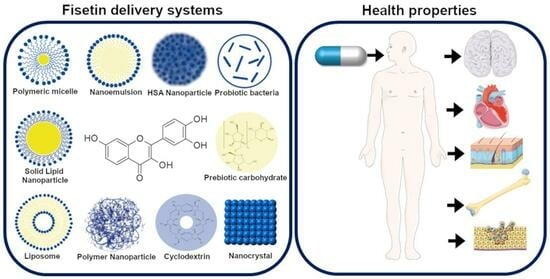
Fisetin—In Search of Better Bioavailability—From Macro to Nano Modifications: A Review
Abstract
:1. Introduction
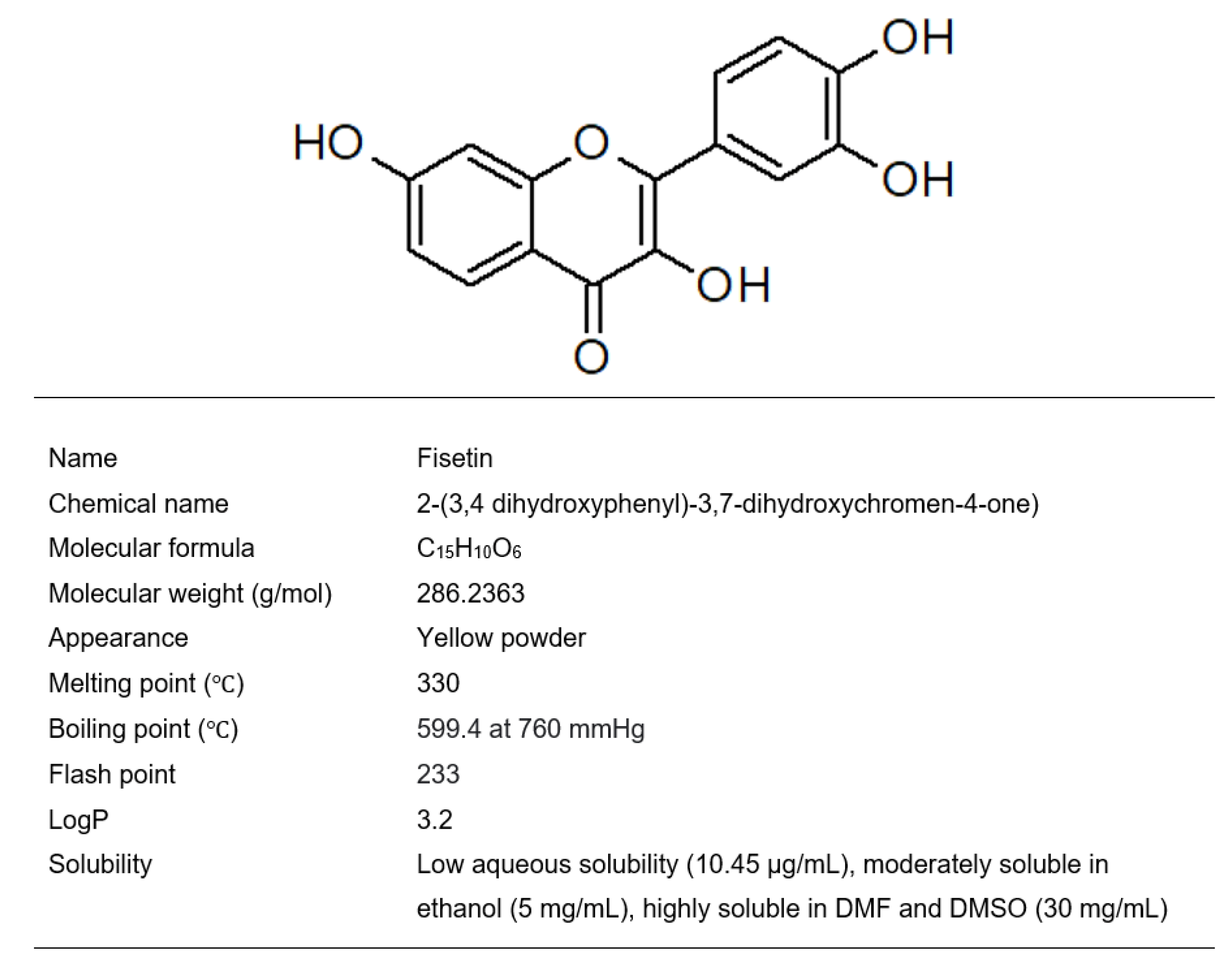
2. Fisetin
3. Bioavailability and Bioefficacy
4. Nanoparticle-Based Delivery Systems
4.1. Polymeric Nanoparticles
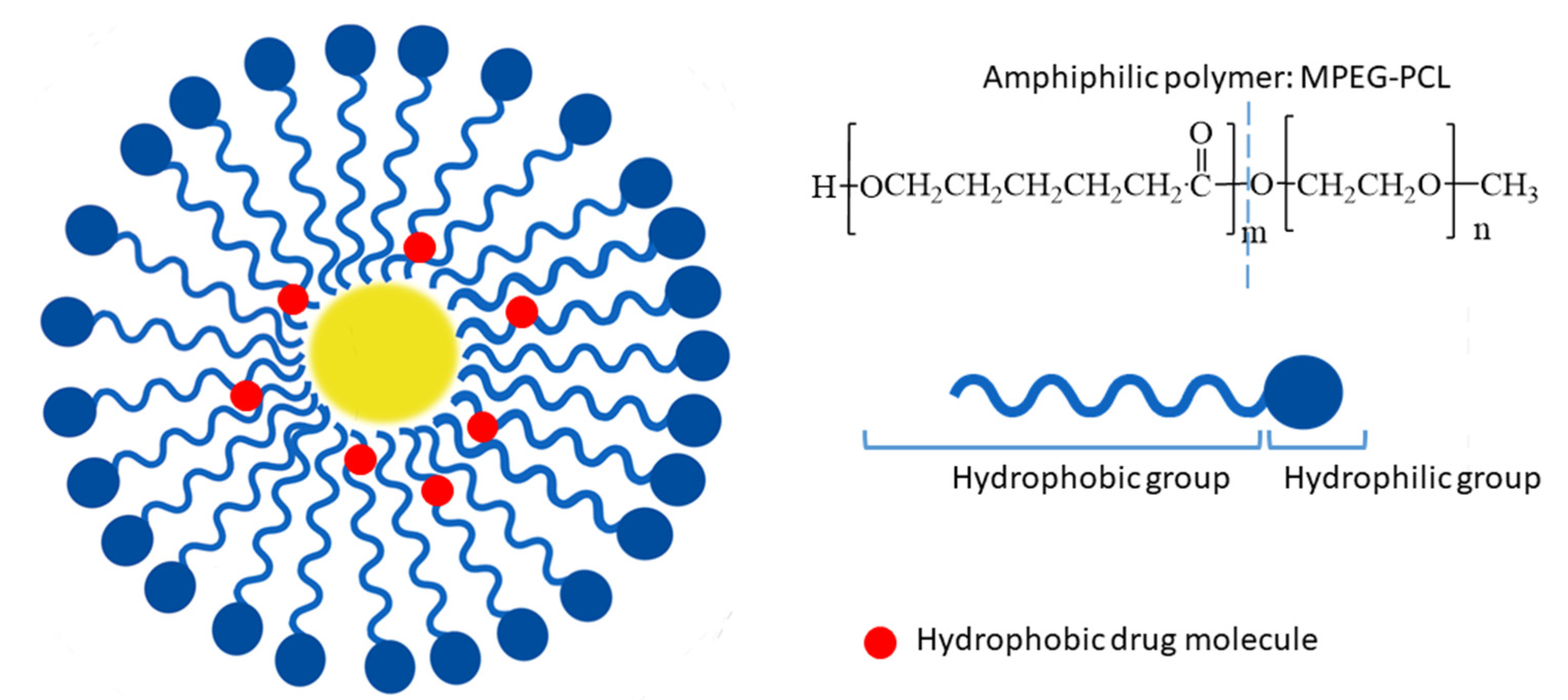
4.2. Polymeric Micelles
4.3. Human Serum Albumin Nanoparticles
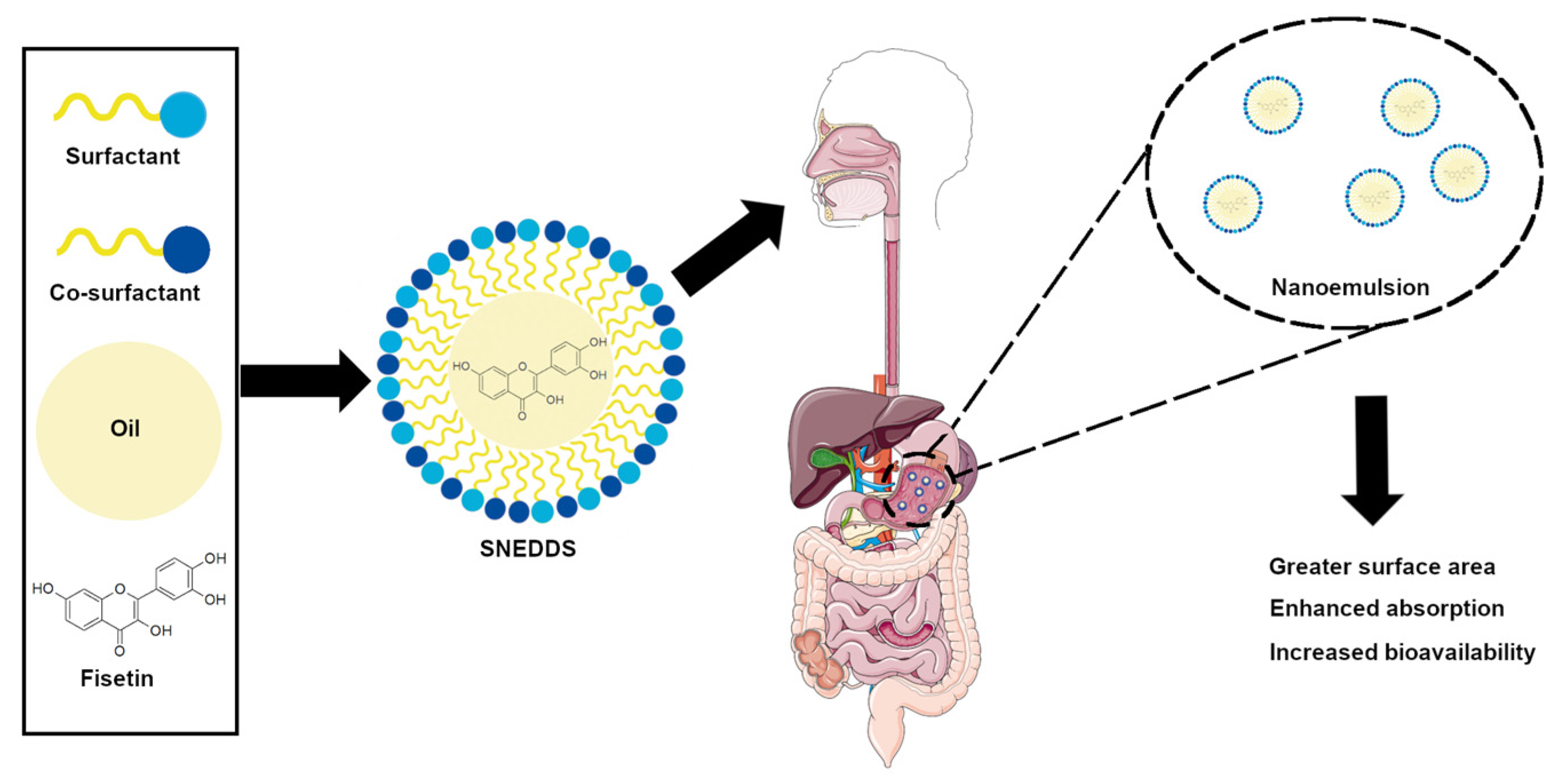
4.4. Nanoemulsions
4.5. Lipid Structures
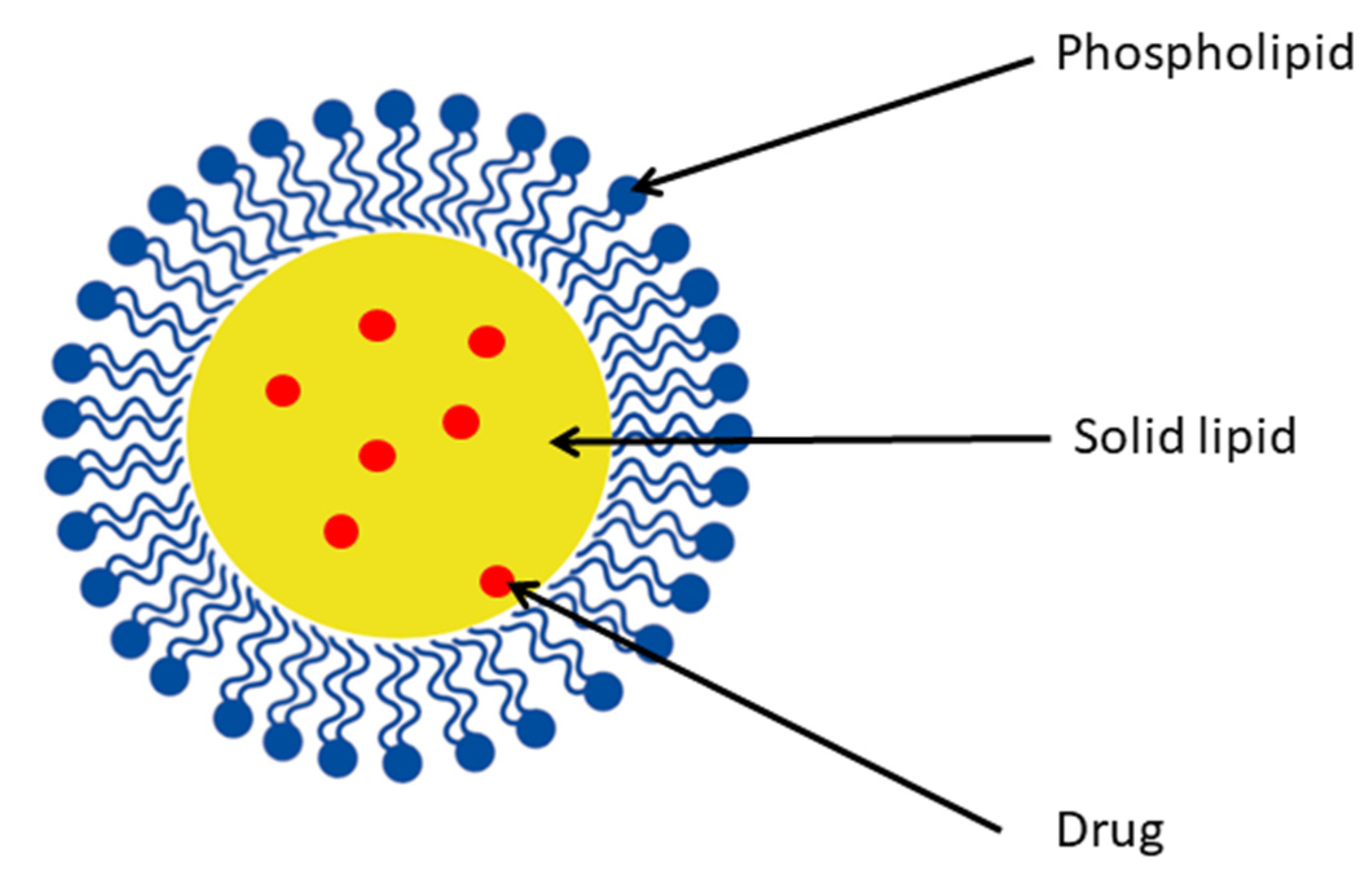
4.5.1. Solid Lipid Nanoparticles
4.5.2. Liposomes
| Lipid Vesicle | Methods of Preparation | Diameter (nm) | Entrapment Efficiency (%) | In Vitro Study | In Vivo Study | Ref. |
|---|---|---|---|---|---|---|
| Liposome (P90 G, DODA-GLY-PEG2000) | Lipid cake formation | 175 | 73 | 3LL, CT26, EAhy 926 cell line | - | [86] |
| Liposome (DOPC, DODA-PEG2000) | Thin-film hydration | 173.5 ± 2.4 | 58 | LLC tumor cells—assessment of apoptosis | Anticancer activity (LLC tumor in mice (i.p.)) | [87] |
| Liposome (DOPC, DODA-GLY-PEG2000) | Thin-film hydration (cisplatin co-encapsulation) | 173 ± 8 | 90–100 | EA.hy926, U-87 MG cell lines | - | [88] |
| Transethosome (Lipoid S 100, sodium cholate) | Thin-film hydration | 74.21 ± 2.65 | 68.31 ± 1.48 | - | Rat skin permeation | [81] |
| Ethosome (P90 G, EtOH, GLY) | Thin-film hydration | 99.89 ± 3.24 | 89.23 ± 2.13 | DPPH assay, TNF-α, IL-1α, lipid peroxidation assay, glutathione assay, catalase enzyme assay | Rat skin permeation | [85] |
| Glycerosome (Lipoid S 100, GLY) | Thin-film hydration | 138.8 ± 4.09 | 86.41 ± 2.95 | - | Rat skin permeation | [82] |
4.5.3. Spherulites
4.5.4. Nanocochleates
5. Nanocrystals
6. Biotechnological Modifications and Combination with Biotechnologically Obtained Structures
6.1. Complex with Cyclodextrin
| CD Used with Fisetin | Method(s) of Preparation/Fisetin/CD Ratio | In Vitro Study | Stability Constant (M−1) | Solubility Enhancement | Ref. |
|---|---|---|---|---|---|
| γ-CD | Co-solvent (water/ethanol) | - | 1.46 × 103 | - | [108] |
| HP-γ-CD | Co-solvent (water/ethanol) | - | 82.3 | No measurable data | [109] |
| γ-CD | Co-solvent (water/ethanol) then Freeze-drying 1:1 | DPPH assay | - | - | [119] |
| β-CD, HP-β-CD, DIMEB, QABCD | - | HepG2 cell line | 3.18 3.63 3.77 4.06 (25 °C) | - | [116] |
| β-CD, γ-CD | Co-solvent (water/ethanol) then freeze-drying 1:1 | Hela and MCF-7 cell line | - | 1.6 fold 2.78 fold | [115] |
| β-CD, SHPβ-CD | Co-solvent (water/methanol) | - | 622.56 2309.13 (25 °C) | - | [107] |
| β-CD, HP-β-CD, SBEβ-CD | Aqueous method; co-solvent (water/ethanol) 1:1 | DPPH assay, A549 cell line | 0.628 2.291 4.474 (37 °C) | - | [117] |
| HP-β-CD in PLGA NPs | Coacervation (water/ethanol) 1:1 | ROS assay, MCF-7 cell line | 1296.03 (37 °C) For FIS-HP-β-CD | 161.9 fold AL type | [114] |
| β-CD, HP-β-CD, RAMEB | Powder mixing; kneading (water/ethanol) 1:2 | A2780, MDA-MB-231, SiHa cell line | - | - | [118] |
| α-CD, β-CD | Co-solvent (water/methanol) 1:1 | - | 1000 (15 °C) 860 (25 °C) 510 (35 °C) 360 (45 °C) For FIS-β-CD | - | [120] |
6.2. Probiotic Bacteria as Vehicle
6.3. Prebiotic Carbohydrates as Vehicles
6.4. Glycosylation Method
7. Comparison of the Effects of Fisetin Pre-Formulation Modifications on the Bioavailability and Bioefficacy Based on In Vivo and In Vitro Models
8. Conclusions
- Based on in vitro and in vivo antitumor activity studies, it can be concluded that the prepared formulations showed improved anticancer activity after encapsulation.
- The fact that the encapsulation caused a slower/controlled release and that the fisetin was released gradually was unquestionably in favor of biological action and may have contributed to a greater outcome.
- Taking into account the potential beneficial impact of fisetin on keratinocyte damage, there is presently limited study on the topical application of fisetin, with most studies focusing on intravenously or intraperitoneally administered doses. However, the results that have been provided open the possibility of carrying out such experiments.
- More research might focus on the bioefficacy of fisetin in liposomes on skin cancer cells based on the notion that liposomal formulations have good skin penetration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| AKT | protein kinase B |
| AUC | area under the curve |
| BACE1 | beta-site amyloid precursor protein cleaving enzyme |
| CAT | catalase |
| α-CD | α-cyclodextrin |
| β-CD | β-cyclodextrin |
| γ-CD | γ-cyclodextrin |
| CDs | cyclodextrins |
| COX-2 | cyclooxygenase 2 |
| CryoTEM | cryogenic transmission electron microscopy |
| DIMEB | heptakis-(2,6-di-O-methyl)-β-cyclodextrin |
| DMPC | dimyristoyl phosphatidylcholine |
| DODA-GLY-PEG2000 | 2-dioctadecylcarbamoyl-methoxyacetylamino) acetic acid (ω-methoxy)-polyethylene glycol 2000 ester |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| DSC | Differential Scanning Calorimetry |
| EMA | European Medicines Agency |
| ERK | extracellular signal-regulated kinases |
| FAK | focal adhesion kinase |
| FDA | Food and Drug Administration |
| FGFR1 | fibroblast growth factor receptor |
| FIS-β-CD | fisetin β-cyclodextrin complex |
| FIS-HP-β-CD | fisetin 2-Hydroxypropyl-β-cyclodextrin complex |
| FRAP | ferric reducing antioxidant power assay |
| GSH | reduced glutathione |
| GSK-3 β | glycogen synthase kinase 3 beta |
| GPx | glutathione peroxidase |
| GRAS | generally recognized as safe |
| HaCaT | Cultured Human Keratinocyte |
| HP-β-CD | 2-Hydroxypropyl-β-cyclodextrin |
| HPC | hydroxypropyl cellulose |
| HPMC | hydroxypropyl methylcellulose |
| HSA-NPs | human serum albumin nanoparticles |
| IL-1α | interleukin-1α |
| IL-1β | interleukin-1β |
| IL-6 | interleukin 6 |
| IL-8 | interleukin 8 |
| iNOS | inducible nitric oxide synthase |
| iRhom2 | the inactive rhomboid protein 2 |
| LDL | low-density lipoprotein |
| LL/2 | Lewis lung carcinoma cell line |
| LLC | Lewis lung carcinoma |
| MAPK | mitogen-activated protein kinases |
| MPEG-PCL | methoxy poly(ethylene glycol)-poly(ε-caprolactone) |
| mTOR | serine-threonine protein kinase |
| mTORC2 | mammalian target of rapamycin complex 2 |
| NLRP3 | the nucleotide-binding oligomerization domain (NOD)-like receptor pyrin domain containing 3 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | nitric oxide |
| NPs | nanoparticles |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| ORAC | oxygen radical absorbance capacity |
| PCL | poly-(ε-caprolactone) |
| PCOS | polycystic ovary syndrome |
| PGE2 | prostaglandin E2 |
| PI3K | phosphoinositide-3-kinase |
| PLA | poly(lactic acid) |
| PLGA | poly(lactic-co-glycolic acid |
| PLGA-PEG-COOH | poly(D,L-lactic-co-glycolic acid)-block-poly(ethylene glycol) carboxylic acid |
| PPAR-γ | the peroxisome proliferators-activated receptors gamma |
| PTEN | phosphatase and tensin homolog deleted on chromosome 10 |
| PVA | polyvinyl alcohol |
| PVP | poly(vinylpyrrolidone) |
| QABCD | quaternary ammonium β-cyclodextrin |
| RAMEB | randomly methylated β-cyclodextrin |
| ROS | reactive oxygen species |
| SBE-β-CD | sulfobutylether β-cyclodextrin |
| SHPβ-CD | succinyl-2-hydroxypropyl β-cyclodextrin |
| SLN | solid lipid nanoparticles |
| SLS | sodium lauryl sulfate |
| SNEDDS | self-nonoemulsifying drug delivery system |
| SOD | superoxide dismutase |
| TLR4 | toll-like receptor 4 |
| TNF-α | tumor necrosis factor-alpha |
| TRAIL | tumor necrosis factor-related apoptosis-inducing ligand |
| VEGF | vascular endothelial growth factor |
References
- Saparbekova, A.A.; Kantureyeva, G.O.; Kudasova, D.E.; Konarbayeva, Z.K.; Latif, A.S. Potential of phenolic compounds from pomegranate (Punica granatum L.) by-product with significant antioxidant and therapeutic effects: A narrative review. Saudi J. Biol. Sci. 2023, 30, 103553. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Zhou, X.; Li, H.; Ma, X.; Zhuang, J. NF-κB inhibitors gifted by nature: The anticancer promise of polyphenol compounds. Biomed. Pharmacother. 2022, 156, 113951. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, A.F.; Alizadeh, M. Antioxidant properties of the flavonoid fisetin: An updated review of in vivo and in vitro studies. Trends Food Sci. Technol. 2017, 70, 34–44. [Google Scholar] [CrossRef]
- Sun, Y.; Qin, H.; Zhang, H.; Feng, X.; Yang, L.; Hou, D.-X.; Chen, J. Fisetin inhibits inflammation and induces autophagy by mediating PI3K/AKT/mTOR signaling in LPS-induced RAW264.7 cells. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Xiao, J. 2-Polyphenols: Absorption, bioavailability, and metabolomics. In Polyphenols: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 45–67. ISBN 978-0-12-813572-3. [Google Scholar]
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Dietary Intakes of Flavonols, Flavones and Isoflavones by Japanese Women and the Inverse Correlation between Quercetin Intake and Plasma LDL Cholesterol Concentration. J. Nutr. 2000, 130, 2243–2250. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Naureen, H.; Zahid, R.; Youssef, L.; Attar, R.; Xu, B. Cancer chemopreventive role of fisetin: Regulation of cell signaling pathways in different cancers. Pharmacol. Res. 2021, 172, 105784. [Google Scholar] [CrossRef]
- Park, H.-H.; Lee, S.; Son, H.-Y.; Park, S.-B.; Kim, M.-S.; Choi, E.-J.; Singh, T.S.K.; Ha, J.-H.; Lee, M.-G.; Kim, J.-E.; et al. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch. Pharm. Res. 2008, 31, 1303–1311. [Google Scholar] [CrossRef]
- Pu, J.-L.; Huang, Z.-T.; Luo, Y.-H.; Mou, T.; Li, T.-T.; Li, Z.-T.; Wei, X.-F.; Wu, Z.-J. Fisetin mitigates hepatic ischemia-reperfusion injury by regulating GSK3β/AMPK/NLRP3 inflammasome pathway. Hepatobiliary Pancreat. Dis. Int. 2021, 20, 352–360. [Google Scholar] [CrossRef]
- Xiao, S.; Lu, Y.; Wu, Q.; Yang, J.; Chen, J.; Zhong, S.; Eliezer, D.; Tan, Q.; Wu, C. Fisetin inhibits tau aggregation by interacting with the protein and preventing the formation of β-strands. Int. J. Biol. Macromol. 2021, 178, 381–393. [Google Scholar] [CrossRef]
- Rane, A.R.; Paithankar, H.; Hosur, R.V.; Choudhary, S. Modulation of α-synuclein fibrillation by plant metabolites, daidzein, fisetin and scopoletin under physiological conditions. Int. J. Biol. Macromol. 2021, 182, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Zhou, Y.; Zhu, Y.; Fei, M. Fisetin alleviates oxidative stress after traumatic brain injury via the Nrf2-ARE pathway. Neurochem. Int. 2018, 118, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, K.; Nelson, A.L.; Nishimura, H.; Rutledge, J.C.; Ravuri, S.K.; Bahney, C.; Philippon, M.J.; Huard, J. The effects of fisetin on bone and cartilage: A systematic review. Pharmacol. Res. 2022, 185, 106504. [Google Scholar] [CrossRef]
- Kim, S.G.; Sung, J.Y.; Kim, J.-R.; Choi, H.C. Fisetin-induced PTEN expression reverses cellular senescence by inhibiting the mTORC2-Akt Ser473 phosphorylation pathway in vascular smooth muscle cells. Exp. Gerontol. 2021, 156, 111598. [Google Scholar] [CrossRef]
- Huang, X.; Shen, H.; Liu, Y.; Qiu, S.; Guo, Y. Fisetin attenuates periodontitis through FGFR1/TLR4/NLRP3 inflammasome pathway. Int. Immunopharmacol. 2021, 95, 107505. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, H.; Huang, G.; Wang, X.; Bu, X. Therapeutic targeting of mechanical stretch-induced FAK/ERK signaling by fisetin in hypertrophic scars. Eur. J. Pharmacol. 2022, 932, 175228. [Google Scholar] [CrossRef]
- Seo, S.-H.; Jeong, G.-S. Fisetin inhibits TNF-α-induced inflammatory action and hydrogen peroxide-induced oxidative damage in human keratinocyte HaCaT cells through PI3K/AKT/Nrf-2-mediated heme oxygenase-1 expression. Int. Immunopharmacol. 2015, 29, 246–253. [Google Scholar] [CrossRef]
- Mihanfar, A.; Nouri, M.; Roshangar, L.; Khadem-Ansari, M.H. Ameliorative effects of fisetin in letrozole-induced rat model of polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 2021, 213, 105954. [Google Scholar] [CrossRef]
- Molagoda, I.M.N.; Kang, C.-H.; Lee, M.-H.; Choi, Y.H.; Lee, C.-M.; Lee, S.; Kim, G.-Y. Fisetin promotes osteoblast differentiation and osteogenesis through GSK-3β phosphorylation at Ser9 and consequent β-catenin activation, inhibiting osteoporosis. Biochem. Pharmacol. 2021, 192, 114676. [Google Scholar] [CrossRef]
- Garg, S.; Khan, S.I.; Malhotra, R.K.; Sharma, M.K.; Kumar, M.; Kaur, P.; Nag, T.C.; RumaRay; Bhatia, J.; Arya, D.S. The molecular mechanism involved in cardioprotection by the dietary flavonoid fisetin as an agonist of PPAR-γ in a murine model of myocardial infarction. Arch. Biochem. Biophys. 2020, 694, 108572. [Google Scholar] [CrossRef]
- Althunibat, O.Y.; Al Hroob, A.M.; Abukhalil, M.H.; Germoush, M.O.; Bin-Jumah, M.; Mahmoud, A.M. Fisetin ameliorates oxidative stress, inflammation and apoptosis in diabetic cardiomyopathy. Life Sci. 2019, 221, 83–92. [Google Scholar] [CrossRef] [PubMed]
- ALTamimi, J.Z.; BinMowyna, M.N.; AlFaris, N.A.; Alagal, R.I.; El-kott, A.F.; AL-Farga, A.M. Fisetin protects against streptozotocin-induced diabetic cardiomyopathy in rats by suppressing fatty acid oxidation and inhibiting protein kinase R. Saudi. Pharm. J. 2021, 29, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Ali, T.; Park, H.Y.; Badshah, H.; Rehman, S.U.; Kim, M.O. Neuroprotective Effect of Fisetin Against Amyloid-Beta-Induced Cognitive/Synaptic Dysfunction, Neuroinflammation, and Neurodegeneration in Adult Mice. Mol. Neurobiol. 2017, 54, 2269–2285. [Google Scholar] [CrossRef] [PubMed]
- Chenxu, G.; Xianling, D.; Qin, K.; Linfeng, H.; Yan, S.; Mingxin, X.; Jun, T.; Minxuan, X. Fisetin protects against high fat diet-induced nephropathy by inhibiting inflammation and oxidative stress via the blockage of iRhom2/NF-κB signaling. Int. Immunopharmacol. 2021, 92, 107353. [Google Scholar] [CrossRef]
- Shin, M.-J.; Cho, Y.; Moon, J.; Jeon, H.J.; Lee, S.-M.; Chung, J.H. Hypocholesterolemic effect of daily fisetin supplementation in high fat fed Sprague–Dawley rats. Food Chem. Toxicol. 2013, 57, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Pawar, A.; Mahadik, K.; Bothiraja, C. Emerging novel drug delivery strategies for bioactive flavonol fisetin in biomedicine. Biomed. Pharmacother. 2018, 106, 1282–1291. [Google Scholar] [CrossRef]
- Shia, C.-S.; Tsai, S.-Y.; Kuo, S.-C.; Hou, Y.-C.; Chao, P.-D.L. Metabolism and Pharmacokinetics of 3,3′,4′,7-Tetrahydroxyflavone (Fisetin), 5-Hydroxyflavone, and 7-Hydroxyflavone and Antihemolysis Effects of Fisetin and Its Serum Metabolites. J. Agric. Food Chem. 2009, 57, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Krasieva, T.B.; Ehren, J.; O’Sullivan, T.; Tromberg, B.J.; Maher, P. Cell and brain tissue imaging of the flavonoid fisetin using label-free two-photon microscopy. Neurochem. Int. 2015, 89, 243–248. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Feng, C.; Yuan, X.; Chu, K.; Zhang, H.; Ji, W.; Rui, M. Preparation and optimization of poly (lactic acid) nanoparticles loaded with fisetin to improve anti-cancer therapy. Int. J. Biol. Macromol. 2019, 125, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liao, J.; Deng, X.; Liang, J.; Long, C.; Xie, C.; Chen, X.; Zhang, L.; Sun, J.; Peng, J.; et al. Anti-Tumor Activity and Safety Evaluation of Fisetin-Loaded Methoxy Poly(ethylene glycol)–Poly(ε-Caprolactone) Nanoparticles. J. Biomed. Nanotechnol. 2014, 10, 580–591. [Google Scholar] [CrossRef]
- Chen, L.-F.; Xu, P.-Y.; Fu, C.-P.; Kankala, R.K.; Chen, A.-Z.; Wang, S.-B. Fabrication of Supercritical Antisolvent (SAS) Process-Assisted Fisetin-Encapsulated Poly (Vinyl Pyrrolidone) (PVP) Nanocomposites for Improved Anticancer Therapy. Nanomaterials 2020, 10, 322. [Google Scholar] [CrossRef]
- Sechi, M.; Syed, D.N.; Pala, N.; Mariani, A.; Marceddu, S.; Brunetti, A.; Mukhtar, H.; Sanna, V. Nanoencapsulation of dietary flavonoid fisetin: Formulation and in vitro antioxidant and α-glucosidase inhibition activities. Mater. Sci. Eng. C 2016, 68, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-Y.; Lin, C.-C.; Hsieh, Y.-S.; Wu, Y.-T. Nanoformulation Development to Improve the Biopharmaceutical Properties of Fisetin Using Design of Experiment Approach. Molecules 2021, 26, 3031. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Q.; Song, L.; He, T.; Li, Y.; Li, L.; Su, W.; Liu, L.; Qian, Z.; Gong, C. Polymeric Micelles Encapsulating Fisetin Improve the Therapeutic Effect in Colon Cancer. ACS Appl. Mater. Interfaces 2015, 7, 534–542. [Google Scholar] [CrossRef]
- Xiao, X.; Zou, J.; Fang, Y.; Meng, Y.; Xiao, C.; Fu, J.; Liu, S.; Bai, P.; Yao, Y. Fisetin and polymeric micelles encapsulating fisetin exhibit potent cytotoxic effects towards ovarian cancer cells. BMC Complement. Altern. Med. 2018, 18, 91. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, D.-Z.; Wang, Y.-X. Bioflavonoid Fisetin Loaded α-Tocopherol-Poly(lactic acid)-Based Polymeric Micelles for Enhanced Anticancer Efficacy in Breast Cancers. Pharm. Res. 2017, 34, 453–461. [Google Scholar] [CrossRef]
- Pawar, A.; Singh, S.; Rajalakshmi, S.; Shaikh, K.; Bothiraja, C. Development of fisetin-loaded folate functionalized pluronic micelles for breast cancer targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 347–361. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef]
- Ghosh, P.; Singha Roy, A.; Chaudhury, S.; Jana, S.K.; Chaudhury, K.; Dasgupta, S. Preparation of albumin based nanoparticles for delivery of fisetin and evaluation of its cytotoxic activity. Int. J. Biol. Macromol. 2016, 86, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M.J. Nano-emulsions. J. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Venkata Ramana Rao, S.; Shao, J. Self-nanoemulsifying drug delivery systems (SNEDDS) for oral delivery of protein drugs. Int. J. Pharm. 2008, 362, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Febi, S.; Kuruvila, F.M. Solid Self Nanoemulsifying Drug Delivery System (Snedds) Devolopment, Applications And Future Perspective: A Review. Indian J. Pharm. Sci. 2017, 4, 651–669. [Google Scholar] [CrossRef]
- Pouton, C.W. Lipid formulations for oral administration of drugs: Non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur. J. Pharm. Sci. 2000, 11, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Khursheed, R.; Kumar, R.; Awasthi, A.; Sharma, N.; Khurana, S.; Kapoor, B.; Khurana, N.; Singh, S.K.; Gowthamarajan, K.; et al. Self-nanoemulsifying drug delivery system of fisetin: Formulation, optimization, characterization and cytotoxicity assessment. J. Drug Deliv. Sci. Technol. 2019, 54, 101252. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, R.; Khurana, N.; Singh, S.K.; Khurana, S.; Verma, S.; Sharma, N.; Kapoor, B.; Vyas, M.; Khursheed, R.; et al. Enhanced oral bioavailability and neuroprotective effect of fisetin through its SNEDDS against rotenone-induced Parkinson’s disease rat model. Food Chem. Toxicol. 2020, 144, 111590. [Google Scholar] [CrossRef]
- Ragelle, H.; Crauste-Manciet, S.; Seguin, J.; Brossard, D.; Scherman, D.; Arnaud, P.; Chabot, G.G. Nanoemulsion formulation of fisetin improves bioavailability and antitumour activity in mice. Int. J. Pharm. 2012, 427, 452–459. [Google Scholar] [CrossRef]
- Gasco, M.R. Method for producing solid lipid microspheres having a narrow size distribution. U.S. Patent 5,250,236, 1993. [Google Scholar]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Kulbacka, J.; Pucek, A.; Kotulska, M.; Dubińska-Magiera, M.; Rossowska, J.; Rols, M.-P.; Wilk, K.A. Electroporation and lipid nanoparticles with cyanine IR-780 and flavonoids as efficient vectors to enhanced drug delivery in colon cancer. Bioelectrochemistry 2016, 110, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B. 2019, 9, 36–48. [Google Scholar] [CrossRef]
- Sheikholeslami, B.; Lam, N.W.; Dua, K.; Haghi, M. Exploring the impact of physicochemical properties of liposomal formulations on their in vivo fate. Life Sci. 2022, 300, 120574. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Grainger, D.W. Lyophilized liposome-based parenteral drug development: Reviewing complex product design strategies and current regulatory environments. Adv. Drug Deliv. Rev. 2019, 151, 56–71. [Google Scholar] [CrossRef]
- Hussain, A.; Singh, S.; Sharma, D.; Webster, T.; Shafaat, K.; Faruk, A. Elastic liposomes as novel carriers: Recent advances in drug delivery. Int. J. Nanomed. 2017, 12, 5087–5108. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Laouini, A.; Jaafar-Maalej, C.; Limayem-Blouza, I.; Sfar, S.; Charcosset, C.; Fessi, H. Preparation, Characterization and Applications of Liposomes: State of the Art. J. Colloid Sci. Biotechnol. 2012, 1, 147–168. [Google Scholar] [CrossRef]
- Chauhan, N.; Vasava, P.; Khan, S.L.; Siddiqui, F.A.; Islam, F.; Chopra, H.; Emran, T.B. Ethosomes: A novel drug carrier. Ann. Med. Surg. 2022, 82, 104595. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Y.; Liu, H.; Zhang, G.; Wu, X. Preparation and In vitro Evaluation of Ethosomal Total Alkaloids of Sophora alopecuroides Loaded by a Transmembrane pH-Gradient Method. AAPS PharmSciTech 2010, 11, 1350–1358. [Google Scholar] [CrossRef]
- Rai, K.; Gupta, Y.; Jain, A.; Jain, S.K. Transfersomes: Self-optimizing carriers for bioactives. PDA J. Pharm. Sci. Technol. 2008, 62, 362–379. [Google Scholar]
- Khoee, S.; Yaghoobian, M. Niosomes: A novel approach in modern drug delivery systems. In Nanostructures for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 207–237. ISBN 978-0-323-46143-6. [Google Scholar]
- Manca, M.L.; Zaru, M.; Manconi, M.; Lai, F.; Valenti, D.; Sinico, C.; Fadda, A.M. Glycerosomes: A new tool for effective dermal and transdermal drug delivery. Int. J. Pharm. 2013, 455, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, P.; Kalpana, B.; Prasanthi, D. Invasomes-novel Vesicular Carriers for Enhanced Skin Permeation. Syst Rev. Pharm. 2013, 4, 26. [Google Scholar] [CrossRef]
- Duangjit, S.; Obata, Y.; Sano, H.; Kikuchi, S.; Onuki, Y.; Opanasopit, P.; Ngawhirunpat, T.; Maitani, Y.; Takayama, K. Menthosomes, Novel Ultradeformable Vesicles for Transdermal Drug Delivery: Optimization and Characterization. Biol. Pharm. Bull. 2012, 35, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Kaddah, S.; Khreich, N.; Kaddah, F.; Charcosset, C.; Greige-Gerges, H. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem. Toxicol. 2018, 113, 40–48. [Google Scholar] [CrossRef]
- Zylberberg, C.; Matosevic, S. Pharmaceutical liposomal drug delivery: A review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef]
- Knudsen, K.B.; Northeved, H.; Kumar EK, P.; Permin, A.; Gjetting, T.; Andresen, T.L.; Larsen, S.; Wegener, K.M.; Lykkesfeldt, J.; Jantzen, K.; et al. In vivo toxicity of cationic micelles and liposomes. Nanomedicine 2015, 11, 467–477. [Google Scholar] [CrossRef]
- Vega-Villa, K.R.; Takemoto, J.K.; Yáñez, J.A.; Remsberg, C.M.; Forrest, M.L.; Davies, N.M. Clinical toxicities of nanocarrier systems. Adv. Drug Deliv. Rev. 2008, 60, 929–938. [Google Scholar] [CrossRef]
- Barenholz, Y. (Chezy) Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Saraf, S.; Jain, A.; Tiwari, A.; Verma, A.; Panda, P.K.; Jain, S.K. Advances in liposomal drug delivery to cancer: An overview. J. Drug Deliv. Sci. Technol. 2020, 56, 101549. [Google Scholar] [CrossRef]
- Hillery, A.M. Supramolecular lipidic drug delivery systems: From laboratory to clinic A review of the recently introduced commercial liposomal and lipid-based formulations of amphotericin B. Adv. Drug Deliv. Rev. 1997, 24, 345–363. [Google Scholar] [CrossRef]
- Alving, C.R.; Beck, Z.; Matyas, G.R.; Rao, M. Liposomal adjuvants for human vaccines. Expert Opin. Drug Deliv. 2016, 13, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Beiranvand, S.; Eatemadi, A.; Karimi, A. New Updates Pertaining to Drug Delivery of Local Anesthetics in Particular Bupivacaine Using Lipid Nanoparticles. Nanoscale Res. Lett. 2016, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Bressler, N.M.; Bressler, S.B. Photodynamic therapy with verteporfin (Visudyne): Impact on ophthalmology and visual sciences. Investig. Ophthalmol. Vis. Sci. 2000, 41, 624–628. [Google Scholar]
- Halevas, E.G.; Avgoulas, D.I.; Katsipis, G.; Pantazaki, A.A. Flavonoid-liposomes formulations: Physico-chemical characteristics, biological activities and therapeutic applications. Eur. J. Med. Chem. 2022, 5, 100059. [Google Scholar] [CrossRef]
- Akhtar, N. Vesicles: A Recently Developed Novel Carrier for Enhanced Topical Drug Delivery. Curr. Drug Deliv. 2014, 11, 87–97. [Google Scholar] [CrossRef]
- Song, C.K.; Balakrishnan, P.; Shim, C.-K.; Chung, S.-J.; Chong, S.; Kim, D.-D. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: Characterization and in vitro/in vivo evaluation. Colloids Surf. B Biointerfaces 2012, 92, 299–304. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Iqbal, B.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Development of transethosomes formulation for dermal fisetin delivery: Box–Behnken design, optimization, in vitro skin penetration, vesicles–skin interaction and dermatokinetic studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 755–765. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M. Preparation and optimization of fisetin loaded glycerol based soft nanovesicles by Box-Behnken design. Int. J. Pharm. 2020, 578, 119125. [Google Scholar] [CrossRef]
- Zaki, R.M.; Alfadhel, M.M.; Alossaimi, M.A.; Elsawaf, L.A.; Devanathadesikan Seshadri, V.; Almurshedi, A.S.; Yusif, R.M.; Said, M. Central Composite Optimization of Glycerosomes for the Enhanced Oral Bioavailability and Brain Delivery of Quetiapine Fumarate. Pharmaceuticals 2022, 15, 940. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Husain, M.; Khan, N.; Alfaleh, M.A.; Asfour, H.Z.; Riadi, Y.; Bilgrami, A.L.; Akhter, M.H. Plumbagin-Loaded Glycerosome Gel as Topical Delivery System for Skin Cancer Therapy. Polymers 2021, 13, 923. [Google Scholar] [CrossRef] [PubMed]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Fisetin loaded binary ethosomes for management of skin cancer by dermal application on UV exposed mice. Int. J. Pharm. 2019, 560, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Mignet, N.; Seguin, J.; Ramos Romano, M.; Brullé, L.; Touil, Y.S.; Scherman, D.; Bessodes, M.; Chabot, G.G. Development of a liposomal formulation of the natural flavonoid fisetin. Int. J. Pharm. 2012, 423, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Seguin, J.; Brullé, L.; Boyer, R.; Lu, Y.M.; Ramos Romano, M.; Touil, Y.S.; Scherman, D.; Bessodes, M.; Mignet, N.; Chabot, G.G. Liposomal encapsulation of the natural flavonoid fisetin improves bioavailability and antitumor efficacy. Int. J. Pharm. 2013, 444, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Renault-Mahieux, M.; Vieillard, V.; Seguin, J.; Espeau, P.; Le, D.T.; Lai-Kuen, R.; Mignet, N.; Paul, M.; Andrieux, K. Co-Encapsulation of Fisetin and Cisplatin into Liposomes for Glioma Therapy: From Formulation to Cell Evaluation. Pharmaceutics 2021, 13, 970. [Google Scholar] [CrossRef]
- Crauste-Manciet, S.; Khawand, K.; Mignet, N. Spherulites: Onion-like vesicles as nanomedicines. Ther. Deliv. 2015, 6, 1377–1385. [Google Scholar] [CrossRef]
- Gulik-Krzywicki, T.; Dedieu, J.C.; Roux, D.; Degert, C.; Laversanne, R. Freeze−Fracture Electron Microscopy of Sheared Lamellar Phase. Langmuir 1996, 12, 4668–4671. [Google Scholar] [CrossRef]
- Crauste-Manciet, S.; Larquet, E.; Khawand, K.; Bessodes, M.; Chabot, G.G.; Brossard, D.; Mignet, N. Lipidic spherulites: Formulation optimisation by paired optical and cryoelectron microscopy. Eur. J. Pharm. Biopharm. 2013, 85, 1088–1094. [Google Scholar] [CrossRef]
- Papahadjopoulos, D.; Vail, W.J.; Jacobson, K.; Poste, G. Cochleate lipid cylinders: Formation by fusion of unilamellar lipid vesicles. Biochim. Biophys. Acta Biomembr. 1975, 394, 483–491. [Google Scholar] [CrossRef]
- Ray, S.; Nayak, A.K. (Eds.) Design and Applications of Theranostic Nanomedicines; Woodhead Publishing: Cambridge, MA, USA, 2023; ISBN 978-0-323-88598-0. [Google Scholar]
- Sankar, V.R.; Reddy, Y.D. Nanocochleate—A new approch in lipid drug delivery. Int. J. Pharm. Pharm. Sci. 2010, 2, 220–223. [Google Scholar]
- Rub, R.; Munot, N.; Wadate, A. Improved Efficacy and Stability of Silymarin Loaded Nanocochleates Over Liposomes for the Treatment of Skin Diseases. J. Pharm. Res. Int. 2021, 33, 163–176. [Google Scholar] [CrossRef]
- El-Melegy, M.G.; Eltaher, H.M.; Gaballah, A.; El-Kamel, A.H. Enhanced oral permeability of Trans-Resveratrol using nanocochleates for boosting anticancer efficacy; in-vitro and ex-vivo appraisal. Eur. J. Pharm. Biopharm. 2021, 168, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Bothiraja, C.; Yojana, B.D.; Pawar, A.P.; Shaikh, K.S.; Thorat, U.H. Fisetin-loaded nanocochleates: Formulation, characterisation, in vitro anticancer testing, bioavailability and biodistribution study. Expert Opin. Drug Deliv. 2014, 11, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Junghanns, J.-U.A.H.; Müller, R.H. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008, 3, 295–309. [Google Scholar] [CrossRef]
- Fernandes, A.R.; Dias-Ferreira, J.; Ferreira-da-Silva, C.; Severino, P.; Martins-Gomes, C.; Silva, A.M.; Souto, E.B. Drug nanocrystals. In Applications of Nanocomposite Materials in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 239–253. ISBN 978-0-12-813741-3. [Google Scholar]
- Dzakwan, M.; Ganet, E.P.; Rachmat, M.; Wikarsa, S. Nanosized and Enhancement of Solubility Fisetin. Asian J. Pharm. Res. Dev. 2019, 7, 6–10. [Google Scholar] [CrossRef]
- Ma, P.; Seguin, J.; Ly, N.K.; Henríquez, L.C.; Plansart, E.; Hammad, K.; Gahoual, R.; Dhôtel, H.; Izabelle, C.; Saubamea, B.; et al. Designing fisetin nanocrystals for enhanced in cellulo anti-angiogenic and anticancer efficacy. Int. J. Pharm. X 2022, 4, 100138. [Google Scholar] [CrossRef]
- Saenger, W.; Jacob, J.; Gessler, K.; Steiner, T.; Hoffmann, D.; Sanbe, H.; Koizumi, K.; Smith, S.M.; Takaha, T. Structures of the Common Cyclodextrins and Their Larger AnaloguessBeyond the Doughnut. Chem. Rev. 1998, 98, 1787–1802. [Google Scholar] [CrossRef]
- Szejtli, J. Cyclodextrin Technology; Springer: Dordrecht, The Netherlands, 1988; Volume 1, ISBN 978-90-481-8427-9. [Google Scholar]
- Szente, L. Highly soluble cyclodextrin derivatives: Chemistry, properties, and trends in development. Adv. Drug Deliv. Rev. 1999, 36, 17–28. [Google Scholar] [CrossRef]
- Ueda, H. Physicochemical Properties and Complex Formation Abilities of Large-Ring Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 53–56. [Google Scholar] [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef]
- Banerjee, A.; Sengupta, P.K. Encapsulation of 3-hydroxyflavone and fisetin in β-cyclodextrins: Excited state proton transfer fluorescence and molecular mechanics studies. Chem. Phys. Lett. 2006, 424, 379–386. [Google Scholar] [CrossRef]
- Pahari, B.; Sengupta, B.; Chakraborty, S.; Thomas, B.; McGowan, D.; Sengupta, P.K. Contrasting binding of fisetin and daidzein in γ-cyclodextrin nanocavity. J. Photochem. Photobiol. B Biol. 2013, 118, 33–41. [Google Scholar] [CrossRef]
- Pahari, B.; Chakraborty, S.; Sengupta, P.K. Molecular insight into the inclusion of the dietary plant flavonol fisetin and its chromophore within a chemically modified γ-cyclodextrin: Multi-spectroscopic, molecular docking and solubility studies. Food Chem. 2018, 260, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Munro, I.C.; Newberne, P.M.; Young, V.R.; Bär, A. Safety assessment of γ-cyclodextrin. Regul. Toxicol. Pharmacol. 2004, 39, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Cyclodextrins Used as Excipients Report Report Published in Support of the ‘Questions and Answers on Cyclodextrins Used as Excipients in Medicinal Products for Human Use’ (EMA/CHMP/495747/2013); European Medicines Agency: London, UK, 2017.
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies related to the safety of alpha-cyclodextrin. EFSA J. 2007, 5, 537. [Google Scholar] [CrossRef]
- Irie, T.; Uekama, K. Pharmaceutical Applications of Cyclodextrins. III. Toxicological Issues and Safety Evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef]
- Kadari, A.; Gudem, S.; Kulhari, H.; Bhandi, M.M.; Borkar, R.M.; Kolapalli, V.R.M.; Sistla, R. Enhanced oral bioavailability and anticancer efficacy of fisetin by encapsulating as inclusion complex with HPβCD in polymeric nanoparticles. Drug Deliv. 2017, 24, 224–232. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, K.; An, K.; Ren, S.-H.; Xie, X.; Jin, Y.; Lin, J. Novel water-soluble fisetin/cyclodextrins inclusion complexes: Preparation, characterization, molecular docking and bioavailability. Carbohydr. Res. 2015, 418, 20–28. [Google Scholar] [CrossRef]
- Sali, N.; Csepregi, R.; Kőszegi, T.; Kunsági-Máté, S.; Szente, L.; Poór, M. Complex formation of flavonoids fisetin and geraldol with β-cyclodextrins. J. Lumin. 2018, 194, 82–90. [Google Scholar] [CrossRef]
- Mohtar, N.; Taylor, K.M.G.; Sheikh, K.; Somavarapu, S. Design and development of dry powder sulfobutylether-β-cyclodextrin complex for pulmonary delivery of fisetin. Eur. J. Pharm. Biopharm. 2017, 113, 1–10. [Google Scholar] [CrossRef]
- Danciu, C.; Citu, C.; Zupko, I.; Ghiulai, R.; Pavel, I.Z.; Popescu, A.; Pinzaru, I.; Simu, G.; Daliborca, V.C. In vitro antiproliferative activity of flavonols: Fisetin, quercetinand kaempferol and their cyclodextrin complexes. Farmacia 2015, 63, 858–864. [Google Scholar]
- Pais, J.M.; Barroca, M.J.; Marques, M.P.M.; Almeida Paz, F.A.; Braga, S.S. Solid-state studies and antioxidant properties of the γ-cyclodextrin·fisetin inclusion compound. Beilstein J. Org. Chem. 2017, 13, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, M.R.; Uemi, M.; Donate, P.M.; Nikolaou, S.; Machado, A.E.H.; Okano, L.T. Study of the Complexation of Fisetin with Cyclodextrins. J. Phys. Chem. A 2006, 110, 10545–10551. [Google Scholar] [CrossRef] [PubMed]
- da Silva Pedrini, M.R.; Dupont, S.; de Anchieta Câmara, A.; Beney, L.; Gervais, P. Osmoporation: A simple way to internalize hydrophilic molecules into yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, E.W.V.; Dupont, S.; Beney, L.; Hoskin, R.T.; da Silva Pedrini, M.R. Osmoporation is a versatile technique to encapsulate fisetin using the probiotic bacteria Lactobacillus acidophilus. Appl. Microbiol. Biotechnol. 2022, 106, 1031–1044. [Google Scholar] [CrossRef]
- Ashwini, A.; Ramya, H.N.; Ramkumar, C.; Reddy, K.R.; Kulkarni, R.V.; Abinaya, V.; Naveen, S.; Raghu, A.V. Reactive mechanism and the applications of bioactive prebiotics for human health: Review. J. Microbiol. Methods 2019, 159, 128–137. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Bangar, S.P.; Chaudhary, V.; Hosseini, E.; Mokhtari, Z.; Karaca, A.C.; Samota, M.K.; Goswami, D.; Krishnan, V.; Askari, G.; et al. Recent advances in oral delivery of bioactive molecules: Focus on prebiotic carbohydrates as vehicle matrices. Carbohydr. Polym. 2022, 298, 120074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, G.; Gao, M.; Liu, X.; Ji, B.; Hua, R.; Zhou, Y.; Yang, Y. RGD-peptide conjugated inulin-ibuprofen nanoparticles for targeted delivery of Epirubicin. Colloids Surf. B Biointerfaces 2016, 144, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Fares, M.M.; Salem, M.S. Dissolution enhancement of curcumin via curcumin–prebiotic inulin nanoparticles. Drug Dev. Ind. Pharm. 2015, 41, 1785–1792. [Google Scholar] [CrossRef]
- Charoenwongpaiboon, T.; Wangpaiboon, K.; Panpetch, P.; Field, R.A.; Barclay, J.E.; Pichyangkura, R.; Kuttiyawong, K. Temperature-dependent inulin nanoparticles synthesized by Lactobacillus reuteri 121 inulosucrase and complex formation with flavonoids. Carbohydr. Polym. 2019, 223, 115044. [Google Scholar] [CrossRef]
- Liang, D.-M.; Liu, J.-H.; Wu, H.; Wang, B.-B.; Zhu, H.-J.; Qiao, J.-J. Glycosyltransferases: Mechanisms and applications in natural product development. Chem. Soc. Rev. 2015, 44, 8350–8374. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Bai, X.; Tan, Y.; Xie, W.; Feng, Y.; Yang, G.-Y. Glycosyltransferases: Mining, engineering and applications in biosynthesis of glycosylated plant natural products. Synth. Syst. Biotechnol. 2022, 7, 602–620. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, H.; Moon, Y.; Kwak, S.; Kang, C.G.; Park, C.; Jo, J.; Kim, S.W.; Pal, K.; Kang, D.H.; et al. Enhancement of the water solubility and antioxidant capacities of mangiferin by transglucosylation using a cyclodextrin glycosyltransferase. Enzym. Microb. Technol. 2022, 159, 110065. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.-J.; Kang, H.-K.; Nguyen, T.T.H.; Kim, G.-E.; Kim, Y.-M.; Park, J.-S.; Kim, D.; Cha, J.; Moon, Y.-H.; Nam, S.-H.; et al. Synthesis and characterization of ampelopsin glucosides using dextransucrase from Leuconostoc mesenteroides B-1299CB4: Glucosylation enhancing physicochemical properties. Enzym. Microb. Technol. 2012, 51, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nguyen, T.T.H.; Kim, N.M.; Moon, Y.-H.; Ha, J.-M.; Park, N.; Lee, D.-G.; Hwang, K.-H.; Park, J.-S.; Kim, D. Functional Properties of Novel Epigallocatechin Gallate Glucosides Synthesized by Using Dextransucrase from Leuconostoc mesenteroides B-1299CB4. J. Agric. Food Chem. 2016, 64, 9203–9213. [Google Scholar] [CrossRef]
- Moon, Y.; Kim, H.; Kang, C.G.; Park, C.; Kim, S.W.; Kim, D. Biochemical characterization of synthesized fisetin glucoside by dextransucrase from Leuconostoc mesenteroides NRRL B-1299CB4 with enhanced water solubility. Enzym. Microb. Technol. 2022, 161, 110111. [Google Scholar] [CrossRef]
- Lorthongpanich, N.; Mahalapbutr, P.; Rungrotmongkol, T.; Charoenwongpaiboon, T.; Prousoontorn, M.H. Fisetin glycosides synthesized by cyclodextrin glycosyltransferase from Paenibacillus sp. RB01: Characterization, molecular docking, and antioxidant activity. PeerJ 2022, 10, e13467. [Google Scholar] [CrossRef]


| Disease | Beneficial Effects | Ref. |
|---|---|---|
| Periodontitis | Attenuation of periodontitis in vitro and in vivo due to inhibition of inflammatory reaction via FGFR1/TLR4/NLRP3 inflammasome pathway. | [16] |
| Hypertrophic scars | In vivo inhibition of stretch-induced profibrotic effect by suppressing the proliferation, activation, and collagen production of fibroblasts. Downregulation of FAK and ERK phosphorylation. | [17] |
| Oxidative skin damage | Increased viability of human keratinocyte HaCaT cells; reduced production of ROS, NO, PGE2, IL-1β, and IL-6; inhibited expression of iNOS and COX-2; and activation of NF-κB. | [18] |
| Polycystic ovary syndrome (PCOS) | In vivo attenuation of clinical features of PCOS (anti-hyperglycemic, anti-hyperlipidemic, and anti-hyperandrogenic activities) as well as increased activity of antioxidant enzymes, i.e., catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx). | [19] |
| Osteoporosis | In vitro inhibition of osteoclastogenesis and promotion of osteoblastogenesis. Osteogenic activity is stimulated through the GSK-3β/β-catenin signaling pathway. | [20] |
| Heart disease | In vivo reduced ischemia; upregulated expression of PPAR-γ in heart tissue leading to cardioprotection. | [21] |
| Diabetic cardiomyopathy | In vivo attenuation of oxidative stress, inflammation, and apoptotic cell death. | [22] |
| In vivo hypoglycemic and insulin-sensitizing effect with improved cardiac glucose oxidation, and anti-inflammatory, anti-fibrotic and anti-apoptotic effect. | [23] | |
| Alzheimer’s disease | Decreased accumulation of amyloid beta, BACE-1 expression, and hyperphosphorylation of tau protein at serine 413, activation of p-PI3K, p-Akt (Ser 473), and p-GSK3β (Ser 9) expression, and protection from neuroinflammation. | [24] |
| High-fat diet-induced nephropathy | Reduction in lipid accumulation in the kidney, suppression of oxidative stress and anti-inflammatory response, and iRhom2/NF-κB signaling pathway inhibition. | [25] |
| Hypercholesterolemia | The modulation of gene expression involved in cholesterol and bile acid metabolism leads to the reduction in total cholesterol, LDL-cholesterol, and hepatic cholesterol content | [26] |
| Fisetin Formulation | Route of Administration | Dose | Subject | Fold of AUC Improvement | Ref. |
|---|---|---|---|---|---|
| Fisetin-PLA Nanoparticles | i.v. | 40 mg/kg | rats | 2.32 | [32] |
| Pluronic F127 copolymer conjugated with folic acid (polymeric micelles) | i.p. | 15 mg/kg | rats | 6.3 | [40] |
| Self-nanoemulsifying drug delivery system (SNEDDS) | oral | 20 mg/kg | rats | 1.52 | [48] |
| Nanoemulsion (Miglyol 812N/Labrasol/Tween 80/Lipoid E80/water) | i.p. | 13 mg/kg | mice | 11.92 | [49] |
| Binary ethosomes gel | topical | - | rats | Dermis 4.14 Epidermis 3.11 | [85] |
| Liposomes (DOPC/DODA-PEG2000) | i.v. i.p. | 13 mg/kg 21 mg/kg | mice | 1.64 4.43 | [87] |
| Nanocochleates (DMPC/cholesterol/calcium ions) | i.p. | 21 mg/kg | mice | 13 | [97] |
| Fisetin Formulation | Type of Study | IC50 [µg/mL] | Dose [mg/kg] | Cell Culture | Biological Effect | Ref. |
|---|---|---|---|---|---|---|
| Fisetin-PLA nanoparticles | in vivo | 40 | 4T1 | Tumor volume reduced to 67% | [32] | |
| in vitro | 29.3 | HCT116 | Cell viability < 40% (200 µg/mL) | |||
| Fisetin-PVP nanoparticles | in vitro | 80 | MDA-MB-231 | Cell viability reduced to 35% | [34] | |
| Fisetin polymeric micelles conjugated with folic acid | in vitro | 4.9 | MCF-7 | GI50 decreased by 65.737% | [40] | |
| Fisetin HSA NPs | in vitro | MCF-7 | Stronger cytotoxic effect than free fisetin | [42] | ||
| Fisetin polymeric micelles PLA-D-α-tocopheryl-PEG1000 | in vivo | MCF-7 | Tumor growth ↓ Induction of apoptosis after 24 h (20%), 48 h (42%) | [39] | ||
| in vitro | 9.68 | Increased cytotoxicity | ||||
| Fisetin nanocochleates (DMPC/cholesterol/calcium ions) | in vitro | 11.2 | MCF-7 | Increased cytotoxicity | [97] | |
| Fisetin-HP-β-CD incorporated into PLGA | in vitro | 22.09 | MCF-7 | Increased cytotoxicity | [114] | |
| Fisetin-MPEG-PCL polymeric micelles | in vivo (i.v.) | 50 | SKOV3 | Tumor growth inhibition of 70.7% | [38] | |
| in vitro | 13.79 | Increased cytotoxicity | ||||
| Fisetin complexed with β-CD, HPβ-CD, RAMEB | in vitro | A2780 MDA-MB-231 SiHa | Free fisetin was more cytotoxic than cyclodextrin inclusions | [118] | ||
| Fisetin-IR-780 co-loaded SLN | in vitro | LoVo | After 24 h cell viability decreased by ca. 50% Increase in p53 and manganese superoxide dismutase expression | [53] | ||
| Fisetin-DIMEB | in vitro | Hep62 | ATP ↓, total protein levels ↓ The presence of BCD, HPBCD, or DIMEB did not modify considerably the effects of fisetin | [116] | ||
| Fisetin-SBEβ-CD | in vitro | 67.97 | A549 | Antioxidant and cytotoxic activity unchanged after encapsulation | [117] | |
| Fisetin-MPEG-PCL polymeric micelles | in vivo (i.v) | 10 | LL/2 | Volume and weight of the tumor ↓ | [33] | |
| in vitro | 16.63 | Increased cytotoxicity | ||||
| Fisetin nanoemulsion (Miglyol 812N/Labrasol/Tween 80/Lipoid E80/water) | in vivo (i.p.) | 36.6 | 3LL | Reduced tumor size by 53% | [49] | |
| Fisetin in liposome (P90 G, DODA-GLY-PEG2000) | in vitro | 17.9 | EA.hy926 | Similar cytotoxicity values as free fisetin | [86] | |
| 15.5 | 3LL | |||||
| 16.7 | CT26 | |||||
| Fisetin in liposome (DOPC, DODA-PEG2000) | in vivo (i.p.) | 21.0 | 3LL | Delayed tumor growth of 3.3 days | [87] | |
| Fisetin nanocrystals | in vitro | 15.8 | EA.hy926 | Increased apoptosis | [101] | |
| 13.5 | 3LL | The same effect as free fisetin on 3LL | ||||
| Fisetin spherulites | in vitro | 25.1 | EA.hy926 | Increased cytotoxicity | [91] | |
| 26.8 | 3LL | |||||
| Fisetin liposome (DOPC, DODA-GLY-PEG2000) | in vitro | 38.64 | EA.hy926 | Increased cytotoxicity | [88] | |
| 12.59 | U-87 MG | |||||
| Fisetin-MPEG-PCL polymeric micelles | in vivo (s.c.) | 50 | CT26 | Extended life of mice by 17 days Suppression of the tumor growth | [37] | |
| In vitro | 7.97 | Increased cytotoxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczak, J.; Cielecka-Piontek, J. Fisetin—In Search of Better Bioavailability—From Macro to Nano Modifications: A Review. Int. J. Mol. Sci. 2023, 24, 14158. https://doi.org/10.3390/ijms241814158
Szymczak J, Cielecka-Piontek J. Fisetin—In Search of Better Bioavailability—From Macro to Nano Modifications: A Review. International Journal of Molecular Sciences. 2023; 24(18):14158. https://doi.org/10.3390/ijms241814158
Chicago/Turabian StyleSzymczak, Joanna, and Judyta Cielecka-Piontek. 2023. "Fisetin—In Search of Better Bioavailability—From Macro to Nano Modifications: A Review" International Journal of Molecular Sciences 24, no. 18: 14158. https://doi.org/10.3390/ijms241814158
APA StyleSzymczak, J., & Cielecka-Piontek, J. (2023). Fisetin—In Search of Better Bioavailability—From Macro to Nano Modifications: A Review. International Journal of Molecular Sciences, 24(18), 14158. https://doi.org/10.3390/ijms241814158