γ-Aminobutyric Acid Priming Alleviates Acid-Aluminum Toxicity to Creeping Bentgrass by Regulating Metabolic Homeostasis
Abstract
:1. Introduction
2. Results
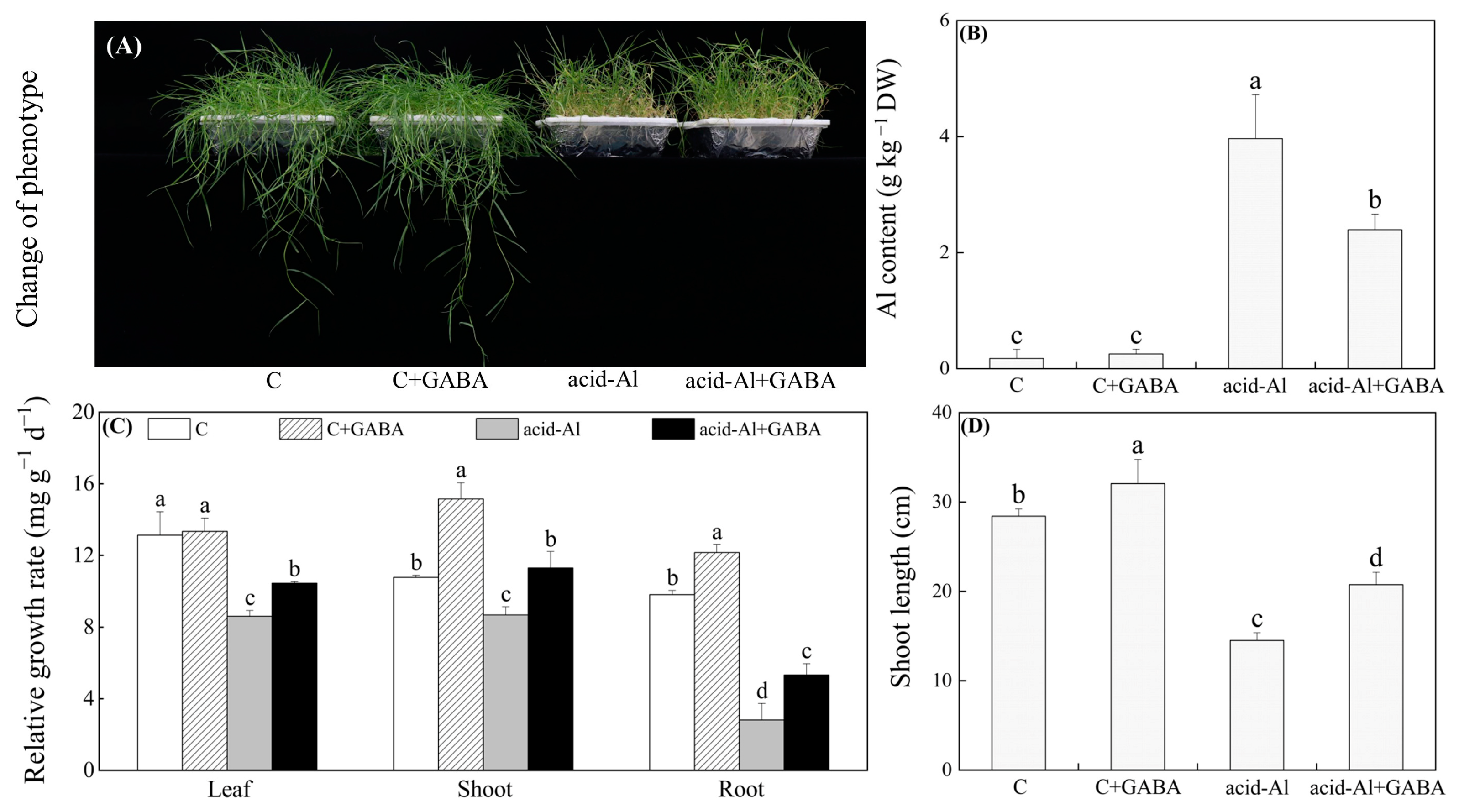
2.1. Effects of GABA Priming on Aluminum Content and Plant Growth under Normal Conditions and Acid-Al Stress
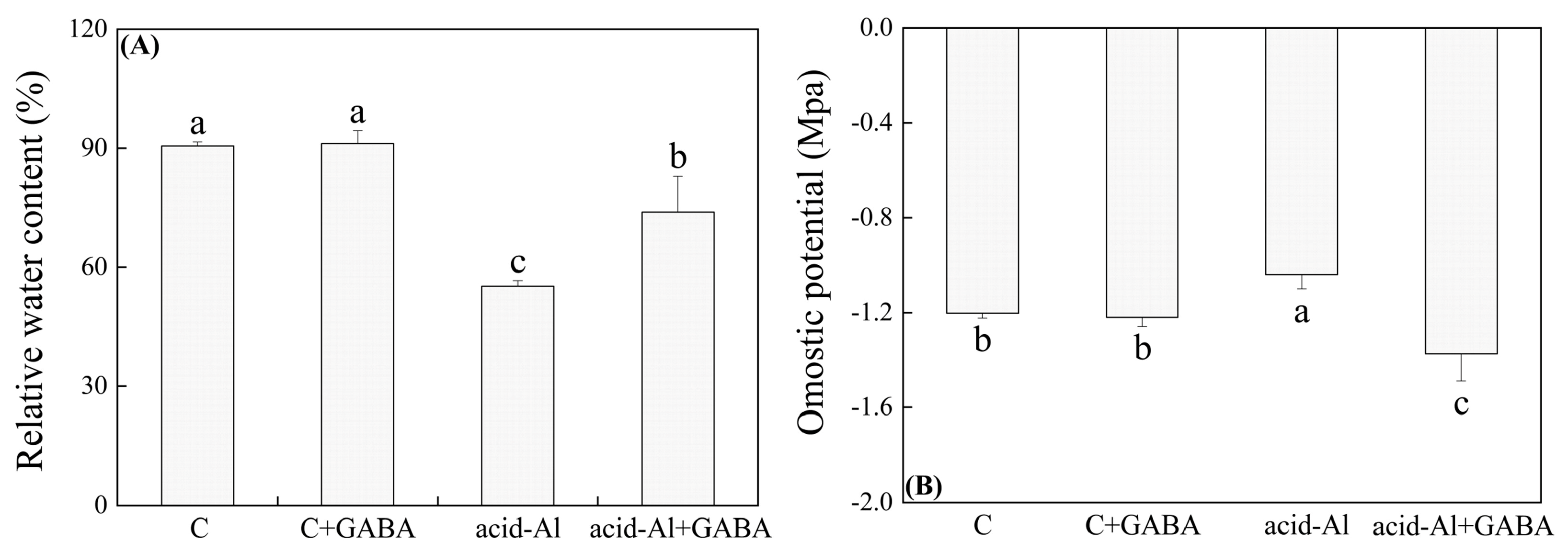
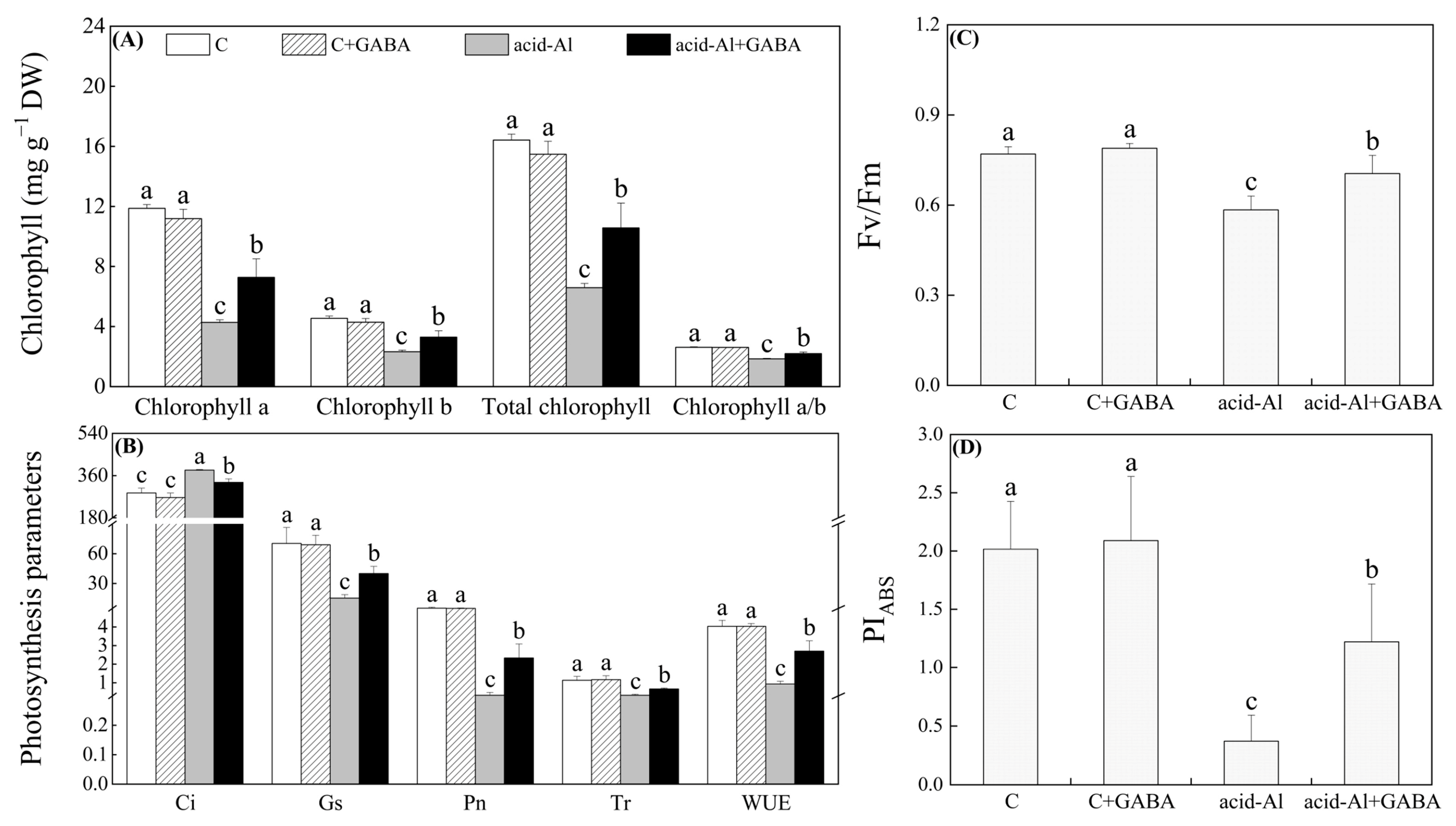
2.2. Effects of GABA Priming on Leaf Water Status and Photosynthesis under Normal Conditions and Acid-Al Stress
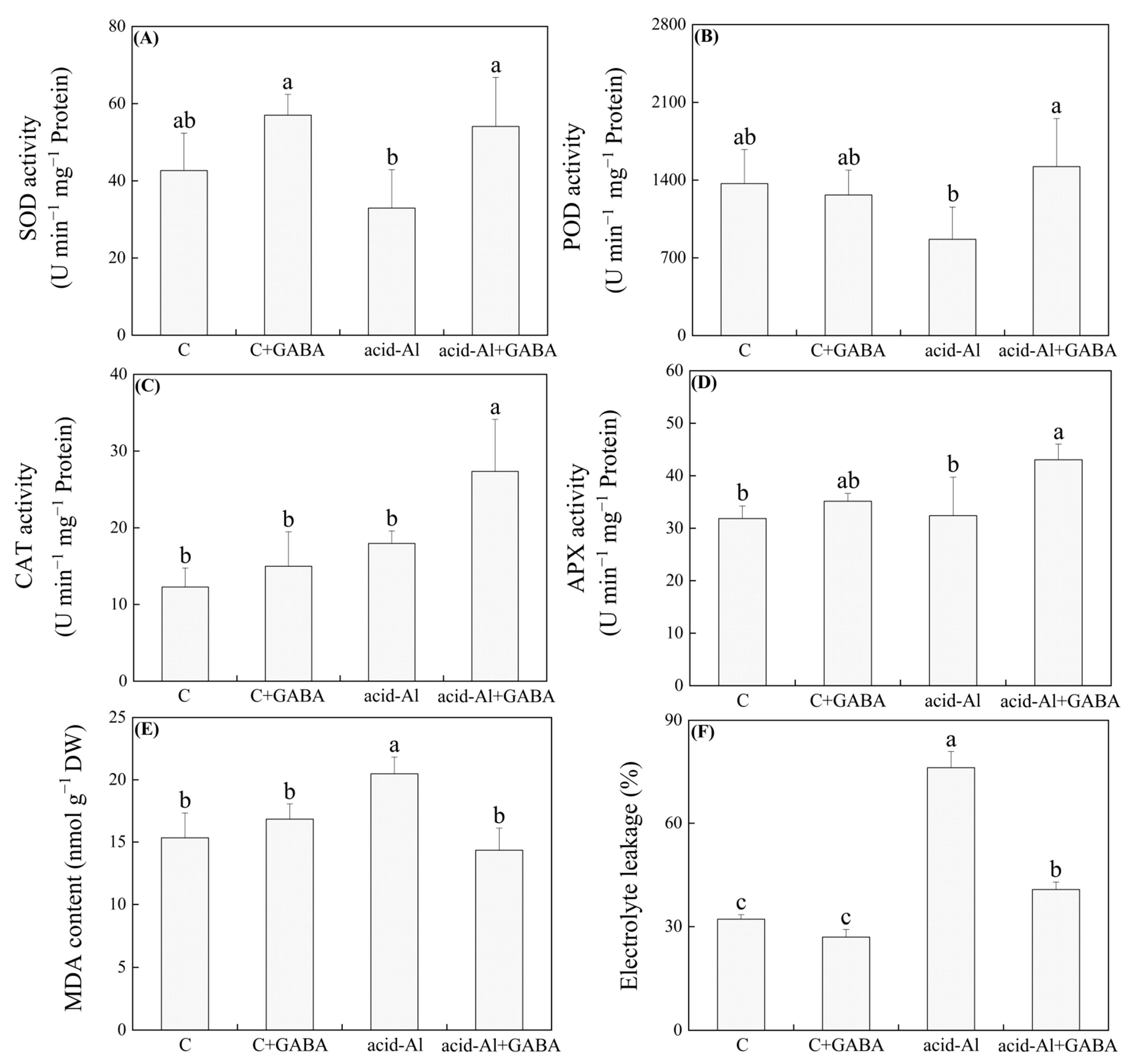
2.3. Effects of GABA Priming on Antioxidant Enzyme Activities and Oxidative Damage under Normal Conditions and Acid-Al Stress
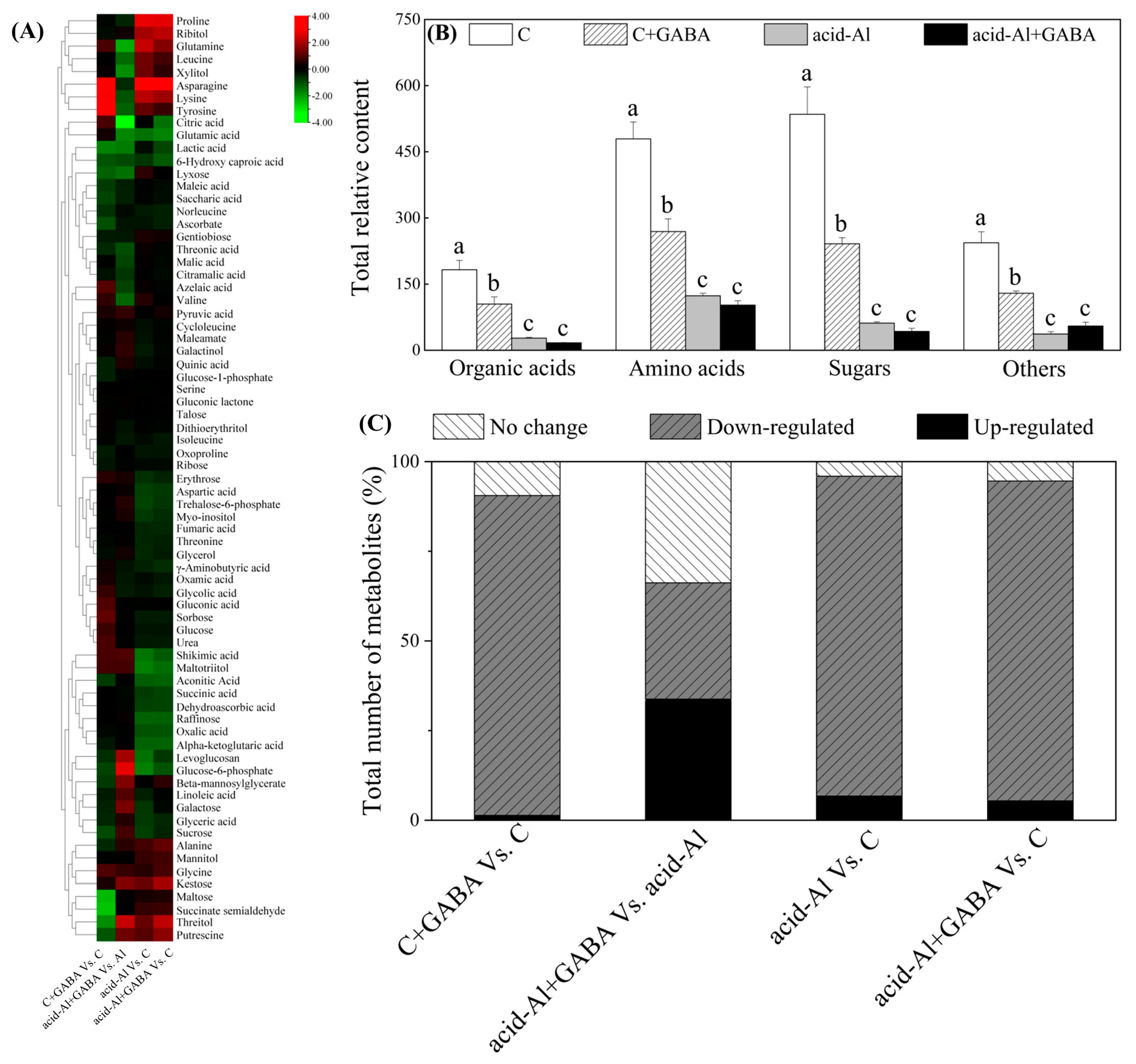
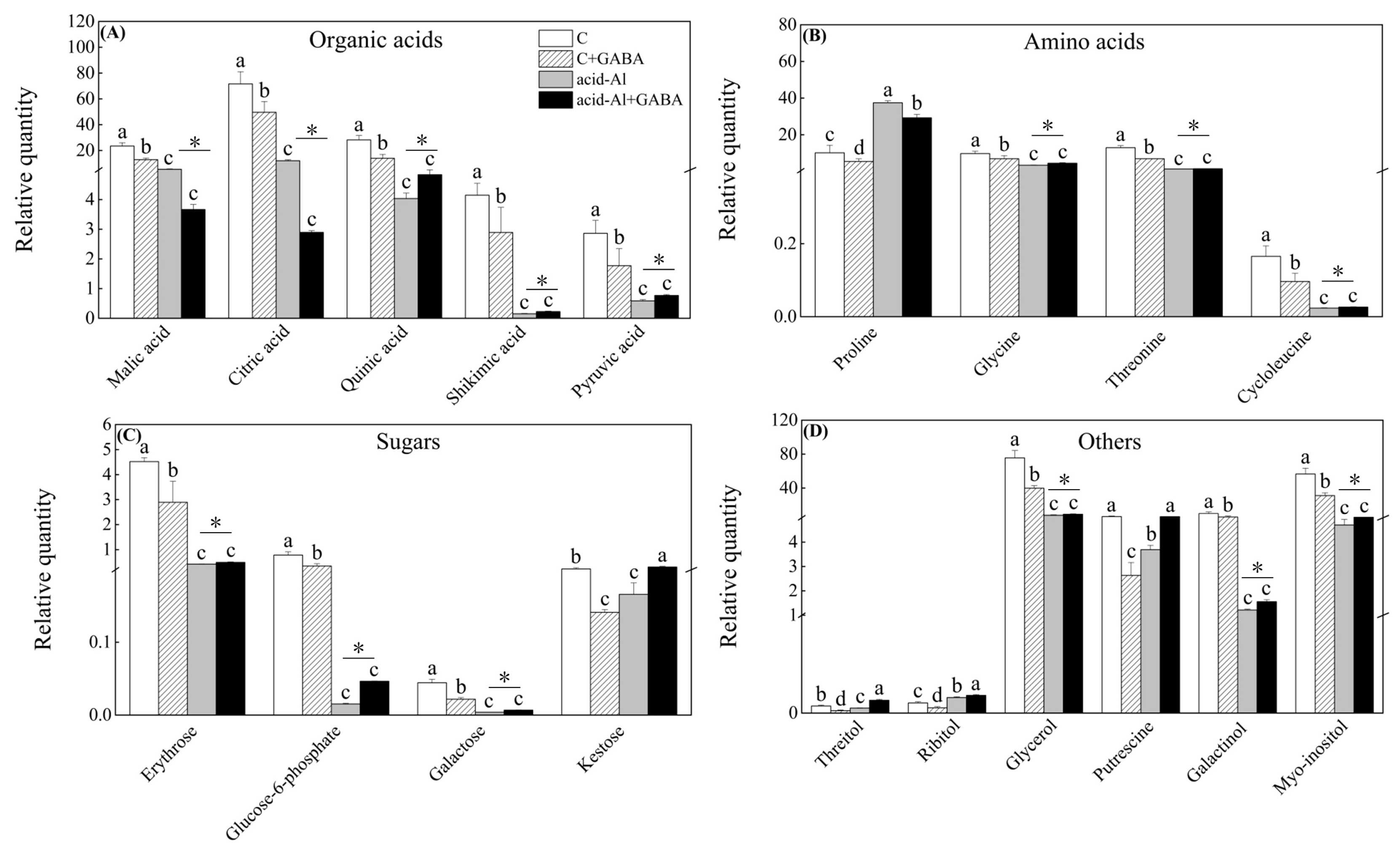
2.4. Effects of GABA Priming on Metabolites Profile under Normal Conditions and Acid-Al Stress
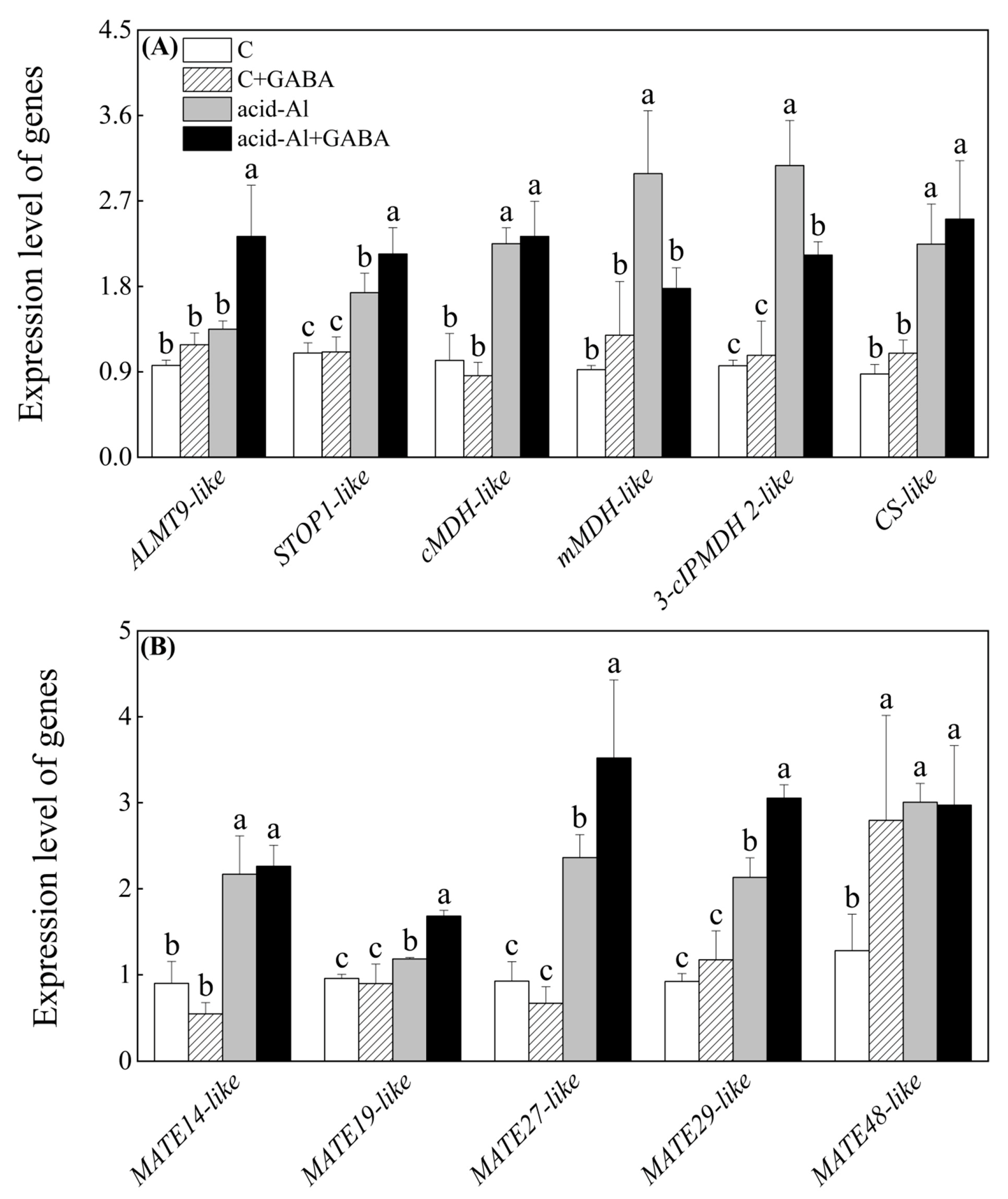
2.5. Effects of GABA Priming on Expression Levels of Genes Involved in Accumulation and Transport of Malic and Citric Acids under Normal Conditions and Acid-Al Stress
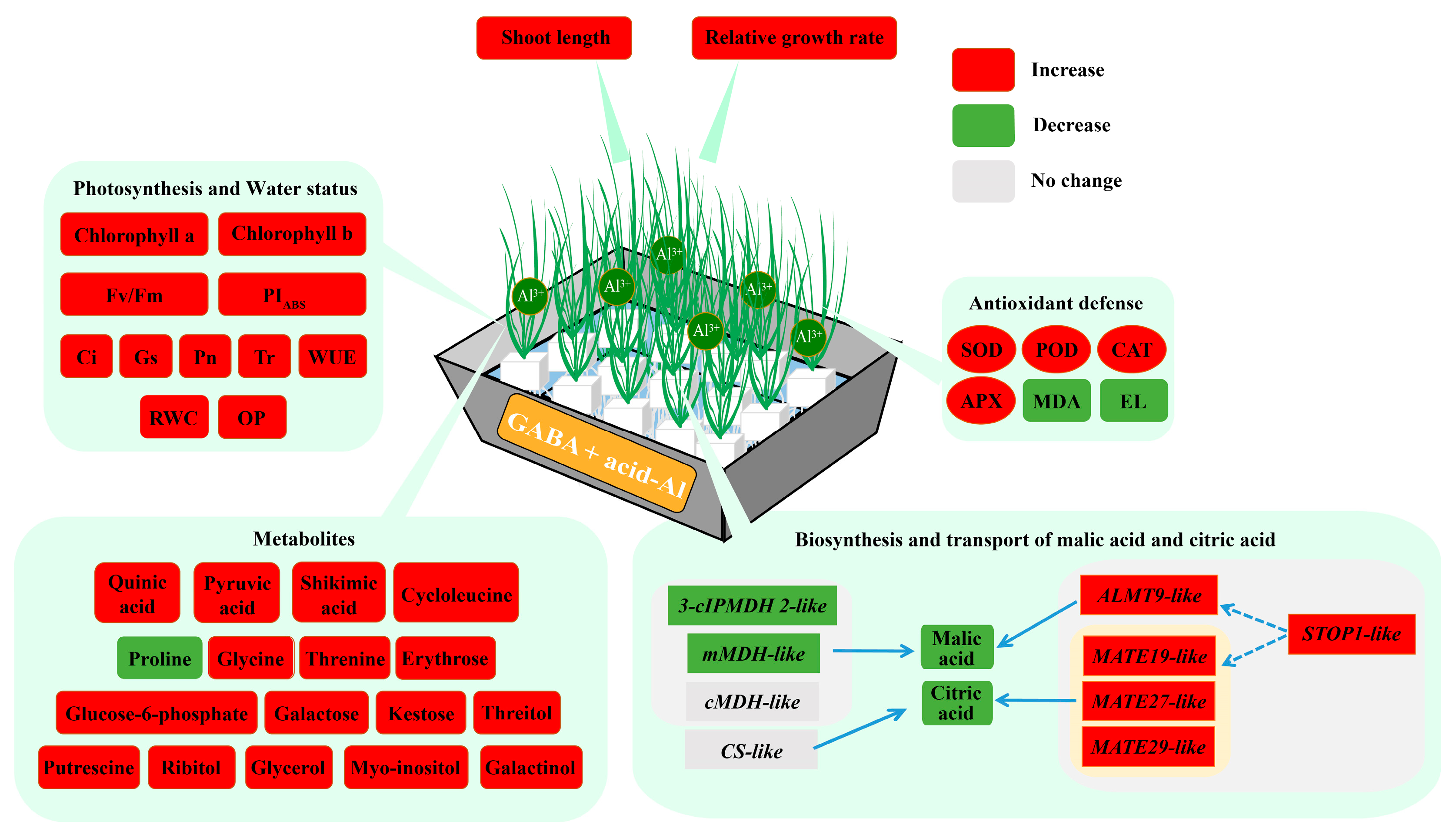
3. Discussion
3.1. GABA-Regulated Tolerance to Acid-Al Stress in Relation to Changes in Al Accumulation, Photosynthesis, and Osmotic Balance
3.2. GABA-Regulated Tolerance to Acid-Al Stress in Relation to Changes in Oxidative Damage and Antioxidant Defense
3.3. GABA-Regulated Tolerance to Acid-Al Stress in Relation to Changes in Accumulation and Transport of Organic Acids
3.4. GABA-Regulated Tolerance to Acid-Al Stress in Relation to Changes in Accumulations of Amino Acids and Other Metabolites
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Measurements of Growth, Water Status, and Aluminum Content
4.3. Measurements of Chlorophyll Content and Photosynthetic Parameters
4.4. Measurements of Antioxidant Enzyme Activity, Oxidative Damage, and Cell Membrane Stability
4.5. Determination of Metabolomics and Gene Expression Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hajiboland, R.; Panda, C.K.; Lastochkina, O.; Gavassi, M.A.; Habermann, G.; Pereira, J.F. Aluminum toxicity in plants: Present and future. J. Plant. Growth Regul. 2023, 42, 3967–3999. [Google Scholar] [CrossRef]
- Qiu, Q.; Wu, J.; Liang, G.; Liu, J.; Chu, G.; Zhou, G.; Zhang, D. Effects of simulated acid rain on soil and soil solution chemistry in a monsoon evergreen broad-leaved forest in southern China. Environ. Monit. Assess. 2015, 187, 272. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Schubert, S.; Mengel, K. Soil pH changes during legume growth and application of plant material. Biol. Fertil. Soils 1996, 23, 236–242. [Google Scholar] [CrossRef]
- Ali, B.; Hasan, S.A.; Hayat, S.; Hayat, Q.; Yadav, S.; Fariduddin, Q.; Ahmad, A. A role for brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiata L. Wilczek). Environ. Exp. Bot. 2008, 62, 153–159. [Google Scholar] [CrossRef]
- Dong, D.; Li, Y.; Jiang, L. Effects of brassinosteroid on photosynthetic characteristics in soybean under aluminum stress. Acta Agron. Sin. 2008, 34, 1673–1678. [Google Scholar] [CrossRef]
- Jiang, H.; Tang, N.; Zheng, J.; Li, Y.; Chen, L. Phosphorus alleviates aluminum-induced inhibition of growth and photosynthesis in Citrus grandis seedlings. Physiol. Plant. 2009, 137, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Senoo, Y.; Kasai, M.; Maeshima, M. Response of the plant root to aluminum stress: Analysis of the inhibition of the root elongation and changes in membrane function. J. Plant Res. 1996, 109, 99–105. [Google Scholar] [CrossRef]
- Mohan, M.A.V.; Patnaik, A.R.; Panda, B.B. Oxidative biomarkers in leaf tissue of barley seedlings in response to aluminum stress. Ecotoxicol. Environ. Saf. 2012, 75, 16–26. [Google Scholar] [CrossRef]
- Moustakas, M.; Ouzounidou, G.; Eleftheriou, E.P.; Lannoye, R. Indirect effects of aluminum stress on the function of the photosynthetic apparatus. Plant Physiol. Biochem. 1996, 34, 553–560. [Google Scholar]
- Kochian, L.V.; Hoekenga, O.A.; Piñeros, M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef]
- Liang, Y.; Bai, T.; Liu, B.; Yu, W.; Teng, W. Different antioxidant regulation mechanisms in response to aluminum-induced oxidative stress in Eucalyptus species. Ecotoxicol. Environ. Saf. 2022, 241, 113748. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xiao, J.; Zheng, K.; Ma, J.; He, M.; Li, J.; Li, M. Transcriptome profiling reveals the effects of nitric oxide on the growth and physiological characteristics of watermelon under aluminum stress. Genes 2021, 12, 1735. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Wang, Y.; Imran, M.; Jiang, C. Boron alleviates the aluminum toxicity in trifoliate orange by regulating antioxidant defense system and reducing root cell injury. J. Environ. Manag. 2018, 208, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Brunner, I.; Sperisen, C. Aluminum exclusion and aluminum tolerance in woody plants. Front. Plant Sci. 2013, 4, 172. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ryan, P.R.; Delhaize, E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Xiao, C.; Xiao, B.; Wang, M.; Liu, J.; Bhanbhro, N.; Khan, A.; Wang, H.; Wang, H.; Yang, C. Proteomic profiling sheds light on alkali tolerance of common wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2019, 138, 58–64. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Li, Y.; Yan, S.; Li, W.; Zhang, J. Isolation and characterization of PbCS2 gene regulated by iron deficiency and auxin-based systemic signals in Pyrus betulifolia. Sci. Hortic. 2016, 205, 25–31. [Google Scholar] [CrossRef]
- Chauhan, D.K.; Yadav, V.; Vaculik, M.; Gassmann, W.; Pike, S.; Arif, N.; Singh, V.P.; Deshmukh, R.; Sahi, S.; Tripathi, D.K. Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit. Rev. Biotechnol. 2021, 41, 715–730. [Google Scholar] [CrossRef]
- Kar, D.; Pradhan, A.A.; Datta, S. The role of solute transporters in aluminum toxicity and tolerance. Physiol. Plant. 2021, 171, 638–652. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, J.; Zhang, J.; Dai, Z.; Zhang, L. Ectopic expression of the chinese cabbage malate dehydrogenase gene promotes growth and aluminum resistance in Arabidopsis. Front. Plant Sci. 2016, 7, 1180. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Kou, J.; Shi, L.; Zhang, C.; Xu, F. Dual effects of transgenic Brassica napus overexpressing CS gene on tolerances to aluminum toxicity and phosphorus deficiency. Plant Soil 2013, 362, 231–246. [Google Scholar] [CrossRef]
- Ma, Q.; Yi, R.; Li, L.; Liang, Z.; Zeng, T.; Zhang, Y.; Huang, H.; Zhang, X.; Yin, X.; Cai, Z.; et al. GsMATE encoding a multidrug and toxic compound extrusion transporter enhances aluminum tolerance in Arabidopsis thaliana. BMC Plant Biol. 2018, 18, 212. [Google Scholar] [CrossRef] [PubMed]
- Daspute, A.A.; Sadhukhan, A.; Tokizawa, M.; Kobayashi, Y.; Panda, S.K.; Koyama, H. Transcriptional regulation of aluminum-tolerance genes in higher plants: Clarifying the underlying molecular mechanisms. Front. Plant Sci. 2017, 8, 1358. [Google Scholar] [CrossRef] [PubMed]
- Le Poder, L.; Mercier, C.; Février, L.; Duong, N.; David, P.; Pluchon, S.; Nussaume, L.; Desnos, T. Uncoupling aluminum toxicity from aluminum signals in the STOP1 pathway. Front. Plant Sci. 2022, 13, 785791. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, S.; Koyama, H.; Iuchi, A.; Kobayashi, Y.; Kitabayashi, S.; Kobayashi, Y.; Ikka, T.; Hirayama, T.; Shinozaki, K.; Kobayashi, M. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc. Natl. Acad. Sci. USA 2007, 104, 9900–9905. [Google Scholar] [CrossRef]
- Jiang, Y. Application of gamma-aminobutyric acid and nitric oxide on turfgrass stress resistance: Current knowledge and perspectives. Grass Res. 2023, 3, 3. [Google Scholar] [CrossRef]
- Suhel, M.; Husain, T.; Pandey, A.; Singh, S.; Dubey, N.K.; Prasad, S.M.; Singh, V.P. An appraisal of ancient molecule GABA in abiotic stress tolerance in plants, and its crosstalk with other signaling molecules. J. Plant Growth Regul. 2023, 42, 614–629. [Google Scholar] [CrossRef]
- Wang, P.; Dong, Y.; Zhu, L.; Hao, Z.; Hu, L.; Hu, X.; Wang, G.; Cheng, T.; Shi, J.; Chen, J. The role of γ-aminobutyric acid in aluminum stress tolerance in a woody plant, Liriodendron chinense × tulipifera. Hortic. Res. 2021, 8, 80. [Google Scholar] [CrossRef]
- Dernoeden, P.H. Creeping Bentgrass Management; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- de Jesus, L.R.; Batista, B.L.; da Silva Lobato, A.K. Silicon reduces aluminum accumulation and mitigates toxic effects in cowpea plants. Acta Physiol. Plant. 2017, 39, 138. [Google Scholar] [CrossRef]
- Ali, S.; Bai, P.; Zeng, F.; Cai, S.; Shamsi, I.H.; Qiu, B.; Wu, F.; Zhang, G. The ecotoxicological and interactive effects of chromium and aluminum on growth, oxidative damage and antioxidant enzymes on two barley genotypes differing in Al tolerance. Environ. Exp. Bot. 2011, 70, 185–191. [Google Scholar] [CrossRef]
- Lyu, M.; Liu, J.; Xu, X.; Liu, C.; Qin, H.; Zhang, X.; Tian, G.; Jiang, H.; Jiang, Y.; Zhu, Z.; et al. Magnesium alleviates aluminum-induced growth inhibition by enhancing antioxidant enzyme activity and carbon-nitrogen metabolism in apple seedlings. Ecotoxicol. Environ. Saf. 2023, 249, 114421. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.A.; Barros, J.A.S.; Dal-Bianco, M.; Martins, S.C.V.; Magalhães, P.C.; Ribeiro, D.M.; DaMatta, F.M.; Araújo, W.L.; Ribeiro, C. Metabolic and physiological adjustments of maize leaves in response to aluminum stress. Theor. Exp. Plant Physiol. 2020, 32, 133–145. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Valentine, A.; Cornejo, P. Role of Curtobacterium herbarum strain CAH5 on aluminum bioaccumulation and enhancement of Lactuca sativa growth under aluminum and drought stresses. Ecotoxicol. Environ. Saf. 2019, 183, 109573. [Google Scholar] [CrossRef] [PubMed]
- Rezaei-Chiyaneh, E.; Seyyedi, S.M.; Ebrahimian, E.; Moghaddam, S.S.; Damalas, C.A. Exogenous application of gamma-aminobutyric acid (GABA) alleviates the effect of water deficit stress in black cumin (Nigella sativa L.). Ind. Crop. Prod. 2018, 112, 741–748. [Google Scholar] [CrossRef]
- Rossi, S.; Chapman, C.; Huang, B. Suppression of heat-induced leaf senescence by γ-aminobutyric acid, proline, and ammonium nitrate through regulation of chlorophyll degradation in creeping bentgrass. Environ. Exp. Bot. 2020, 177, 104116. [Google Scholar] [CrossRef]
- Zeng, W.; Hassan, M.J.; Kang, D.; Peng, Y.; Li, Z. Photosynthetic maintenance and heat shock protein accumulation relating to γ-aminobutyric acid (GABA)-regulated heat tolerance in creeping bentgrass (Agrostis stolonifera). S. Afr. J. Bot. 2021, 141, 405–413. [Google Scholar] [CrossRef]
- Li, Z.; Yong, B.; Cheng, B.; Wu, X.; Zhang, X.; Peng, Y. Nitric oxide, γ-aminobutyric acid, and mannose pretreatment influence metabolic profiles in white clover under water stress. J. Integr. Plant. Biol. 2019, 61, 1255–1273. [Google Scholar] [CrossRef] [PubMed]
- Gavassi, M.A.; Silva, G.S.; da Silva, C.d.M.S.; Thompson, A.J.; Macleod, K.; Oliveira, P.M.R.; Cavalheiro, M.F.; Domingues, D.S.; Habermann, G. NCED expression is related to increased ABA biosynthesis and stomatal closure under aluminum stress. Environ. Exp. Bot. 2021, 185, 104404. [Google Scholar] [CrossRef]
- Han, X.; Tang, S.; An, Y.; Zheng, D.; Xia, X.; Yin, W. Overexpression of the poplar NF-YB7 transcription factor confers drought tolerance and improves water-use efficiency in Arabidopsis. J. Exp. Bot. 2013, 64, 4589–4601. [Google Scholar] [CrossRef]
- Hassan, M.J.; Qi, H.; Cheng, B.; Hussain, S.; Peng, Y.; Liu, W.; Feng, G.; Zhao, J.; Li, Z. Enhanced adaptability to limited water supply regulated by diethyl aminoethyl hexanoate (DA-6) associated with lipidomic reprogramming in two white clover genotypes. Front. Plant Sci. 2022, 13, 879331. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, G.; Kang, H.; Zhou, S.; Wang, W. TaPUB1, a putative E3 ligase gene from wheat, enhances salt stress tolerance in transgenic Nicotiana benthamiana. Plant Cell Physiol. 2017, 58, 1673–1688. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Sinha, R.; Sharma, T.R.; Pattanayak, A.; Singh, A.K. Alleviating aluminum toxicity in plants: Implications of reactive oxygen species signalling and crosstalk with other signaling pathways. Physiol. Plant. 2021, 173, 1765–1784. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xiao, Q.; Ding, L.; Chen, M.; Yin, L.; Li, J.; Zhou, S.; He, G. Differential responses of lipid peroxidation and antioxidants in Alternanthera philoxeroides and Oryza sativa subjected to drought stress. Plant Growth Regul. 2008, 56, 89–95. [Google Scholar] [CrossRef]
- Li, Z.; Tang, M.; Hassan, M.J.; Zhang, Y.; Han, L.; Peng, Y. Adaptability to high temperature and stay-green genotypes associated with variations in antioxidant, chlorophyll metabolism, and γ-aminobutyric acid accumulation in creeping bentgrass species. Front. Plant Sci. 2021, 12, 750728. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, M.; Ye, B.; Liu, L.; Ji, S. Calcium ion improves cold resistance of green peppers (Capsicum annuum L.) by regulating the activity of protective enzymes and membrane lipid composition. Sci. Hortic. 2021, 277, 109789. [Google Scholar] [CrossRef]
- Bowler, C.; Van Camp, W.; Van Montagu, M.; Inzé, D.; Asada, K. Superoxide dismutase in plants. Crit. Rev. Plant Sci. 1994, 13, 199–218. [Google Scholar] [CrossRef]
- Fujita, M.; Hasanuzzaman, M. Approaches to enhancing antioxidant defense in plants. Antioxidants 2022, 11, 925. [Google Scholar] [CrossRef]
- Shen, X.; Xiao, X.; Dong, Z.; Chen, Y. Silicon effects on antioxidative enzymes and lipid peroxidation in leaves and roots of peanut under aluminum stress. Acta Physiol. Plant. 2014, 36, 3063–3069. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Wang, M.; Sun, M.; Gu, Z.; Yang, R. GABA mediates phenolic compounds accumulation and the antioxidant system enhancement in germinated hulless barley under NaCl stress. Food Chem. 2019, 270, 593–601. [Google Scholar] [CrossRef]
- Li, D.; Zhang, D.; Zhang, Z.; Xing, Y.; Sun, N.; Wang, S.; Cai, H. Exogenous application of GABA alleviates alkali damage in alfalfa by increasing the activities of antioxidant enzymes. Agronomy 2022, 12, 1577. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Y.; Ma, Y.; Zhang, Z.; Yue, H.; Wang, L.; Li, J.; Jiao, Y. Effects of exogenous γ-aminobutyric acid (GABA) on photosynthesis and antioxidant system in pepper (Capsicum annuum L.) seedlings under low light stress. J. Plant Growth Regul. 2017, 36, 436–449. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.-P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef] [PubMed]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Perlikowski, D.; Czyżniejewski, M.; Marczak, Ł.; Augustyniak, A.; Kosmala, A. Water deficit affects primary metabolism differently in two Lolium multiflorum/Festuca arundinacea introgression forms with a distinct capacity for photosynthesis and membrane regeneration. Front. Plant Sci. 2016, 7, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.M.; Bibi, S.; et al. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Genet. Eng. 2022, 19, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, N.; Kayano, S.-i.; Kikuzaki, H.; Sumino, K.; Katagiri, K.; Mitani, T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.). J. Agric. Food Chem. 2000, 48, 5512–5516. [Google Scholar] [CrossRef]
- Jardine, K.J.; Sommer, E.D.; Saleska, S.R.; Huxman, T.E.; Harley, P.C.; Abrell, L. Gas phase measurements of pyruvic acid and its volatile metabolites. Environ. Sci. Technol. 2010, 44, 2454–2460. [Google Scholar] [CrossRef]
- Yu, H.; Du, X.; Zhang, F.; Zhang, F.; Hu, Y.; Liu, S.; Jiang, X.; Wang, G.; Liu, D. A mutation in the E2 subunit of the mitochondrial pyruvate dehydrogenase complex in Arabidopsis reduces plant organ size and enhances the accumulation of amino acids and intermediate products of the TCA Cycle. Planta 2012, 236, 387–399. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Ding, Z.; Song, L.; Li, Y.; Ma, D.; Wang, Y.; Shen, J.; Jia, S.; Sun, H.; et al. Aluminum induced metabolic responses in two tea cultivars. Plant. Physiol. Biochem. 2016, 101, 162–172. [Google Scholar] [CrossRef]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Jiang, C. Mechanisms of organic acids and boron induced tolerance of aluminum toxicity: A review. Ecotoxicol. Environ. Saf. 2018, 165, 25–35. [Google Scholar] [CrossRef]
- Ye, J.; Wang, X.; Hu, T.; Zhang, F.; Wang, B.; Li, C.; Yang, T.; Li, H.; Lu, Y.; Giovannoni, J.J. An InDel in the promoter of Al-ACTIVATED MALATE TRANSPORTER9 selected during tomato domestication determines fruit malate contents and aluminum tolerance. Plant Cell 2017, 29, 2249–2268. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.; Vinecky, F.; Duarte, K.E.; Santiago, T.R.; das Chagas Noqueli Casari, R.A.; Hell, A.F.; da Cunha, B.A.D.B.; Martins, P.K.; da Cruz Centeno, D.; de Oliveira Molinari, P.A.; et al. Enhanced aluminum tolerance in sugarcane: Evaluation of SbMATE overexpression and genome-wide identification of ALMTs in Saccharum spp. BMC Plant Biol. 2021, 21, 300. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Gao, J.; You, J.; Liang, Y.; Guan, K.; Yan, S.; Zhan, M.; Yang, Z. Identification of STOP1-like proteins associated with aluminum tolerance in sweet sorghum (Sorghum bicolor L.). Front. Plant Sci. 2018, 9, 258–270. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, D.; Wang, J.; Wang, Z.; Zhang, J.; Bai, R.; Wang, X.; Rubio Wilhelmi, M.D.M.; Blumwald, E.; Zhang, N.; et al. A zinc finger protein SlSZP1 protects SlSTOP1 from SlRAE1-mediated degradation to modulate aluminum resistance. New Phytol. 2022, 236, 165–181. [Google Scholar] [CrossRef]
- Almaghamsi, A.; Nosarzewski, M.; Kanayama, Y.; Archbold, D.D. Effects of abiotic stresses on sorbitol biosynthesis and metabolism in tomato (Solanum lycopersicum). Funct. Plant Biol. 2021, 48, 286–297. [Google Scholar] [CrossRef] [PubMed]
- González-Hernández, A.I.; Scalschi, L.; Vicedo, B.; Marcos-Barbero, E.L.; Morcuende, R.; Camañes, G. Putrescine: A key metabolite involved in plant development, tolerance and resistance responses to stress. Int. J. Mol. Sci. 2022, 23, 2971. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; Joung, J.-G.; Fei, Z.; Jander, G. Interdependence of threonine, methionine and isoleucine metabolism in plants: Accumulation and transcriptional regulation under abiotic stress. Amino Acids 2010, 39, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef]
- Noctor, G.; Arisi, A.-C.M.; Jouanin, L.; Valadier, M.-H.; Roux, Y.; Foyer, C.H. The role of glycine in determining the rate of glutathione synthesis in poplar. Possible implications for glutathione production during stress. Physiol. Plant. 1997, 100, 255–263. [Google Scholar] [CrossRef]
- Shen, B.; Hohmann, S.; Jensen, R.G.; Bohnert, J.H. Roles of sugar alcohols in osmotic stress adaptation. Replacement of glycerol by mannitol and sorbitol in yeast. Plant Physiol. 1999, 121, 45–52. [Google Scholar] [CrossRef]
- Valluru, R.; Van den Ende, W. Myo-inositol and beyond-emerging networks under stress. Plant Sci. 2011, 181, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Van den Ende, W.; De Coninck, B.; Van Laere, A. Plant fructan exohydrolases: A role in signaling and defense? Trends Plant Sci. 2004, 9, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, R.; Zhang, C.; Tang, K.; Sun, M.; Yan, G.; Liu, Q. Manipulation of the rice L-galactose pathway: Evaluation of the effects of transgene overexpression on ascorbate accumulation and abiotic stress tolerance. PLoS ONE 2015, 10, e0125870. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jin, C.; Sun, C.; Wang, J.; Ye, Y.; Zhou, W.; Lu, L.; Lin, X. Inhibition of ethylene production by putrescine alleviates aluminium-induced root inhibition in wheat plants. Sci. Rep. 2016, 6, 18888. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhou, W.; Liang, X.; Zhou, K.; Lin, X. Increased bound putrescine accumulation contributes to the maintenance of antioxidant enzymes and higher aluminum tolerance in wheat. Environ. Pollut. 2019, 252, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Mandal, C.; Ghosh, N.; Maiti, S.; Das, K.; Gupta, S.; Dey, N.; Adak, M.K. Antioxidative responses of Salvinia (Salvinia natans Linn.) to aluminium stress and it’s modulation by polyamine. Physiol. Mol. Biol. Plants 2013, 19, 91–103. [Google Scholar] [CrossRef]
- Al-Mushhin, A.A.M.; Qari, S.H.; Fakhr, M.A.; Alnusairi, G.S.H.; Alnusaire, T.S.; ALrashidi, A.A.; Latef, A.A.H.A.; Ali, O.M.; Khan, A.A.; Soliman, M.H. Exogenous myo-inositol alleviates salt stress by enhancing antioxidants and membrane stability via the upregulation of stress responsive genes in Chenopodium quinoa L. Plants 2021, 10, 2416. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, K.; Ren, G.; Yang, S.; Liu, Y.; Zhang, Z.; Li, Y.; Gong, X.; Ma, F. Myo-inositol mediates reactive oxygen species-induced programmed cell death via salicylic acid-dependent and ethylene-dependent pathways in apple. Hortic. Res. 2020, 7, 138. [Google Scholar] [CrossRef]
- Meng, P.H.; Raynaud, C.; Tcherkez, G.; Blanchet, S.; Massoud, K.; Domenichini, S.; Henry, Y.; Soubigou-Taconnat, L.; Lelarge-Trouverie, C.; Saindrenan, P.; et al. Crosstalks between myo-Inositol metabolism, programmed cell death and basal immunity in Arabidopsis. PLoS ONE 2009, 4, e7364. [Google Scholar] [CrossRef]
- Hoagland, C.R.; Arnon, D.I. The solution-culture method for growing plants without soil. Calif. Agric. Exp. Circ. 1950, 347, 357–359. [Google Scholar]
- Li, Z.; Peng, Y.; Zhang, X.Q.; Pan, M.H.; Yan, Y.H. Exogenous spermidine improves water stress tolerance of white clover (Trifolium repens L.) involved in antioxidant defence, gene expression and proline metabolism. Plant Omics 2014, 7, 517–526. [Google Scholar]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment and growth of barley genotypes under drought stress. Crop. Sci. 1989, 29, 230–233. [Google Scholar] [CrossRef]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Li, Z.; Fu, J.; Shi, D.; Peng, Y. Myo-inositol enhances drought tolerance in creeping bentgrass through alteration of osmotic adjustment, photosynthesis, and antioxidant defense. Crop. Sci. 2020, 60, 2149–2158. [Google Scholar] [CrossRef]
- Cheng, B.; Zhou, M.; Tang, T.; Hassan, M.J.; Zhou, J.; Tan, M.; Li, Z.; Peng, Y. A Trifolium repens flavodoxin-like quinone reductase 1 (TrFQR1) improves plant adaptability to high temperature associated with oxidative homeostasis and lipids remodeling. Plant J. 2023, 115, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Constantine, N.G.; Ries, S.K. Superoxide dismutases: I. occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop. Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.; Peng, Y.; Huang, B. Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci. Rep. 2016, 6, 30338. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Su, M.; Liu, Y.; Chen, M.; Gu, J.; Zhang, J.; Jia, W. Application of ethyl chloroformate derivatization for gas chromatography-mass spectrometry based metabonomic profiling. Anal. Chim. Acta 2007, 583, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Yuan, Y.; Lin, J.; Lin, L.; Zhou, J.; Li, Z. γ-Aminobutyric Acid Priming Alleviates Acid-Aluminum Toxicity to Creeping Bentgrass by Regulating Metabolic Homeostasis. Int. J. Mol. Sci. 2023, 24, 14309. https://doi.org/10.3390/ijms241814309
Zhou M, Yuan Y, Lin J, Lin L, Zhou J, Li Z. γ-Aminobutyric Acid Priming Alleviates Acid-Aluminum Toxicity to Creeping Bentgrass by Regulating Metabolic Homeostasis. International Journal of Molecular Sciences. 2023; 24(18):14309. https://doi.org/10.3390/ijms241814309
Chicago/Turabian StyleZhou, Min, Yan Yuan, Junnan Lin, Long Lin, Jianzhen Zhou, and Zhou Li. 2023. "γ-Aminobutyric Acid Priming Alleviates Acid-Aluminum Toxicity to Creeping Bentgrass by Regulating Metabolic Homeostasis" International Journal of Molecular Sciences 24, no. 18: 14309. https://doi.org/10.3390/ijms241814309
APA StyleZhou, M., Yuan, Y., Lin, J., Lin, L., Zhou, J., & Li, Z. (2023). γ-Aminobutyric Acid Priming Alleviates Acid-Aluminum Toxicity to Creeping Bentgrass by Regulating Metabolic Homeostasis. International Journal of Molecular Sciences, 24(18), 14309. https://doi.org/10.3390/ijms241814309






