FGF2 Rescued Cisplatin-Injured Granulosa Cells through the NRF2-Autophagy Pathway
Abstract
:1. Introduction
2. Results
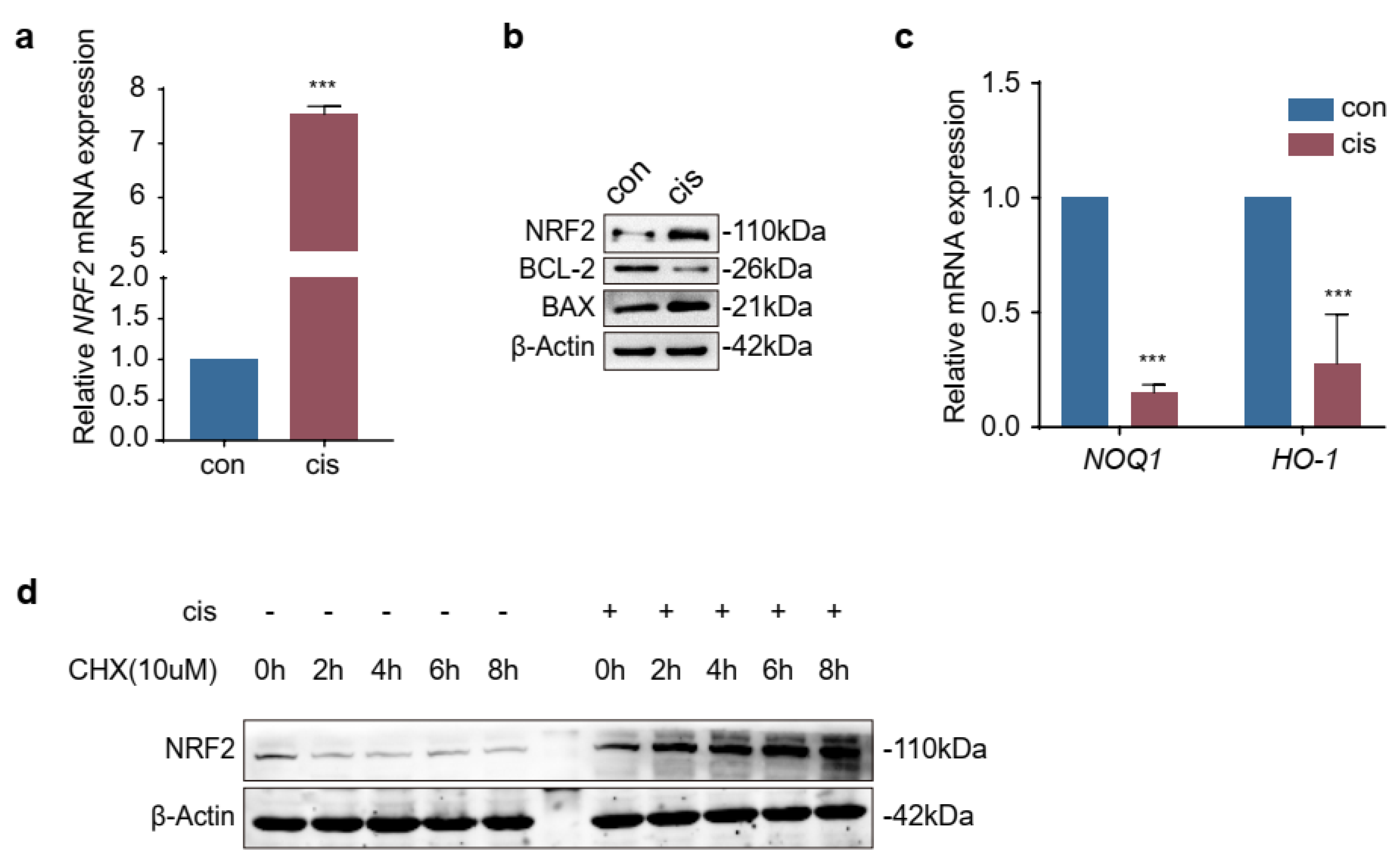
2.1. Expression of NRF2 Was Increased in Cisplatin-Induced Granulosa Cells
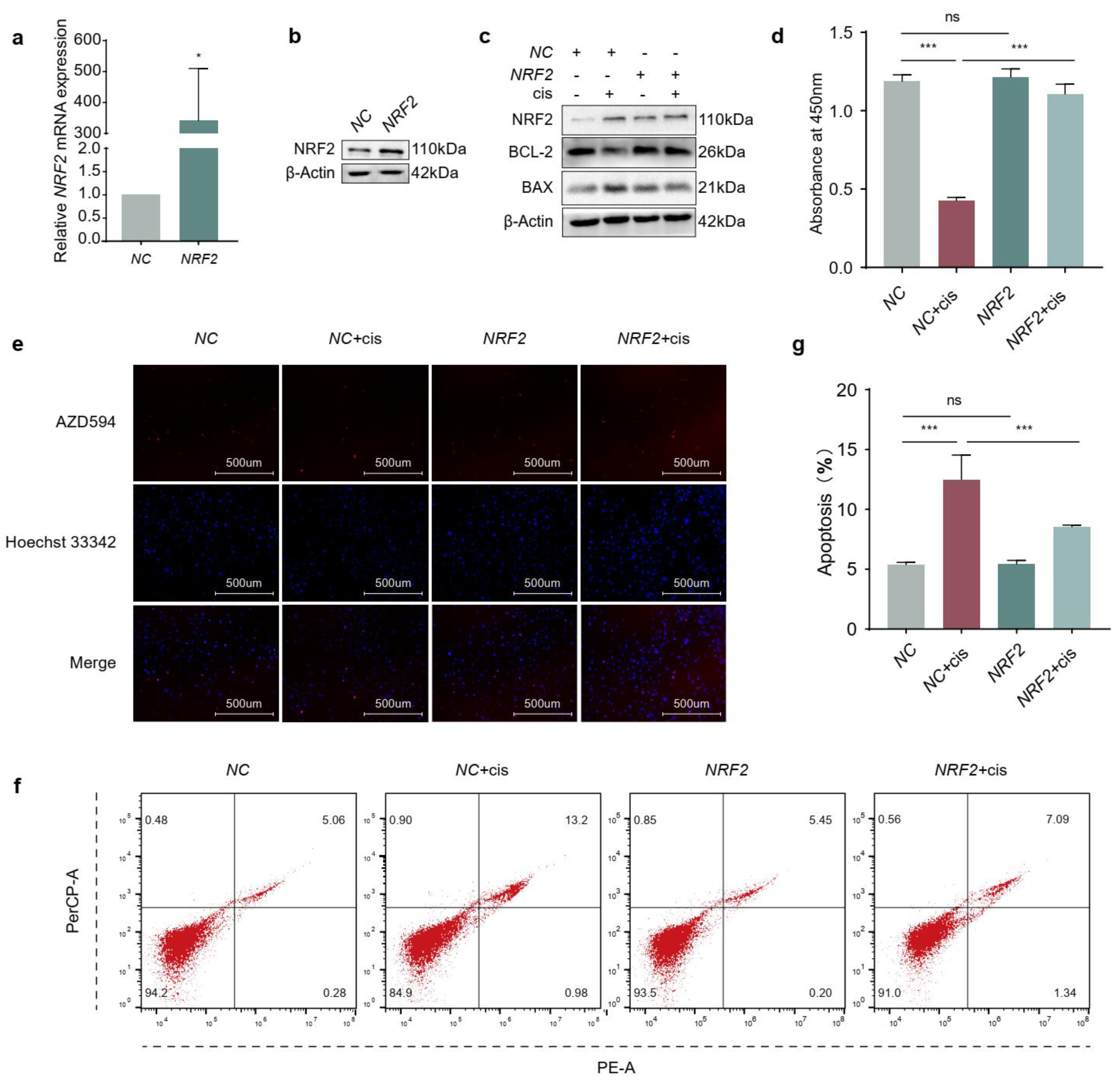
2.2. Overexpression of NRF2 Promotes Proliferation and Attenuates Apoptosis of Cisplatin-Induced Granulosa Cells
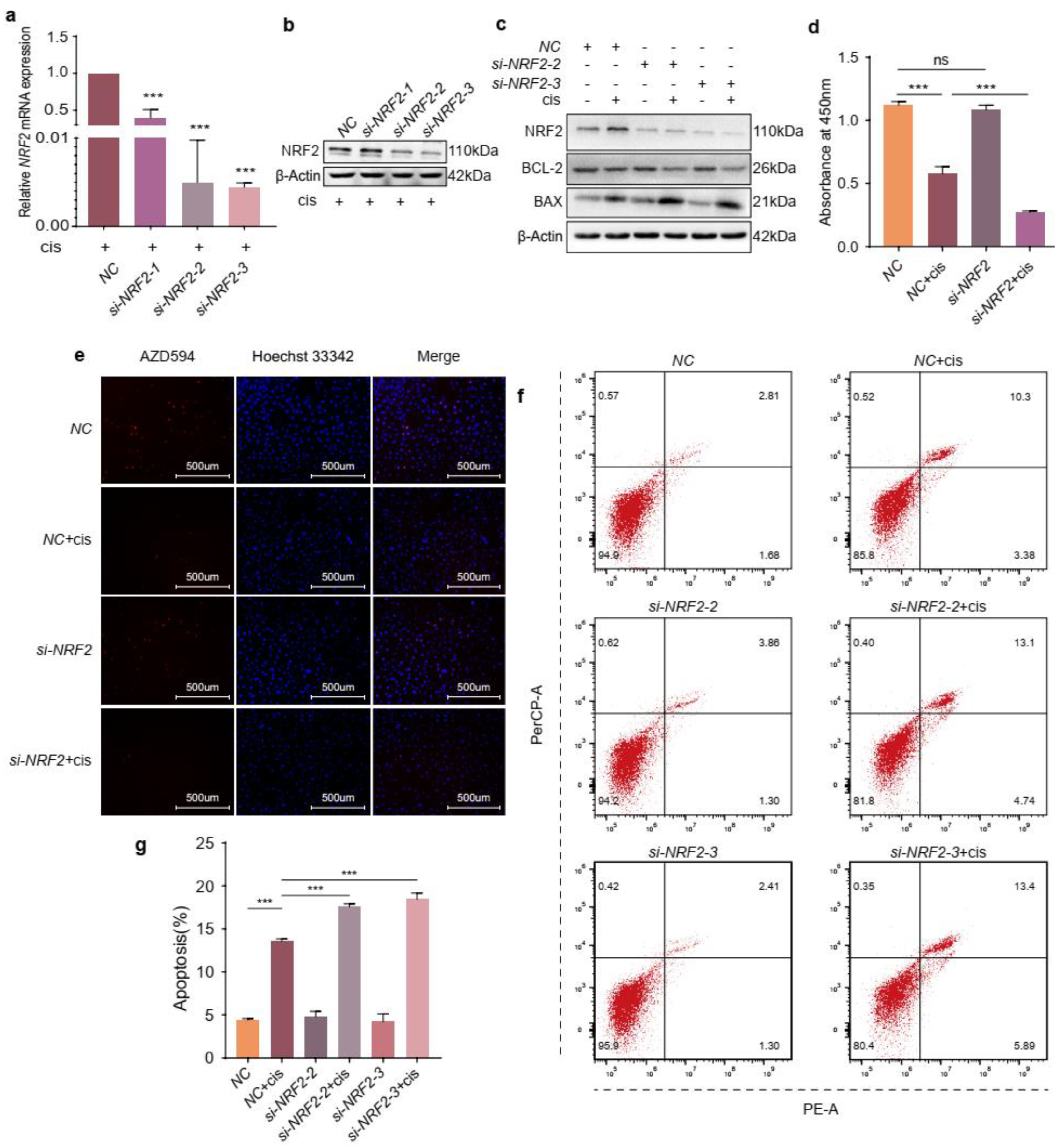
2.3. NRF2 Knockdown Exacerbates the Effects of Cisplatin on Granulosa Cells
2.4. The Increased Expression of NRF2 Is Compensatory in Cisplatin-Induced Granulosa Cells
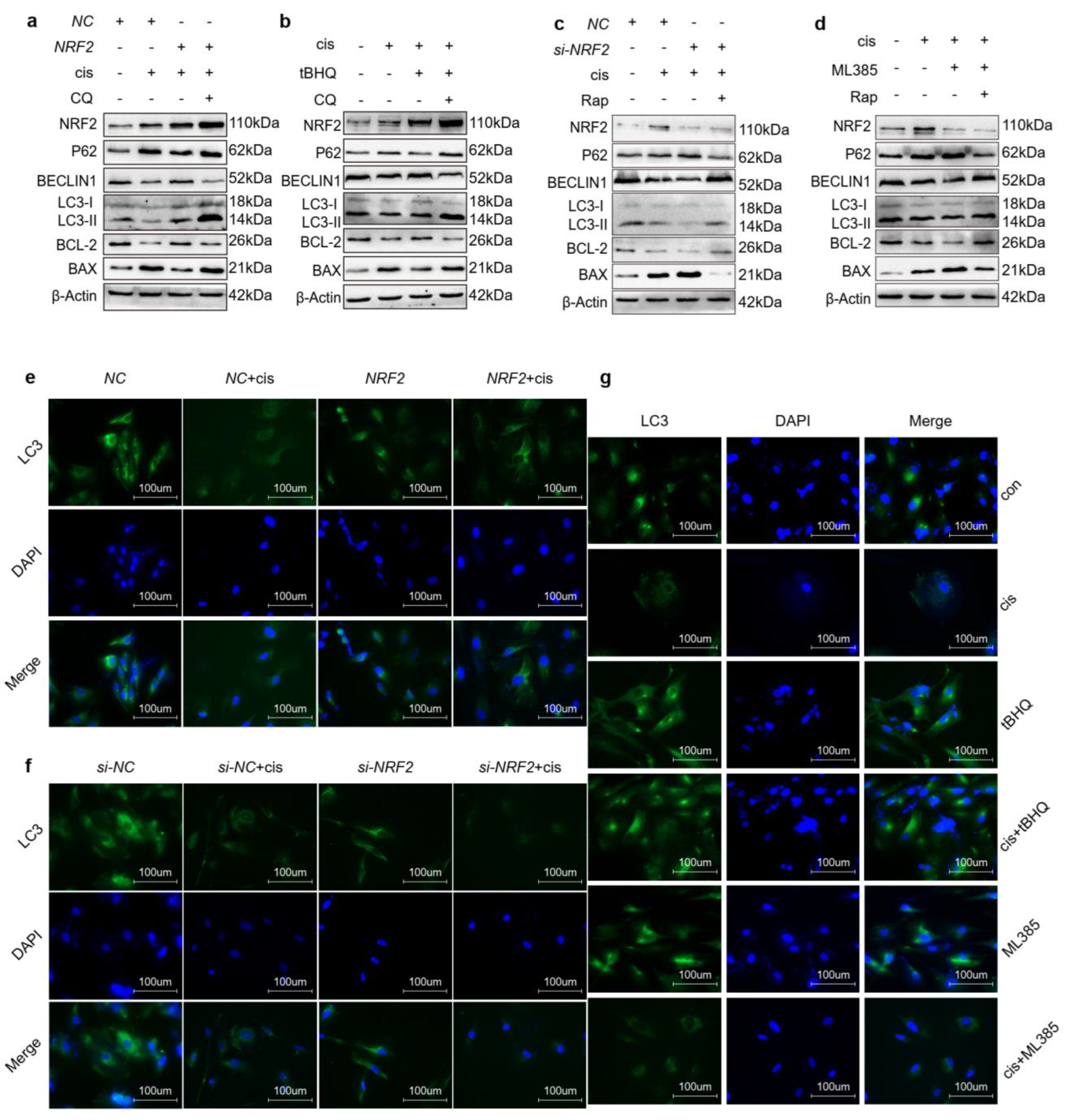
2.5. Upregulation of NRF2 Activates Autophagy and Downregulation of NRF2 Inhibits Autophagy
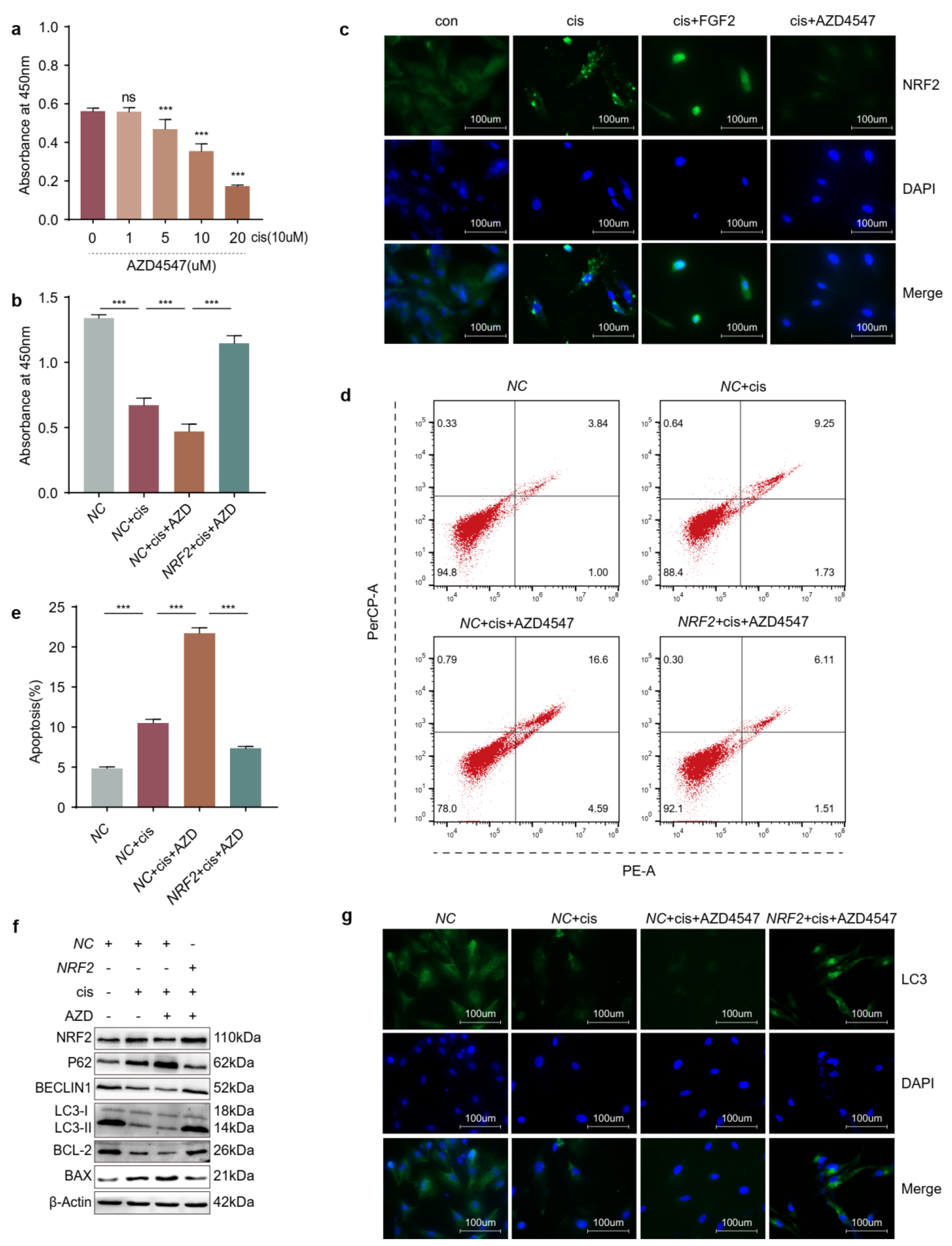
2.6. Overexpression of NRF2 Counteracts the Effects Caused by Blocking FGFR
2.7. Knockdown of NRF2 Counteracts the Effects Caused by Exogenous Addition of FGF2
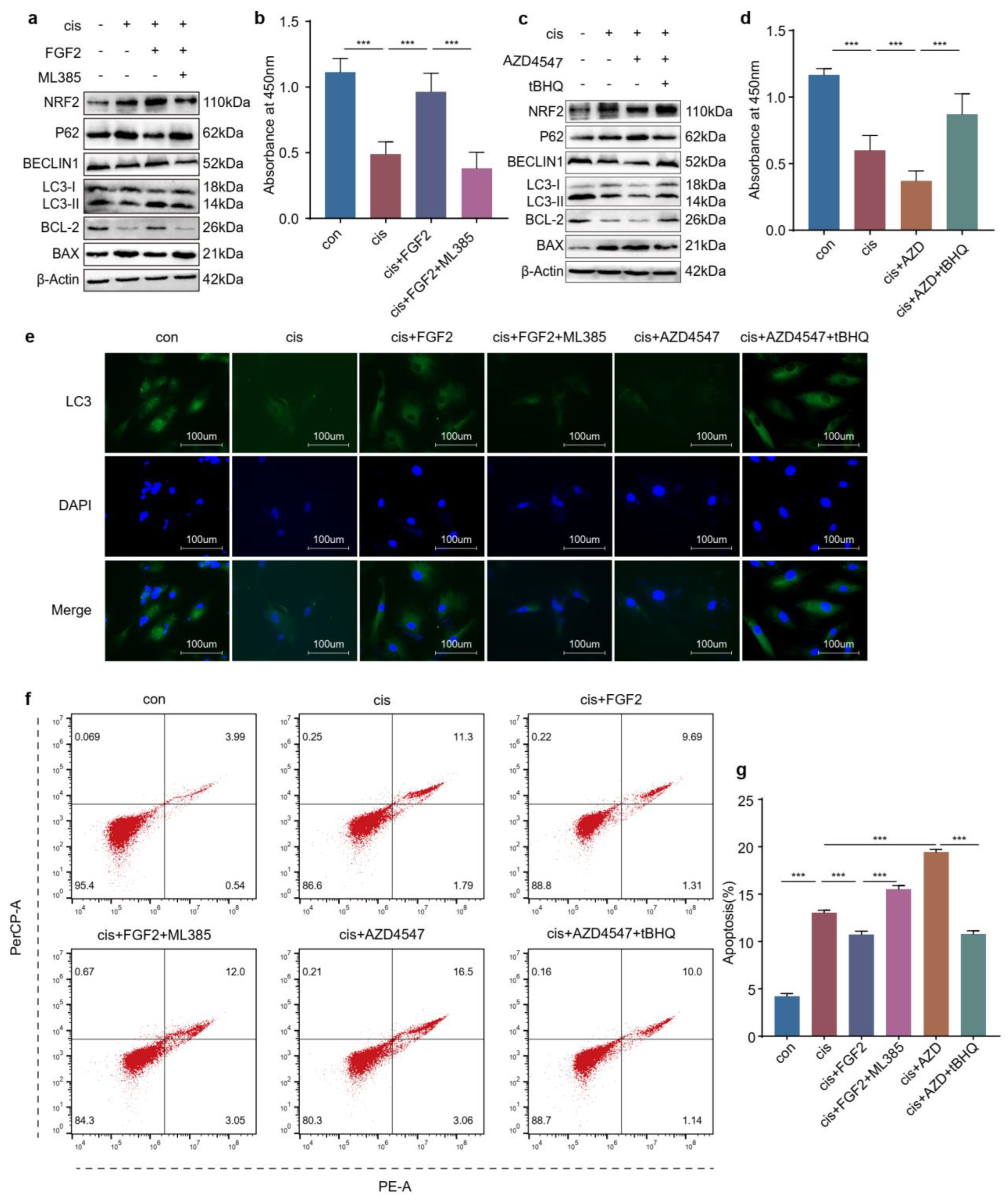
2.8. FGF2 Protects Cisplatin-Induced Granulosa Cells through the NRF2-Autophagy Pathway
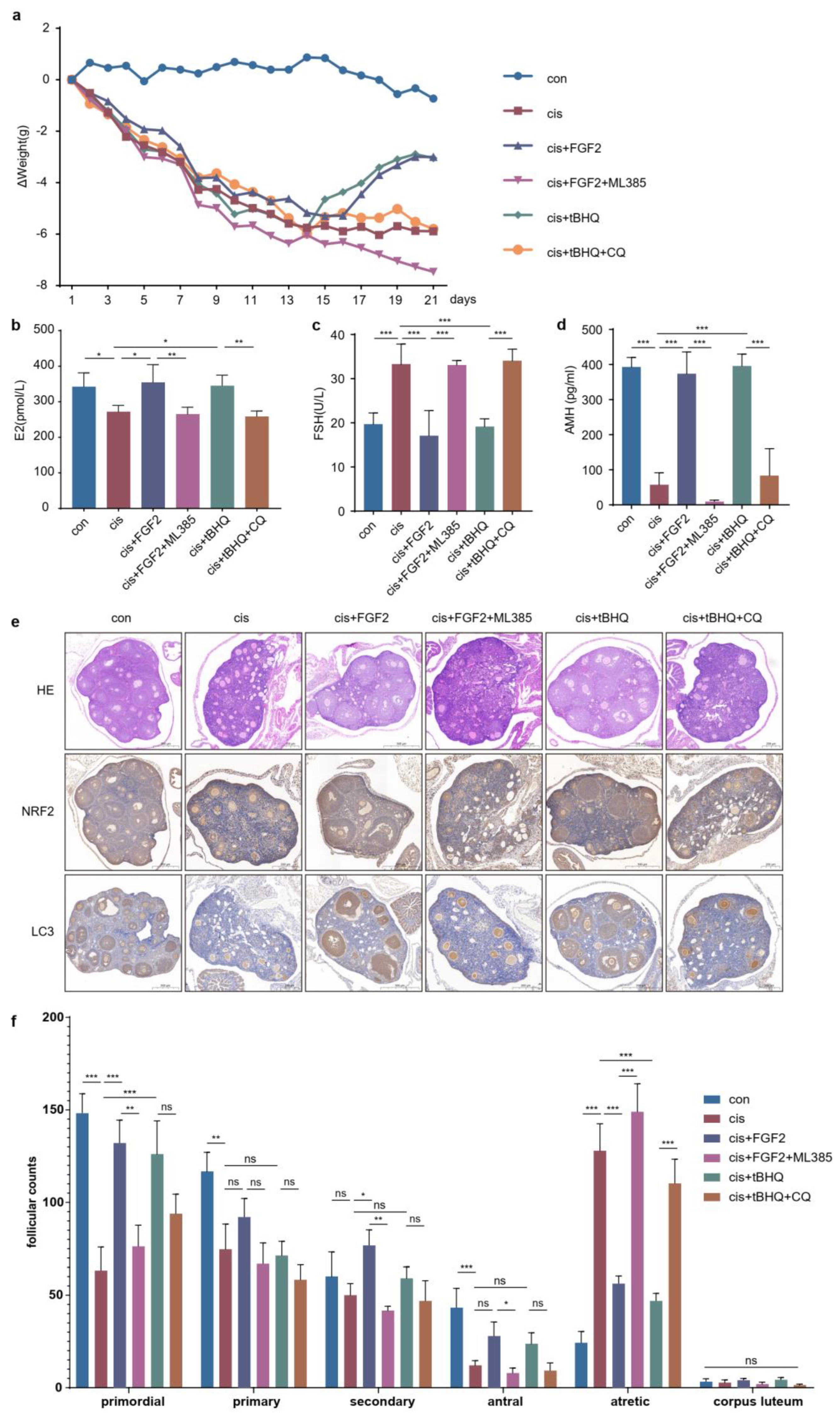
2.9. FGF2 Protects Cisplatin-Induced Mice Models through the NRF2-Autophagy Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Plasmids and Small Interfering RNA Transfection
4.3. Reagents
4.4. Cell Counting Kit-8 Assay (CCK-8)
4.5. Western Blotting Analysis
4.6. Total RNA Extraction and Quantitative Real-Time PCR (RT-qPCR)
4.7. EdU Labelling
4.8. Immunofluorescence
4.9. Flow Cytometry Analysis
4.10. POF Mice Model and Treatments
4.11. Enzyme Linked Immunosorbent Assay (ELISA)
4.12. Hematoxylin-Eosin Staining
4.13. Immunohistochemistry
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghahremani-Nasab, M.; Ghanbari, E.; Jahanbani, Y.; Mehdizadeh, A.; Yousefi, M. Premature ovarian failure and tissue engineering. J. Cell Physiol. 2020, 235, 4217–4226. [Google Scholar] [CrossRef]
- Chon, S.J.; Umair, Z.; Yoon, M.S. Premature ovarian insufficiency: Past, present, and future. Front. Cell Dev. Biol. 2021, 9, 672890. [Google Scholar] [CrossRef]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef]
- Fu, X.; He, Y.; Wang, X.; Peng, D.; Chen, X.; Li, X.; Wang, Q. Overexpression of mir-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting pdcd4 and pten to inhibit granulosa cell apoptosis. Stem Cell Res. Ther. 2017, 8, 187. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Spears, N.; Lopes, F.; Stefansdottir, A.; Rossi, V.; De Felici, M.; Anderson, R.A.; Klinger, F.G. Ovarian damage from chemotherapy and current approaches to its protection. Hum. Reprod. Update 2019, 25, 673–693. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Yang, T.; Li, J.; Yang, X. Study of the reparative effects of menstrual-derived stem cells on premature ovarian failure in mice. Stem Cell Res. Ther. 2017, 8, 11. [Google Scholar] [CrossRef]
- Mirzaei, S.; Mohammadi, A.T.; Gholami, M.H.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Hushmandi, K.; Makvandi, P.; Samec, M.; Liskova, A.; et al. Nrf2 signaling pathway in cisplatin chemotherapy: Potential involvement in organ protection and chemoresistance. Pharmacol. Res. 2021, 167, 105575. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. Nrf2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Verma, S.; Crawford, D.; Khateb, A.; Feng, Y.; Sergienko, E.; Pathria, G.; Ma, C.T.; Olson, S.H.; Scott, D.; Murad, R.; et al. Nrf2 mediates melanoma addiction to gcdh by modulating apoptotic signalling. Nat. Cell Biol. 2022, 24, 1422–1432. [Google Scholar] [CrossRef]
- Akino, N.; Wada-Hiraike, O.; Terao, H.; Honjoh, H.; Isono, W.; Fu, H.; Hirano, M.; Miyamoto, Y.; Tanikawa, M.; Harada, M.; et al. Activation of nrf2 might reduce oxidative stress in human granulosa cells. Mol. Cell. Endocrinol. 2018, 470, 96–104. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, X.; Li, D.; Ma, N.; Wei, Z.; Ci, X.; Zhang, S. Nrf2 signaling pathway mediates the protective effects of daphnetin against d-galactose induced-premature ovarian failure. Front. Pharmacol. 2022, 13, 810524. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Yin, H.; Hu, C.; Zeng, J.; Shi, X.; Chen, S.; Zhang, K.; Zheng, W.; Wu, W.; Liu, S. Tilapia skin peptides restore cyclophosphamide-induced premature ovarian failure via inhibiting oxidative stress and apoptosis in mice. Food Funct. 2022, 13, 1668–1679. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Wang, L.; Cui, Z.; Cheng, F.; Wang, W.; Yang, X. Fgf2 is protective towards cisplatin-induced kgn cell toxicity by promoting fto expression and autophagy. Front. Endocrinol. 2022, 13, 890623. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, L.; Liu, P.; Yu, T.; Lin, C.; Yan, C.; Hu, Y.; Zhou, W.; Sun, Y.; Panayi, A.C.; et al. All-in-one: Multifunctional hydrogel accelerates oxidative diabetic wound healing through timed-release of exosome and fibroblast growth factor. Small 2022, 18, e2104229. [Google Scholar] [CrossRef]
- Xie, C.; Zhou, X.; Liang, C.; Li, X.; Ge, M.; Chen, Y.; Yin, J.; Zhu, J.; Zhong, C. Apatinib triggers autophagic and apoptotic cell death via vegfr2/stat3/pd-l1 and ros/nrf2/p62 signaling in lung cancer. J. Exp. Clin. Cancer Res. 2021, 40, 266. [Google Scholar] [CrossRef]
- Jiang, T.; Harder, B.; Rojo de la Vega, M.; Wong, P.K.; Chapman, E.; Zhang, D.D. P62 links autophagy and nrf2 signaling. Free Radic. Biol. Med. 2015, 88 Pt B, 199–204. [Google Scholar] [CrossRef]
- Podfigurna-Stopa, A.; Czyzyk, A.; Grymowicz, M.; Smolarczyk, R.; Katulski, K.; Czajkowski, K.; Meczekalski, B. Premature ovarian insufficiency: The context of long-term effects. J. Endocrinol. Investig. 2016, 39, 983–990. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Chen, J.; Han, Y.; Shi, W.; Yan, X.; Shi, Y.; Yang, Y.; Gao, H.; Li, Y. Ovarian tissue bank for fertility preservation and anti-menopause hormone replacement. Front. Endocrinol. 2022, 13, 950297. [Google Scholar] [CrossRef]
- Almeida, C.P.; Ferreira, M.C.F.; Silveira, C.O.; Campos, J.R.; Borges, I.T.; Baeta, P.G.; Silva, F.H.S.; Reis, F.M.; Del Puerto, H.L. Clinical correlation of apoptosis in human granulosa cells-a review. Cell Biol. Int. 2018, 42, 1276–1281. [Google Scholar] [CrossRef]
- Tatone, C.; Amicarelli, F. The aging ovary—The poor granulosa cells. Fertil. Steril. 2013, 99, 12–17. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative stress in oocyte aging and female reproduction. J. Cell. Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef]
- Lim, J.; Ali, S.; Liao, L.S.; Nguyen, E.S.; Ortiz, L.; Reshel, S.; Luderer, U. Antioxidant supplementation partially rescues accelerated ovarian follicle loss, but not oocyte quality, of glutathione-deficient micedagger. Biol. Reprod. 2020, 102, 1065–1079. [Google Scholar] [CrossRef]
- Khadrawy, O.; Gebremedhn, S.; Salilew-Wondim, D.; Taqi, M.O.; Neuhoff, C.; Tholen, E.; Hoelker, M.; Schellander, K.; Tesfaye, D. Endogenous and exogenous modulation of nrf2 mediated oxidative stress response in bovine granulosa cells: Potential implication for ovarian function. Int. J. Mol. Sci. 2019, 20, 1635. [Google Scholar] [CrossRef]
- Yan, Z.; Dai, Y.; Fu, H.; Zheng, Y.; Bao, D.; Yin, Y.; Chen, Q.; Nie, X.; Hao, Q.; Hou, D.; et al. Curcumin exerts a protective effect against premature ovarian failure in mice. J. Mol. Endocrinol. 2018, 60, 261–271. [Google Scholar] [CrossRef]
- Ding, H.; Li, Z.; Li, X.; Yang, X.; Zhao, J.; Guo, J.; Lu, W.; Liu, H.; Wang, J. Fto alleviates cdcl(2)-induced apoptosis and oxidative stress via the akt/nrf2 pathway in bovine granulosa cells. Int. J. Mol. Sci. 2022, 23, 4948. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Li, L.; Bai, L.; Wang, Y.; Zhang, J.; Wang, H. Inhibition of nicotinamide adenine dinucleotide phosphate oxidase 4 attenuates cell apoptosis and oxidative stress in a rat model of polycystic ovary syndrome through the activation of nrf-2/ho-1 signaling pathway. Mol. Cell. Endocrinol. 2022, 550, 111645. [Google Scholar] [CrossRef]
- Ji, R.; Jia, F.-Y.; Chen, X.; Wang, Z.-H.; Jin, W.-Y.; Yang, J. Salidroside alleviates oxidative stress and apoptosis via ampk/nrf2 pathway in dht-induced human granulosa cell line kgn. Arch. Biochem. Biophys. 2022, 715, 109094. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Park, J.S.; Lee, Y.S.; Han, J.; Lee, D.K.; Kwon, S.W.; Han, D.H.; Lee, Y.H.; Bae, S.H. Sqstm1/p62 activates nfe2l2/nrf2 via ulk1-mediated autophagic keap1 degradation and protects mouse liver from lipotoxicity. Autophagy 2020, 16, 1949–1973. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Lim, J.; Wang, Q.; Purtell, K.; Wu, S.; Palomo, G.M.; Tan, H.; Manfredi, G.; Zhao, Y.; Peng, J.; et al. Als-ftld-linked mutations of sqstm1/p62 disrupt selective autophagy and nfe2l2/nrf2 anti-oxidative stress pathway. Autophagy 2020, 16, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Dolivo, D.M.; Larson, S.A.; Dominko, T. Fibroblast growth factor 2 as an antifibrotic: Antagonism of myofibroblast differentiation and suppression of pro-fibrotic gene expression. Cytokine Growth Factor Rev. 2017, 38, 49–58. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Li, J.; Chen, W. Ethyl carbamate triggers ferroptosis in liver through inhibiting gsh synthesis and suppressing nrf2 activation. Redox Biol. 2022, 53, 102349. [Google Scholar] [CrossRef] [PubMed]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef] [PubMed]
- Xian, P.; Hei, Y.; Wang, R.; Wang, T.; Yang, J.; Li, J.; Di, Z.; Liu, Z.; Baskys, A.; Liu, W.; et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics 2019, 9, 5956–5975. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Cheng, F.; Pan, R.; Cui, Z.; She, J.; Zhang, Y.; Yang, X. FGF2 Rescued Cisplatin-Injured Granulosa Cells through the NRF2-Autophagy Pathway. Int. J. Mol. Sci. 2023, 24, 14215. https://doi.org/10.3390/ijms241814215
Wang L, Cheng F, Pan R, Cui Z, She J, Zhang Y, Yang X. FGF2 Rescued Cisplatin-Injured Granulosa Cells through the NRF2-Autophagy Pathway. International Journal of Molecular Sciences. 2023; 24(18):14215. https://doi.org/10.3390/ijms241814215
Chicago/Turabian StyleWang, Lihui, Feiyan Cheng, Rumeng Pan, Zhiwei Cui, Jing She, Yidan Zhang, and Xinyuan Yang. 2023. "FGF2 Rescued Cisplatin-Injured Granulosa Cells through the NRF2-Autophagy Pathway" International Journal of Molecular Sciences 24, no. 18: 14215. https://doi.org/10.3390/ijms241814215
APA StyleWang, L., Cheng, F., Pan, R., Cui, Z., She, J., Zhang, Y., & Yang, X. (2023). FGF2 Rescued Cisplatin-Injured Granulosa Cells through the NRF2-Autophagy Pathway. International Journal of Molecular Sciences, 24(18), 14215. https://doi.org/10.3390/ijms241814215



