The Role of the Circadian Rhythm in Dyslipidaemia and Vascular Inflammation Leading to Atherosclerosis
Abstract
1. Introduction
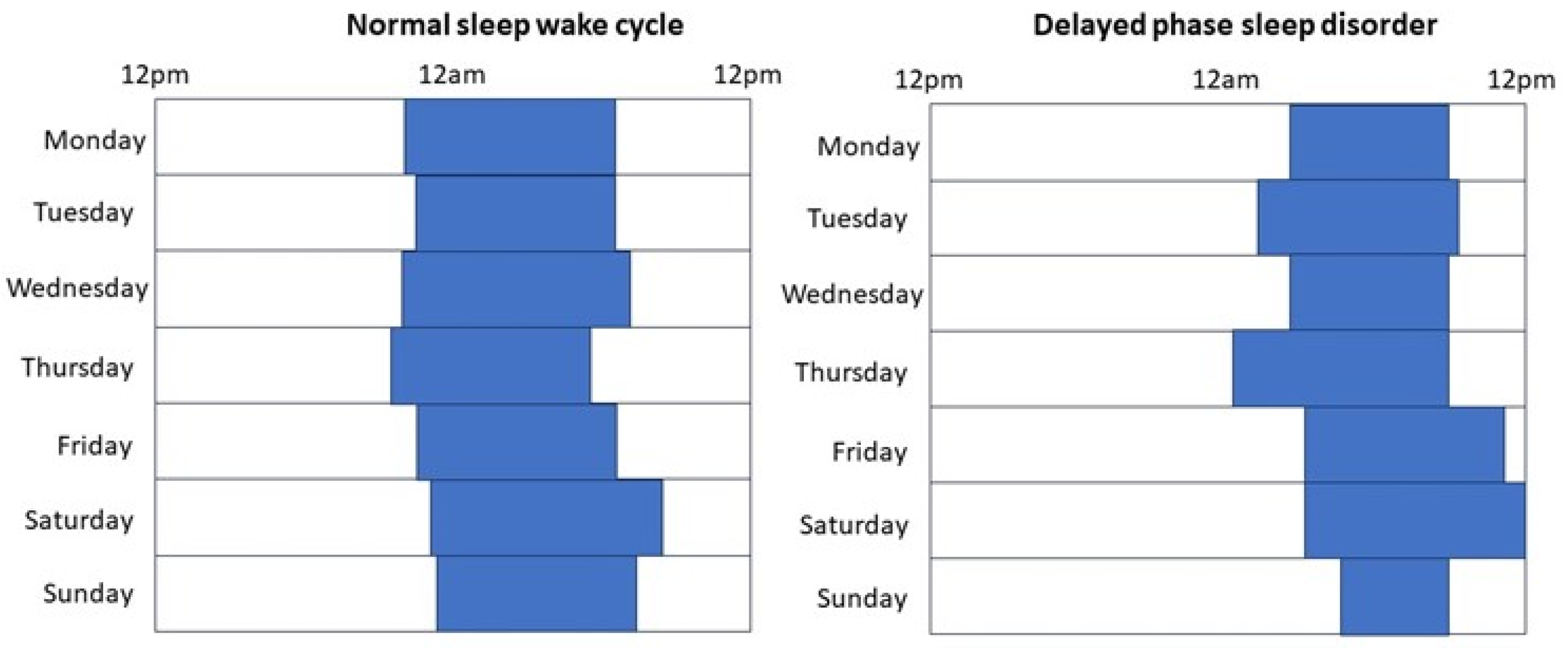
2. Circadian Rhythm Sleep-Wake Disorders and Cardiovascular Diseases
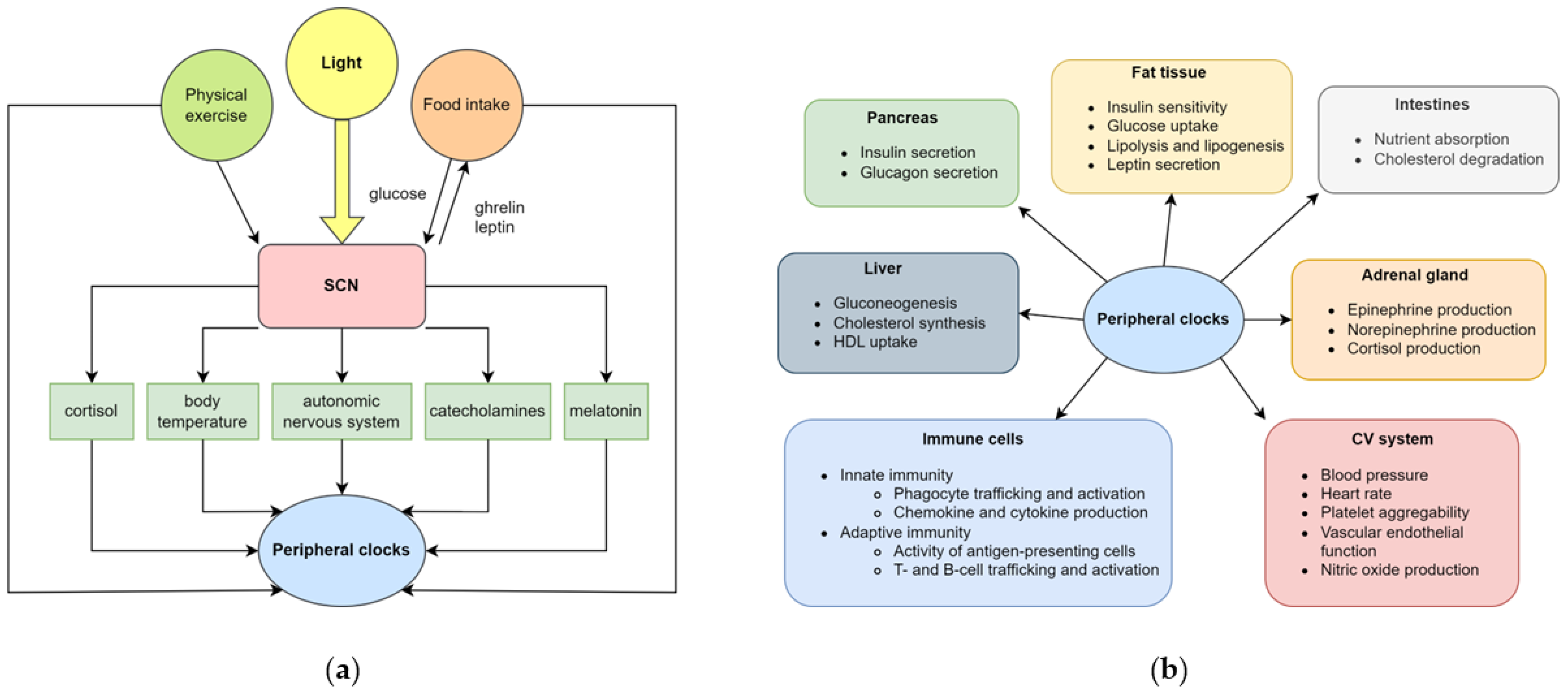
2.1. Normal Within-Day Variations in CV Functions
2.2. Clinical Consequences of Circadian Disorders on Cardiovascular Health
3. The Role of Circadian Rhythm and Dyslipidaemia
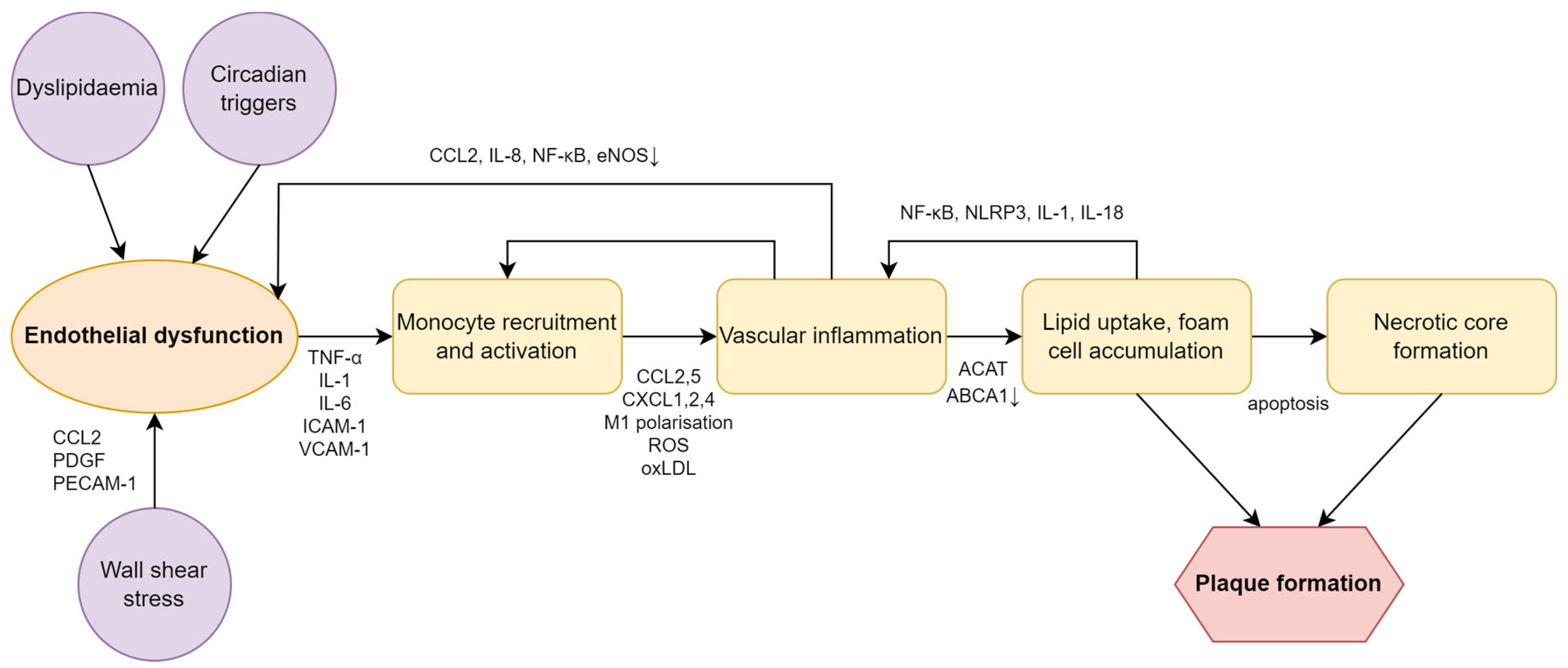
4. The Role of Circadian Rhythm and Vascular Inflammation
4.1. Circadian Control of Immune Functions
4.2. Circadian Misalignment and Vascular Inflammation
5. Therapeutic Considerations
5.1. Chronotherapy
5.2. The Effect of Melatonin
5.3. The Effect of Bright Light
5.4. Experimental Therapies
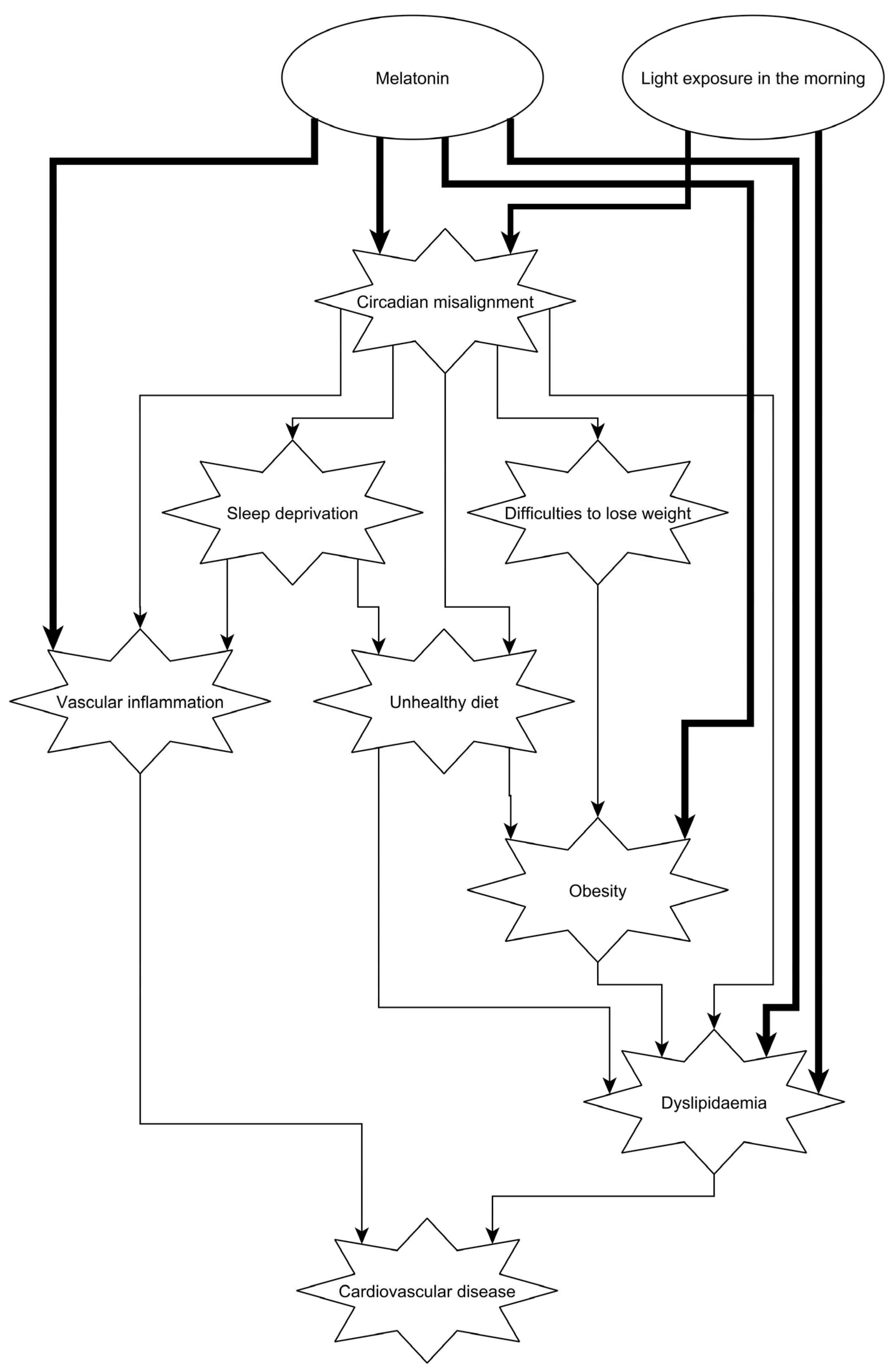
6. Conclusions and Suggestions for Further Research and Clinical Practice
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Killick, R.; Stranks, L.; Hoyos, C.M. Sleep Deficiency and Cardiometabolic Disease. Sleep Med. Clin. 2023, 18, 331–347. [Google Scholar] [CrossRef]
- Redline, S.; Azarbarzin, A.; Peker, Y. Obstructive sleep apnoea heterogeneity and cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 560–573. [Google Scholar] [CrossRef] [PubMed]
- Wipper, B.; Winkelman, J.W. The Long-Term Psychiatric and Cardiovascular Morbidity and Mortality of Restless Legs Syndrome and Periodic Limb Movements of Sleep. Sleep Med. Clin. 2021, 16, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Stenvers, D.J.; Scheer, F.; Schrauwen, P.; la Fleur, S.E.; Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef]
- Dijk, D.J.; Lockley, S.W. Integration of human sleep-wake regulation and circadian rhythmicity. J. Appl. Physiol. 2002, 92, 852–862. [Google Scholar] [CrossRef]
- Buhr, E.D.; Takahashi, J.S. Molecular components of the Mammalian circadian clock. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–27. [Google Scholar] [CrossRef]
- Sato, T.; Sato, S. Circadian Regulation of Metabolism: Commitment to Health and Diseases. Endocrinology 2023, 164, bqad086. [Google Scholar] [CrossRef]
- Meszaros, M.; Bikov, A. Obstructive Sleep Apnoea and Lipid Metabolism: The Summary of Evidence and Future Perspectives in the Pathophysiology of OSA-Associated Dyslipidaemia. Biomedicines 2022, 10, 2754. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-Garcia, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martin, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Ziegler, K.A.; Ahles, A.; Dueck, A.; Esfandyari, D.; Pichler, P.; Weber, K.; Kotschi, S.; Bartelt, A.; Sinicina, I.; Graw, M.; et al. Immune-mediated denervation of the pineal gland underlies sleep disturbance in cardiac disease. Science 2023, 381, 285–290. [Google Scholar] [CrossRef]
- Degaute, J.P.; van de Borne, P.; Linkowski, P.; Van Cauter, E. Quantitative analysis of the 24-hour blood pressure and heart rate patterns in young men. Hypertension 1991, 18, 199–210. [Google Scholar] [CrossRef]
- Shea, S.A.; Hilton, M.F.; Hu, K.; Scheer, F.A. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ. Res. 2011, 108, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Prinz, P.N.; Halter, J.; Benedetti, C.; Raskind, M. Circadian variation of plasma catecholamines in young and old men: Relation to rapid eye movement and slow wave sleep. J. Clin. Endocrinol. Metab. 1979, 49, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Turton, M.B.; Deegan, T. Circadian variations of plasma catecholamine, cortisol and immunoreactive insulin concentrations in supine subjects. Clin. Chim. Acta 1974, 55, 389–397. [Google Scholar] [CrossRef]
- Otto, M.E.; Svatikova, A.; Barretto, R.B.; Santos, S.; Hoffmann, M.; Khandheria, B.; Somers, V. Early morning attenuation of endothelial function in healthy humans. Circulation 2004, 109, 2507–2510. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Michelson, A.D.; Frelinger, A.L., 3rd; Evoniuk, H.; Kelly, E.E.; McCarthy, M.; Doamekpor, L.A.; Barnard, M.R.; Shea, S.A. The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS ONE 2011, 6, e24549. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Vujovic, N.; Williams, J.S.; Scheer, F. Impact of Circadian Disruption on Cardiovascular Function and Disease. Trends Endocrinol. Metab. 2019, 30, 767–779. [Google Scholar] [CrossRef]
- Muller, J.E. Circadian variation in cardiovascular events. Am. J. Hypertens. 1999, 12, 35S–42S. [Google Scholar] [CrossRef][Green Version]
- Elliott, W.J. Circadian variation in the timing of stroke onset: A meta-analysis. Stroke 1998, 29, 992–996. [Google Scholar] [CrossRef]
- Muller, J.E.; Stone, P.H.; Turi, Z.G.; Rutherford, J.D.; Czeisler, C.A.; Parker, C.; Poole, W.K.; Passamani, E.; Roberts, R.; Robertson, T.; et al. Circadian variation in the frequency of onset of acute myocardial infarction. N. Engl. J. Med. 1985, 313, 1315–1322. [Google Scholar] [CrossRef]
- Willich, S.N.; Goldberg, R.J.; Maclure, M.; Perriello, L.; Muller, J.E. Increased onset of sudden cardiac death in the first three hours after awakening. Am. J. Cardiol. 1992, 70, 65–68. [Google Scholar] [CrossRef]
- Minors, D.S.; Waterhouse, J.M. The use of constant routines in unmasking the endogenous component of human circadian rhythms. Chronobiol. Int. 1984, 1, 205–216. [Google Scholar] [CrossRef]
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Thosar, S.S.; Butler, M.P.; Shea, S.A. Role of the circadian system in cardiovascular disease. J. Clin. Investig. 2018, 128, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Dolan, E.; Stanton, A.; Thijs, L.; Hinedi, K.; Atkins, N.; McClory, S.; Den Hond, E.; McCormack, P.; Staessen, J.A.; O’Brien, E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: The Dublin outcome study. Hypertension 2005, 46, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.A.; Drayer, J.I.; Nakamura, D.K.; Wyle, F.A. The circadian blood pressure pattern in ambulatory normal subjects. Am. J. Cardiol. 1984, 54, 115–119. [Google Scholar] [CrossRef]
- Seo, H.S.; Kang, T.S.; Park, S.; Choi, E.Y.; Ko, Y.G.; Choi, D.; Ha, J.; Rim, S.J.; Chung, N. Non-dippers are associated with adverse cardiac remodeling and dysfunction (R1). Int. J. Cardiol. 2006, 112, 171–177. [Google Scholar] [CrossRef]
- Zweiker, R.; Eber, B.; Schumacher, M.; Toplak, H.; Klein, W. “Non-dipping” related to cardiovascular events in essential hypertensive patients. Acta Med. Austriaca 1994, 21, 86–89. [Google Scholar]
- Kario, K.; Shimada, K.; Pickering, T.G. Clinical implication of morning blood pressure surge in hypertension. J. Cardiovasc. Pharmacol. 2003, 42 (Suppl. S1), S87–S91. [Google Scholar] [CrossRef]
- Sogunuru, G.P.; Kario, K.; Shin, J.; Chen, C.H.; Buranakitjaroen, P.; Chia, Y.C.; Divinagracia, R.; Nailes, J.; Park, S.; Siddique, S.; et al. Morning surge in blood pressure and blood pressure variability in Asia: Evidence and statement from the HOPE Asia Network. J. Clin. Hypertens. 2019, 21, 324–334. [Google Scholar] [CrossRef]
- Scheer, F.A.; Hu, K.; Evoniuk, H.; Kelly, E.E.; Malhotra, A.; Hilton, M.F.; Shea, S.A. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc. Natl. Acad. Sci. USA 2010, 107, 20541–20546. [Google Scholar] [CrossRef]
- Man, K.; Loudon, A.; Chawla, A. Immunity around the clock. Science 2016, 354, 999–1003. [Google Scholar] [CrossRef]
- Tofler, G.H.; Brezinski, D.; Schafer, A.I.; Czeisler, C.A.; Rutherford, J.D.; Willich, S.N.; Gleason, R.E.; Williams, G.H.; Muller, J.E. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N. Engl. J. Med. 1987, 316, 1514–1518. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Shea, S.A. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood 2014, 123, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Maemura, K.; de la Monte, S.M.; Chin, M.T.; Layne, M.D.; Hsieh, C.M.; Yet, S.F.; Perrella, M.A.; Lee, M.E. CLIF, a novel cycle-like factor, regulates the circadian oscillation of plasminogen activator inhibitor-1 gene expression. J. Biol. Chem. 2000, 275, 36847–36851. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, L.; Lazar, M.A. The orphan nuclear receptor Rev-erbα regulates circadian expression of plasminogen activator inhibitor type 1. J. Biol. Chem. 2006, 281, 33842–33848. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Manson, J.E.; Buring, J.E.; Muller, J.E.; Hennekens, C.H. Circadian variation of acute myocardial infarction and the effect of low-dose aspirin in a randomized trial of physicians. Circulation 1990, 82, 897–902. [Google Scholar] [CrossRef]
- Jamwal, S.; Sharma, S. Vascular endothelium dysfunction: A conservative target in metabolic disorders. Inflamm. Res. 2018, 67, 391–405. [Google Scholar] [CrossRef]
- Man, A.W.C.; Li, H.; Xia, N. Circadian Rhythm: Potential Therapeutic Target for Atherosclerosis and Thrombosis. Int. J. Mol. Sci. 2021, 22, 676. [Google Scholar] [CrossRef]
- Halcox, J.P.; Schenke, W.H.; Zalos, G.; Mincemoyer, R.; Prasad, A.; Waclawiw, M.A.; Nour, K.R.; Quyyumi, A.A. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002, 106, 653–658. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. The discovery of nitric oxide as the endogenous nitrovasodilator. Hypertension 1988, 12, 365–372. [Google Scholar] [CrossRef]
- Palmer, R.M.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Radomski, M.W.; Palmer, R.M.; Moncada, S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 1987, 2, 1057–1058. [Google Scholar] [CrossRef] [PubMed]
- Bunting, S.; Moncada, S.; Vane, J.R. Antithrombotic properties of vascular endothelium. Lancet 1977, 2, 1075–1076. [Google Scholar] [CrossRef] [PubMed]
- Sagripanti, A.; Carpi, A. Antithrombotic and prothrombotic activities of the vascular endothelium. Biomed. Pharmacother. 2000, 54, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Elherik, K.; Khan, F.; McLaren, M.; Kennedy, G.; Belch, J.J. Circadian variation in vascular tone and endothelial cell function in normal males. Clin. Sci. 2002, 102, 547–552. [Google Scholar] [CrossRef]
- Jones, H.; Lewis, N.C.; Thompson, A.; Marrin, K.; Green, D.J.; Atkinson, G. Diurnal variation in vascular function: Role of sleep. Chronobiol. Int. 2012, 29, 271–277. [Google Scholar] [CrossRef]
- Kawano, H.; Motoyama, T.; Yasue, H.; Hirai, N.; Waly, H.M.; Kugiyama, K.; Ogawa, H. Endothelial function fluctuates with diurnal variation in the frequency of ischemic episodes in patients with variant angina. J. Am. Coll. Cardiol. 2002, 40, 266–270. [Google Scholar] [CrossRef]
- Viswambharan, H.; Carvas, J.M.; Antic, V.; Marecic, A.; Jud, C.; Zaugg, C.E.; Ming, X.F.; Montani, J.P.; Albrecht, U.; Yang, Z. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation 2007, 115, 2188–2195. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wen, M.S.; Wang, H.W.; Hsieh, I.C.; Li, Y.; Liu, P.Y.; Lin, F.C.; Liao, J.K. Increased vascular senescence and impaired endothelial progenitor cell function mediated by mutation of circadian gene Per2. Circulation 2008, 118, 2166–2173. [Google Scholar] [CrossRef]
- Burford, N.G.; Webster, N.A.; Cruz-Topete, D. Hypothalamic-Pituitary-Adrenal Axis Modulation of Glucocorticoids in the Cardiovascular System. Int. J. Mol. Sci. 2017, 18, 2150. [Google Scholar] [CrossRef]
- Storch, K.F.; Lipan, O.; Leykin, I.; Viswanathan, N.; Davis, F.C.; Wong, W.H.; Weitz, C.J. Extensive and divergent circadian gene expression in liver and heart. Nature 2002, 417, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Martino, T.; Arab, S.; Straume, M.; Belsham, D.D.; Tata, N.; Cai, F.; Liu, P.; Trivieri, M.; Ralph, M.; Sole, M.J. Day/night rhythms in gene expression of the normal murine heart. J. Mol. Med. 2004, 82, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Hergenhan, S.; Holtkamp, S.; Scheiermann, C. Molecular Interactions Between Components of the Circadian Clock and the Immune System. J. Mol. Biol. 2020, 432, 3700–3713. [Google Scholar] [CrossRef] [PubMed]
- Lefta, M.; Campbell, K.S.; Feng, H.Z.; Jin, J.P.; Esser, K.A. Development of dilated cardiomyopathy in Bmal1-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H475–H485. [Google Scholar] [CrossRef] [PubMed]
- Young, M.E.; Brewer, R.A.; Peliciari-Garcia, R.A.; Collins, H.E.; He, L.; Birky, T.L.; Peden, B.W.; Thompson, E.G.; Ammons, B.J.; Bray, M.S.; et al. Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart. J. Biol. Rhythm. 2014, 29, 257–276. [Google Scholar] [CrossRef]
- Schroder, E.A.; Lefta, M.; Zhang, X.; Bartos, D.C.; Feng, H.Z.; Zhao, Y.; Patwardhan, A.; Jin, J.P.; Esser, K.A.; Delisle, B.P. The cardiomyocyte molecular clock, regulation of Scn5a, and arrhythmia susceptibility. Am. J. Physiol. Cell Physiol. 2013, 304, C954–C965. [Google Scholar] [CrossRef]
- Xie, Z.; Su, W.; Liu, S.; Zhao, G.; Esser, K.; Schroder, E.A.; Lefta, M.; Stauss, H.M.; Guo, Z.; Gong, M.C. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J. Clin. Investig. 2015, 125, 324–336. [Google Scholar] [CrossRef]
- Doi, M.; Takahashi, Y.; Komatsu, R.; Yamazaki, F.; Yamada, H.; Haraguchi, S.; Emoto, N.; Okuno, Y.; Tsujimoto, G.; Kanematsu, A.; et al. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat. Med. 2010, 16, 67–74. [Google Scholar] [CrossRef]
- Gumz, M.L.; Stow, L.R.; Lynch, I.J.; Greenlee, M.M.; Rudin, A.; Cain, B.D.; Weaver, D.R.; Wingo, C.S. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J. Clin. Investig. 2009, 119, 2423–2434. [Google Scholar] [CrossRef]
- Hermann, D.M.; Bassetti, C.L. Sleep-related breathing and sleep-wake disturbances in ischemic stroke. Neurology 2009, 73, 1313–1322. [Google Scholar] [CrossRef]
- Morris, C.J.; Purvis, T.E.; Hu, K.; Scheer, F.A. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E1402–E1411. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Purvis, T.E.; Mistretta, J.; Hu, K.; Scheer, F. Circadian Misalignment Increases C-Reactive Protein and Blood Pressure in Chronic Shift Workers. J. Biol. Rhythm. 2017, 32, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Han, J.; Laden, F.; Pan, A.; Caporaso, N.E.; Stampfer, M.J.; Kawachi, I.; Rexrode, K.M.; Willett, W.C.; Hankinson, S.E.; et al. Total and cause-specific mortality of U.S. nurses working rotating night shifts. Am. J. Prev. Med. 2015, 48, 241–252. [Google Scholar] [CrossRef]
- Knutsson, A.; Akerstedt, T.; Jonsson, B.G.; Orth-Gomer, K. Increased risk of ischaemic heart disease in shift workers. Lancet 1986, 2, 89–92. [Google Scholar] [CrossRef]
- Wegrzyn, L.R.; Tamimi, R.M.; Rosner, B.A.; Brown, S.B.; Stevens, R.G.; Eliassen, A.H.; Laden, F.; Willett, W.C.; Hankinson, S.E.; Schernhammer, E.S. Rotating Night-Shift Work and the Risk of Breast Cancer in the Nurses’ Health Studies. Am. J. Epidemiol. 2017, 186, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Lotti, S.; Pagliai, G.; Colombini, B.; Sofi, F.; Dinu, M. Chronotype Differences in Energy Intake, Cardiometabolic Risk Parameters, Cancer, and Depression: A Systematic Review with Meta-Analysis of Observational Studies. Adv. Nutr. 2022, 13, 269–281. [Google Scholar] [CrossRef]
- Irwin, M.R.; Wang, M.; Campomayor, C.O.; Collado-Hidalgo, A.; Cole, S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch. Intern. Med. 2006, 166, 1756–1762. [Google Scholar] [CrossRef]
- van Leeuwen, W.M.; Lehto, M.; Karisola, P.; Lindholm, H.; Luukkonen, R.; Sallinen, M.; Harma, M.; Porkka-Heiskanen, T.; Alenius, H. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS ONE 2009, 4, e4589. [Google Scholar] [CrossRef]
- Nagai, M.; Hoshide, S.; Kario, K. Sleep duration as a risk factor for cardiovascular disease- a review of the recent literature. Curr. Cardiol. Rev. 2010, 6, 54–61. [Google Scholar] [CrossRef]
- Briancon-Marjollet, A.; Weiszenstein, M.; Henri, M.; Thomas, A.; Godin-Ribuot, D.; Polak, J. The impact of sleep disorders on glucose metabolism: Endocrine and molecular mechanisms. Diabetol. Metab. Syndr. 2015, 7, 25. [Google Scholar] [CrossRef]
- Sauvet, F.; Leftheriotis, G.; Gomez-Merino, D.; Langrume, C.; Drogou, C.; Van Beers, P.; Bourrilhon, C.; Florence, G.; Chennaoui, M. Effect of acute sleep deprivation on vascular function in healthy subjects. J. Appl. Physiol. 2010, 108, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, D.; Carter, J.R.; Van Cauter, E.; Leproult, R. Adverse Impact of Sleep Restriction and Circadian Misalignment on Autonomic Function in Healthy Young Adults. Hypertension 2016, 68, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Leproult, R.; Holmback, U.; Van Cauter, E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 2014, 63, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, R.; Fabbian, F.; De Giorgi, A.; Zucchi, B.; Cappadona, R.; Signani, F.; Katsiki, N.; Mikhailidis, D.P. Daylight saving time and myocardial infarction: Should we be worried? A review of the evidence. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 750–755. [Google Scholar] [CrossRef]
- Erren, T.C.; Lewis, P. Can yesterday’s smoking research inform today’s shiftwork research? Epistemological consequences for exposures and doses due to circadian disruption at and off work. J. Occup. Med. Toxicol. 2017, 12, 29. [Google Scholar] [CrossRef][Green Version]
- Huang, T.; Mariani, S.; Redline, S. Sleep Irregularity and Risk of Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis. J. Am. Coll. Cardiol. 2020, 75, 991–999. [Google Scholar] [CrossRef]
- Mason, I.C.; Grimaldi, D.; Reid, K.J.; Warlick, C.D.; Malkani, R.G.; Abbott, S.M.; Zee, P.C. Light exposure during sleep impairs cardiometabolic function. Proc. Natl. Acad. Sci. USA 2022, 119, e2113290119. [Google Scholar] [CrossRef]
- Alibhai, F.J.; Tsimakouridze, E.V.; Chinnappareddy, N.; Wright, D.C.; Billia, F.; O’Sullivan, M.L.; Pyle, W.G.; Sole, M.J.; Martino, T.A. Short-term disruption of diurnal rhythms after murine myocardial infarction adversely affects long-term myocardial structure and function. Circ. Res. 2014, 114, 1713–1722. [Google Scholar] [CrossRef]
- Mondola, P.; Gambardella, P.; Santangelo, F.; Santillo, M.; Greco, A.M. Circadian rhythms of lipid and apolipoprotein pattern in adult fasted rats. Physiol. Behav. 1995, 58, 175–180. [Google Scholar] [CrossRef]
- Kent, B.A.; Rahman, S.A.; St Hilaire, M.A.; Grant, L.K.; Rüger, M.; Czeisler, C.A.; Lockley, S.W. Circadian lipid and hepatic protein rhythms shift with a phase response curve different than melatonin. Nat. Commun. 2022, 13, 681. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metab. Clin. Exp. 2018, 84, 11–27. [Google Scholar] [CrossRef]
- Kyle, J.E.; Bramer, L.M.; Claborne, D.; Stratton, K.G.; Bloodsworth, K.J.; Teeguarden, J.G.; Gaddameedhi, S.; Metz, T.O.; Van Dongen, H.P.A. Simulated Night-Shift Schedule Disrupts the Plasma Lipidome and Reveals Early Markers of Cardiovascular Disease Risk. Nat. Sci. Sleep 2022, 14, 981–994. [Google Scholar] [CrossRef]
- Scheer, F.A.; Morris, C.J.; Shea, S.A. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity 2013, 21, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Moro Chao, D.; León-Mercado, L.; Foppen, E.; Guzmán-Ruiz, M.; Basualdo, M.C.; Escobar, C.; Buijs, R.M. The Suprachiasmatic Nucleus Modulates the Sensitivity of Arcuate Nucleus to Hypoglycemia in the Male Rat. Endocrinology 2016, 157, 3439–3451. [Google Scholar] [CrossRef] [PubMed]
- Blancas-Velazquez, A.; Mendoza, J.; Garcia, A.N.; la Fleur, S.E. Diet-Induced Obesity and Circadian Disruption of Feeding Behavior. Front. Neurosci. 2017, 11, 23. [Google Scholar] [CrossRef]
- Cha, M.C.; Chou, C.J.; Boozer, C.N. High-fat diet feeding reduces the diurnal variation of plasma leptin concentration in rats. Metab. Clin. Exp. 2000, 49, 503–507. [Google Scholar] [CrossRef]
- Qian, J.; Morris, C.J.; Caputo, R.; Garaulet, M.; Scheer, F. Ghrelin is impacted by the endogenous circadian system and by circadian misalignment in humans. Int. J. Obes. 2019, 43, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Galindo Muñoz, J.S.; Jiménez Rodríguez, D.; Hernández Morante, J.J. Diurnal rhythms of plasma GLP-1 levels in normal and overweight/obese subjects: Lack of effect of weight loss. J. Physiol. Biochem. 2015, 71, 17–28. [Google Scholar] [CrossRef]
- Hill, B.R.; De Souza, M.J.; Williams, N.I. Characterization of the diurnal rhythm of peptide YY and its association with energy balance parameters in normal-weight premenopausal women. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E409–E415. [Google Scholar] [CrossRef]
- Schade, R.; Vick, K.; Ott, T.; Sohr, R.; Pfister, C.; Bellach, J.; Golor, G.; Lemmer, B. Circadian rhythms of dopamine and cholecystokinin in nucleus accumbens and striatum of rats—Influence on dopaminergic stimulation. Chronobiol. Int. 1995, 12, 87–99. [Google Scholar] [CrossRef]
- Hoogerwerf, W.A. Role of clock genes in gastrointestinal motility. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G549–G555. [Google Scholar] [CrossRef] [PubMed]
- Glasbrenner, B.; Dürrschnabel, L.; Büchler, M.; Malfertheiner, P. Nonparallel patterns of circadian pancreatic and biliary secretions in fasting rats. Int. J. Pancreatol. Off. J. Int. Assoc. Pancreatol. 1992, 11, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Geiger, S.S.; Traba, J.; Richoz, N.; Farley, T.K.; Brooks, S.R.; Petermann, F.; Wang, L.; Gonzalez, F.J.; Sack, M.N.; Siegel, R.M. Feeding-induced resistance to acute lethal sepsis is dependent on hepatic BMAL1 and FXR signalling. Nat. Commun. 2021, 12, 2745. [Google Scholar] [CrossRef] [PubMed]
- Le Martelot, G.; Claudel, T.; Gatfield, D.; Schaad, O.; Kornmann, B.; Lo Sasso, G.; Moschetta, A.; Schibler, U. REV-ERBα participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009, 7, e1000181. [Google Scholar] [CrossRef]
- He, C.; Shen, W.; Chen, C.; Wang, Q.; Lu, Q.; Shao, W.; Jiang, Z.; Hu, H. Circadian Rhythm Disruption Influenced Hepatic Lipid Metabolism, Gut Microbiota and Promoted Cholesterol Gallstone Formation in Mice. Front. Endocrinol. 2021, 12, 723918. [Google Scholar] [CrossRef]
- Hussain, M.M. Regulation of intestinal lipid absorption by clock genes. Annu. Rev. Nutr. 2014, 34, 357–375. [Google Scholar] [CrossRef]
- Pan, X.; Jiang, X.C.; Hussain, M.M. Impaired cholesterol metabolism and enhanced atherosclerosis in clock mutant mice. Circulation 2013, 128, 1758–1769. [Google Scholar] [CrossRef]
- Pan, X.; Bradfield, C.A.; Hussain, M.M. Global and hepatocyte-specific ablation of Bmal1 induces hyperlipidaemia and enhances atherosclerosis. Nat. Commun. 2016, 7, 13011. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, Y.; Wang, L.; Hussain, M.M. Diurnal regulation of MTP and plasma triglyceride by CLOCK is mediated by SHP. Cell Metab. 2010, 12, 174–186. [Google Scholar] [CrossRef]
- Pan, X.; Hussain, M.M. Bmal1 regulates production of larger lipoproteins by modulating cAMP-responsive element-binding protein H and apolipoprotein AIV. Hepatology 2022, 76, 78–93. [Google Scholar] [CrossRef]
- Delezie, J.; Dumont, S.; Dardente, H.; Oudart, H.; Gréchez-Cassiau, A.; Klosen, P.; Teboul, M.; Delaunay, F.; Pévet, P.; Challet, E. The nuclear receptor REV-ERBα is required for the daily balance of carbohydrate and lipid metabolism. FASEB J. 2012, 26, 3321–3335. [Google Scholar] [CrossRef] [PubMed]
- Schoonjans, K.; Peinado-Onsurbe, J.; Lefebvre, A.M.; Heyman, R.A.; Briggs, M.; Deeb, S.; Staels, B.; Auwerx, J. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 1996, 15, 5336–5348. [Google Scholar] [CrossRef] [PubMed]
- Shimba, S.; Ishii, N.; Ohta, Y.; Ohno, T.; Watabe, Y.; Hayashi, M.; Wada, T.; Aoyagi, T.; Tezuka, M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 12071–12076. [Google Scholar] [CrossRef] [PubMed]
- Benavides, A.; Siches, M.; Llobera, M. Circadian rhythms of lipoprotein lipase and hepatic lipase activities in intermediate metabolism of adult rat. Am. J. Physiol. 1998, 275, R811–R817. [Google Scholar] [CrossRef]
- Ma, D.; Liu, T.; Chang, L.; Rui, C.; Xiao, Y.; Li, S.; Hogenesch, J.B.; Chen, Y.E.; Lin, J.D. The Liver Clock Controls Cholesterol Homeostasis through Trib1 Protein-mediated Regulation of PCSK9/Low Density Lipoprotein Receptor (LDLR) Axis. J. Biol. Chem. 2015, 290, 31003–31012. [Google Scholar] [CrossRef]
- Eberlé, D.; Hegarty, B.; Bossard, P.; Ferré, P.; Foufelle, F. SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef]
- Matsumoto, E.; Ishihara, A.; Tamai, S.; Nemoto, A.; Iwase, K.; Hiwasa, T.; Shibata, S.; Takiguchi, M. Time of day and nutrients in feeding govern daily expression rhythms of the gene for sterol regulatory element-binding protein (SREBP)-1 in the mouse liver. J. Biol. Chem. 2010, 285, 33028–33036. [Google Scholar] [CrossRef]
- Edwards, P.A.; Muroya, H.; Gould, R.G. In vivo demonstration of the circadian thythm of cholesterol biosynthesis in the liver and intestine of the rat. J. Lipid Res. 1972, 13, 396–401. [Google Scholar] [CrossRef]
- Cella, L.K.; Van Cauter, E.; Schoeller, D.A. Diurnal rhythmicity of human cholesterol synthesis: Normal pattern and adaptation to simulated “jet lag”. Am. J. Physiol. 1995, 269, E489–E498. [Google Scholar] [CrossRef]
- Inoue, I.; Shinoda, Y.; Ikeda, M.; Hayashi, K.; Kanazawa, K.; Nomura, M.; Matsunaga, T.; Xu, H.; Kawai, S.; Awata, T.; et al. CLOCK/BMAL1 is involved in lipid metabolism via transactivation of the peroxisome proliferator-activated receptor (PPAR) response element. J. Atheroscler. Thromb. 2005, 12, 169–174. [Google Scholar] [CrossRef]
- Adamovich, Y.; Rousso-Noori, L.; Zwighaft, Z.; Neufeld-Cohen, A.; Golik, M.; Kraut-Cohen, J.; Wang, M.; Han, X.; Asher, G. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 2014, 19, 319–330. [Google Scholar] [CrossRef]
- Aggarwal, A.; Costa, M.J.; Rivero-Gutiérrez, B.; Ji, L.; Morgan, S.L.; Feldman, B.J. The Circadian Clock Regulates Adipogenesis by a Per3 Crosstalk Pathway to Klf15. Cell Rep. 2017, 21, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Shostak, A.; Meyer-Kovac, J.; Oster, H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes 2013, 62, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Saini, C.; Petrenko, V.; Pulimeno, P.; Giovannoni, L.; Berney, T.; Hebrok, M.; Howald, C.; Dermitzakis, E.T.; Dibner, C. A functional circadian clock is required for proper insulin secretion by human pancreatic islet cells. Diabetes Obes. Metab. 2016, 18, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Shimba, S.; Ogawa, T.; Hitosugi, S.; Ichihashi, Y.; Nakadaira, Y.; Kobayashi, M.; Tezuka, M.; Kosuge, Y.; Ishige, K.; Ito, Y.; et al. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLoS ONE 2011, 6, e25231. [Google Scholar] [CrossRef]
- Buckley, T.M.; Schatzberg, A.F. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: Normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J. Clin. Endocrinol. Metab. 2005, 90, 3106–3114. [Google Scholar] [CrossRef]
- Brindley, D.N. Role of glucocorticoids and fatty acids in the impairment of lipid metabolism observed in the metabolic syndrome. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1995, 19 (Suppl. S1), S69–S75. [Google Scholar]
- Ramracheya, R.D.; Muller, D.S.; Squires, P.E.; Brereton, H.; Sugden, D.; Huang, G.C.; Amiel, S.A.; Jones, P.M.; Persaud, S.J. Function and expression of melatonin receptors on human pancreatic islets. J. Pineal Res. 2008, 44, 273–279. [Google Scholar] [CrossRef]
- Willoughby, J.O.; Martin, J.B. The suprachiasmatic nucleus synchronizes growth hormone secretory rhythms with the light-dark cycle. Brain Res. 1978, 151, 413–417. [Google Scholar] [CrossRef]
- Møller, N.; Gjedsted, J.; Gormsen, L.; Fuglsang, J.; Djurhuus, C. Effects of growth hormone on lipid metabolism in humans. Growth Horm. IGF Res. Off. J. Growth Horm. Res. Soc. Int. IGF Res. Soc. 2003, 13 (Suppl. S1), S18–S21. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kipnis, D.M.; Daughaday, W.H. Growth hormone secretion during sleep. J. Clin. Investig. 1968, 47, 2079–2090. [Google Scholar] [CrossRef] [PubMed]
- Steffens, S.; Winter, C.; Schloss, M.J.; Hidalgo, A.; Weber, C.; Soehnlein, O. Circadian Control of Inflammatory Processes in Atherosclerosis and Its Complications. Arter. Thromb. Vasc. Biol. 2017, 37, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Arter. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Fentress, S.J.; Qiu, Y.; Yun, K.; Cox, J.S.; Chawla, A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 2013, 341, 1483–1488. [Google Scholar] [CrossRef]
- Early, J.O.; Menon, D.; Wyse, C.A.; Cervantes-Silva, M.P.; Zaslona, Z.; Carroll, R.G.; Palsson-McDermott, E.M.; Angiari, S.; Ryan, D.G.; Corcoran, S.E.; et al. Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc. Natl. Acad. Sci. USA 2018, 115, E8460–E8468. [Google Scholar] [CrossRef]
- Curtis, A.M.; Fagundes, C.T.; Yang, G.; Palsson-McDermott, E.M.; Wochal, P.; McGettrick, A.F.; Foley, N.H.; Early, J.O.; Chen, L.; Zhang, H.; et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc. Natl. Acad. Sci. USA 2015, 112, 7231–7236. [Google Scholar] [CrossRef]
- Spengler, M.L.; Kuropatwinski, K.K.; Comas, M.; Gasparian, A.V.; Fedtsova, N.; Gleiberman, A.S.; Gitlin, II; Artemicheva, N.M.; Deluca, K.A.; Gudkov, A.V.; et al. Core circadian protein CLOCK is a positive regulator of NF-κB-mediated transcription. Proc. Natl. Acad. Sci. USA 2012, 109, E2457–E2465. [Google Scholar] [CrossRef]
- Liu, J.; Malkani, G.; Shi, X.; Meyer, M.; Cunningham-Runddles, S.; Ma, X.; Sun, Z.S. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect. Immun. 2006, 74, 4750–4756. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Z.; Yang, P.; Xia, L.; Zhou, M.; Wang, S.; Du, J.; Zhang, J. PER1 prevents excessive innate immune response during endotoxin-induced liver injury through regulation of macrophage recruitment in mice. Cell Death Dis. 2016, 7, e2176. [Google Scholar] [CrossRef]
- Hashiramoto, A.; Yamane, T.; Tsumiyama, K.; Yoshida, K.; Komai, K.; Yamada, H.; Yamazaki, F.; Doi, M.; Okamura, H.; Shiozawa, S. Mammalian clock gene Cryptochrome regulates arthritis via proinflammatory cytokine TNF-α. J. Immunol. 2010, 184, 1560–1565. [Google Scholar] [CrossRef]
- Narasimamurthy, R.; Hatori, M.; Nayak, S.K.; Liu, F.; Panda, S.; Verma, I.M. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 12662–12667. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.E.; Blaikley, J.; Beesley, S.; Matthews, L.; Simpson, K.D.; Boyce, S.H.; Farrow, S.N.; Else, K.J.; Singh, D.; Ray, D.W.; et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Pourcet, B.; Zecchin, M.; Ferri, L.; Beauchamp, J.; Sitaula, S.; Billon, C.; Delhaye, S.; Vanhoutte, J.; Mayeuf-Louchart, A.; Thorel, Q.; et al. Nuclear Receptor Subfamily 1 Group D Member 1 Regulates Circadian Activity of NLRP3 Inflammasome to Reduce the Severity of Fulminant Hepatitis in Mice. Gastroenterology 2018, 154, 1449–1464.e1420. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Sakurai, T.; Ogasawara, J.; Takahashi, M.; Izawa, T.; Imaizumi, K.; Taniguchi, N.; Ohno, H.; Kizaki, T. A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J. Immunol. 2014, 192, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Serbina, N.V.; Pamer, E.G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006, 7, 311–317. [Google Scholar] [CrossRef]
- Delerive, P.; Monte, D.; Dubois, G.; Trottein, F.; Fruchart-Najib, J.; Mariani, J.; Fruchart, J.C.; Staels, B. The orphan nuclear receptor RORα is a negative regulator of the inflammatory response. EMBO Rep. 2001, 2, 42–48. [Google Scholar] [CrossRef]
- Dzhagalov, I.; Giguere, V.; He, Y.W. Lymphocyte development and function in the absence of retinoic acid-related orphan receptor α. J. Immunol. 2004, 173, 2952–2959. [Google Scholar] [CrossRef]
- Swirski, F.K.; Libby, P.; Aikawa, E.; Alcaide, P.; Luscinskas, F.W.; Weissleder, R.; Pittet, M.J. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Investig. 2007, 117, 195–205. [Google Scholar] [CrossRef]
- Tacke, F.; Alvarez, D.; Kaplan, T.J.; Jakubzick, C.; Spanbroek, R.; Llodra, J.; Garin, A.; Liu, J.; Mack, M.; van Rooijen, N.; et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Investig. 2007, 117, 185–194. [Google Scholar] [CrossRef]
- McAlpine, C.S.; Swirski, F.K. Circadian Influence on Metabolism and Inflammation in Atherosclerosis. Circ. Res. 2016, 119, 131–141. [Google Scholar] [CrossRef]
- Huo, M.; Huang, Y.; Qu, D.; Zhang, H.; Wong, W.T.; Chawla, A.; Huang, Y.; Tian, X.Y. Myeloid Bmal1 deletion increases monocyte recruitment and worsens atherosclerosis. FASEB J. 2017, 31, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Meng, D.; Sun, N.; Zhu, Z.; Zhao, R.; Lu, C.; Chen, S.; Hua, L.; Qian, R. Clock upregulates intercellular adhesion molecule-1 expression and promotes mononuclear cells adhesion to endothelial cells. Biochem. Biophys. Res. Commun. 2014, 443, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Mazuch, J.; Abraham, U.; Eom, G.D.; Herzog, E.D.; Volk, H.D.; Kramer, A.; Maier, B. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. USA 2009, 106, 21407–21412. [Google Scholar] [CrossRef] [PubMed]
- Anea, C.B.; Cheng, B.; Sharma, S.; Kumar, S.; Caldwell, R.W.; Yao, L.; Ali, M.I.; Merloiu, A.M.; Stepp, D.W.; Black, S.M.; et al. Increased superoxide and endothelial NO synthase uncoupling in blood vessels of Bmal1-knockout mice. Circ. Res. 2012, 111, 1157–1165. [Google Scholar] [CrossRef]
- Anea, C.B.; Zhang, M.; Stepp, D.W.; Simkins, G.B.; Reed, G.; Fulton, D.J.; Rudic, R.D. Vascular disease in mice with a dysfunctional circadian clock. Circulation 2009, 119, 1510–1517. [Google Scholar] [CrossRef]
- Sitaula, S.; Billon, C.; Kamenecka, T.M.; Solt, L.A.; Burris, T.P. Suppression of atherosclerosis by synthetic REV-ERB agonist. Biochem. Biophys. Res. Commun. 2015, 460, 566–571. [Google Scholar] [CrossRef]
- Yang, L.; Chu, Y.; Wang, L.; Wang, Y.; Zhao, X.; He, W.; Zhang, P.; Yang, X.; Liu, X.; Tian, L.; et al. Overexpression of CRY1 protects against the development of atherosclerosis via the TLR/NF-κB pathway. Int. Immunopharmacol. 2015, 28, 525–530. [Google Scholar] [CrossRef]
- Full, K.M.; Huang, T.; Shah, N.A.; Allison, M.A.; Michos, E.D.; Duprez, D.A.; Redline, S.; Lutsey, P.L. Sleep Irregularity and Subclinical Markers of Cardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2023, 12, e027361. [Google Scholar] [CrossRef]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian timing of food intake contributes to weight gain. Obesity 2009, 17, 2100–2102. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef]
- Mukherji, A.; Kobiita, A.; Damara, M.; Misra, N.; Meziane, H.; Champy, M.F.; Chambon, P. Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, E6691–E6698. [Google Scholar] [CrossRef]
- Tsurudome, Y.; Akamine, T.; Horiguchi, M.; Wada, Y.; Fujimura, A.; Ushijima, K. Potential mechanism of hepatic lipid accumulation during a long-term rest phase restricted feeding in mice. Chronobiol. Int. 2022, 39, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Vujović, N.; Piron, M.J.; Qian, J.; Chellappa, S.L.; Nedeltcheva, A.; Barr, D.; Heng, S.W.; Kerlin, K.; Srivastav, S.; Wang, W.; et al. Late isocaloric eating increases hunger, decreases energy expenditure, and modifies metabolic pathways in adults with overweight and obesity. Cell Metab. 2022, 34, 1486–1498.e1487. [Google Scholar] [CrossRef] [PubMed]
- Romon, M.; Le Fur, C.; Lebel, P.; Edmé, J.L.; Fruchart, J.C.; Dallongeville, J. Circadian variation of postprandial lipemia. Am. J. Clin. Nutr. 1997, 65, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; McStay, M.; Gabel, K.; Varady, K.A. Time restricted eating for the prevention of type 2 diabetes. J. Physiol. 2022, 600, 1253–1264. [Google Scholar] [CrossRef]
- Liu, L.; Chen, W.; Wu, D.; Hu, F. Metabolic Efficacy of Time-Restricted Eating in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2022, 107, 3428–3441. [Google Scholar] [CrossRef]
- Cederroth, C.R.; Albrecht, U.; Bass, J.; Brown, S.A.; Dyhrfjeld-Johnsen, J.; Gachon, F.; Green, C.B.; Hastings, M.H.; Helfrich-Förster, C.; Hogenesch, J.B.; et al. Medicine in the Fourth Dimension. Cell Metab. 2019, 30, 238–250. [Google Scholar] [CrossRef]
- Hermida, R.C.; Ayala, D.E. Chronotherapy with the angiotensin-converting enzyme inhibitor ramipril in essential hypertension: Improved blood pressure control with bedtime dosing. Hypertension 2009, 54, 40–46. [Google Scholar] [CrossRef]
- Hermida, R.C.; Ayala, D.E.; Chayan, L.; Mojon, A.; Fernandez, J.R. Administration-time-dependent effects of olmesartan on the ambulatory blood pressure of essential hypertension patients. Chronobiol. Int. 2009, 26, 61–79. [Google Scholar] [CrossRef]
- Hermida, R.C.; Ayala, D.E.; Fernández, J.R.; Calvo, C. Comparison of the efficacy of morning versus evening administration of telmisartan in essential hypertension. Hypertension 2007, 50, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Hermida, R.C.; Ayala, D.E.; Mojón, A.; Fernández, J.R. Chronotherapy with nifedipine GITS in hypertensive patients: Improved efficacy and safety with bedtime dosing. Am. J. Hypertens. 2008, 21, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Yoshida, S.; Nakaya, N.; Hata, Y.; Goto, Y. Comparison between morning and evening doses of simvastatin in hyperlipidemic subjects. A double-blind comparative study. Arterioscler. Thromb. J. Vasc. Biol. 1991, 11, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, M.K.; Seo, H.S.; Hyun, M.S.; Han, K.R.; Cho, S.W.; Kim, Y.K.; Hoon Park, S. Efficacy and safety of morning versus evening dose of controlled-release simvastatin tablets in patients with hyperlipidemia: A randomized, double-blind, multicenter phase III trial. Clin. Ther. 2013, 35, 1350–1360.e1351. [Google Scholar] [CrossRef]
- Yoon, H.S.; Kim, S.H.; Kim, J.K.; Ko, S.H.; Ko, J.E.; Park, S.J.; Park, M.G.; Lee, J.H.; Hyon, M.S. Comparison of effects of morning versus evening administration of ezetimibe/simvastatin on serum cholesterol in patients with primary hypercholesterolemia. Ann. Pharmacother. 2011, 45, 841–849. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, C.; Zhang, T.; Wu, B. Identification of rhythmic human CYPs and their circadian regulators using synchronized hepatoma cells. Xenobiotica Fate Foreign Compd. Biol. Syst. 2020, 50, 1052–1063. [Google Scholar] [CrossRef]
- Awad, K.; Serban, M.C.; Penson, P.; Mikhailidis, D.P.; Toth, P.P.; Jones, S.R.; Rizzo, M.; Howard, G.; Lip, G.Y.H.; Banach, M. Effects of morning vs evening statin administration on lipid profile: A systematic review and meta-analysis. J. Clin. Lipidol. 2017, 11, 972–985.e979. [Google Scholar] [CrossRef]
- Ayala, D.E.; Ucieda, R.; Hermida, R.C. Chronotherapy with low-dose aspirin for prevention of complications in pregnancy. Chronobiol. Int. 2013, 30, 260–279. [Google Scholar] [CrossRef]
- Bonten, T.N.; Snoep, J.D.; Assendelft, W.J.; Zwaginga, J.J.; Eikenboom, J.; Huisman, M.V.; Rosendaal, F.R.; van der Bom, J.G. Time-dependent effects of aspirin on blood pressure and morning platelet reactivity: A randomized cross-over trial. Hypertension 2015, 65, 743–750. [Google Scholar] [CrossRef]
- Winter, C.; Silvestre-Roig, C.; Ortega-Gomez, A.; Lemnitzer, P.; Poelman, H.; Schumski, A.; Winter, J.; Drechsler, M.; de Jong, R.; Immler, R.; et al. Chrono-pharmacological Targeting of the CCL2-CCR2 Axis Ameliorates Atherosclerosis. Cell Metab. 2018, 28, 175–182.e175. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; BaHammam, A.S.; Ojike, N.I.; Akinseye, O.A.; Kendzerska, T.; Buttoo, K.; Dhandapany, P.S.; Brown, G.M.; Cardinali, D.P. Melatonin and Human Cardiovascular Disease. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Arangino, S.; Cagnacci, A.; Angiolucci, M.; Vacca, A.M.; Longu, G.; Volpe, A.; Melis, G.B. Effects of melatonin on vascular reactivity, catecholamine levels, and blood pressure in healthy men. Am. J. Cardiol. 1999, 83, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Cagnacci, A.; Arangino, S.; Angiolucci, M.; Maschio, E.; Longu, G.; Melis, G.B. Potentially beneficial cardiovascular effects of melatonin administration in women. J. Pineal Res. 1997, 22, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.J.; Sletten, T.; Savic, N.; Gilbert, S.S.; Dawson, D. Effects of bright light and melatonin on sleep propensity, temperature, and cardiac activity at night. J. Appl. Physiol. 2001, 91, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K.; Yasue, H.; Moriyama, Y.; Tsunoda, R.; Ogawa, H.; Yoshimura, M.; Kugiyama, K. Acute effects of melatonin administration on cardiovascular autonomic regulation in healthy men. Am. Heart J. 2001, 141, E9. [Google Scholar] [CrossRef]
- Weekley, L.B. Effects of melatonin on isolated pulmonary artery and vein: Role of the vascular endothelium. Pulm. Pharmacol. 1993, 6, 149–154. [Google Scholar] [CrossRef]
- Vandewalle, G.; Middleton, B.; Rajaratnam, S.M.; Stone, B.M.; Thorleifsdottir, B.; Arendt, J.; Dijk, D.J. Robust circadian rhythm in heart rate and its variability: Influence of exogenous melatonin and photoperiod. J. Sleep Res. 2007, 16, 148–155. [Google Scholar] [CrossRef]
- Lusardi, P.; Preti, P.; Savino, S.; Piazza, E.; Zoppi, A.; Fogari, R. Effect of bedtime melatonin ingestion on blood pressure of normotensive subjects. Blood Press. Monit. 1997, 2, 99–103. [Google Scholar]
- Scheer, F.A.; Van Montfrans, G.A.; van Someren, E.J.; Mairuhu, G.; Buijs, R.M. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension 2004, 43, 192–197. [Google Scholar] [CrossRef]
- Grossman, E.; Laudon, M.; Yalcin, R.; Zengil, H.; Peleg, E.; Sharabi, Y.; Kamari, Y.; Shen-Orr, Z.; Zisapel, N. Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am. J. Med. 2006, 119, 898–902. [Google Scholar] [CrossRef]
- Lemoine, P.; Wade, A.G.; Katz, A.; Nir, T.; Zisapel, N. Efficacy and safety of prolonged-release melatonin for insomnia in middle-aged and elderly patients with hypertension: A combined analysis of controlled clinical trials. Integr. Blood Press. Control 2012, 5, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Rahbari-Oskoui, F.F.; Abramson, J.L.; Bruckman, A.M.; Chapman, A.B.; Cotsonis, G.A.; Johnson, S.A.; Bliwise, D.L. Nighttime administration of high-dose, sustained-release melatonin does not decrease nocturnal blood pressure in African-American patients: Results from a preliminary randomized, crossover trial. Complement. Ther. Med. 2019, 43, 157–164. [Google Scholar] [CrossRef]
- Lusardi, P.; Piazza, E.; Fogari, R. Cardiovascular effects of melatonin in hypertensive patients well controlled by nifedipine: A 24-hour study. Br. J. Clin. Pharmacol. 2000, 49, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Możdżan, M.; Możdżan, M.; Chałubiński, M.; Wojdan, K.; Broncel, M. The effect of melatonin on circadian blood pressure in patients with type 2 diabetes and essential hypertension. Arch. Med. Sci. 2014, 10, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Rechciński, T.; Trzos, E.; Wierzbowska-Drabik, K.; Krzemińska-Pakuła, M.; Kurpesa, M. Melatonin for nondippers with coronary artery disease: Assessment of blood pressure profile and heart rate variability. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2010, 33, 56–61. [Google Scholar] [CrossRef]
- Gubin, D.G.; Gubin, G.D.; Gapon, L.I.; Weinert, D. Daily Melatonin Administration Attenuates Age-Dependent Disturbances of Cardiovascular Rhythms. Curr. Aging Sci. 2016, 9, 5–13. [Google Scholar] [CrossRef]
- Koziróg, M.; Poliwczak, A.R.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Broncel, M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J. Pineal Res. 2011, 50, 261–266. [Google Scholar] [CrossRef]
- Cavallo, A.; Daniels, S.R.; Dolan, L.M.; Bean, J.A.; Khoury, J.C. Blood pressure-lowering effect of melatonin in type 1 diabetes. J. Pineal Res. 2004, 36, 262–266. [Google Scholar] [CrossRef]
- Bazyar, H.; Zare Javid, A.; Bavi Behbahani, H.; Moradi, F.; Moradi Poode, B.; Amiri, P. Consumption of melatonin supplement improves cardiovascular disease risk factors and anthropometric indices in type 2 diabetes mellitus patients: A double-blind, randomized, placebo-controlled trial. Trials 2021, 22, 231. [Google Scholar] [CrossRef]
- Raygan, F.; Ostadmohammadi, V.; Bahmani, F.; Reiter, R.J.; Asemi, Z. Melatonin administration lowers biomarkers of oxidative stress and cardio-metabolic risk in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 191–196. [Google Scholar] [CrossRef]
- Bahrami, M.; Cheraghpour, M.; Jafarirad, S.; Alavinejad, P.; Asadi, F.; Hekmatdoost, A.; Mohammadi, M.; Yari, Z. The effect of melatonin on treatment of patients with non-alcoholic fatty liver disease: A randomized double blind clinical trial. Complement. Ther. Med. 2020, 52, 102452. [Google Scholar] [CrossRef]
- Ekmekcioglu, C. Melatonin receptors in humans: Biological role and clinical relevance. Biomed. Pharmacother. 2006, 60, 97–108. [Google Scholar] [CrossRef]
- Hoyos, M.; Guerrero, J.M.; Perez-Cano, R.; Olivan, J.; Fabiani, F.; Garcia-Pergañeda, A.; Osuna, C. Serum cholesterol and lipid peroxidation are decreased by melatonin in diet-induced hypercholesterolemic rats. J. Pineal Res. 2000, 28, 150–155. [Google Scholar] [CrossRef]
- Albreiki, M.S.; Middleton, B.; Hampton, S.M. The effect of melatonin on glucose tolerance, insulin sensitivity and lipid profiles after a late evening meal in healthy young males. J. Pineal Res. 2021, 71, e12770. [Google Scholar] [CrossRef]
- Garfinkel, D.; Zorin, M.; Wainstein, J.; Matas, Z.; Laudon, M.; Zisapel, N. Efficacy and safety of prolonged-release melatonin in insomnia patients with diabetes: A randomized, double-blind, crossover study. Diabetes Metab. Syndr. Obes. Targets Ther. 2011, 4, 307–313. [Google Scholar] [CrossRef]
- Wakatsuki, A.; Okatani, Y.; Ikenoue, N.; Kaneda, C.; Fukaya, T. Effects of short-term melatonin administration on lipoprotein metabolism in normolipidemic postmenopausal women. Maturitas 2001, 38, 171–177. [Google Scholar] [CrossRef]
- Marqueze, E.C.; Nogueira, L.F.R.; Vetter, C.; Skene, D.J.; Cipolla-Neto, J.; Moreno, C.R.C. Exogenous melatonin decreases circadian misalignment and body weight among early types. J. Pineal Res. 2021, 71, e12750. [Google Scholar] [CrossRef]
- Müller-Wieland, D.; Behnke, B.; Koopmann, K.; Krone, W. Melatonin inhibits LDL receptor activity and cholesterol synthesis in freshly isolated human mononuclear leukocytes. Biochem. Biophys. Res. Commun. 1994, 203, 416–421. [Google Scholar] [CrossRef]
- Tengattini, S.; Reiter, R.J.; Tan, D.X.; Terron, M.P.; Rodella, L.F.; Rezzani, R. Cardiovascular diseases: Protective effects of melatonin. J. Pineal Res. 2008, 44, 16–25. [Google Scholar] [CrossRef]
- Antolín, I.; Rodríguez, C.; Saínz, R.M.; Mayo, J.C.; Uría, H.; Kotler, M.L.; Rodríguez-Colunga, M.J.; Tolivia, D.; Menéndez-Peláez, A. Neurohormone melatonin prevents cell damage: Effect on gene expression for antioxidant enzymes. FASEB J. 1996, 10, 882–890. [Google Scholar] [CrossRef]
- Marchetti, C.; Sidahmed-Adrar, N.; Collin, F.; Jore, D.; Gardès-Albert, M.; Bonnefont-Rousselot, D. Melatonin protects PLPC liposomes and LDL towards radical-induced oxidation. J. Pineal Res. 2011, 51, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, A.; Okatani, Y.; Ikenoue, N.; Shinohara, K.; Watanabe, K.; Fukaya, T. Melatonin protects against oxidized low-density lipoprotein-induced inhibition of nitric oxide production in human umbilical artery. J. Pineal Res. 2001, 31, 281–288. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Guerrero, J.M.; Lardone, P.J.; Reiter, R.J. A review of the multiple actions of melatonin on the immune system. Endocrine 2005, 27, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, V.; Singh, V.; Kulkarni, S.K.; Agrewala, J.N. Melatonin enhances Th2 cell mediated immune responses: Lack of sensitivity to reversal by naltrexone or benzodiazepine receptor antagonists. Mol. Cell. Biochem. 2001, 221, 57–62. [Google Scholar] [CrossRef]
- Di Stefano, A.; Paulesu, L. Inhibitory effect of melatonin on production of IFN gamma or TNF alpha in peripheral blood mononuclear cells of some blood donors. J. Pineal Res. 1994, 17, 164–169. [Google Scholar] [CrossRef]
- Liu, F.; Ng, T.B.; Fung, M.C. Pineal indoles stimulate the gene expression of immunomodulating cytokines. J. Neural Transm. 2001, 108, 397–405. [Google Scholar] [CrossRef]
- Garcia-Mauriño, S.; Gonzalez-Haba, M.G.; Calvo, J.R.; Rafii-El-Idrissi, M.; Sanchez-Margalet, V.; Goberna, R.; Guerrero, J.M. Melatonin enhances IL-2, IL-6, and IFN-gamma production by human circulating CD4+ cells: A possible nuclear receptor-mediated mechanism involving T helper type 1 lymphocytes and monocytes. J. Immunol. 1997, 159, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Tan, D.X.; Hardeland, R.; Leon, J.; Rodriguez, C.; Reiter, R.J. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J. Neuroimmunol. 2005, 165, 139–149. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, X.; Zhang, R.; Ma, B.; Niu, S.; Di, X.; Ni, L.; Liu, C. Melatonin attenuates smoking-induced atherosclerosis by activating the Nrf2 pathway via NLRP3 inflammasomes in endothelial cells. Aging 2021, 13, 11363–11380. [Google Scholar] [CrossRef]
- Del Zar, M.M.; Martinuzzo, M.; Falcón, C.; Cardinali, D.P.; Carreras, L.O.; Vacas, M.I. Inhibition of human platelet aggregation and thromboxane-B2 production by melatonin: Evidence for a diurnal variation. J. Clin. Endocrinol. Metab. 1990, 70, 246–251. [Google Scholar] [CrossRef]
- Santhi, N.; Thorne, H.C.; van der Veen, D.R.; Johnsen, S.; Mills, S.L.; Hommes, V.; Schlangen, L.J.; Archer, S.N.; Dijk, D.J. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J. Pineal Res. 2012, 53, 47–59. [Google Scholar] [CrossRef]
- Rüger, M.; Gordijn, M.C.; Beersma, D.G.; de Vries, B.; Daan, S. Time-of-day-dependent effects of bright light exposure on human psychophysiology: Comparison of daytime and nighttime exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1413–R1420. [Google Scholar] [CrossRef]
- Scheer, F.A.; van Doornen, L.J.; Buijs, R.M. Light and diurnal cycle affect human heart rate: Possible role for the circadian pacemaker. J. Biol. Rhythm. 1999, 14, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Kitamura, S.; Kozaki, T.; Yasukouchi, A. Inhibition of heart rate variability during sleep in humans by 6700 K pre-sleep light exposure. J. Physiol. Anthropol. 2007, 26, 39–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cajochen, C.; Münch, M.; Kobialka, S.; Kräuchi, K.; Steiner, R.; Oelhafen, P.; Orgül, S.; Wirz-Justice, A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J. Clin. Endocrinol. Metab. 2005, 90, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Sene-Fiorese, M.; Duarte, F.O.; de Aquino Junior, A.E.; Campos, R.M.; Masquio, D.C.; Tock, L.; de Oliveira Duarte, A.C.; Dâmaso, A.R.; Parizotto, N.A.; Bagnato, V.S. The potential of phototherapy to reduce body fat, insulin resistance and “metabolic inflexibility” related to obesity in women undergoing weight loss treatment. Lasers Surg. Med. 2015, 47, 634–642. [Google Scholar] [CrossRef]
- Danilenko, K.V.; Mustafina, S.V.; Pechenkina, E.A. Bright light for weight loss: Results of a controlled crossover trial. Obes. Facts 2013, 6, 28–38. [Google Scholar] [CrossRef]
- Dunai, A.; Novak, M.; Chung, S.A.; Kayumov, L.; Keszei, A.; Levitan, R.; Shapiro, C.M. Moderate exercise and bright light treatment in overweight and obese individuals. Obesity 2007, 15, 1749–1757. [Google Scholar] [CrossRef]
- Leppämäki, S.J.; Partonen, T.T.; Hurme, J.; Haukka, J.K.; Lönnqvist, J.K. Randomized trial of the efficacy of bright-light exposure and aerobic exercise on depressive symptoms and serum lipids. J. Clin. Psychiatry 2002, 63, 316–321. [Google Scholar] [CrossRef]
- Versteeg, R.I.; Stenvers, D.J.; Visintainer, D.; Linnenbank, A.; Tanck, M.W.; Zwanenburg, G.; Smilde, A.K.; Fliers, E.; Kalsbeek, A.; Serlie, M.J.; et al. Acute Effects of Morning Light on Plasma Glucose and Triglycerides in Healthy Men and Men with Type 2 Diabetes. J. Biol. Rhythm. 2017, 32, 130–142. [Google Scholar] [CrossRef]
- Obayashi, K.; Saeki, K.; Iwamoto, J.; Okamoto, N.; Tomioka, K.; Nezu, S.; Ikada, Y.; Kurumatani, N. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: A cross-sectional analysis of the HEIJO-KYO study. J. Clin. Endocrinol. Metab. 2013, 98, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Pinchasov, B.B.; Shurgaja, A.M.; Grischin, O.V.; Putilov, A.A. Mood and energy regulation in seasonal and non-seasonal depression before and after midday treatment with physical exercise or bright light. Psychiatry Res. 2000, 94, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, A.; Courville, A.B.; Chen, K.Y. The Complex Effects of Light on Metabolism in Humans. Nutrients 2023, 15, 1391. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.E.; McBride, A.A.; Balba, N.M.; Thomas, S.V.; Pattinson, C.L.; Morasco, B.J.; Wilkerson, A.; Gill, J.M.; Lim, M.M. Feasibility and preliminary efficacy for morning bright light therapy to improve sleep and plasma biomarkers in US Veterans with TBI. A prospective, open-label, single-arm trial. PLoS ONE 2022, 17, e0262955. [Google Scholar] [CrossRef]
- Leu, S.J.; Shiah, I.S.; Yatham, L.N.; Cheu, Y.M.; Lam, R.W. Immune-inflammatory markers in patients with seasonal affective disorder: Effects of light therapy. J. Affect. Disord. 2001, 63, 27–34. [Google Scholar] [CrossRef]
- He, B.; Nohara, K.; Park, N.; Park, Y.S.; Guillory, B.; Zhao, Z.; Garcia, J.M.; Koike, N.; Lee, C.C.; Takahashi, J.S.; et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016, 23, 610–621. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Assini, J.M.; Lee, J.K.; Allister, E.M.; Sutherland, B.G.; Koppes, J.B.; Sawyez, C.G.; Edwards, J.Y.; Telford, D.E.; Charbonneau, A.; et al. Nobiletin attenuates VLDL overproduction, dyslipidemia, and atherosclerosis in mice with diet-induced insulin resistance. Diabetes 2011, 60, 1446–1457. [Google Scholar] [CrossRef]
- Montaigne, D.; Marechal, X.; Modine, T.; Coisne, A.; Mouton, S.; Fayad, G.; Ninni, S.; Klein, C.; Ortmans, S.; Seunes, C.; et al. Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbα antagonism: A single-centre propensity-matched cohort study and a randomised study. Lancet 2018, 391, 59–69. [Google Scholar] [CrossRef]
- Merikanto, I.; Lahti, T.; Puolijoki, H.; Vanhala, M.; Peltonen, M.; Laatikainen, T.; Vartiainen, E.; Salomaa, V.; Kronholm, E.; Partonen, T. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol. Int. 2013, 30, 470–477. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csoma, B.; Bikov, A. The Role of the Circadian Rhythm in Dyslipidaemia and Vascular Inflammation Leading to Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 14145. https://doi.org/10.3390/ijms241814145
Csoma B, Bikov A. The Role of the Circadian Rhythm in Dyslipidaemia and Vascular Inflammation Leading to Atherosclerosis. International Journal of Molecular Sciences. 2023; 24(18):14145. https://doi.org/10.3390/ijms241814145
Chicago/Turabian StyleCsoma, Balazs, and Andras Bikov. 2023. "The Role of the Circadian Rhythm in Dyslipidaemia and Vascular Inflammation Leading to Atherosclerosis" International Journal of Molecular Sciences 24, no. 18: 14145. https://doi.org/10.3390/ijms241814145
APA StyleCsoma, B., & Bikov, A. (2023). The Role of the Circadian Rhythm in Dyslipidaemia and Vascular Inflammation Leading to Atherosclerosis. International Journal of Molecular Sciences, 24(18), 14145. https://doi.org/10.3390/ijms241814145





