Metabolic Responses of the Microalga Neochloris oleoabundans to Extracellular Self- and Nonself-DNA
Abstract
:1. Introduction
2. Results
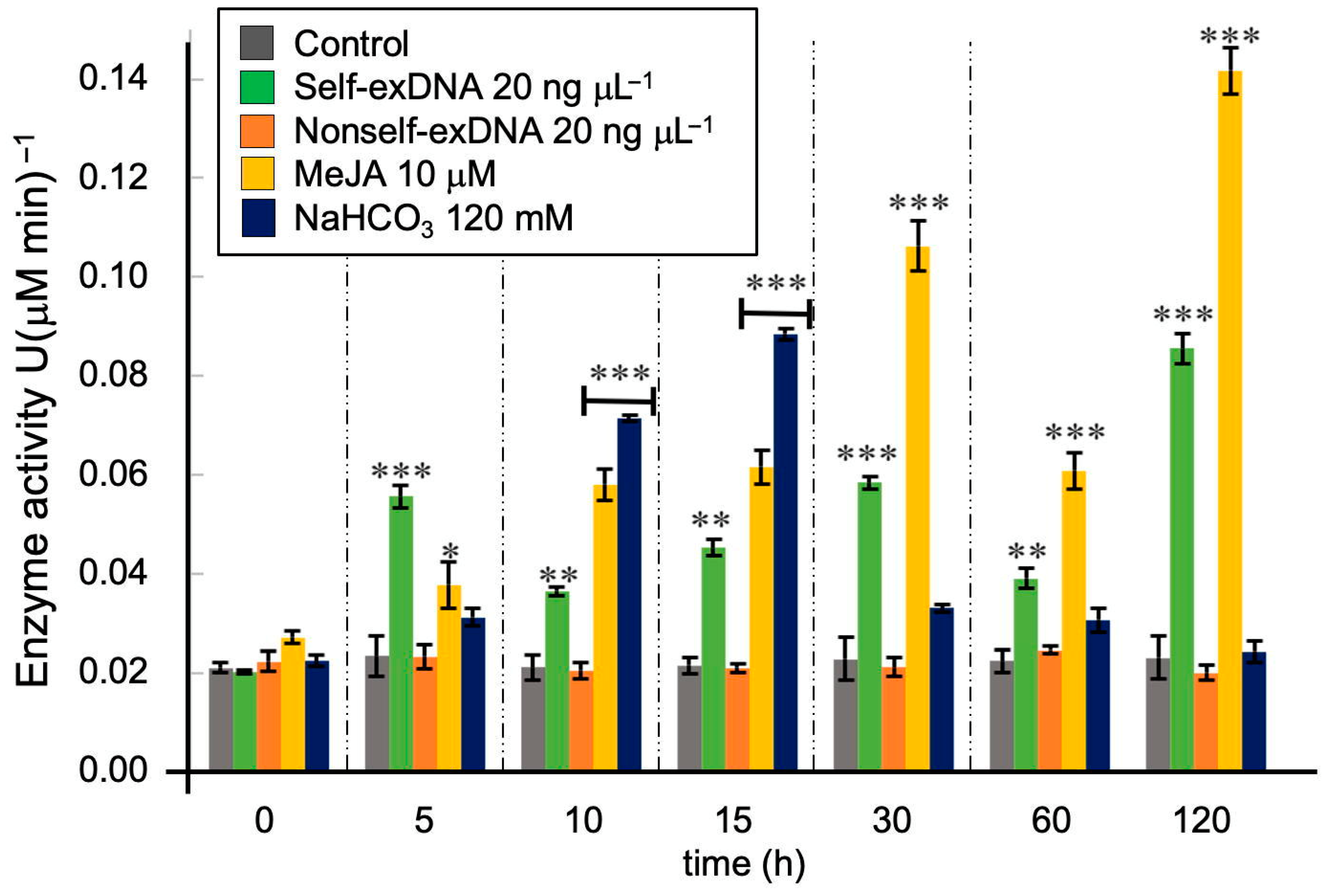
2.1. Assay of the N. oleabundans Peroxidase Enzyme Activity after Treatments
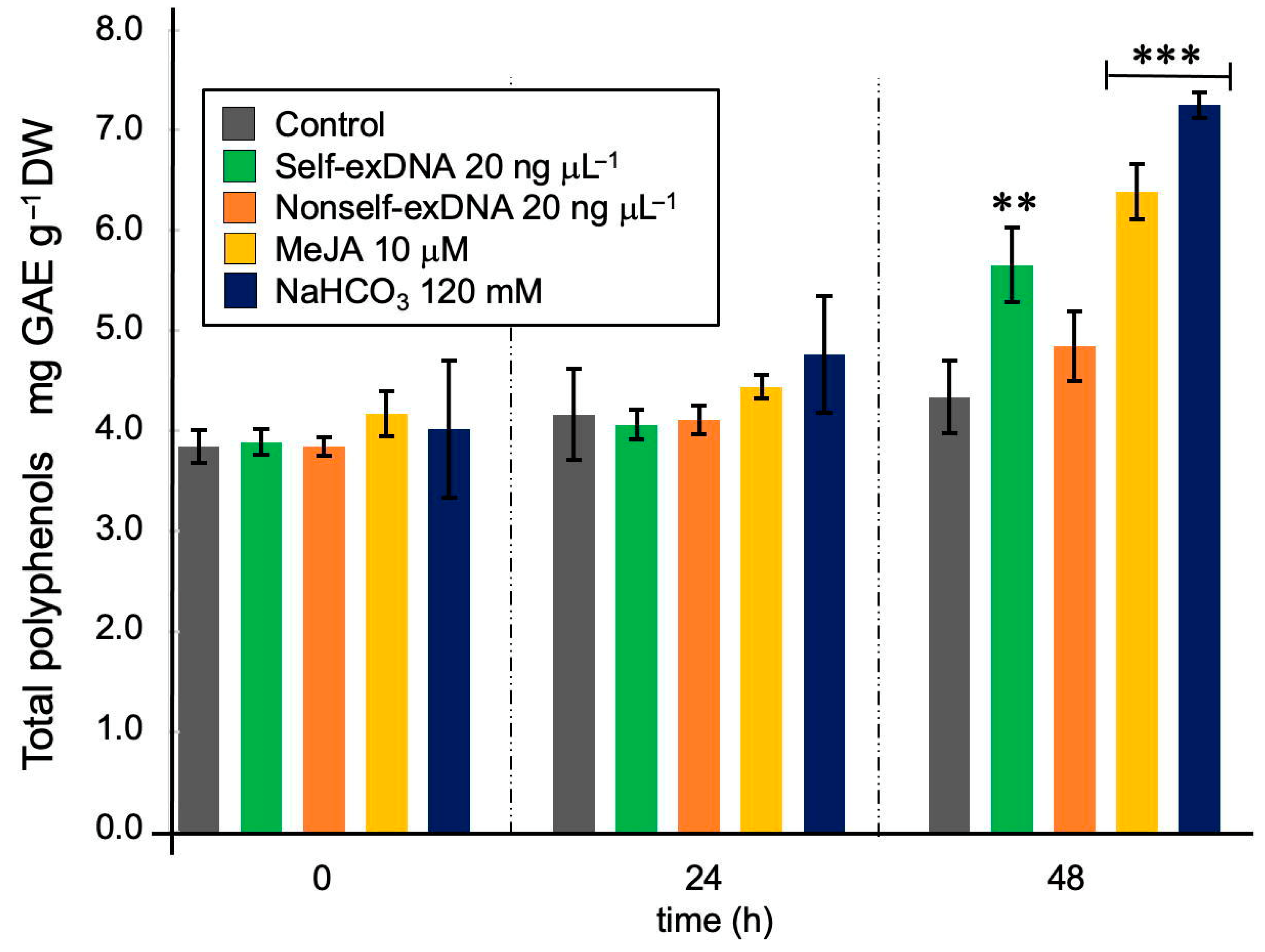
2.2. Effect of Elicitors on the Production of Total Polyphenols in N. oleoabundans
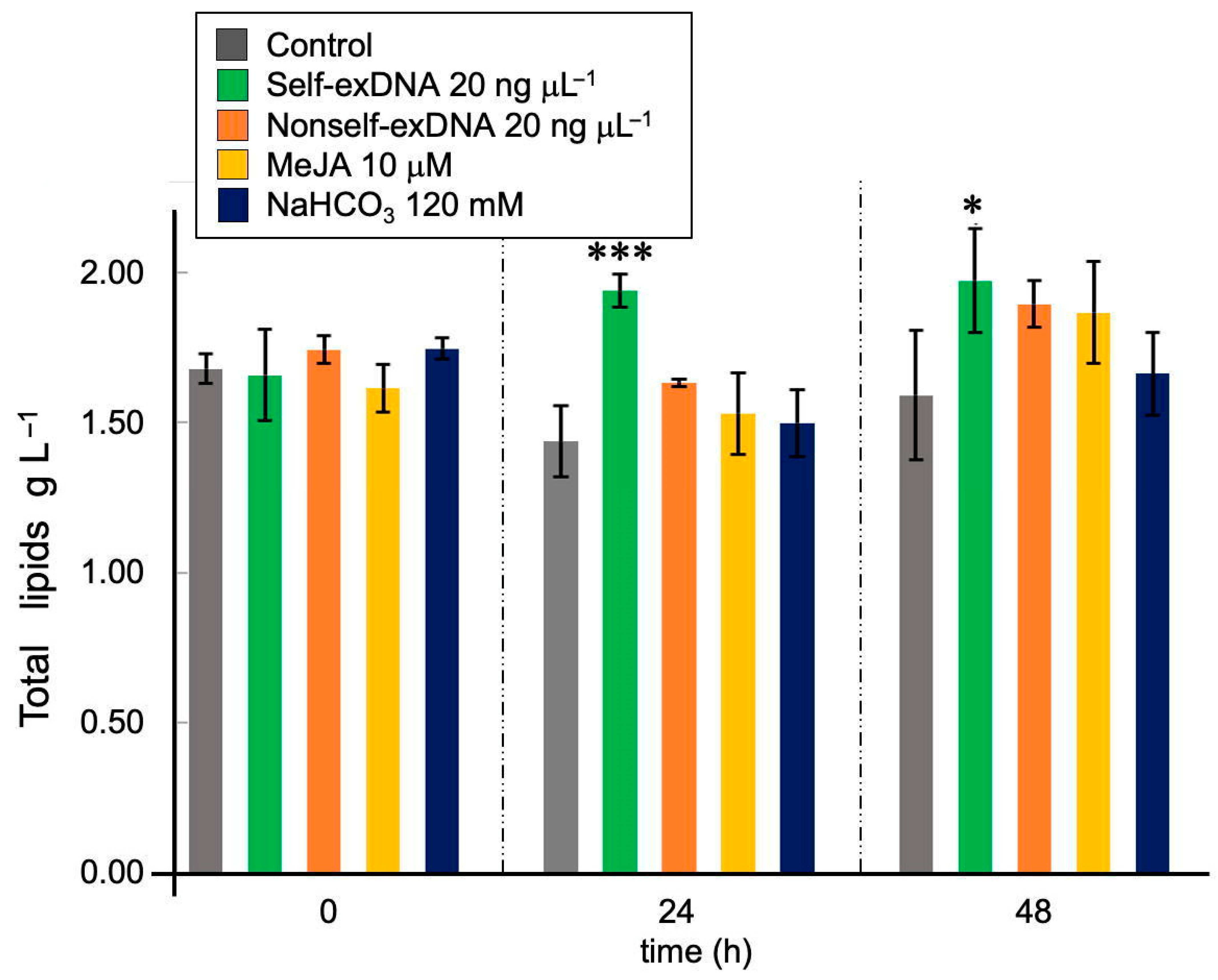
2.3. Effect of Inducers on the Production of Total Lipids in N. oleoabundans
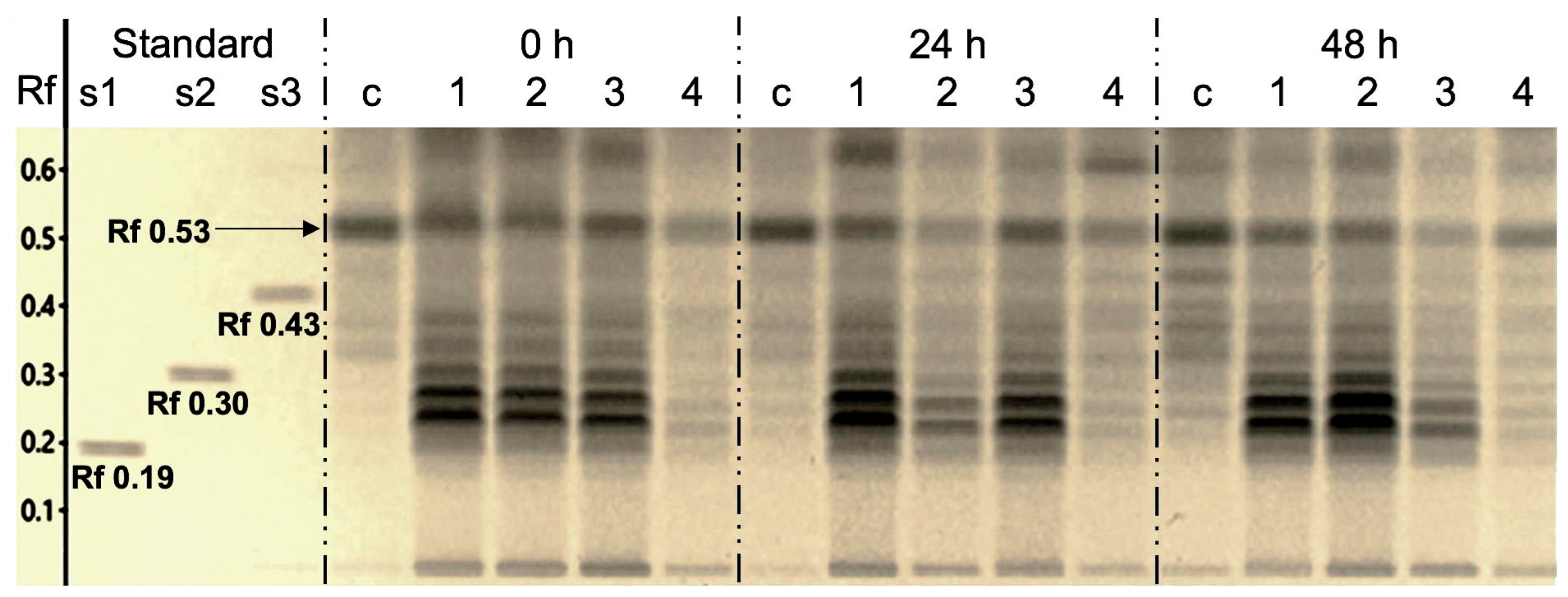
2.4. Effects of Elicitors on Triacylglycerols Production in N. oleoabundans
2.5. Effect of Elicitors on the Production of Phytohormones in Neochloris oleoabundans by UHPLC-MS
3. Discussion
4. Materials and Methods
4.1. Strain and Crops
4.2. Microalgal DNA Extraction
4.3. DNA Fragmentation
4.4. Treatments
4.5. Peroxidase Enzyme Activity Assay
4.6. Determination of Total Polyphenols
4.7. Gravimetric Determination of Total Lipids
4.8. Qualitative Analysis of Triacylglycerols by HPTLC
4.9. Extraction, Purification, and UHPLC-ESI-MS/MS Analysis of Phytohormones
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanguankiattichai, N.; Buscaill, P.; Preston, G.M. How bacteria overcome flagellin pattern recognition in plants. Curr. Opin. Plant Biol. 2022, 67, 102224. [Google Scholar] [CrossRef] [PubMed]
- Boutrot, F.; Zipfel, C. Function, Discovery, and Exploitation of Plant Pattern Recognition Receptors for Broad-Spectrum Disease Resistance. Annu. Rev. Phytopathol. 2017, 55, 257–286. [Google Scholar] [CrossRef]
- Quintana-Rodriguez, E.; Durán-Flores, D.; Heil, M.; Camacho-Coronel, X. Damage-associated molecular patterns (DAMPs) as future plant vaccines that protect crops from pests. Sci. Hortic. 2018, 237, 207–220. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Si, J.; Han, Z.; Chen, D. Action Mechanisms of Effectors in Plant-Pathogen Interaction. Int. J. Mol. Sci. 2022, 23, 6758. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Manohar, M.; Manosalva, P.; Tian, M.; Moreau, M.; Klessig, D.F. Activation of plant innate immunity by extracellular high mobility group box 3 and its inhibition by salicylic acid. PLoS Pathog. 2016, 12, e1005518. [Google Scholar] [CrossRef]
- Gust, A.A.; Pruitt, R.; Nürnberger, T. Sensing danger: Key to activating plant immunity. Trends Plant Sci. 2017, 22, 779–791. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Jander, G.; Bhawal, R.; Zhang, S.; Liu, Z.; Oakley, A.; Hua, J. AIG2A & AIG2B limit the activation of salicylic acid-regulated defenses by tryptophan-derived secondary metabolism in Arabidopsis. Plant Cell 2022, 34, 4641–4660. [Google Scholar] [CrossRef]
- Duran-Flores, D.; Heil, M. Sources of specificity in plant damaged-self recognition. Curr. Opin. Plant Biol. 2016, 32, 77–87. [Google Scholar] [CrossRef]
- DeFalco, T.A.; Zipfel, C. Molecular mechanisms of early plant pattern-triggered immune signaling. Mol. Cell 2021, 81, 3449–3467. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Bonanomi, G.; Incerti, G.; Chiusano, M.L.; Termolino, P.; Mingo, A.; Senatore, M.; Giannino, F.; Fabrizio, G.; Rietkerk, M.; et al. Inhibitory and toxic effects of extracellular self-DNA in litter: A mechanism for negative plant–soil feedbacks? New Phytol. 2015, 205, 1195–1210. [Google Scholar] [CrossRef]
- Bonanomi, G.; Zotti, M.; Idbella, M.; Termolino, P.; De Micco, V.; Mazzoleni, S. Field evidence for litter and self-DNA inhibitory effects on Alnus glutinosa roots. New Phytol. 2022, 236, 399–412. [Google Scholar] [CrossRef]
- Barbero, F.; Guglielmotto, M.; Capuzzo, A.; Maffei, M.E. Extracellular self-DNA (esDNA), but not heterologous plant or insect DNA (etDNA), induces plasma membrane depolarization and calcium signaling in lima bean (Phaseolus lunatus) and maize (Zea mays). Int. J. Mol. Sci. 2016, 17, 1659. [Google Scholar] [CrossRef]
- Duran-Flores, D.; Heil, M. Extracellular self-DNA as a damage-associated molecular pattern (DAMP), that triggers self-specific immunity induction in plants. Brain Behav. Immun. 2018, 72, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Geng, S.; Feng, T.; Wu, H. Effects of Ascophyllum nodosum extract on growth and antioxidant defense systems of two freshwater microalgae. J. Appl. Phycol. 2018, 30, 851–859. [Google Scholar] [CrossRef]
- Torres, J.; Rivera, A.; Clark, G.; Roux, S.J. Participation of Extracellular Nucleotides in the Wound Response of Dasycladus vermicularis and Acetabularia acetabulum (Dasycladales, Cholorophyta) 1. J. Phycol. 2008, 44, 1504–1511. [Google Scholar] [CrossRef]
- Cornejo-Corona, I.; Thapa, H.R.; Browne, D.R.; Devarenne, T.P.; Lozoya-Gloria, E. Stress responses of the oil-producing green microalga Botryococcus braunii Race B. PeerJ 2016, 4, e2748. [Google Scholar] [CrossRef]
- Palomba, E.; Chiaiese, P.; Termolino, P.; Paparo, R.; Filippone, E.; Mazzoleni, S.; Chiusano, M.A. Effects of Extracellular Self- and Nonself-DNA on the Freshwater Microalga Chlamydomonas reinhardtii and on the Marine Microalga Nannochloropsis gaditana. Plants 2022, 11, 1436. [Google Scholar] [CrossRef]
- Popovich, C.A.; Damiani, C.; Constenla, D.; Martínea, A.M.; Freije, H.; Giovanardi, M.; Pancaldi, S.; Leonardi, P. Neochloris oleoabundans grown in enriched natural seawater for biodiesel feedstock: Evaluation of its growth and biochemical composition. Bioresour. Technol. 2012, 114, 287–293. [Google Scholar] [CrossRef]
- Gatenby, C.M.; Orcutt, D.M.; Kreeger, D.A.; Parker, B.C.; Jones, V.A.; Neves, R.J. Biochemical composition of three algal species proposed as food for captive freshwater mussels. J. Appl. Phycol. 2003, 15, 1–11. [Google Scholar] [CrossRef]
- Safi, C.; Pollio, A.; Olivieri, G. Neochloris oleoabundans from nature to industry: A comprehensive review. Rev. Environ. Sci. Bio/Technol. 2021, 20, 943–958. [Google Scholar] [CrossRef]
- Tornabene, T.G.; Holzer, G.; Lien, S.; Burris, N. Lipid composition of the nitrogen starved green alga Neochloris oleoabundans. Enzym. Microb. Technol. 1983, 5, 435–440. [Google Scholar] [CrossRef]
- Rashidi, B.; Trindade, L.M. Detailed biochemical and morphologic characteristics of the green microalga Neochloris oleoabundans cell wall. Algal Res. 2018, 35, 152–159. [Google Scholar] [CrossRef]
- Minhas, A.K.; Hodgson, P.; Barrow Colin, J.; Sashidar, B.; Adholeya, A. The isolation and identification of new microalgal strains producing oil and carotenoid simultaneously with biofuel potential. Bioresour. Technol. 2016, 211, 556–565. [Google Scholar] [CrossRef]
- Chungjatupornchai, W.; Areerat, K.; Fa-Aroonsawat, S. Increased triacylglycerol production in oleaginous microalga Neochloris oleoabundans by overexpression of plastidial lysophosphatidic acid acyltransferase. Microb. Cell Factories 2019, 18, 53. [Google Scholar] [CrossRef]
- Mangold, H.K. Aliphatic lipids. II. Thin-layer chromatography of lipids. 1a. Neutral lipids and their hydrolysis products. In Thin-Layer Chromatography. A Laboratory Handbook, 2nd ed.; Stahl, E., Ed.; Springer: Berlin, Germany, 1969; p. 375. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Raman, V.; Ravi, S. Effect of salicylic acid and methyl jasmonate on antioxidant systems of Haematococcus pluvialis. Acta Physiol. Plant. 2011, 33, 1043–1049. [Google Scholar] [CrossRef]
- Cui, H.; Yang, F.; Li, Y. Exogenous methyl jasmonate enhances lipid production in Isochrysis galbana under nitrogen deprivation and high light. Algal Res. 2021, 58, 102406. [Google Scholar] [CrossRef]
- Magwell PF, R.; Djoudjeu, K.T.; Minyaka, E.; Tavea, M.F.; Fotsop, O.W.; Tagnikeu, R.F.; Fofou, A.M.; Vinnie-Darelle, C.K.; Dzokouo-Dzoyem, C.U.; Lehman, L.G. Sodium Bicarbonate (NaHCO3) Increases Growth, Protein and Photosynthetic Pigments Production and Alters Carbohydrate Production of Spirulina platensis. Curr. Microbiol. 2023, 80, 63. [Google Scholar] [CrossRef]
- Duval, B.; Shetty, K.; Thomas, W.H. Phenolic compounds and antioxidant properties in the snow alga Chlamydomonas nivalis after exposure to UV light. J. Appl. Phycol. 1999, 11, 559–566. [Google Scholar] [CrossRef]
- Li, H.B.; Cheng, K.W.; Wong, C.C.; Fan, K.W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Goh, S.H.; Yusoff, F.M.; Loh, S.P. A Comparison of the Antioxidant Properties and Total Phenolic Content in a Diatom, Chaetoceros sp. and a Green Microalga, Nannochloropsis sp. J. Agric. Sci. 2010, 2, 123–130. [Google Scholar] [CrossRef]
- Hajimahmoodi, M.; Faramarzi, F.A.; Mohammadi, N.; Soltani, N.; Oveizi, M.R.; Nafissi-Varcheh, N. Evaluation of antioxidant properties and total phenolic contents of some strains of microalgae. J. Appl. Phycol. 2010, 22, 43–50. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J. Appl. Phycol. 2015, 27, 1109–1119. [Google Scholar] [CrossRef]
- El-Baky HH, A.; El Baz, F.K.; El-Baroty, G.S. Production of phenolic compounds from Spirulina maxima microalgae and its protective effects. Afr. J. Biotechnol. 2009, 8, 7059–7067. [Google Scholar]
- Di Caprio, F.; Pipitone, L.M.; Altimari, P.; Pagnanelli, F. Extracellular and intracellular phenol production by microalgae during photoautotrophic batch cultivation. New Biotechnol. 2021, 62, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, A.; Tornabene, T.G.; Thomas, W.H. Chemical profile of selected species of microalgae with emphasis on lipids 1. J. Phycol. 1985, 21, 72–81. [Google Scholar] [CrossRef]
- Widjaja, A.; Chien, C.C.; Ju, Y.H. Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. J. Taiwan Inst. Chem. Eng. 2009, 40, 13–20. [Google Scholar] [CrossRef]
- Yeesang, C.; Cheirsilp, B. Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour. Technol. 2011, 102, 3034–3040. [Google Scholar] [CrossRef]
- Wu, L.F.; Chen, P.C.; Lee, C.M. The effects of nitrogen sources and temperature on cell growth and lipid accumulation of microalgae. Int. Biodeterior. Biodegrad. 2013, 85, 506–510. [Google Scholar] [CrossRef]
- Tornabene, T.G.; Bourne, T.F.; Raziuddin, S.; Ben-Amotz, A. Lipid and Lipopolysaccharide Constituents of Cyanobacterium Spirulina platensis (Cyanophyceae, Nostocales); Marine Ecology Progress Series; Peck, M.A., Snelgrove, P., Richardson, K., Wilson, R.P., Eds.; Inter-Research: Oldendorf, Germany, 1985; Volume 22, pp. 121–125. Available online: https://www.int-res.com/articles/meps/22/m022p121.pdf (accessed on 24 November 2022).
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef]
- Gigova, L.G.; Ivanova, N.J. Microalgae respond differently to nitrogen availability during culturing. J. Biosci. 2015, 40, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Reddy CR, K.; Jha, B. Methyl jasmonate-induced lipidomic and biochemical alterations in the intertidal macroalga Gracilaria dura (Gracilariaceae, Rhodophyta). Plant Cell Physiol. 2015, 56, 1877–1889. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Dhup, S.; Jamsheer, K.M. Addition of methyl jasmonate and rutin hydrate at harvest time elicits lipid production in Scenedesmus. Botany 2021, 99, 167–173. [Google Scholar] [CrossRef]
- Ayothi, P.; Muthu, A.; Shanmugam, K. Iron and methyl jasmonate increase high-value PUFA production by elevating the expression of desaturase genes in marine microalga Isochrysis sp. J. Appl. Microbiol. 2022, 132, 2042–2053. [Google Scholar] [CrossRef]
- Sangela, V.; Kumar, M.; Choudhary, S.; Gour, V.S.; Meena, M.; Vinayak, V. Effect of nitrogen, phosphorus and sodium bicarbonate on lipid production and fatty acid profile in Coelastrella terrestris. Biocatal. Agric. Biotechnol. 2022, 45, 102518. [Google Scholar] [CrossRef]
- Singh, R.P.; Yadav, P.; Kumar, A.; Hashem, A.; Al-Arjani, A.-B.F.; Abd_Allah, E.F.; Rodríguez-Dorantes, A.; Gupta, R.K. Physiological and biochemical responses of bicarbonate supplementation on biomass and lipid content of green algae Scenedesmus sp. BHU1 isolated from wastewater for renewable biofuel feedstock. Front. Microbiol. 2022, 13, 839800. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.A.; El-Naeb, E.H.; Hifney, A.F.; Adam, M.S.; Gomaa, M. Growth behavior, phenol removal and lipid productivity of microalgae in mixotrophic and heterotrophic conditions under synergistic effect of phenol and bicarbonate for biodiesel production. J. Appl. Phycol. 2022, 34, 2981–2994. [Google Scholar] [CrossRef]
- Udayan, A.; Sabapathy, H.; Arumugam, M. Stress hormones mediated lipid accumulation and modulation of specific fatty acids in Nannochloropsis oceanica CASA CC201. Bioresour. Technol. 2020, 310, 123437. [Google Scholar] [CrossRef]
- Stirk, W.A.; van Staden, J. Potential of phytohormones as a strategy to improve microalgae productivity for biotechnological applications. Biotechnol. Adv. 2020, 44, 107612. [Google Scholar] [CrossRef]
- Leliaert, F.; Smith, D.R.; Moreau, H.; Herron, M.D.; Verbruggen, H.; Delwiche, C.F.; De Clerck, O. Phylogeny and molecular evolution of the green algae. Crit. Rev. Plant Sci. 2012, 31, 1–46. [Google Scholar] [CrossRef]
- Umen, J.G. Green algae and the origins of multicellularity in the plant kingdom. Cold Spring Harb. Perspect. Biol. 2014, 6, a016170. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, S.; Hardtke, C.S. Hormone signalling crosstalk in plant growth regulation. Curr. Biol. 2011, 21, R365–R373. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, A.A.; Tarachovskaya, E.R.; Shishova, M.F. Biosynthesis of phytohormones in algae. Russ. J. Plant Physiol. 2012, 59, 595–610. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, J. Phytohormones in microalgae: A new opportunity for microalgal biotechnology? Trends Plant Sci. 2015, 20, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Jirásková, D.; Poulíčková, A.; Novák, O.; Sedlakova, K.; Hradecká, V.; Strnad, M. High-throughput screening technology for monitoring phytohormone production in microalgae 1. J. Phycol. 2009, 45, 108–118. [Google Scholar] [CrossRef]
- Frebort, I.; Kowalska, M.; Hluska, T.; Frébortová, J.; Galuszka, P. Evolution of cytokinin biosynthesis and degradation. J. Exp. Bot. 2011, 62, 2431–2452. [Google Scholar] [CrossRef]
- Stirk, W.A.; Bálint, P.; Tarkowská, D.; Novák, O.; Strnad, M.; Ördög, V.; Van Staden, J. Hormone profiles in microalgae: Gibberellins and brassinosteroids. Plant Physiol. Biochem. 2013, 70, 348–353. [Google Scholar] [CrossRef]
- Blanc, G.; Duncan, G.; Agarkova, I.; Borodovsky, M.; Gurnon, J.; Kuo, A.; Lindquist, E.; Lucas, S.; Pangilinan, J.; Polle, J.; et al. The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 2010, 22, 2943–2955. [Google Scholar] [CrossRef]
- Liu, Y.H.; Alimujiang, A.; Wang, X.; Luo, S.W.; Balamurugan, S.; Yang, W.D.; Liu, J.S.; Zhang, L.; Li, H.Y. Ethanol induced jasmonate pathway promotes astaxanthin hyperaccumulation in Haematococcus pluvialis. Bioresour. Technol. 2019, 289, 121720. [Google Scholar] [CrossRef]
- Horváth, E.; Szalai, G.; Janda, T. Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Stirk, W.A.; Tarkowská, D.; Gruz, J.; Strnad, M.; Ördög, V.; van Staden, J. Effect of gibberellins on growth and biochemical constituents in Chlorella minutissima (Trebouxiophyceae). S. Afr. J. Bot. 2019, 126, 92–98. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Hedbavny, J.; Bačkor, M. Effect of copper and salicylic acid on phenolic metabolites and free amino acids in Scenedesmus quadricauda (Chlorophyceae). Plant Sci. 2010, 178, 307–311. [Google Scholar] [CrossRef]
- Fu, L.; Li, Q.; Chen, C.; Zhang, Y.; Liu, Y.; Xu, L.; Zhou, Y.; Li, C.; Zhou, D.; Rittmann, B.E. Benzoic and salicylic acid are the signaling molecules of Chlorella cells for improving cell growth. Chemosphere 2021, 265, 129084. [Google Scholar] [CrossRef]
- Chiusano, M.L.; Incerti, G.; Colantuono, C.; Termolino, P.; Palomba, E.; Monticolo, F.; Benvenuto, G.; Foscari, A.; Esposito, A.; Marti, L.; et al. Arabidopsis thaliana response to extracellular DNA: Self versus nonself exposure. Plants 2021, 10, 1744. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Ryu, C.M. Plant perceptions of extracellular DNA and RNA. Mol. Plant 2016, 9, 956–958. [Google Scholar] [CrossRef]
- Tanaka, K.; Heil, M. Damage-associated molecular patterns (DAMPs). in plant innate immunity: Applying the danger model and evolutionary perspectives. Annu. Rev. Phytopathol. 2021, 59, 53–75. [Google Scholar] [CrossRef]
- Li, C.; Wang, K.; Zou, Y.; Lei, C.; Chen, Z.; Zheng, Y. Extracellular self-DNA induced a PTI-related local defence against Rhizopus rot in postharvest peach fruit. Postharvest Biol. Technol. 2023, 200, 112306. [Google Scholar] [CrossRef]
- Ge, D.; Yeo, I.C.; Shan, L. Knowing me, knowing you: Self and non-self recognition in plant immunity. Essays Biochem. 2022, 66, 447–458. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, H.; Zhang, X.; Khashi u Rahman, M.; Mazzoleni, S.; Du, M.; Wu, F. Plant extracellular self-DNA inhibits growth and induces immunity via the jasmonate signaling pathway. Plant Physiol. 2023, 192, 2475–2491. [Google Scholar] [CrossRef]
- Monticolo, F.; Palomba, E.; Termolino, P.; Chiaiese, P.; De Alteriis, E.; Mazzoleni, S.; Chiusano, M.L. The role of DNA in the extracellular environment: A focus on NETs, RETs and biofilms. Front. Plant Sci. 2020, 11, 589837. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Y.; Xu, H.; Liu, Y.; Sun, J.; Qiao, D.; Cao, Y. Effect of nitrogen-starvation, light intensity and iron on triacylglyceride/carbohydrate production and fatty acid profile of Neochloris oleoabundans HK-129 by a two-stage process. Bioresource Technology. 2014, 155, 204–212. [Google Scholar] [CrossRef]
- Bischoff, W.H.; Bold, C.H. Some soil algae from Enchanted Rock and related algal species. Phycological Studies IV. Univ. Tex. Publ. 1963, 6318, 1–95. [Google Scholar]
- Hemeda, H.M.; Klein, B.P. Effects of Naturally Occurring Antioxidants on Peroxidase Activity of Vegetable Extracts. J. Food Sci. 1990, 55, 184–185. [Google Scholar] [CrossRef]
- Weng, Z.; Hendrickx, M.; Maesman, G.; Gebruers, K.; Tobback, P. Thermostability of Soluble and Immobilized Horseradish Peroxidase. J. Food Sci. 1991, 56, 574–578. [Google Scholar] [CrossRef]
- Saraiva, J.; Oliveira, J.C.; Lemos, A.; Hendrickx, M. Analysis of the kinetic patterns of horseradish peroxidase thermal inactivation in sodium phosphate buffer solutions of different ionic strength. Int. J. Food Sci. Technol. 1996, 31, 223–231. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1964, 16, 144–158. [Google Scholar] [CrossRef]
- Šimura, J.; Antoniadi, I.; Široká, J.; Tarkowská, D.; Strnad, M.; Ljung, K.; Novák, O. Plant Hormonomics: Multiple Phytohormone Profiling by Targeted Metabolomics. Plant Physiol. 2018, 177, 476–489. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zárate-López, M.A.; Quintana-Rodríguez, E.; Orona-Tamayo, D.; Aguilar-Hernández, V.; Araujo-León, J.A.; Brito-Argáez, L.; Molina-Torres, J.; Hernández-Flores, J.L.; Loyola-Vargas, V.M.; Lozoya-Pérez, N.E.; et al. Metabolic Responses of the Microalga Neochloris oleoabundans to Extracellular Self- and Nonself-DNA. Int. J. Mol. Sci. 2023, 24, 14172. https://doi.org/10.3390/ijms241814172
Zárate-López MA, Quintana-Rodríguez E, Orona-Tamayo D, Aguilar-Hernández V, Araujo-León JA, Brito-Argáez L, Molina-Torres J, Hernández-Flores JL, Loyola-Vargas VM, Lozoya-Pérez NE, et al. Metabolic Responses of the Microalga Neochloris oleoabundans to Extracellular Self- and Nonself-DNA. International Journal of Molecular Sciences. 2023; 24(18):14172. https://doi.org/10.3390/ijms241814172
Chicago/Turabian StyleZárate-López, Mónica A., Elizabeth Quintana-Rodríguez, Domancar Orona-Tamayo, Víctor Aguilar-Hernández, Jesús A. Araujo-León, Ligia Brito-Argáez, Jorge Molina-Torres, José Luis Hernández-Flores, Víctor M. Loyola-Vargas, Nancy E. Lozoya-Pérez, and et al. 2023. "Metabolic Responses of the Microalga Neochloris oleoabundans to Extracellular Self- and Nonself-DNA" International Journal of Molecular Sciences 24, no. 18: 14172. https://doi.org/10.3390/ijms241814172
APA StyleZárate-López, M. A., Quintana-Rodríguez, E., Orona-Tamayo, D., Aguilar-Hernández, V., Araujo-León, J. A., Brito-Argáez, L., Molina-Torres, J., Hernández-Flores, J. L., Loyola-Vargas, V. M., Lozoya-Pérez, N. E., & Lozoya-Gloria, E. (2023). Metabolic Responses of the Microalga Neochloris oleoabundans to Extracellular Self- and Nonself-DNA. International Journal of Molecular Sciences, 24(18), 14172. https://doi.org/10.3390/ijms241814172







