1. Introduction
Accompanying the rapid progress in nanotechnology toward biofunctionalized particles, tremendous efforts have been made in recent years to optimize core and core/shell magnetic nanoparticles (MNPs). The core/shell type nanoparticles can be broadly defined as comprising a core (inner material) and a shell (outer layer material). Because both terminologies, “shell@core” and “core/shell”, became equally popular in the literature, the authors of the paper decided to use the former. Currently, magnetic nanoparticles are of particular interest as a diagnostic and therapeutic tool, which, due to their small size, can overcome most biological barriers, allowing their diffusion and distribution into most tissues [
1,
2]. Well-controlled preparation of pure and doped nanomagnetites allows for modeling their properties, which results in many benefits in various fields of science [
3,
4,
5]. Notably, different types of shell@core nanoparticles, surface stabilizer@inorganic nanoparticles, and, more specifically, magnetic core materials coated with other inorganic materials, are extensively studied because of their wide biomedical applications [
6,
7]. In addition, biocompatible polymers, such as polyethylene glycol, are used extensively with a view to increasing the biocompatibility of the core [
8]. Superparamagnetic iron oxide nanoparticles (SPIO NPs) can transform both cancer diagnostics [
9,
10,
11] and therapeutics [
12,
13]. The interest in them stems from relatively easy synthesis, good corrosion resistance, high magnetization, and relatively low cytotoxicity [
14]. The US Federal Drug Administration (FDA) has approved iron oxide nanoparticles for use in human therapeutics and diagnostics [
15,
16].
Surface modification and functionalization is an essential aspect of SPIO NP synthesis. Thermal decomposition is a well-known method for the successive synthesis of ferrite core–shell nanoparticles [
17]. Such a “heating-up” method involves the decomposition of precursors in the presence of organic surfactants to produce the desired particles. The most commonly used non-magnetic precursors are acetylacetonates and surfactants. The impacts of the surfactant quantity and quality on the growth regime of iron oxide nanoparticles are extremely important. Usually, disperse SPIO NPs with sizes from 3 to 20 nm are prepared at 265 °C in the presence of Fe(acac)
3, phenyl ether, 1,2-hexadecanediol as a reducing agent, oleic acid, and oleylamine [
18]. This process offers yield quantity, particle size control, fine size distribution, crystallinity, and dispersibility to synthesized shell@core nanoparticles. A simplified notation of the composition of nanoparticles was adopted in this work, i.e., M@Ga
x and Ga
x@M, where M means magnetite with the formula Fe
3O
4, while x means a dopant of Ga in gallium ferrite.
Magnetites doped with bi- and trivalent cations form a group of innovative materials [
19,
20,
21,
22,
23,
24,
25]. Among these, gallium nanoferrites seem to be promising candidates for use in magnetic hyperthermia [
12].
Because of their simple crystal structure and high tendency to form homogeneous compounds, gallium-modified magnetites represent an ideal system to study the influence of ionic order on the thermomagnetic features of nanoparticles [
26]. A large family of ferrite binary oxides with the spinel structure (Fd-3m no. 227) is characterized as a ferrimagnetic with strong dependence of magnetic properties on the state of chemical order and on the cation distribution between the two crystallographically distinct tetrahedral (Td) and octahedral (Oh) sites. Notably, the crystal ordering for the system of interest is defined according to Formula (1).
It can be easily seen that any
condition leads to the inverse spinel structure of Ga
xFe
3−xO
4 (for simplicity, hereinafter referred to as Ga
x). Simultaneously, under the
condition, Ga will only occupy the Oh site, while the
condition leads to total Td site preference (see, respectively, red and blue dots shown in
Figure 1a). One of the problems of contemporary nanomaterial physics currently being solved is the creation of magnetic nanoparticles that conclusively exhibit magnetism that is weak enough to prevent agglomeration but strong enough to preserve superparamagnetic fluctuations up to temperatures well above 300 K. The magnetic ordering of the doped system is illustrated in
Figure 1b. The appropriate spin configuration of the gallium nanoferrite is sensitive to the content and location of the Ga dopant [
27,
28].
The site that is partially occupied by non-magnetic gallium ions will exhibit weaker magnetic exchange interactions. If the gallium prefers any of the spinel sublattice, this will weaken the compensation of the antiferromagnetically oriented magnetic moments of the site Td with respect to Oh (see, respectively, smaller and larger arrows shown in
Figure 1b). As a result, the magnetic moment of the single domain (nanoparticle) would increase. The gallium doping of Fe
3O
4 may be a simple route of tuning magnetic properties, with direct consequences on superparamagnetic behavior. Gallium-doped magnetite nanoparticles are soft magnetic materials with a phase transition temperature above 350 K [
26]. Therefore, it was decided to use the properties of gallium as an admixture of nanoferrites in the presented studies.
Certain properties of magnetic nanocrystals, such
as the blocking temperature
TB, magnetic
saturation, and permanent magnetization, are all dependent on particle size,
but the coercivity of the nanocrystals totally depends on the particle shape
because of surface anisotropy effects [
29]. By
simple modeling both radius and spherical shape dependences versus
(Equation (2)) it becomes simpler to predict some other parameters that optimize superparamagnetism.
where:
r—radius of particle,
V—volume of spherical particle,
—Boltzmann constant,
—blocking temperature, and
K—anisotropy constant.
Subsequent modification on the core or shell of spherical nanoparticle influences, in different ways, the magnetic and thermal behavior of the systems.
The superparamagnetic behavior is exhibited by correspondingly small particles. If they are too small, almost all the atoms are on the surface, leading to thermal and magnetic properties strongly modified with respect to the bulk materials. The typical values for spherical particles are about 8–18 nm for Fe
3O
4 [
27,
28,
30,
31,
32]. Notably, with decreasing particle size, the anisotropy energy decreases. For a grain size equal to or lower than a characteristic value, this energy may become even lower than the thermal energy. This implies that the energy barrier for magnetization reversal may be overcome, and the total magnetic moment of the particle can then thermally fluctuate. Finally, the entire spin system may be rotated, and the spins within the single-domain particles remaining magnetically coupled can disclose superparamagnetic behavior. Obviously, the blocking temperature for a magnetic particle increases with increasing size and for a given size increases with decreasing measuring time, and the observation of a superparamagnetic or blocked state then depends on the experimental technique. For example, the techniques used to study the superparamagnetic relaxation are (1) dc susceptibility, where
is estimated to be around
; (2)
57Fe Mössbauer spectroscopy with the time window range of
; and (3) neutron diffraction with time window of
, depending on the type of experiments.
Blocking temperatures determined from RT measurements using the Mössbauer effect (ME) or from neutron diffraction (ND) are significantly higher compared to those from RT dc magnetization (MAG). The reason for this is the much shorter measuring time in these experimental methods. Assuming the longest possible times of , , and , the superparamagnetic blocking temperatures relate to each other as , i.e., 1:5.5:11.
As an example, for Fe
3O
4 (for simplicity, hereinafter referred to as M) the characteristic grain diameter below which superparamagnetic relaxations and above which magnetically ordered states are observed is 17 nm for dc susceptibility measurements, while it is 9 nm for Mössbauer spectroscopy experiments, which have a much shorter measuring time [
30].
Additionally, in the measurements of saturation magnetization or blocking temperature, it is difficult, in fact, to determine the mass of magnetic nanoparticles in a colloidal ferrifluid suspension (nanoparticles often surrounded by an organic surfactant in a dispersing medium). From a clinical point of view, the toxicity of nanoparticles, their stability under physiological conditions, and their ability to be removed from the body are essential issues [
1]. The main disadvantage of nanoparticles in biomedicine is their instability and tendency to form aggregates in aqueous media due to magnetic dipole–dipole and van der Waals interactions [
33].
The objective of this work is to study the influence of the location and quantity of gallium dopants on the crystal and magnetic properties of spherical and nearly spherical shell@core nanoparticles of Gax@M and M@Gax series based on diffraction, spectroscopy, microscopy, and cytotoxic data. In addition, ferrimagnetism, associated with superparamagnetic behavior, is investigated with Mössbauer spectroscopy and low-temperature neutron data. The research is aimed at checking whether the superparamagnetism and specific absorption rate coefficients with high biocompatibility and nontoxicity are in an easily accessible range for potential biomedical applications.
3. Materials and Methods
In this study, two series of shell@core magnetite nanoparticles, Gax@M and M@Gax, with 2.85–20 at. % gallium doping (x = 0.2–1.4) were used, where in one case the gallium ferrite coated the magnetite core, and in the other case the magnetite coated the gallium ferrite core. In order to prepare the magnetite core, 4 mmol of Fe(acac)3 was mixed with 1,2-hexadecanediol, oleylamine, and oleic acid in a high-boiling solvent (diphenyl ether). This mixture was heated to 259 °C (boiling point of diphenyl ether) in the presence of argon flow. In the next step, when the mixture was cooled down, a gallium ferrite shell was prepared on the resulting Fe3O4 core, e.g., with the stoichiometry of Ga0.6Fe2.4O4. For this purpose, 0.8 mmol of Ga(acac)3, 3.2 mmol of Fe(acac)3, oleylamine, and oleic acid were added to the obtained magnetite cores. The mixture was again heated to 259 °C in the presence of argon flow. When the solution cooled, the nanoparticle precipitation process was started by adding an excess of deoxygenated acetone. Acetone was exchanged 3 times, and then the precipitate was dried in the vacuum evaporator. In the case of coating gallium ferrite cores with magnetite, the procedure was analogous, with an appropriate change in the order of adding the substrates.
3.1. X-ray and Neutron Dyffraction
Room temperature (RT) XRD measurements were performed using Empyrean PANalytical powder diffractometer located at the Faculty of Physics University of Bialystok (FP, UB). A diffractometer was used working at 40 KV and 40 mA, using Mo
Kα radiation, λ= 0.7093187 Å in Bragg–Brentano geometry. A scattered intensity was recorded using a PixCel1D strip detector within a 2θ range from 5° to 55° and in a step-scanning mode with a step of Δ2θ = 0.026°. Pure LaB
6 (660 C) powder standard sample was used to correct the data for instrumental broadening. Neutron diffraction spectra obtained using a time-of-flight instrument RTD (IBR-2, JINR, Dubna) [
51] were normalized by calibrated scattering from a standard. To determine the shape of an incident neutron spectrum, the scattering from a vanadium sample was used. The diffraction patterns were analyzed using a Rietveld-type profile refinement method using the FullProf program [
52] and HighScore software package 4.0 [
53]. The full-profile Rietveld method leads to the average structure determination from the Bragg peak positions and intensities, and the microstructure determination (size–strain analysis) from the peak profile analysis [
52,
53]. The FullProf program allows the estimation of the average crystalline size and microstrain by averaging the values obtained for apparent size and maximum strain calculated for each crystal direction [hkl]. X-ray powder diffraction data peak profiles were modeled by the Thomson–Cox–Hasting modified pseudo-Voight function. Such peak profiles and the instrumental resolution function were used to obtain the apparent crystallite size.
3.2. TEM Images
Transmission electron microscopy (TEM) images were obtained at the Center of Synthesis and Analytical BioNanoTechno Methods at the Faculty of Chemistry University of Bialystok (FCh UB). The morphology and microstructure characterization of the particles were carried out using a FEI Tecnai G2 electron microscope operating at 200 kV.
3.3. SANS Investigations
SANS data were collected using a YuMO spectrometer operating on IBR-2. All samples were measured at four temperature points in the range between 20 °C and 50 °C. The particles’ shapes, sizes, dispersion coefficients, and morphologies were analyzed using the SAS-view program [
54]. In order to most precisely determine SANS data descriptions, the necessity to use three different shape models was presented and justified, as described in detail in
Section 2.3.
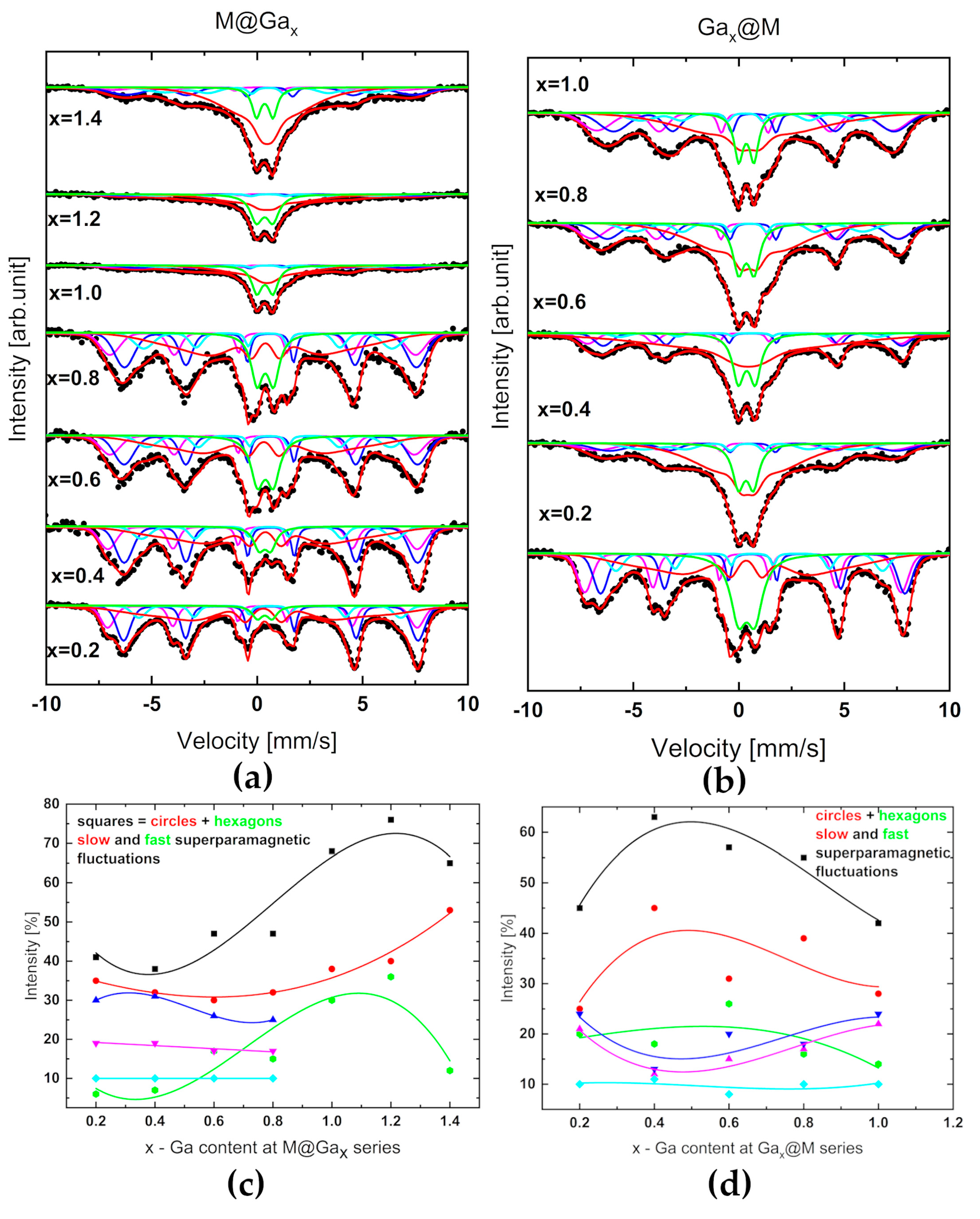
3.4. Mössbauer Spectroscpy
Mössbauer measurements were performed at room temperature on a standard spectrometer operating in the constant acceleration mode with a
57Co gamma source in an Rh matrix (FP, UB). The velocity scale was calibrated using α-Fe standard foil, also at room temperature. Mössbauer spectra were analyzed using the commercially available NORMOS package [
55] using Voigt profile convolution of Lorenz and Gaussian shapes as a lines shape.
3.5. SAR and Heat Cacapcity Measurements
Calorimetric experiments under nonadiabatic conditions were performed using the magneTherm system (nanoTherics, Staffordshire, UK), while the analysis was performed by corrected slope method (CSM) [
56,
57]. According to the experimental apparatus, inside a 100.5 mL Styrofoam jacket, a 3 mL Eppendorf tube was filled with 2 mL of a ferrifluid sample. The frequency f = 532 kHz and intensity H = 15 kA/m were selected as the optimal parameters of the alternating magnetic field. The concentration of nanoparticles in the tested samples was 10 mg/mL. The solvent was diphenyl ether.
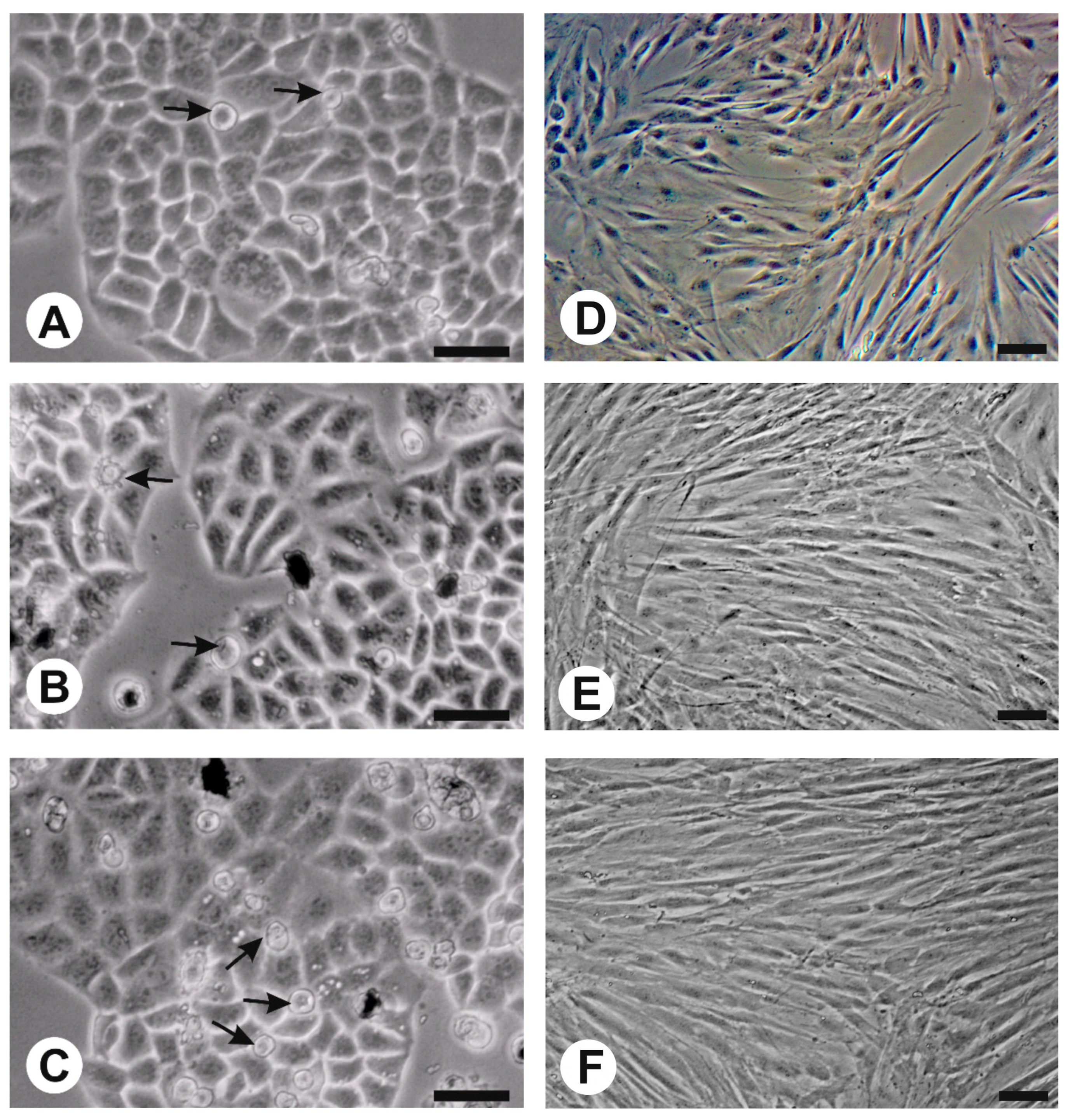
3.6. Effects of Nanferrites on Fibroblasts and HeLa Cells in In Vitro Culture
For evaluation of the impact of nanoparticles on cells, the in vitro culture method was used with HeLa (ATCC-CCL-2) cells as the cancer model and fibroblast cells (ATCC-CRL-2106) as the normal cells model. Before being added to the culture medium, nanoparticle powders were sterilized for 30 min in 70% ethanol and evaporated overnight to dryness at 60 °C. For preparation of nanoparticle suspension in culture medium, an ultrasonic processor Hielscher UP50H was used (0.5 s cycle, 100% amplitude by 60 s/3 min in ice). The content of both kinds of nanoparticles in separate culture medium was 0.1 mg/mL, 0.05 mg/mL, and 0.01 mg/mL. Control cultures of HeLa and fibroblast cells did not contain nanoparticles. All cultures were maintained in a NuAire NU-5820E incubator (37 °C, 5% CO2, 95% humidity) on 2 mL MEM (Sigma-Aldrich ref. no. M4655) medium, with 10% fetal bovine serum (Sigma-Aldrich ref. no. F7524) and antibiotics (penicillin 50 U/mL, streptomycin 0.05 mg/mL, Sigma-Aldrich ref. no. P0781). The initial inoculum was 1.5 × 105 cells per well. Control cultures (without nanoparticles) and experimental variants (with nanoparticles) were grown in 12-well plates until the control variant reached 90% confluence (3 days in the case of HeLa cells and 7 days for fibroblasts). In the case of fibroblasts, the medium was changed after 3 days of culture. During the experiment, in vivo observations of cultures were carried out using an Olympus inverted contrast phase microscope (CKX 41).
For cell counting and viability estimation, an EVE
MT automatic cell counter (NanoEnTec Inc., Seoul, Republic of Korea) was used, with Trypan-blue staining [
58] at a final concentration of 0.2% for 3 min.
The impact of nanoparticles on cell metabolism was evaluated using the MTT test [
59] using tetrazolium salt (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma-Aldrich ref. no. M2128) at a final concentration in DPBS of 0.25 mg/mL (30 min incubation in 37 °C). The absorbance of the obtained solution was measured at λ = 570 nm using a Lambda E MWG AG BIOTECH plate reader. The results are presented as percent of control.
Data from six independent experiments were used for statistical evaluation. The results obtained from individual research methods were analyzed using the Shapiro–Wilk W-test to identify the normal distribution of data and the Levene L-test for verifying if the variances were homoscedastic. Since the obtained data showed a normal distribution and homoscedastic variances, the parametric Student’s t-test for a single sample = 1 (100%) was used to compare data expressed as percent of control. When data did not show a normal distribution, a nonparametric Mann–Whitney U-test was used to compare the average values of control and experimental groups.
4. Conclusions
Core-shell gallium nanoferrites were prepared from acetylacetonates. Using various techniques, it was possible to find a number of correlations between the morphological, structural, and magnetic parameters of the Gax@M and M@Gax systems with x increasing to 1.4. Optimal biomaterials were selected.
All obtained nanostructures crystallize in the cubic inverse spinel type structure. Diffraction studies confirmed the homogeneity, ordering, and evident low-temperature magnetism of nanoparticles (neutron diffraction). TEM images of Gax@M series disclosed a relatively weaker agglomeration effect, and in consequence, more effective dispersion in the diphenyl ether. Mostly, gallium-doped magnetite nanoparticles kept spherically symmetrical shapes in the whole range of the studied admixtures. A core–shell model was used successfully to develop the most SANS data. From this point of view, shape, size, and TB values of gallium ferrites with a Ga content in the range of 0.6–0.8 are optimal. Complementary analysis of the diffraction intensities of (220) and (311) reflections indicate the preference for the location of gallium in the Td sublattice and presence of oxygen vacancies as the volume rather than the surface effect. Analysis of crystallite size as a function of gallium content in both series showed a consistent trend, with a minimum in the range above gallium content of 0.8.
In both series, systems with the highest concentration of Ga, i.e., M@Ga1.4 and Ga1.0@M, disclose reconstruction of magnetic order. The strongest contribution of superparamagnetism in the series of Gax@M is observed for systems with a Ga content in the range of 0.4–0.8.
Calorimetric measurements of the studied nanosystems showed that these particles, subjected to an alternating magnetic field, have the ability to heat up quickly. In the case of M@Gax series, the system with x = 0.6 heated up the fastest and reached the highest SAR (68.32 ± 0.25) W/g. The increase in gallium content in the shell is accompanied by a downward trend in SAR.
Considering the biological properties of gallium-doped nanoparticles that were found, as well as their effects on both cancer cells (slight growth inhibition) and normal fibroblasts (small increase of growth rate), it can be concluded that the use of gallium as a dopant in the nanoparticle structure is a promising research direction. The results obtained indicate that, of the nanostructures obtained, Ga0.6@M show the highest potential for use in cancer thermotherapy. The physical and biological properties of these nanostructures make them an alternative to classical iron nanoparticles. Gallium-containing nanoparticles, while maintaining their magnetic properties, can have a lesser effect on the free radical damage to cells due to their limited iron content.