Analysis of MicroRNA Signature Differentially Expressed in Pancreatic Islet Cells Treated with Pancreatic Cancer-Derived Exosomes
Abstract
1. Introduction
2. Results
2.1. DEmiRNAs
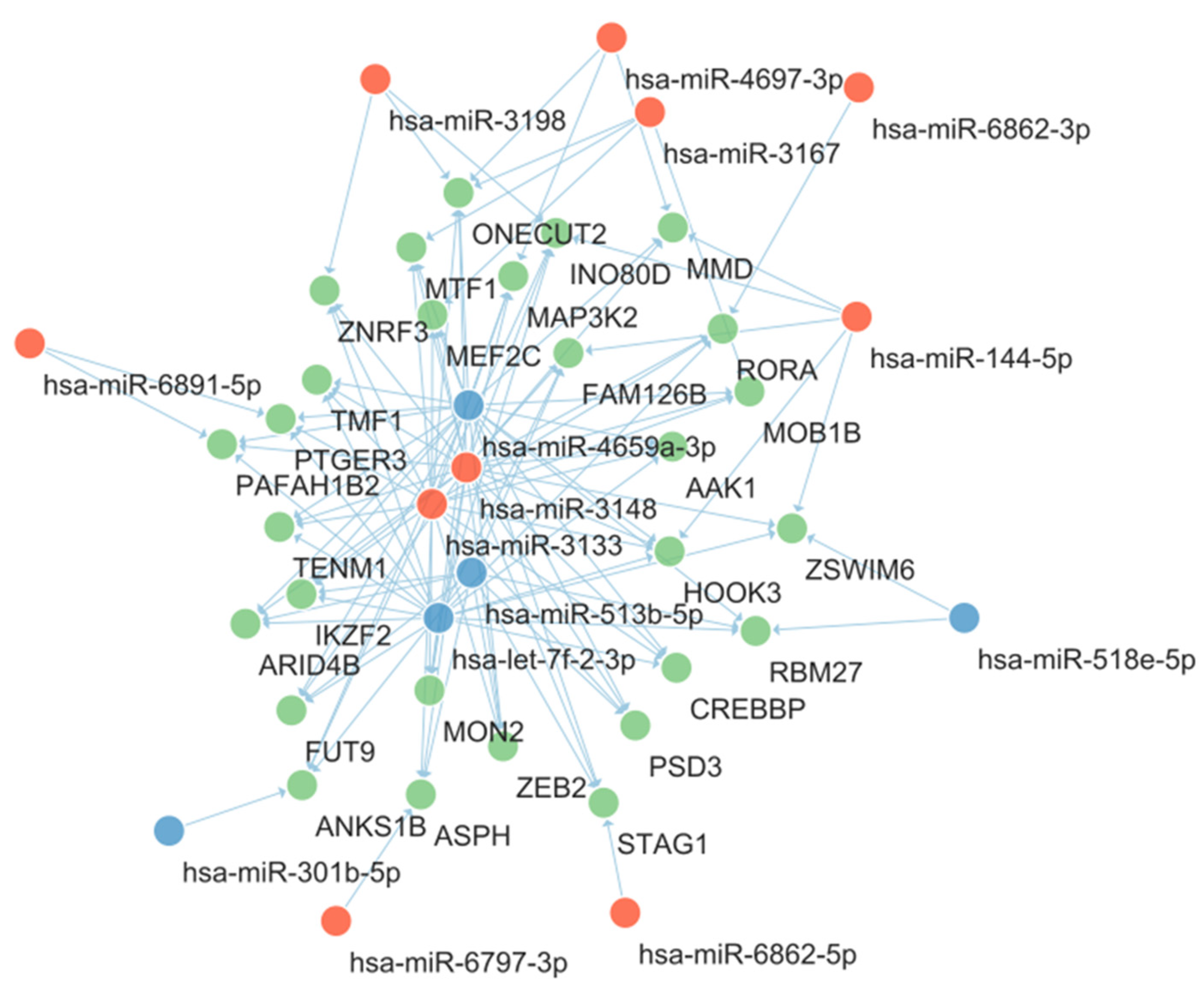
2.2. KEGG Pathway Enrichment Analysis
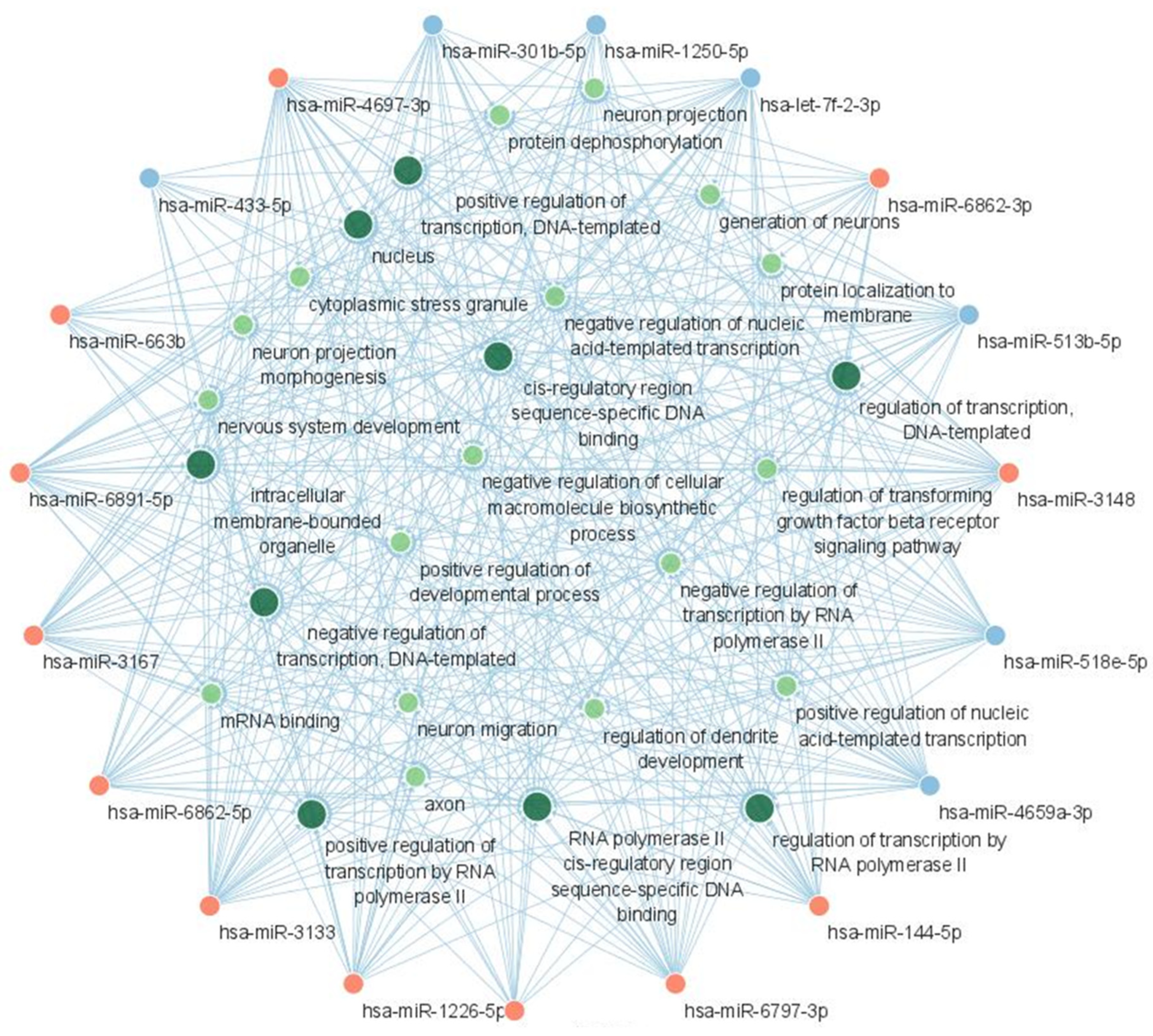
2.3. GO Function Enrichment Analysis
2.4. Expression Pattern of Candidate miRNA Markers in Non-Treated Cancer Cell Lines
3. Discussion
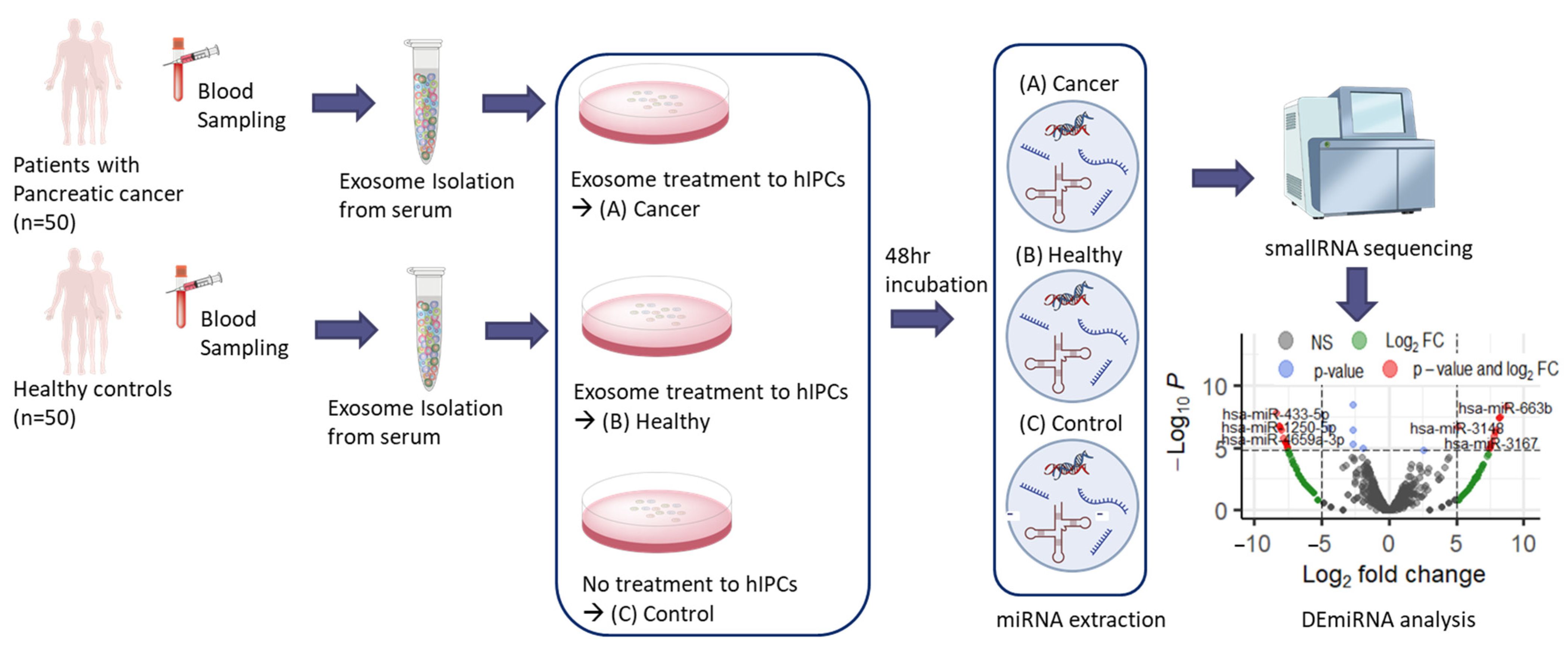
4. Materials and Methods
4.1. Participants
4.2. Exosome Isolation
4.3. Culture and Exosome Treatment of Human Pancreatic Islet-Derived Precursor Cells (hIPCs)
4.4. miRNA Extraction
4.5. miRNA Sequencing and Bioinformatic Process
4.6. Differentially Expressed miRNA (DEmiRNA) Analysis
4.7. Pathway and Gene Ontology (GO) Analysis
4.8. Non-Treated Cancer Cell Lines Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Wood, L.D.; Canto, M.I.; Jaffee, E.M.; Simeone, D.M. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology 2022, 163, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Fahrmann, J.F.; Schmidt, C.M.; Mao, X.; Irajizad, E.; Loftus, M.; Zhang, J.; Patel, N.; Vykoukal, J.; Dennison, J.B.; Long, J.P.; et al. Lead-Time Trajectory of CA19-9 as an Anchor Marker for Pancreatic Cancer Early Detection. Gastroenterology 2020, 160, 1373–1383.e6. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A.; Richards, D.; Cohn, A.; Tummala, M.; Lapham, R.; Cosgrove, D.; Chung, G.; Clement, J.; Gao, J.; Hunkapiller, N.; et al. Clinical validation of a targeted methyla-tion-based multi-cancer early detection test using an independent validation set. Ann. Oncol. 2021, 32, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.; Kühberger, S.; Lazzeri, I.; Vlachos, G.; Heitzer, E. Bridging biological cfDNA features and machine learning approaches. Trends Genet. 2023, 39, 285–307. [Google Scholar] [CrossRef]
- Yu, W.; Hurley, J.; Roberts, D.; Chakrabortty, S.K.; Enderle, D.; Noerholm, M.; Breakefield, X.O.; Skog, J.K. Exo-some-based liquid biopsies in cancer: Opportunities and challenges. Ann. Oncol. 2021, 32, 466–477. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Peferoen, L.; Amor, S. Extracellular vesicles as modulators of cell-to-cell communication in the healthy and diseased brain. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130516. [Google Scholar] [CrossRef]
- Guo, X.-Y.; Xiao, F.; Li, J.; Zhou, Y.-N.; Zhang, W.-J.; Sun, B.; Wang, G. Exosomes and pancreatic diseases: Status, challenges, and hopes. Int. J. Biol. Sci. 2019, 15, 1846–1860. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, X.; Shi, H.; Wu, L.; Qian, H.; Xu, W. Exosomes in cancer: Small particle, big player. J. Hematol. Oncol. 2015, 8, 83. [Google Scholar] [CrossRef]
- Han, Q.F.; Li, W.J.; Hu, K.S.; Gao, J.; Zhai, W.L.; Yang, J.H.; Zhang, S.J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 2022, 21, 207. [Google Scholar] [CrossRef]
- Yan, Y.; Fu, G.; Ming, L. Role of exosomes in pancreatic cancer (Review). Oncol. Lett. 2018, 15, 7479–7488. [Google Scholar] [CrossRef] [PubMed]
- Ariston Gabriel, A.N.; Wang, F.; Jiao, Q.; Yvette, U.; Yang, X.; Al-Ameri, S.A.; Du, L.; Wang, Y.S.; Wang, C. The involvement of exosomes in the diagnosis and treatment of pancreatic cancer. Mol. Cancer 2020, 19, 132. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tang, H.; Li, Q.; Chen, G.; Li, D. Exosomes and their roles in the chemoresistance of pancreatic cancer. Cancer Med. 2022, 11, 4979–4988. [Google Scholar] [CrossRef]
- Zhang, H.; Xing, J.; Dai, Z.; Wang, D.; Tang, D. Exosomes: The key of sophisticated cell–cell communication and targeted metastasis in pancreatic cancer. Cell Commun. Signal. 2022, 20, 9. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Montgomery, K.C.; Jiang, L.; Lyon, C.J.; Hu, T.Y. Advanced technologies for molecular diagnosis of cancer: State of pre-clinical tumor-derived exosome liquid biopsies. Mater. Today Bio 2023, 18, 100538. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Fujiya, M.; Konishi, H.; Sasajima, J.; Fujibayashi, S.; Hayashi, A.; Utsumi, T.; Sato, H.; Iwama, T.; Ijiri, M.; et al. An elevated expression of serum exosomal microRNA-191, -21, -451a of pancreatic neoplasm is considered to be efficient diagnostic marker. BMC Cancer 2018, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.; Ansary-Moghaddam, A.; Berrington de Gonzalez, A.; Barzi, F.; Woodward, M. Type-II diabetes and pancreatic cancer: A meta-analysis of 36 studies. Br. J. Cancer 2005, 92, 2076–2083. [Google Scholar] [CrossRef]
- Javeed, N.; Sagar, G.; Dutta, S.K.; Smyrk, T.C.; Lau, J.S.; Bhattacharya, S.; Truty, M.; Petersen, G.M.; Kaufman, R.J.; Chari, S.T.; et al. Pancreatic Cancer-Derived Exosomes Cause Paraneoplastic beta-cell Dysfunction. Clin. Cancer Res. 2015, 21, 1722–1733. [Google Scholar] [CrossRef]
- Hart, P.A.; Bellin, M.D.; Andersen, D.K.; Bradley, D.; Cruz-Monserrate, Z.; Forsmark, C.E.; Goodarzi, M.O.; Habtezion, A.; Korc, M.; Kudva, Y.C.; et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol. Hepatol. 2016, 1, 226–237. [Google Scholar] [CrossRef]
- Andersen, D.K.; Korc, M.; Petersen, G.M.; Eibl, G.; Li, D.; Rickels, M.R.; Chari, S.T.; Abbruzzese, J.L. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes 2017, 66, 1103–1110. [Google Scholar] [CrossRef]
- Popovic, K.; Smolović, B.; Martinović, M.; Vučković, L. The Relationship between Diabetes Mellitus and Pancreatic Cancer—Diabetes Mellitus as a Red Flag for Pancreatic Cancer. Cancer Epidemiol. Biomark. Prev. 2023, 32, 298–305. [Google Scholar] [CrossRef]
- Sah, R.P.; Nagpal, S.J.S.; Mukhopadhyay, D.; Chari, S.T. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 423–433. [Google Scholar] [CrossRef]
- Zhou, W.; Deng, Y.; Zhao, H.; Zhang, C. Current Status of Serum Insulin and C-Peptide Measurement in Clinical La-boratories: Experience from 94 Laboratories in China. Ann. Lab. Med. 2022, 42, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Korc, M. Pancreatic cancer-associated diabetes is an “exosomopathy”. Clin. Cancer Res. 2015, 21, 1508–1510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, S.; Li, P.; Chen, Q.; Li, Y.; Zhou, Y.; Wang, L.; Kang, M.; Zhang, B.; Yang, B.; et al. Pan-creatic cancer-derived exosomes suppress the production of GIP and GLP-1 from STC-1cells in vitro by down-regulating the PCSK1/3. Cancer Lett. 2018, 431, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Pang, W.; Zhang, A.; Li, L.; Yao, W.; Dai, X. Exosomal miR-19a decreases insulin production by targeting Neurod1 in pancreatic cancer associated diabetes. Mol. Biol. Rep. 2022, 49, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, B.; Zheng, W.; Kang, M.; Chen, Q.; Qin, W.; Li, C.; Zhang, Y.; Shao, Y.; Wu, Y. Exosomes derived from pancreatic cancer cells induce insulin resistance in C2C12 myotube cells through the PI3K/Akt/FoxO1 pathway. Sci. Rep. 2017, 7, 5384. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef]
- Rahimi, R.; Malek, I.; Lerrer-Goldshtein, T.; Elkis, Y.; Shoval, I.; Jacob, A.; Shpungin, S.; Nir, U. TMF1 is upregulated by insulin and is required for a sustained glucose homeostasis. FASEB J. 2021, 35, e21295. [Google Scholar] [CrossRef]
- Kimple, M.E.; Keller, M.P.; Rabaglia, M.R.; Pasker, R.L.; Neuman, J.C.; Truchan, N.A.; Brar, H.K.; Attie, A.D. Prostaglandin E2 Receptor, EP3, Is Induced in Diabetic Islets and Negatively Regulates Glucose- and Hormone-Stimulated Insulin Secretion. Diabetes 2013, 62, 1904–1912. [Google Scholar] [CrossRef]
- Perera, C.J.; Falasca, M.; Chari, S.T.; Greenfield, J.R.; Xu, Z.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Role of Pancreatic Stellate Cell-Derived Exosomes in Pancreatic Cancer-Related Diabetes: A Novel Hypothesis. Cancers 2021, 13, 5224. [Google Scholar] [CrossRef] [PubMed]
- Binang, H.B.; Perera, C.J.; Apte, M.V. Role of Pancreatic Tumour-Derived Exosomes and Their Cargo in Pancreatic Cancer-Related Diabetes. Int. J. Mol. Sci. 2023, 24, 10203. [Google Scholar] [CrossRef]
- Navarro, A.; Karolina, D.S.; Armugam, A.; Tavintharan, S.; Wong, M.T.K.; Lim, S.C.; Sum, C.F.; Jeyaseelan, K. Mi-croRNA 144 Impairs Insulin Signaling by Inhibiting the Expression of Insulin Receptor Substrate 1 in Type 2 Diabetes Mellitus. PLoS ONE 2011, 6, e22839. [Google Scholar]
- Raitoharju, E.; Seppala, I.; Oksala, N.; Lyytikainen, L.P.; Raitakari, O.; Viikari, J.; Ala-Korpela, M.; Soininen, P.; Kangas, A.J.; Waldenberger, M.; et al. Blood microRNA profile associates with the levels of serum lipids and metabolites associated with glucose metabolism and insulin resistance and pinpoints pathways underlying metabolic syndrome: The cardiovascular risk in Young Finns Study. Mol. Cell. Endocrinol. 2014, 391, 41–49. [Google Scholar] [CrossRef]
- Wang, X.; Sundquist, J.; Zöller, B.; Memon, A.A.; Palmér, K.; Sundquist, K.; Bennet, L. Determination of 14 Circulating microRNAs in Swedes and Iraqis with and without Diabetes Mellitus Type 2. PLoS ONE 2014, 9, e86792. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gao, J.; Huang, Q.; Jin, Y.; Wei, Z. Downregulating microRNA-144 mediates a metabolic shift in lung cancer cells by regulating GLUT1 expression. Oncol. Lett. 2016, 11, 3772–3776. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, J.; Chen, Y.; Lei, M. Biomarkers Associated with Ischemic Stroke in Diabetes Mellitus Patients. Cardiovasc. Toxicol. 2016, 16, 213–222. [Google Scholar] [CrossRef]
- Mononen, N.; Lyytikäinen, L.-P.; Seppälä, I.; Mishra, P.P.; Juonala, M.; Waldenberger, M.; Klopp, N.; Illig, T.; Leiviskä, J.; Loo, B.-M.; et al. Whole blood microRNA levels associate with glycemic status and correlate with target mRNAs in pathways important to type 2 diabetes. Sci. Rep. 2019, 9, 8887. [Google Scholar] [CrossRef]
- Xiang, X.; Zhang, C.; Long, D. Palmitic Acid Regulates miRNA-3148 via Insulin Receptor Substrate-1 and is Involved in Insulin Resistance. J. Biomater. Tissue Eng. 2021, 11, 767–771. [Google Scholar] [CrossRef]
- Rovira-Llopis, S.; Díaz-Rúa, R.; Valle, C.G.-D.; Iannantuoni, F.; Abad-Jimenez, Z.; Bosch-Sierra, N.; Panadero-Romero, J.; Victor, V.M.; Rocha, M.; Morillas, C.; et al. Characterization of Differentially Expressed Circulating miRNAs in Metabolically Healthy versus Unhealthy Obesity. Biomedicines 2021, 9, 321. [Google Scholar] [CrossRef]
- Liu, G.; Lei, Y.; Luo, S.; Huang, Z.; Chen, C.; Wang, K.; Yang, P.; Huang, X. MicroRNA expression profile and identifi-cation of novel microRNA biomarkers for metabolic syndrome. Bioengineered 2021, 12, 3864–3872. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, G.; Besharat, Z.M.; Chiacchiarini, M.; Abballe, L.; Sabato, C.; Vacca, A.; Borgiani, P.; Dotta, F.; Tesauro, M.; Po, A.; et al. Circulating MicroRNAs in Elderly Type 2 Diabetic Patients. Int. J. Endocrinol. 2018, 2018, 6872635. [Google Scholar] [CrossRef] [PubMed]
- Alaaeldin, R.; Abdel-Rahman, I.A.M.; Hassan, H.A.; Youssef, N.; Allam, A.E.; Abdelwahab, S.F.; Zhao, Q.L.; Fathy, M. Carpachromene Ameliorates Insulin Resistance in HepG2 Cells via Modulating IR/IRS1/PI3k/Akt/GSK3/FoxO1 Pathway. Molecules 2021, 26, 7629. [Google Scholar] [CrossRef] [PubMed]
- Lyu, M.; Wang, X.; Meng, X.; Qian, H.; Li, Q.; Ma, B.; Zhang, Z.; Xu, K. chi-miR-487b-3p Inhibits Goat Myoblast Prolif-eration and Differentiation by Targeting IRS1 through the IRS1/PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2021, 23, 115. [Google Scholar] [CrossRef]
- Davani, B.; Ikonomou, L.; Raaka, B.M.; Geras-Raaka, E.; Morton, R.A.; Marcus-Samuels, B.; Gershengorn, M.C. Human Islet-Derived Precursor Cells Are Mesenchymal Stromal Cells That Differentiate and Mature to Hormone-Expressing Cells In Vivo. Stem Cells 2007, 25, 3215–3222. [Google Scholar] [CrossRef][Green Version]
- Skelin, M.; Rupnik, M.; Cencič, A. Pancreatic beta cell lines and their applications in diabetes mellitus research. AL-TEX-Altern. Anim. Exp. 2010, 27, 105–113. [Google Scholar] [CrossRef]
- Miyazaki, S.; Tashiro, F.; Tsuchiya, T.; Sasaki, K.; Miyazaki, J.I. Establishment of a long-term stable beta-cell line and its application to analyze the effect of Gcg expression on insulin secretion. Sci. Rep. 2021, 11, 477. [Google Scholar] [CrossRef]
- Villard, O.; Armanet, M.; Couderc, G.; Bony, C.; Moreaux, J.; Noel, D.; De Vos, J.; Klein, B.; Veyrune, J.L.; Wojtusciszyn, A. Characterization of immortalized human islet stromal cells reveals a MSC-like profile with pancreatic features. Stem Cell Res. Ther. 2020, 11, 158. [Google Scholar] [CrossRef]
- Park, J.; Go, E.-B.; Oh, J.S.; Lee, J.K.; Lee, S.-Y. Multiple-Cycle Polymeric Extracellular Vesicle Precipitation and Its Evaluation by Targeted Mass Spectrometry. Int. J. Mol. Sci. 2021, 22, 4311. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, F.; Wu, J.; Schubert, J.; Li, M.M. Application of Next Generation Sequencing in Laboratory Medicine. Ann. Lab. Med. 2021, 41, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2013, 42, D68–D73. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
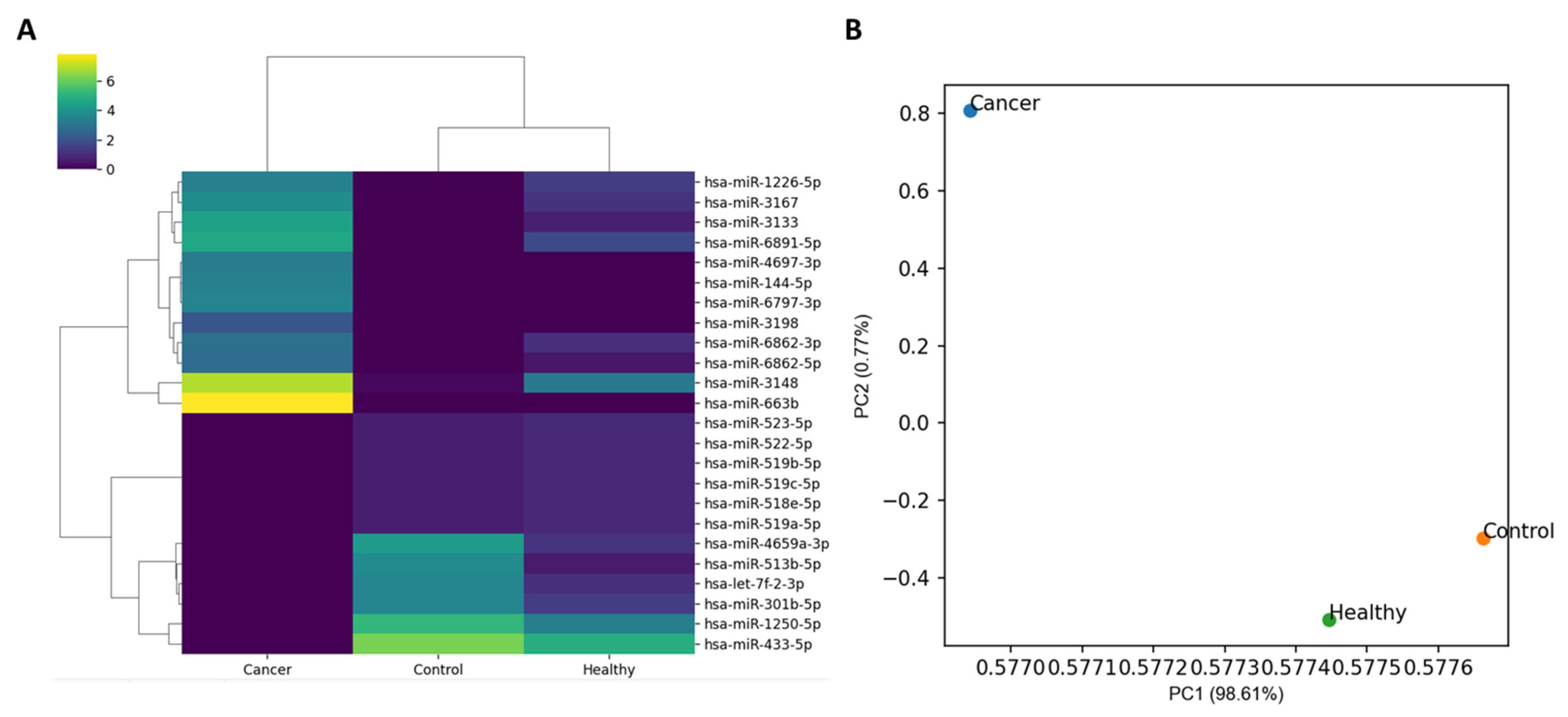




| miRNA | log2FC | log2CPM | p-Value | Upregulated or Downregulated | Degree in miRNA-Gene Network |
|---|---|---|---|---|---|
| hsa-let-7f-2-3p | −7.57 | 0.93 | 0.0000083 | Downregulated | 17 |
| hsa-miR-1226-5p | 7.50 | 1.10 | 0.0000083 | Upregulated | 0 |
| hsa-miR-1250-5p | −8.11 | 1.42 | 0.0000002 | Downregulated | 0 |
| hsa-miR-144-5p | 7.50 | 1.10 | 0.0000083 | Upregulated | 5 |
| hsa-miR-301b-5p | −7.57 | 0.93 | 0.0000083 | Downregulated | 1 |
| hsa-miR-3133 | 7.89 | 1.43 | 0.0000005 | Upregulated | 20 |
| hsa-miR-3148 | 5.14 | 2.02 | 0.0000002 | Upregulated | 25 |
| hsa-miR-3167 | 7.66 | 1.24 | 0.0000036 | Upregulated | 4 |
| hsa-miR-3198 | 7.80 | 1.36 | 0.0000011 | Upregulated | 3 |
| hsa-miR-433-5p | −8.38 | 1.67 | <0.0000001 | Downregulated | 0 |
| hsa-miR-4659a-3p | −7.83 | 1.17 | 0.0000016 | Downregulated | 22 |
| hsa-miR-4697-3p | 7.44 | 1.06 | 0.0000127 | Upregulated | 3 |
| hsa-miR-513b-5p | −7.64 | 1.00 | 0.0000054 | Downregulated | 10 |
| hsa-miR-518e-5p | −7.71 | 1.06 | 0.0000036 | Downregulated | 2 |
| hsa-miR-519a-5p | −7.71 | 1.06 | 0.0000036 | Downregulated | NA |
| hsa-miR-519b-5p | −7.71 | 1.06 | 0.0000036 | Downregulated | NA |
| hsa-miR-519c-5p | −7.71 | 1.06 | 0.0000036 | Downregulated | NA |
| hsa-miR-522-5p | −7.71 | 1.06 | 0.0000036 | Downregulated | NA |
| hsa-miR-523-5p | −7.71 | 1.06 | 0.0000036 | Downregulated | NA |
| hsa-miR-663b | 8.70 | 2.16 | <0.0000001 | Upregulated | 0 |
| hsa-miR-6797-3p | 7.55 | 1.15 | 0.0000054 | Upregulated | 1 |
| hsa-miR-6862-3p | 8.26 | 1.76 | <0.0000001 | Upregulated | 1 |
| hsa-miR-6862-5p | 8.19 | 1.70 | <0.0000001 | Upregulated | 1 |
| hsa-miR-6891-5p | 7.97 | 1.50 | 0.0000003 | Upregulated | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-g.; Park, J.; Park, E.Y.; Kim, S.-M.; Lee, S.-Y. Analysis of MicroRNA Signature Differentially Expressed in Pancreatic Islet Cells Treated with Pancreatic Cancer-Derived Exosomes. Int. J. Mol. Sci. 2023, 24, 14301. https://doi.org/10.3390/ijms241814301
Kim Y-g, Park J, Park EY, Kim S-M, Lee S-Y. Analysis of MicroRNA Signature Differentially Expressed in Pancreatic Islet Cells Treated with Pancreatic Cancer-Derived Exosomes. International Journal of Molecular Sciences. 2023; 24(18):14301. https://doi.org/10.3390/ijms241814301
Chicago/Turabian StyleKim, Young-gon, Jisook Park, Eun Young Park, Sang-Mi Kim, and Soo-Youn Lee. 2023. "Analysis of MicroRNA Signature Differentially Expressed in Pancreatic Islet Cells Treated with Pancreatic Cancer-Derived Exosomes" International Journal of Molecular Sciences 24, no. 18: 14301. https://doi.org/10.3390/ijms241814301
APA StyleKim, Y.-g., Park, J., Park, E. Y., Kim, S.-M., & Lee, S.-Y. (2023). Analysis of MicroRNA Signature Differentially Expressed in Pancreatic Islet Cells Treated with Pancreatic Cancer-Derived Exosomes. International Journal of Molecular Sciences, 24(18), 14301. https://doi.org/10.3390/ijms241814301






