platelets, Inflammation and Anti-Inflammatory Effects of Antiplatelet Drugs in ACS and CAD. Thromb. Haemost. 2015, 114, 498–518. [Google Scholar] [CrossRef]
- Santoro, F.; Nuñez-Gil, I.J.; Vitale, E.; Viana-Llamas, M.C.; Reche-Martinez, B.; Romero-Pareja, R.; Feltez Guzman, G.; Fernandez Rozas, I.; Uribarri, A.; Becerra-Muñoz, V.M.; et al. Antiplatelet Therapy and Outcome in COVID-19: The Health Outcome Predictive Evaluation Registry. Heart 2022, 108, 130–136. [Google Scholar] [CrossRef]
- Meizlish, M.L.; Goshua, G.; Liu, Y.; Fine, R.; Amin, K.; Chang, E.; DeFilippo, N.; Keating, C.; Liu, Y.; Mankbadi, M.; et al. Intermediate-dose Anticoagulation, Aspirin, and In-hospital Mortality in COVID-19: A Propensity Score-matched Analysis. Am. J. Hematol. 2021, 96, 471–479. [Google Scholar] [CrossRef]
- Chow, J.H.; Khanna, A.K.; Kethireddy, S.; Yamane, D.; Levine, A.; Jackson, A.M.; McCurdy, M.T.; Tabatabai, A.; Kumar, G.; Park, P.; et al. Aspirin Use Is Associated with Decreased Mechanical Ventilation, Intensive Care Unit Admission, and In-Hospital Mortality in Hospitalized Patients with Coronavirus Disease 2019. Anesth. Analg. 2021, 132, 930–941. [Google Scholar] [CrossRef]
- Sexton, T.R.; Zhang, G.; Macaulay, T.E.; Callahan, L.A.; Charnigo, R.; Vsevolozhskaya, O.A.; Li, Z.; Smyth, S. Ticagrelor Reduces Thromboinflammatory Markers in Patients with Pneumonia. JACC Basic Transl. Sci. 2018, 3, 435–449. [Google Scholar] [CrossRef]
- Maes, M.; Higginson, E.; Pereira-Dias, J.; Curran, M.D.; Parmar, S.; Khokhar, F.; Cuchet-Lourenço, D.; Lux, J.; Sharma-Hajela, S.; Ravenhill, B.; et al. Ventilator-Associated Pneumonia in Critically Ill Patients with COVID-19. Crit. Care 2021, 25, 25. [Google Scholar] [CrossRef]
- Chandra, A.; Chakraborty, U.; Ghosh, S.; Dasgupta, S. Anticoagulation in COVID-19: Current Concepts and Controversies. Postgrad. Med. J. 2022, 98, 395–402. [Google Scholar] [CrossRef]
- Ebrahimi Chaharom, F.; Pourafkari, L.; Ebrahimi Chaharom, A.A.; Nader, N.D. Effects of Corticosteroids on Covid-19 Patients: A Systematic Review and Meta-Analysis on Clinical Outcomes. Pulm. Pharmacol. Ther. 2022, 72, 102107. [Google Scholar] [CrossRef]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory Action of Glucocorticoids—New Mechanisms for Old Drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef]
- Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021, 384, 1491–1502. [CrossRef]
- Chan, K.H.; Patel, B.; Podel, B.; Szablea, M.E.; Shaaban, H.S.; Guron, G.; Slim, J. Tocilizumab and Thromboembolism in COVID-19: A Retrospective Hospital-Based Cohort Analysis. Cureus 2021, 5, e15208. [Google Scholar] [CrossRef]
- Stallmach, A.; Kortgen, A.; Gonnert, F.; Coldewey, S.M.; Reuken, P.; Bauer, M. Infliximab against Severe COVID-19-Induced Cytokine Storm Syndrome with Organ Failure—A Cautionary Case Series. Crit. Care 2020, 24, 444. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Xu, X.; Gao, Y.; Yu, Y.; Li, C. Crosstalk between Platelets and SARS-CoV-2: Implications in Thrombo-Inflammatory Complications in COVID-19. Int. J. Mol. Sci. 2023, 24, 14133. https://doi.org/10.3390/ijms241814133
Zhao J, Xu X, Gao Y, Yu Y, Li C. Crosstalk between Platelets and SARS-CoV-2: Implications in Thrombo-Inflammatory Complications in COVID-19. International Journal of Molecular Sciences. 2023; 24(18):14133. https://doi.org/10.3390/ijms241814133
Chicago/Turabian StyleZhao, Junyi, Xiafan Xu, Yifei Gao, Yijing Yu, and Conglei Li. 2023. "Crosstalk between Platelets and SARS-CoV-2: Implications in Thrombo-Inflammatory Complications in COVID-19" International Journal of Molecular Sciences 24, no. 18: 14133. https://doi.org/10.3390/ijms241814133
APA StyleZhao, J., Xu, X., Gao, Y., Yu, Y., & Li, C. (2023). Crosstalk between Platelets and SARS-CoV-2: Implications in Thrombo-Inflammatory Complications in COVID-19. International Journal of Molecular Sciences, 24(18), 14133. https://doi.org/10.3390/ijms241814133



