Amygdalin as a Promising Anticancer Agent: Molecular Mechanisms and Future Perspectives for the Development of New Nanoformulations for Its Delivery
Abstract
:1. Introduction
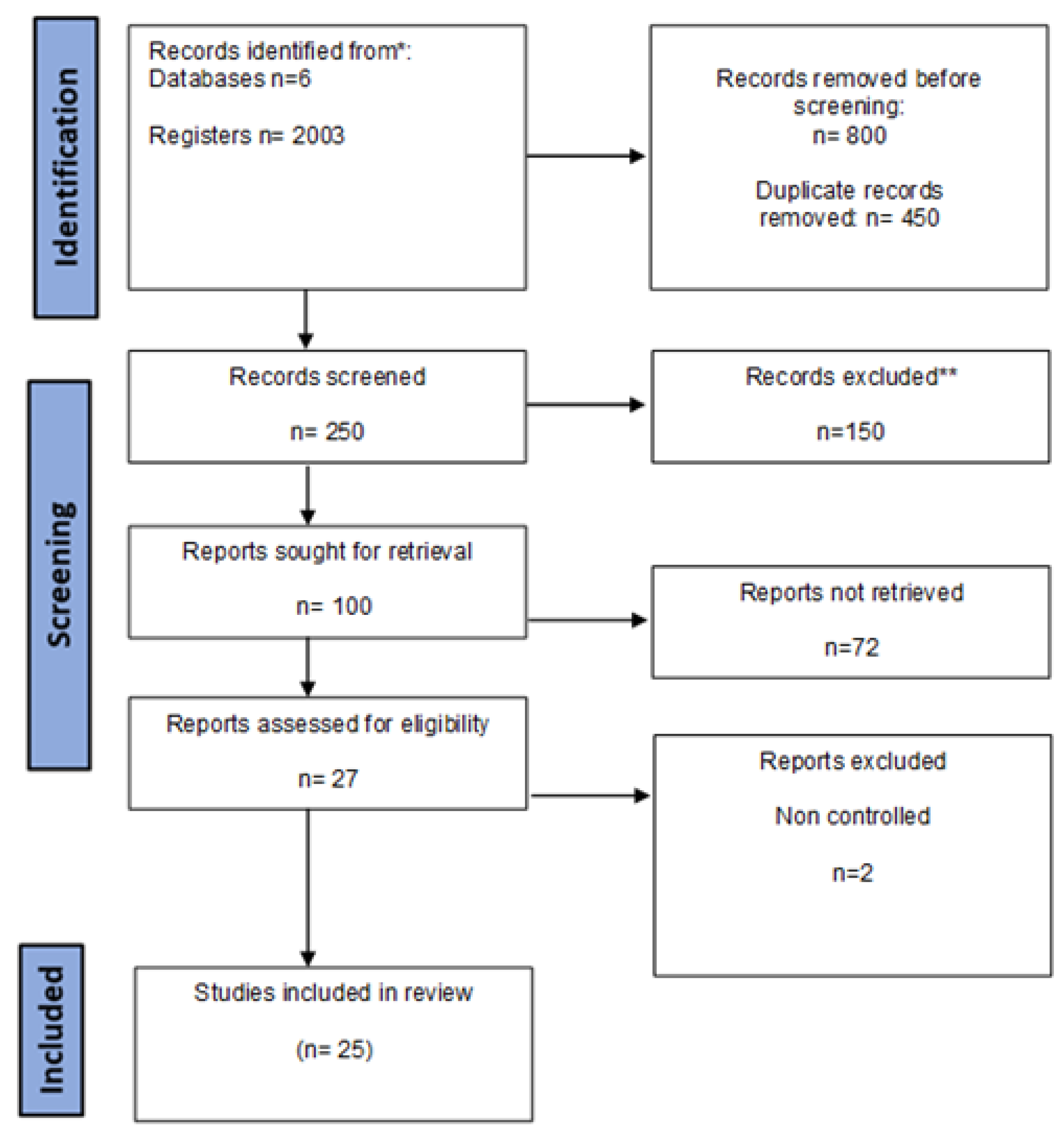
2. Methods
3. Results
3.1. Amygdalin: Basic Information and Properties
3.2. Anticancer Effects and Molecular Mechanisms of Amygdalin: In Vitro and In Vivo Evidence
3.2.1. Lung Cancer
3.2.2. Breast Cancer
3.2.3. Prostate Cancer
3.2.4. Colorectal Cancer
3.2.5. Cervical Cancer
3.2.6. Gastrointestinal Cancer
3.2.7. Other Tumor Malignancies
| Study Design | Dosage of Amygdalin | Action of Amygdalin | References |
|---|---|---|---|
| Animal study (in vitro and in vivo) N = 18 | 1.25, 2.5, 5, 10, and 20 mg/mL, intraperitoneal injection, cytotoxic, ↓ viability of HeLa cells in a dose-dependent manner. | ↑ Apoptosis by ↑ activity of caspase-3, ↑ Bax, and ↓ Bcl-2 protein in cervical cancer cells (HeLa). | [22] |
| Test-tube lab on human cells N = (5 × 105 cells/well) | 1, 2, 5, 10, and 20 mg/mL, cytotoxic to HL-60 cells (6.4 mg/mL) in the presence of 250 nM β-glucosidase. | ↑ Apoptosis in human promyelocytic leukemia cells (HL-60, ATCC CCL240). | [58] |
| Clinical trial N = 178 patients | 3 g (intravenous administration) and 0.5 g (oral use), symptoms of cyanide toxicity or by blood cyanide levels approaching the lethal range. | No substantive benefits in terms of cure, improvement or stabilization of cancer, improvement of symptoms related to cancer, or extension of life span. | [59] |
| Test-tube lab in vitro N = (1 × 105 cells/mL) | 10 mg/mL, no cytotoxic effects were detected in vitro. | ↓ Tumor cell growth, ↓ P19 and P27 expression in renal cancer cells (Caki-1, KTC-26, and A498). | [35] |
| Animal study (in vitro and in vivo) N = 20 | 40 mg/kg and 80 mg/kg amygdalin in saline, intragastrically, no significant toxic effects were observed. | ↑ Apoptosis and proliferation inhibition of lung cancer cells (A549, PC9, H1299, and PA). | [42] |
| Animal study (in vivo), N = 30 | 10 mg/kg (nano-extract of P. persica) and 15 mg/kg (nano-extract solution of P. persica seed extract). | No differences in the histological structure of the liver, central veins, and hepatic chords in rat liver tissues. | [60] |
| Test-tube lab on human cells (in vitro) N = (2 × 104 cells/cm2) | 4, 8, 16, 32, and 65 mmol/L of an amygdalin solution, different cytotoxic effects between the two cell lines. | ↓ Oxidative stress in human breast cancer cell lines (MCF-7 and T47D). | [46] |
| Test-tube lab (in vitro) N = (1 × 105 cells/mL) | 10, 20, and 40 mg/mL, incubation in serum-free medium. | ↑ Apoptosis, ↑ Bax proteins, and caspase-3 activity, ↓ Bcl-2 protein in breast cancer cells (Hs578T, MCF-7, and MDA-MB-231) Data demonstrated that amygdalin exerted cytotoxic effect. | [43,44,45,46] |
| Test-tube lab on human cells, N = (1 × 105 cells/well) | 0.25, 0.5, 2.5, and 5 mg/mL for 24 h, no cytotoxic effects were detected. | Apoptosis-related genes downregulated by amygdalin in human colon cancer cells (SNU-C4). | [51] |
| Test-tube lab on human cells, N = (2 × 104 cells/cm2) | 0.01 mg/mL, 0.1 mg/mL, 1 mg/L, and 10 mg/mL, amygdalin exerted a dose-dependent cytotoxic effect | ↓ Tumor cell growth, ↑ apoptosis, ↓ metastatic spread by α6 integrin, ↑ Bax, and ↓ Bcl-2 protein in prostate cancer cells (DU145 and LNCaP). | [47,48,49] |
| Clinical trial N = 6 patients | 4.5 g/sq m/day (intravenously for 21 days) and 0.5 g three times daily, no evidence of toxic reaction. | Slight ↑ of thiocyanate levels in plasma in 50% of patients with malignant disease. | [32] |
| N = 21 healthy volunteers: Chinese adults | Single doses (3, 6, and 9 g) and multiple doses (6 g, once daily) were administrated intravenously and the safety profiles were evaluated. | No adverse effects and a good tolerability were observed. | [61] |
| Test-tube lab, N = (5 × 104 cells/mL) | 5, 10, 15, or 20 μg/mL, no toxic effects were reported. | ↓ Bcl-2, ↑ caspase-3 expression in hepatocellular cancer cells (HepG2 and EAC). | [54,55,56] |
| Test-tube lab on human cells (in vitro) | 3, 6, and 9 μg/mL, slight ↓ of cell survival (80%), suggesting a weak genotoxic effect. | Anticancer effect in colorectal cancer cells (HepG2, HT-29, BALB/3T3, clone A31, and BJ). | [50] |
| Animal study (in vivo, five groups of nude mice) | 0.5 mL/daily, no toxic effects were reported. | No change of tumor volume in prostate cancer cell lines (PC3). | [10] |
| Test-tube lab (in vitro) N = (1 × 104 cells/mL) | 1.25–10 mg/mL, no signs of toxicity were shown. | ↓ Bladder cancer cell growth by ↓ cyclin A, CKD2, and P27 protein. | [57] |
| In vitro | Nanoformulation of amygdalin by β-CD | β-CD-Amygdalin exerted greater effects than amygdalin. The nanoformulation of amygdalin with β-CD ↑ breast cancer cells mitigation, gene mitigation, and oxidative stress. | [62] |
| In vitro | Silver nanoparticles encapsulated with amygdalin, in breast cancer cell lines, cross-linking to microcapsules charged with chitosan. | Controlled release of amygdalin led to overcoming of low cytotoxic effects in high doses, showing anticancer activity. | [63] |
| In vitro | Alginate–chitosan nanoparticles (were used as drug administration for amygdalin encapsulation and its delivery to tumor cells lines (H1299). | ACNPs demonstrated greater antitumor effect on H1299 cell lines than free amygdalin, suggesting greater cellular uptake of nanoparticle compound. | [64] |
| In vitro | Nanoparticles demonstrated sustained amygdalin–folic acid release properties and obvious selectivity to cells | Cell cycle blocking. Malignancy growth suppressing. ↓ Iron levels, and mitogen-activated protein kinases (MAPK/P38) by ↓ iron levels and MAPK/P38. Inhibition of differentiation complex (CD4 and CD80) expression. ↓ Transformation of growth factor beta interleukin-6, interferon-gamma, interleukin-2, and vascular endothelial growth factor (VEGF) expression in the signaling pathway. Modulation of gene expression of CD8 and the natural killer group 2D. ↓ Proliferation of breast cancer cells. | [11] |
3.3. Toxicity of Amygdalin
3.4. Amygdalin/Laetrile Clinical Studies in Human Tumor Malignancies in the 20th Century
3.5. Nanoparticles and Amygdalin in the 21st Century
3.6. Nutritional Supplements with Amygdalin for Cancer Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Breast Cancer Initiative Implementation Framework: Assessing, Strengthening and Scaling up of Services for the Early Detection and Management of Breast Cancer: Executive Summary; World Health Organization: Geneva, Switzerland, 2023. Available online: https://apps.who.int/iris/handle/10665/365784 (accessed on 12 June 2023).
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Sedeta, E.; Sung, H.; Laversanne, M.; Bray, F.; Jemal, A. Recent Mortality Patterns and Time Trends for the Major Cancers in 47 Countries Worldwide. Cancer Epidemiol. Biomark. Prev. 2023, 32, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, M.; Santucci, C.; Boffetta, P.; Collatuzzo, G.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2023 with focus on lung cancer. Ann. Oncol. 2023, 34, 410–419. [Google Scholar] [CrossRef]
- Dalmartello, M.; La Vecchia, C.; Bertuccio, P.; Boffetta, P.; Levi, F.; Negri, E.; Malvezzi, M. European cancer mortality predictions for the year 2022 with focus on ovarian cancer. Ann. Oncol. 2022, 33, 330–339. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Alwan, A.M.; Rokaya, D.; Kathayat, G.; Afshari, J.T. Onco-immunity and therapeutic application of amygdalin: A review. J. Oral Biol. Craniofac. Res. 2023, 13, 155–163. [Google Scholar] [CrossRef]
- Alwan, A.M.; Afshari, J.T. In Vivo Growth Inhibition of Human Caucasian Prostate Adenocarcinoma in Nude Mice Induced by Amygdalin with Metabolic Enzyme Combinations. Biomed. Res. Int. 2022, 2022, 4767621. [Google Scholar] [CrossRef]
- Askar, M.A.; El-Sayyad, G.S.; Guida, M.S.; Khalifa, E.; Shabana, E.S.; Abdelrahman, I.Y. Amygdalin-folic acid-nanoparticles inhibit the proliferation of breast cancer and enhance the effect of radiotherapy through the modulation of tumor-promoting factors/immunosuppressive modulators in vitro. BMC Complement Med. Ther. 2023, 23, 162. [Google Scholar] [CrossRef]
- Saleem, M.; Asif, J.; Asif, M.; Saleem, U. Amygdalin from Apricot Kernels Induces Apoptosis and Causes Cell Cycle Arrest in Cancer Cells: An Updated Review. Anticancer Agents Med. Chem. 2018, 18, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Liczbiński, P.; Bukowska, B. Molecular mechanism of amygdalin action in vitro: Review of the latest research. Immunopharmacol. Immunotoxicol. 2018, 40, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Lehmane, H.; Kohonou, A.N.; Tchogou, A.P.; Ba, R.; Dah-Nouvlessounon, D.; Didagbé, O.; Sina, H.; Senou, M.; Adjanohoun, A.; Baba-Moussa, L. Antioxidant, Anti-Inflammatory, and Anti-Cancer Properties of Amygdalin Extracted from Three Cassava Varieties Cultivated in Benin. Molecules 2023, 28, 4548. [Google Scholar] [CrossRef] [PubMed]
- Barakat, H.; Aljutaily, T.; Almujaydil, M.S.; Algheshairy, R.M.; Alhomaid, R.M.; Abdulkarim, S.; Almutairi, A.S.; Alshimali, S.I.; Abdellatif, A.A.H. Amygdalin: A Review on Its Characteristics, Antioxidant Potential, Gastrointestinal Microbiota Intervention, Anticancer Therapeutic and Mechanisms, Toxicity, and Encapsulation. Biomolecules 2022, 12, 1514. [Google Scholar] [CrossRef]
- Elderdery, A.Y.; Alzahrani, B.; Hamza, S.M.A.; Mostafa-Hedeab, G.; Mok, P.L.; Subbiah, S.K. Synthesis, Characterization, and Antiproliferative Effect of CuO-TiO2-Chitosan-Amygdalin Nanocomposites in Human Leukemic MOLT4 Cells. Bioinorg. Chem. Appl. 2022, 2022, 1473922. [Google Scholar] [CrossRef]
- Erikel, E.; Yuzbasioglu, D.; Unal, F. A study on Amygdalin’s genotoxicological safety and modulatory activity in human peripheral lymphocytes in vitro. Environ. Mol. Mutagen. 2023, 64, 291–308. [Google Scholar] [CrossRef]
- El-Desouky, M.A.; Fahmi, A.A.; Nasraldin, K.M. The Postulated Mechanism of Action of Amygdalin (Vitamin B17) on Cancer Cells. Anticancer Agents Med. Chem. 2023, 23, 894–899. [Google Scholar] [CrossRef]
- Shi, J.; Qianqian Chen, Q.; Xu, M.; Xia, Q.; Tiansheng Zheng, T.; Teng, J.; Li, M.; Fan, L. Recent updates and future perspectives about amygdalin as a potential anticancer agent: A review. Cancer Med. 2019, 8, 3004–3011. [Google Scholar] [CrossRef]
- Lee, H.M.; Moon, A. Amygdalin Regulates Apoptosis and Adhesion in Hs578T Triple-Negative Breast Cancer Cells. Biomol. Ther. 2016, 24, 62–66. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Zhang, M.; Wei, S.; Li, R.; Gao, Y.; Peng, W.; Chunjie Wu, C. Apoptosis Induction of Fibroblast-Like Synoviocytes Is an Important Molecular-Mechanism for Herbal Medicine along with its Active Components in Treating Rheumatoid Arthritis. Biomolecules 2019, 9, 795. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, J.; Wang, F.; Hu, J.; Cui, A.; Wei, C.; Yang, Q.; Li, F. Amygdalin induces apoptosis in human cervical cancer cell line HeLa cells. Immunopharmacol. Immunotoxicol. 2013, 35, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Aamazadeh, F.; Ostadrahimi, A.; Saadat, T.R.; Barar, J. Bitter apricot ethanolic extract induces apoptosis through increasing expression of Bax/Bcl-2 ratio and caspase-3 in PANC-1 pancreatic cancer cells. Mol. Biol. Rep. 2020, 47, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Aydın, D.; Özkan, K.; Aydın, A. The Combination of Amygdalin with Some Anticancer, Antiparasitic, and Antigout Drugs Against MG63, Saos2, SW1353, and FL Cells In Vitro. J. Med. Food 2021, 24, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-K.; Shin, M.-S.; Yang, H.-Y.; Lee, J.W.; Kim, Y.-S.; Lee, M.-H.; Kim, J.; Kim, K.H.; Kim, C.-J. Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol. Pharm. Bull. 2006, 29, 1597–1602. [Google Scholar] [CrossRef]
- Chen, Y.; Al-Ghamdi, A.A.; Elshikh, M.S.; Shah, M.H.; Al-Dosary, M.A.; Abbasi, A.M. Phytochemical profiling, antioxidant and HepG2 cancer cells’ antiproliferation potential in the kernels of apricot cultivars. Saudi J. Biol. Sci. 2020, 27, 163–172. [Google Scholar] [CrossRef]
- Curran, W.J. Law-medicine notes. Laetrile for the terminally ill: Supreme Court stops the nonsense. N. Engl. J. Med. 1980, 302, 619–621. [Google Scholar] [CrossRef]
- Milazzo, S.; Horneber, M.; Ernst, E. Laetrile treatment for cancer. Cochrane Database Syst. Rev. 2015, 2015, CD005476. [Google Scholar] [CrossRef]
- Jaszczak-Wilke, E.; Polkowska, Z.; Koprowski, M.; Owsianik, K.; Mitchell, A.E.; Bałczewski, P. Amygdalin: Toxicity, Anticancer Activity and Analytical Procedures for Its Determination in Plant Seeds. Molecules 2021, 26, 2253. [Google Scholar] [CrossRef]
- Song, Z.; Xu, X. Advanced research on anti-tumor effects of amygdalin. J. Cancer Res. Ther. 2014, 10 (Suppl. S1), 3–7. [Google Scholar] [CrossRef]
- Rauws, A.G.; Olling, M.; Timmerman, A. The pharmacokinetics of amygdalin. Arch. Toxicol. 1982, 49, 311–319. [Google Scholar] [CrossRef]
- Moertel, C.G.; Ames, M.M.; Kovach, J.S.; Moyer, T.P.; Rubin, J.R.; Tinker, J.H. A pharmacologic and toxicological study of amygdalin. JAMA 1981, 245, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.P.; Trissel, L.A.; Kleinman, L.M. Pharmaceutical assessment of amygdalin (Laetrile) products. Cancer Treat Rep. 1978, 62, 99–104. [Google Scholar] [PubMed]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Amygdalin content of seeds, kernels and food products commercially available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Juengel, E.; Thomas, A.; Rutz, J.; Makarevic, J.; Tsaur, I.; Nelson, K.; Haferkamp, A.; Blaheta, R.A. Amygdalin inhibits the growth of renal cell carcinoma cells in vitro. Int. J. Mol. Med. 2016, 37, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Salama, R.H.; El Rahman Ramadan, A.G.; Alsanory, T.A.; Herdan, M.O.; Fathallah, O.M.; Alsanory, A.A. Experimental and Therapeutic Trials of Amygdalin. Int. J. Biochem. Pharmacol. 2019, 1, 21–26. [Google Scholar] [CrossRef]
- Figurová, D.; Tokárová, K.; Greifová, H.; Knížatová, N.; Kolesárová, A.; Lukáč, N. Inflammation, It’s Regulation and Antiphlogistic Effect of the Cyanogenic Glycoside Amygdalin. Molecules 2021, 26, 5972. [Google Scholar] [CrossRef] [PubMed]
- He, X.-Y.; Wu, L.-J.; Wang, W.-X.; Xie, P.-J.; Chen, Y.-H.; Wang, F. Amygdalin—A pharmacological and toxicological review. J. Ethnopharmacol. 2020, 254, 112717. [Google Scholar] [CrossRef]
- Li, Y.-L.; Li, Q.-X.; Liu, R.-J.; Shen, X.-Q. Chinese Medicine Amygdalin and β-Glucosidase Combined with Antibody Enzymatic Prodrug System as A Feasible Antitumor Therapy. Chin. J. Integr. Med. 2018, 24, 237–240. [Google Scholar] [CrossRef]
- Luo, D.; Wang, Q.; Meng, Y.; Cao, L.; Feng, N.; Du, W.; Li, H.; Dong, X.; Xiumin Ma, X.; Luo, L. Effects of amygdalin on type II collagen-induced arthritis in rats. Biologia 2020, 75, 423–430. [Google Scholar] [CrossRef]
- Lin, S.; Wen, J.; Xu, X.; Shi, J.; Zhang, W.; Zheng, T.; Hou, Y.; Zhang, Y.; Li, Z.; Wang, K.; et al. Amygdalin Induced Mitochondria-Mediated Apoptosis of Lung Cancer Cells via Regulating NFκB-1/NFκB Signaling Cascade in Vitro and in Vivo. Am. J. Chin. Med. 2022, 50, 1361–1386. [Google Scholar] [CrossRef]
- Qian, L.; Xie, B.; Wang, Y.; Qian, J. Amygdalin-mediated inhibition of non-small cell lung cancer cell invasion in vitro. Int. J. Clin. Exp. Pathol. 2015, 8, 5363–5370. [Google Scholar] [PubMed]
- Mosayyebi, B.; Mohammadi, L.; Kalantary-Charvadeh, A.; Rahmati, M. Amygdalin Decreases Adhesion and Migration of MDA-MB-231 and MCF-7 Breast Cancer Cell Lines. Curr. Mol. Pharmacol. 2021, 14, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Cecarini, V.; Salima Selmi, S.; Cuccioloni, M.; Gong, C.; Bonfili, L.; Zheng, Y.; Cortese, M.; Angeletti, M.; Kilani, S.; Eleuteri, A.M. Targeting Proteolysis with Cyanogenic Glycoside Amygdalin Induces Apoptosis in Breast Cancer Cells. Molecules 2022, 27, 7591. [Google Scholar] [CrossRef]
- Moradipoodeh, B.; Jamalan, M.; Zeinali, M.; Fereidoonnezhad, M.; Mohammadzadeh, G. Specific targeting of HER2-positive human breast carcinoma SK-BR-3 cells by amygdaline-Z(HER2) affibody conjugate. Mol. Biol. Rep. 2020, 47, 7139–7151. [Google Scholar] [CrossRef]
- Abboud, M.M.; Al Awaida, W.; Alkhateeb, H.H.; Abu-Ayyad, A.N. Antitumor Action of Amygdalin on Human Breast Cancer Cells by Selective Sensitization to Oxidative Stress. Nutr. Cancer 2019, 71, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Tsaur, I.; Thomas, A.; Monecke, M.M.; Zugelder, M.; Rutz, J.; Grein, T.; Maxeiner, S.; Xie, H.; Chun, F.K.-H.; Florian Rothweiler, F.; et al. Amygdalin Exerts Antitumor Activity in Taxane-Resistant Prostate Cancer Cells. Cancers 2022, 14, 3111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hou, J.; Rao, J.; Zhou, C.; Liu, Y.; Gao, W. Magnetically Directed Enzyme/Prodrug Prostate Cancer Therapy Based on β-Glucosidase/Amygdalin. Int. J. Nanomed. 2020, 15, 4639–4657. [Google Scholar] [CrossRef] [PubMed]
- Mani, J.; Neuschäfer, J.; Resch, C.; Rutz, J.; Maxeiner, S.; Roos, F.; Chun, F.K.; Juengel, E.; Blaheta, R.A. Amygdalin Modulates Prostate Cancer Cell Adhesion and Migration In Vitro. Nutr. Cancer 2020, 72, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, M.; Iliev, I.; Bardarov, K.; Georgieva, D.; Todorova, T. Phytochemical characterization and biological activity of apricot kernels’ extract in yeast-cell based tests and hepatocellular and colorectal carcinoma cell lines. J. Ethnopharmacol. 2021, 279, 114333. [Google Scholar] [CrossRef]
- Park, H.J.; Yoon, S.H.; Han, L.S.; Zheng, L.T.; Jung, K.H.; Uhm, Y.K.; Lee, J.H.; Jeong, J.S.; Joo, W.S.; Yim, S.V.; et al. Amygdalin inhibits genes related to cell cycle in SNU-C4 human colon cancer cells. World J. Gastroenterol. 2005, 11, 5156–5161. [Google Scholar]
- Cassiem, W.; de Kock, M. The anti-proliferative effect of apricot and peach kernel extracts on human colon cancer cells in vitro. BMC Complement Altern Med. 2019, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Asselin, E.; Mills, G.B.; Tsang, B.K. XIAP regulates Akt activity and caspase-3-dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Res. 2001, 61, 1862–1868. [Google Scholar]
- Hosny, S.; Sahyon, H.; Youssef, M.; Negm, A.; Prunus Armeniaca, L. Seed Extract and Its Amygdalin Containing Fraction Induced Mitochondrial-Mediated Apoptosis and Autophagy in Liver Carcinogenesis. Anticancer Agents Med. Chem. 2021, 21, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Mamdouh, A.M.; Khodeer, D.M.; Tantawy, M.A.; Moustafa, Y.M. In-vitro and in-vivo investigation of amygdalin, metformin, and combination of both against doxorubicin on hepatocellular carcinoma. Life Sci. 2021, 285, 119961. [Google Scholar] [CrossRef] [PubMed]
- El-Desouky, M.A.; Fahmi, A.A.; Abdelkader, I.Y.; Nasraldin, K.M. Anticancer Effect of Amygdalin (Vitamin B-17) on Hepatocellular Carcinoma Cell Line (HepG2) in the Presence and Absence of Zinc. Anticancer. Agents Med. Chem. 2020, 20, 486–494. [Google Scholar] [CrossRef]
- Makarević, J.; Rutz, J.; Juengel, E.; Kaulfuss, S.; Reiter, M.; Tsaur, I.; Bartsch, G.; Haferkamp, A.; Blaheta, R.A. Amygdalin blocks bladder cancer cell growth in vitro by diminishing cyclin A and cdk2. PLoS ONE 2014, 9, e105590. [Google Scholar] [CrossRef]
- Koeffler, H.P.; Lowe, L.; Golde, D.W. Amygdalin (Laetrile): Effect on clonogenic cells from human myeloid leukemia cell lines and normal human marrow. Cancer Treat. Rep. 1980, 64, 105–109. [Google Scholar]
- Moertel, C.G.; Fleming, T.R.; Rubin, J.; Kvols, L.K.; Sarna, G.; Koch, R.; Currie, V.E.; Young, C.W.; Jones, S.E.; Davignon, J.P. A clinical trial of amygdalin (Laetrile) in the treatment of human cancer. N. Engl. J. Med. 1982, 306, 201–206. [Google Scholar] [CrossRef]
- Falah, Z.; Alaridhi, J. ‘Effects of Zinc Oxide Nanoparticles of the Alcoholic Extract of Prunus Persica and Prunus Armeniaca Seeds in the Histological Structure of Liver of Albino Rats. NeuroQuantology 2022, 20, 1831–1835. [Google Scholar]
- Li, X.; Shi, F.; Zhang, R.; Sun, C.; Gong, C.; Jian, L.; Ding, L. Pharmacokinetics, Safety, and Tolerability of Amygdalin and Paeoniflorin After Single and Multiple Intravenous Infusions of Huoxue-Tongluo Lyophilized Powder for Injection in Healthy Chinese Volunteers. Clin. Ther. 2016, 38, 327–337. [Google Scholar] [CrossRef]
- Mosayyebi, B.; Mahsa Imani, M.; Rahmati, M.; Akbarzadeh, A.; Zarghami, N.; Alizadeh, E.; Rahmati, M. Comparison Between β-Cyclodextrin-Amygdalin Nanoparticle and Amygdalin Effects on Migration and Apoptosis of MCF-7 Breast Cancer Cell Line. J. Clust. Sci. 2022, 33, 935–947. [Google Scholar] [CrossRef]
- Pandey, A.; Ali, S.A.; Negi, Y. Synthesis of polygonal chitosan microcapsules for the delivery of amygdalin loaded silver nanoparticles in breast cancer therapy. Mater. Today Proc. 2021, 43, 3744–3748. [Google Scholar] [CrossRef]
- Sohail, R.; Abbas, S.R. Evaluation of amygdalin-loaded alginate-chitosan nanoparticles as biocompatible drug delivery carriers for anticancerous efficacy. Int. J. Biol. Macromol. 2020, 153, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, V.; Palanivelu, J.; Ramalingam, C. Effects of the Gut microbiota on Amygdalin and its use as an anti-cancer therapy: Substantial review on the key components involved in altering dose efficacy and toxicity. Biochem. Biophys. Rep. 2018, 14, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Newton, G.W.; Schmidt, E.S.; Lewis, J.P.; Conn, E.; Lawrence, R. Amygdalin toxicity studies in rats predict chronic cyanide poisoning in humans. West. J. Med. 1981, 134, 97–103. [Google Scholar]
- Meredith, T.J. Epidemiology of poisoning. Pharmacol. Ther. 1993, 59, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Arshi, A.; Hosseini, S.M.; Hosseini, F.S.K.; Amiri, Z.Y.; Hosseini, F.S.; Lavasani, M.L.; Kerdarian, H.; Dehkordi, M.S. The anti-cancer effect of amygdalin on human cancer cell lines. Mol. Biol. Rep. 2019, 46, 2059–2066. [Google Scholar] [CrossRef]
- National Cancer Institute begins laetril clinical trial. JAMA 1980, 244, 538. [CrossRef]
- Ames, M.M.; Kovach, J.S.; Flora, K.P. Initial pharmacologic studies of amygdalin (laetrile) in man. Res. Commun. Chem. Pathol. Pharmacol. 1978, 22, 175–185. [Google Scholar]
- Ronis, M.J.J.; Pedersen, K.B.; Watt, J. Adverse Effects of Nutraceuticals and Dietary Supplements. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 583–601. [Google Scholar] [CrossRef]
- El-Ela, F.I.A.; Gamal, A.; Elbanna, H.A.; ElBanna, A.H.; Salem, H.F.; Tulbah, A.S. In Vitro and In Vivo Evaluation of the Effectiveness and Safety of Amygdalin as a Cancer Therapy. Pharmaceuticals 2022, 15, 1306. [Google Scholar] [CrossRef]
- Dang, T.; Nguyen, C.; Tran, P.N. Physician Beware: Severe Cyanide Toxicity from Amygdalin Tablets Ingestion. Case Rep. Emerg. Med. 2017, 2017, 4289527. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.F.; Breitbach, Z.S.; Armstrong, D.W.; Strattan, R.; Berthod, A. Problems and Pitfalls in the Analysis of Amygdalin and Its Epimer. J. Agric. Food Chem. 2015, 63, 8966–8973. [Google Scholar] [CrossRef] [PubMed]
- Cmorej, P.; Bruthans, P.; Halamka, J.; Voriskova, I.; Peran, D. Life-Threatening Cyanide Intoxication after Ingestion of Amygdalin in Prehospital Care. Prehosp. Emerg. Care 2022, 26, 455–458. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, B.; Quigg, C.; Leong, T. Severe cyanide toxicity from “vitamin supplements. Eur. J. Emerg. Med. 2005, 12, 257–258. [Google Scholar] [CrossRef]
- Sauer, H.; Wollny, C.; Oster, I.; Tutdibi, E.; Gortnerm, L.; Gottschling, S.; Meyer, S. Severe cyanide poisoning from an alternative medicine treatment with amygdalin and apricot kernels in a 4-year-old child. Wien Med. Wochenschr. 2015, 165, 185–188. [Google Scholar] [CrossRef]
- Shively, R.M.; Harding, S.A.; Hoffman, R.S.; Hill, A.D.; Astua, A.J.; Manini, A.F. Rebound metabolic acidosis following intentional amygdalin supplement overdose. Clin. Toxicol. 2020, 58, 290–293. [Google Scholar] [CrossRef]
- Charen, E.; Harbord, N. Toxicity of Herbs, Vitamins, and Supplements. Adv. Chronic. Kidney Dis. 2020, 27, 67–71. [Google Scholar] [CrossRef]
- Boháčová, I.; Procházková, S.; Halko, R. Separation and determination of amygdalin and unnatural neoamygdalin in natural food supplements by HPLC-DAD. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 1445–1452. [Google Scholar] [CrossRef]
- Kapoor, S. Safety of studies analysing clinical benefit of amygdalin. Immunopharmacol. Immunotoxicol. 2014, 36, 87. [Google Scholar] [CrossRef]
- Qadir, M.; Fatima, K. Review on Pharmacological Activity of Amygdalin. Arch. Cancer Res. 2017, 5, 160. [Google Scholar] [CrossRef]
- Kwon, H.-Y.; Hong, S.-P.; Hahn, D.-H.; Kim, J.H. Apoptosis induction of Persicae Semen extract in human promyelocytic leukemia (HL-60) cells. Arch. Pharm. Res. 2003, 26, 157–161. [Google Scholar] [CrossRef]
- Newmark, J.; Brady, R.O.; Grimley, P.M.; Gal, A.E.; Waller, S.G.; Thistlethwaite, J.R. Amygdalin (Laetrile) and prunasin beta-glucosidases: Distribution in germ-free rat and in human tumor tissue. Proc. Natl. Acad. Sci. USA 1981, 78, 6513–6516. [Google Scholar] [CrossRef]
- Makarević, J.; Tsaur, I.; Juengel, E.; Borgmann, H.; Nelson, K.; Thomas, C.; Bartsch, G.; Haferkamp, A.; Blaheta, R.A. Amygdalin delays cell cycle progression and blocks growth of prostate cancer cells in vitro. Life Sci. 2016, 147, 137–142. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, D.; Sun, K.; Peng, J.; Zhu, W.; Yin, S.; Tang, D.; Wu, Y. Amygdalin promotes the activity of T cells to suppress the progression of HBV-related hepatocellular carcinoma via the JAK2/STAT3 signaling pathway. BMC Infect. Dis. 2021, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Laetril/Amygdalin (PDQ®)–Health Professional Version. Available online: https://www.cancer.gov/about-cancer/treatment/cam/hp/laetrile-pdq (accessed on 9 September 2023).
- Turzo, S.B.A.; Hantz, E.R.; Lindert, S. Applications of machine learning in computer-aided drug discovery. QRB Discov. 2022, 3, e14. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, R.; Irfan, M.; Gondal, T.M.; Khan, S.; Wu, J.; Hadi, M.U.; Heymach, J.; Le, X.; Yan, H.; Alam, T. AI in drug discovery and its clinical relevance. Heliyon 2023, 9, e17575. [Google Scholar] [CrossRef]
- Pyrkov, A.; Aliper, A.; Bezrukov, D.; Lin, Y.C.; Polykovskiy, D.; Kamya, P.; Ren, F.; Zhavoronkov, A. Quantum computing for near-term applications in generative chemistry and drug discovery. Drug Discov. Toda. 2023, 28, 103675. [Google Scholar] [CrossRef]
- Montgomery, A.P.; Joyce, J.M.; Danon, J.J.; Kassiou, M. An update on late-stage functionalization in today’s drug discovery. Expert. Opin. Drug Discov. 2023, 18, 597–613. [Google Scholar] [CrossRef]
- Sadybekov, A.V.; Katritch, V. Computational approaches streamlining drug discovery. Nature 2023, 616, 673–685. [Google Scholar] [CrossRef]
- Tsopelas, F.; Giaginis, C.; Tsantili-Kakoulidou, A. Lipophilicity and biomimetic properties to support drug discovery. Expert. Opin. Drug Discov. 2017, 12, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Giaginis, C.; Tsantili-Kakoulidou, A. Alternative measures of lipophilicity: From octanol-water partitioning to IAM retention. J. Pharm. Sci. 2008, 97, 2984–3004. [Google Scholar] [CrossRef] [PubMed]
- Al-Khafaji, K.; Taskin Tok, T. Amygdalin as multi-target anticancer drug against targets of cell division cycle: Double docking and molecular dynamics simulation. J. Biomol. Struct. Dyn. 2021, 39, 1965–1974. [Google Scholar] [CrossRef] [PubMed]
- Al-Khafaji, K.; Taskin Tok, T.T. Understanding the mechanism of amygdalin’s multifunctional anti-cancer action using computational approach. J. Biomol. Struct. Dyn. 2021, 39, 1600–1610. [Google Scholar] [CrossRef] [PubMed]
- Al-Khafaji, K.; Taskin Tok, T. Molecular dynamics simulation, free energy landscape and binding free energy computations in exploration the anti-invasive activity of amygdalin against metastasis. Comput. Methods Programs Biomed. 2020, 195, 105660. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanoudaki, M.; Stoumpou, S.; Papadopoulou, S.K.; Karafyllaki, D.; Solovos, E.; Papadopoulos, K.; Giannakoula, A.; Giaginis, C. Amygdalin as a Promising Anticancer Agent: Molecular Mechanisms and Future Perspectives for the Development of New Nanoformulations for Its Delivery. Int. J. Mol. Sci. 2023, 24, 14270. https://doi.org/10.3390/ijms241814270
Spanoudaki M, Stoumpou S, Papadopoulou SK, Karafyllaki D, Solovos E, Papadopoulos K, Giannakoula A, Giaginis C. Amygdalin as a Promising Anticancer Agent: Molecular Mechanisms and Future Perspectives for the Development of New Nanoformulations for Its Delivery. International Journal of Molecular Sciences. 2023; 24(18):14270. https://doi.org/10.3390/ijms241814270
Chicago/Turabian StyleSpanoudaki, Maria, Sofia Stoumpou, Sousana K. Papadopoulou, Dimitra Karafyllaki, Evangelos Solovos, Konstantinos Papadopoulos, Anastasia Giannakoula, and Constantinos Giaginis. 2023. "Amygdalin as a Promising Anticancer Agent: Molecular Mechanisms and Future Perspectives for the Development of New Nanoformulations for Its Delivery" International Journal of Molecular Sciences 24, no. 18: 14270. https://doi.org/10.3390/ijms241814270
APA StyleSpanoudaki, M., Stoumpou, S., Papadopoulou, S. K., Karafyllaki, D., Solovos, E., Papadopoulos, K., Giannakoula, A., & Giaginis, C. (2023). Amygdalin as a Promising Anticancer Agent: Molecular Mechanisms and Future Perspectives for the Development of New Nanoformulations for Its Delivery. International Journal of Molecular Sciences, 24(18), 14270. https://doi.org/10.3390/ijms241814270








