βPix Guanine Nucleotide Exchange Factor Regulates Regeneration of Injured Peripheral Axons
Abstract
:1. Introduction
2. Results
2.1. βPix Protein Levels Increase in the Proximal Stump of the Injured Sciatic Nerve
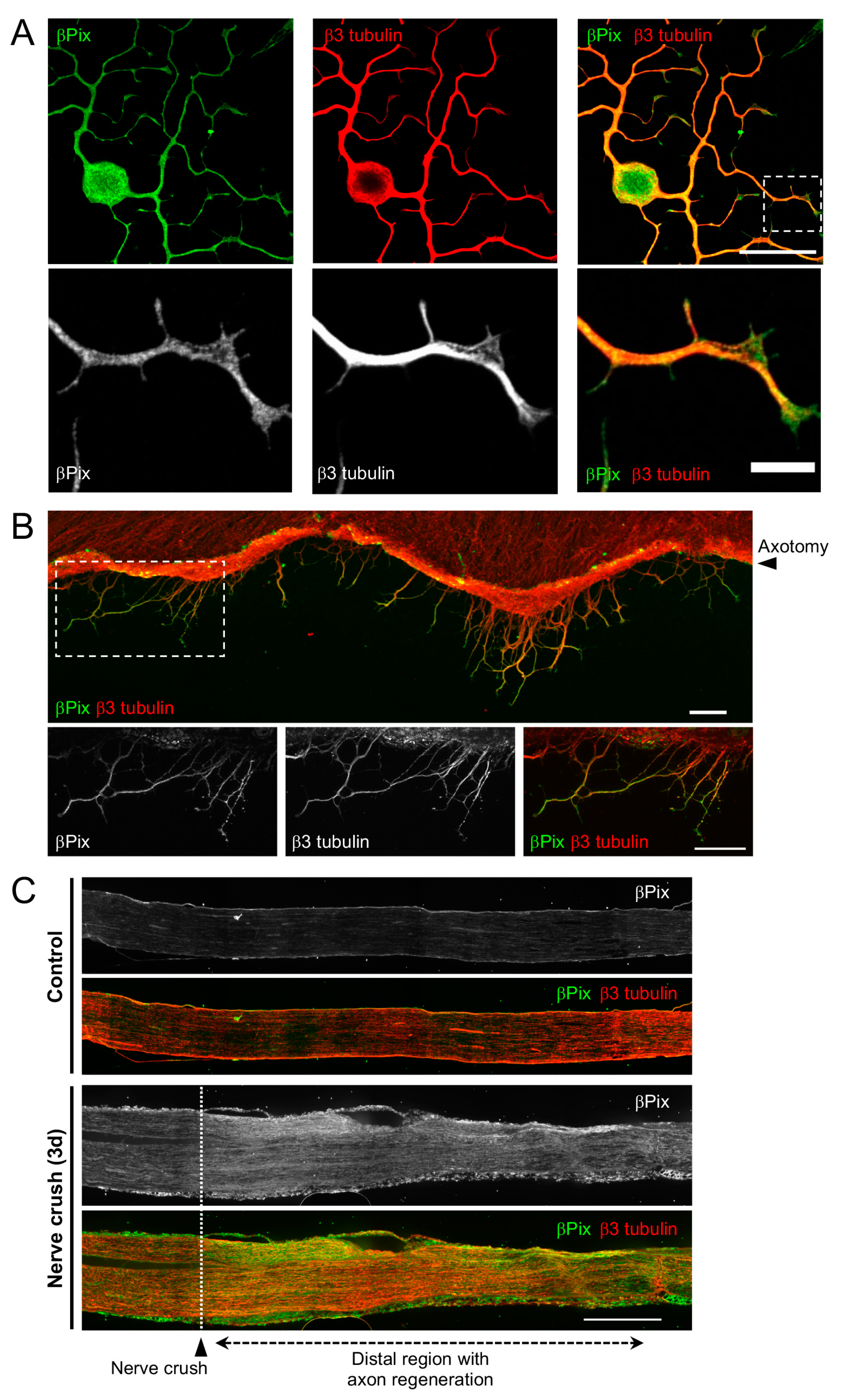
2.2. βPix Protein Is Localized to the Regenerating Axon Tip
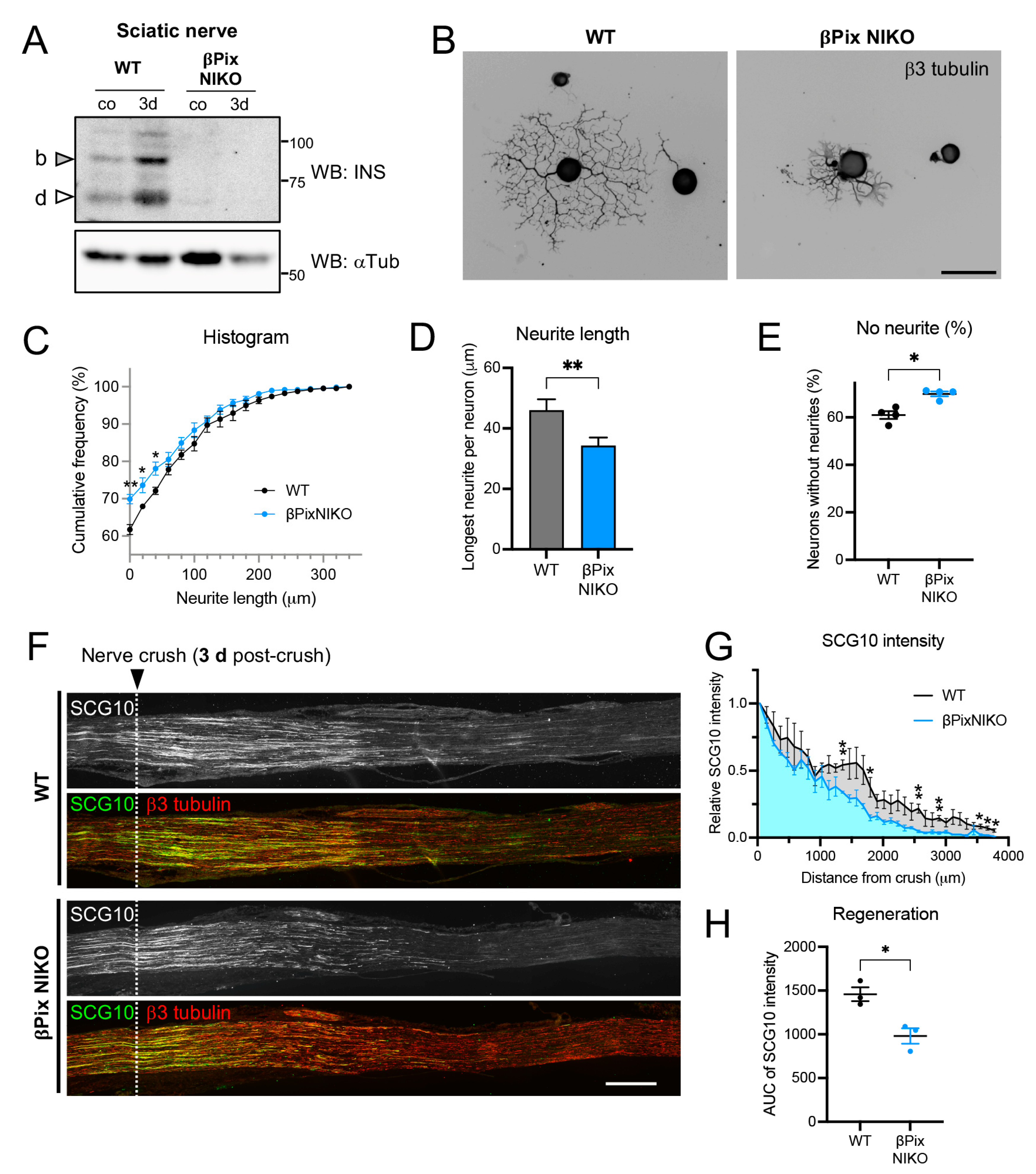
2.3. Axon Regeneration Is Impaired in Mice Deficient for the Neuronal Isoforms of βPix
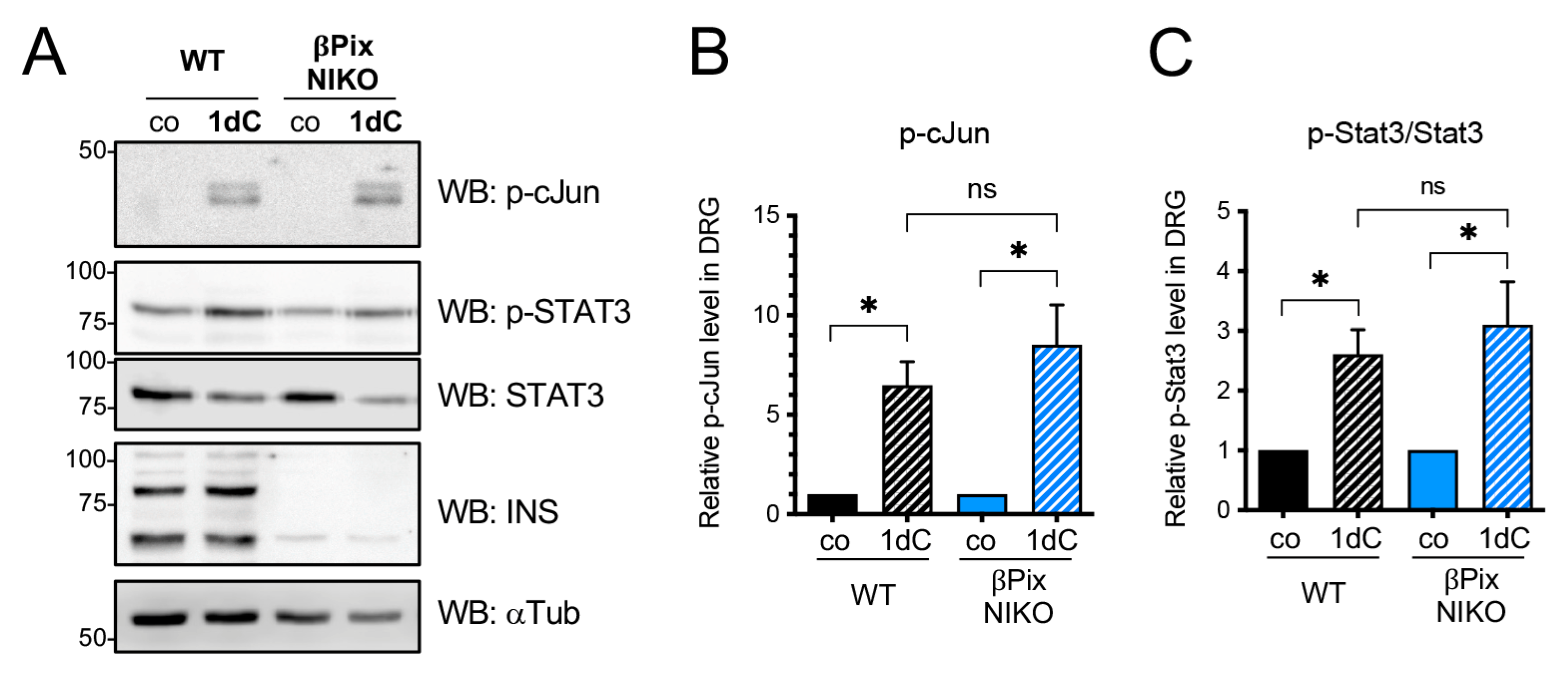
2.4. βPix Neuronal Isoforms Are Not Required for a Conditioning Injury Effect
2.5. Inhibition of Src Kinase Impairs Axonal Outgrowth in DRG Neurons
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Mice and Surgery
4.3. Primary DRG Neuron Cultures
4.4. In Vitro Axon Outgrowth Assay and IF
4.5. In Vivo Axon Regeneration Assay and IF
4.6. Immunoblotting
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary/Abbreviations
| αTub | alpha-tubulin |
| AP-1 | activator protein 1 |
| AUC | area under curve |
| ax | axotomized |
| βPix | PAK-interacting exchange factor β; Arhgef7 |
| CDC42 | cell division cycle 42; cell division control protein 42 homolog |
| cJun | Jun proto-oncogene, ap-1 transcription factor subunit |
| co | control |
| DB | Dbl homology domain |
| DLK | dual leucine zipper kinase |
| DMEM | Dulbecco’s modified eagle medium |
| DMSO | dimethyl sulfoxide |
| DRG | dorsal root ganglion |
| EDTA | ethylenediamine tetraacetic acid |
| EGFR | epidermal growth factor receptor |
| FAK | focal adhesion kinase |
| GBD | Git1-binding domain |
| GDP | guanosine diphosphate |
| GEF | guanine nucleotide exchange factor |
| Gna13 | guanine nucleotide-binding protein subunit alpha-13 |
| GTP | guanosine triphosphate |
| GTPase | guanosine triphosphatase |
| IF | immunofluorescence |
| INS | insert region of βPix |
| JNK | Jun N-terminal kinase |
| LZ | leucine zipper domain |
| MAP3K | mitogen-activated protein kinase kinase kinase |
| NIKO | neuronal isoform knockout |
| PAK | p21 (Rac1) activated kinase |
| PH | pleckstrin homology domain |
| PNS | peripheral nervous system |
| PP2 | an inhibitor of the Src family of protein tyrosine kinases |
| PR | proline-rich domain |
| Rac1 | Rac family small GTPase 1 |
| SCG10 | superior cervical ganglion-10 |
| SDS | sodium dodecyl sulfate |
| SH3 | Src homology 3 domain |
| Src | proto-oncogene c-Src |
| STAT3 | signal transducer and activator of transcription 3 |
| TC10 | Rho-related GTP-binding protein RhoQ |
| WASP | Wiskott-Aldrich syndrome protein |
| WT | wild-type |
References
- Mahar, M.; Cavalli, V. Intrinsic Mechanisms of Neuronal Axon Regeneration. Nat. Rev. Neurosci. 2018, 19, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.E.; Geisler, S.; DiAntonio, A. Dynamic Regulation of SCG10 in Regenerating Axons after Injury. Exp. Neurol. 2014, 252, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huebner, E.A.; Strittmatter, S.M. Axon Regeneration in the Peripheral and Central Nervous Systems. In Results and Problems in Cell Differentiation; Springer: Berlin/Heidelberg, Germany, 2009; Volume 48, pp. 339–351. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T. Peripheral Nerve Regeneration and Muscle Reinnervation. Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef]
- Gangadharan, V.; Zheng, H.; Taberner, F.J.; Landry, J.; Nees, T.A.; Pistolic, J.; Agarwal, N.; Männich, D.; Benes, V.; Helmstaedter, M.; et al. Neuropathic Pain Caused by Miswiring and Abnormal End Organ Targeting. Nature 2022, 606, 137–145. [Google Scholar] [CrossRef]
- Ma, C.H.E.; Omura, T.; Cobos, E.J.; Latrémolière, A.; Ghasemlou, N.; Brenner, G.J.; van Veen, E.; Barrett, L.; Sawada, T.; Gao, F.; et al. Accelerating Axonal Growth Promotes Motor Recovery after Peripheral Nerve Injury in Mice. J. Clin. Investig. 2011, 121, 4332–4347. [Google Scholar] [CrossRef]
- Scheib, J.; Höke, A. Advances in Peripheral Nerve Regeneration. Nat. Rev. Neurol. 2013, 9, 668–676. [Google Scholar] [CrossRef]
- Gumy, L.F.; Yeo, G.S.H.; Tung, Y.-C.L.; Zivraj, K.H.; Willis, D.; Coppola, G.; Lam, B.Y.H.; Twiss, J.L.; Holt, C.E.; Fawcett, J.W. Transcriptome Analysis of Embryonic and Adult Sensory Axons Reveals Changes in MRNA Repertoire Localization. RNA 2011, 17, 85–98. [Google Scholar] [CrossRef]
- Tedeschi, A.; Dupraz, S.; Laskowski, C.J.; Xue, J.; Ulas, T.; Beyer, M.; Schultze, J.L.; Bradke, F. The Calcium Channel Subunit Alpha2delta2 Suppresses Axon Regeneration in the Adult CNS. Neuron 2016, 92, 419–434. [Google Scholar] [CrossRef]
- Zou, H.; Ho, C.; Wong, K.; Tessier-Lavigne, M. Axotomy-Induced Smad1 Activation Promotes Axonal Growth in Adult Sensory Neurons. J. Neurosci. 2009, 29, 7116–7123. [Google Scholar] [CrossRef]
- Patodia, S.; Raivich, G. Role of Transcription Factors in Peripheral Nerve Regeneration. Front. Mol. Neurosci. 2012, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.B.-T.; Doron-mandel, E.; Iavnilovitch, E.; Rishal, I.; Dagan, S.Y.; Tsoory, M.; Coppola, G.; McDonald, M.K.; Gomes, C.; Geschwind, D.H.; et al. Subcellular Knockout of Importin Β1 Perturbs Axonal Retrograde Signaling. Neuron 2012, 75, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Chandran, V.; Coppola, G.; Nawabi, H.; Omura, T.; Versano, R.; Huebner, E.A.; Zhang, A.; Costigan, M.; Yekkirala, A.; Barrett, L.; et al. A Systems-Level Analysis of the Peripheral Nerve Intrinsic Axonal Growth Program. Neuron 2016, 89, 956–970. [Google Scholar] [CrossRef] [PubMed]
- Michaelevski, I.; Segal-Ruder, Y.; Rozenbaum, M.; Medzihradszky, K.F.; Shalem, O.; Coppola, G.; Horn-Saban, S.; Ben-Yaakov, K.; Dagan, S.Y.; Rishal, I.; et al. Signaling to Transcription Networks in the Neuronal Retrograde Injury Response. Sci. Signal. 2010, 3, ra53. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xue, C.; Yuan, Y.; Zhang, R.; Wang, Y.; Wang, Y.; Yu, B.; Liu, J.; Ding, F.; Yang, Y.; et al. The Transcriptional Landscape of Dorsal Root Ganglia after Sciatic Nerve Transection. Sci. Rep. 2015, 5, 16888. [Google Scholar] [CrossRef] [PubMed]
- Stiess, M.; Bradke, F. Neuronal Polarization: The Cytoskeleton Leads the Way. Dev. Neurobiol. 2011, 71, 430–444. [Google Scholar] [CrossRef]
- Bradke, F.; Fawcett, J.W.; Spira, M.E. Assembly of a New Growth Cone after Axotomy: The Precursor to Axon Regeneration. Nat. Rev. Neurosci. 2012, 13, 183–193. [Google Scholar] [CrossRef]
- Hur, E.-M.; Saijilafu; Zhou, F.-Q. Growing the Growth Cone: Remodeling the Cytoskeleton to Promote Axon Regeneration. Trends Neurosci. 2011, 35, 1–11. [Google Scholar] [CrossRef]
- Ng, J.; Nardine, T.; Harms, M.; Tzu, J.; Goldstein, A.; Sun, Y.; Dietzl, G.; Dickson, B.J.; Luo, L. Rac GTPases Control Axon Growth, Guidance and Branching. Nature 2002, 416, 442–447. [Google Scholar] [CrossRef]
- Stankiewicz, T.R.; Linseman, D.A.; Moccia, F. Rho Family GTPases: Key Players in Neuronal Development, Neuronal Survival, and Neurodegeneration. Front. Cell. Neurosci. 2014, 8, 314. [Google Scholar] [CrossRef]
- Rossman, K.L.; Der, C.J.; Sondek, J. GEF Means Go: Turning on RHO GTPases with Guanine Nucleotide-Exchange Factors. Nat. Rev. Mol. Cell Biol. 2005, 6, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Debant, A. Function and Regulation of the Rho Guanine Nucleotide Exchange Factor Trio. Small GTPases 2014, 5, e983880. [Google Scholar] [CrossRef] [PubMed]
- Joo, E.; Olson, M.F. Regulation and Functions of the RhoA Regulatory Guanine Nucleotide Exchange Factor GEF-H1. Small GTPases 2021, 12, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Lee, S.J.; Lee, E.; Kim, D.; Park, D. ΒPix Heterozygous Mice Have Defects in Neuronal Morphology and Social Interaction. Biochem. Biophys. Res. Commun. 2019, 516, 1204–1210. [Google Scholar] [CrossRef]
- Kang, T.; Lee, S.J.; Kwon, Y.; Park, D. Loss of ΒPix Causes Defects in Early Embryonic Development, and Cell Spreading and Platelet-Derived Growth Factor-Induced Chemotaxis in Mouse Embryonic Fibroblasts. Mol. Cells 2019, 42, 589–596. [Google Scholar] [CrossRef]
- Kwon, Y.; Jeon, Y.W.; Kwon, M.; Cho, Y.; Park, D.; Shin, J.E. ΒPix-d Promotes Tubulin Acetylation and Neurite Outgrowth through a PAK/Stathmin1 Signaling Pathway. PLoS ONE 2020, 15, e0230814. [Google Scholar] [CrossRef]
- López Tobón, A.; Suresh, M.; Jin, J.; Vitriolo, A.; Pietralla, T.; Tedford, K.; Bossenz, M.; Mahnken, K.; Kiefer, F.; Testa, G.; et al. The Guanine Nucleotide Exchange Factor Arhgef7/ΒPix Promotes Axon Formation Upstream of TC10. Sci. Rep. 2018, 8, 8811. [Google Scholar] [CrossRef]
- Park, E.; Na, M.; Choi, J.; Kim, S.; Lee, J.R.; Yoon, J.; Park, D.; Sheng, M.; Kim, E. The Shank Family of Postsynaptic Density Proteins Interacts with and Promotes Synaptic Accumulation of the ΒPIX Guanine Nucleotide Exchange Factor for Rac1 and Cdc42. J. Biol. Chem. 2003, 278, 19220–19229. [Google Scholar] [CrossRef]
- Saneyoshi, T.; Wayman, G.; Fortin, D.; Davare, M.; Hoshi, N.; Nozaki, N.; Natsume, T.; Soderling, T.R. Activity-Dependent Synaptogenesis: Regulation by a CaM-Kinase Kinase/CaM-Kinase I/ΒPIX Signaling Complex. Neuron 2008, 57, 94–107. [Google Scholar] [CrossRef]
- Shin, M.-S.; Song, S.-H.; Shin, J.E.; Lee, S.-H.; Huh, S.-O.; Park, D. Src-Mediated Phosphorylation of ΒPix-b Regulates Dendritic Spine Morphogenesis. J. Cell Sci. 2019, 132, jcs224980. [Google Scholar] [CrossRef]
- Kim, S.; Kim, T.; Lee, D.; Park, S.H.; Kim, H.; Park, D. Molecular Cloning of Neuronally Expressed Mouse ΒPix Isoforms. Biochem. Biophys. Res. Commun. 2000, 272, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Park, D. Molecular Cloning and Characterization of a Novel Mouse Pix Isoform. Mol. Cells 2001, 11, 89–94. [Google Scholar] [PubMed]
- Shin, J.E.; Ha, H.; Kim, Y.K.; Cho, Y.; DiAntonio, A. DLK Regulates a Distinctive Transcriptional Regeneration Program after Peripheral Nerve Injury. Neurobiol. Dis. 2019, 127, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yang, S.J.; Kim, D.H.; Pak, J.H.; Lee, K.H.; Choi, K.H.; Park, D.; Rhee, S. Interaction of Microtubules and Actin with the N-Terminus of Βpix-BL Directs Cellular Pinocytosis. Mol. Cell. Biochem. 2011, 351, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Avraham, O.; Deng, P.Y.; Jones, S.; Kuruvilla, R.; Semenkovich, C.F.; Klyachko, V.A.; Cavalli, V. Satellite Glial Cells Promote Regenerative Growth in Sensory Neurons. Nat. Commun. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Rishal, I.; Fainzilber, M. Axon–Soma Communication in Neuronal Injury. Nat. Rev. Neurosci. 2014, 15, 32–42. [Google Scholar] [CrossRef]
- Shin, J.E.; Cho, Y.; Beirowski, B.; Milbrandt, J.; Cavalli, V.; DiAntonio, A. Dual Leucine Zipper Kinase Is Required for Retrograde Injury Signaling and Axonal Regeneration. Neuron 2012, 74, 1015–1022. [Google Scholar] [CrossRef]
- Raivich, G.; Bohatschek, M.; Da Costa, C.; Iwata, O.; Galiano, M.; Hristova, M.; Nateri, A.S.; Makwana, M.; Riera-Sans, L.; Wolfer, D.P.; et al. The AP-1 Transcription Factor c-Jun Is Required for Efficient Axonal Regeneration. Neuron 2004, 43, 57–67. [Google Scholar] [CrossRef]
- Ben-Yaakov, K.; Dagan, S.Y.; Segal-Ruder, Y.; Shalem, O.; Vuppalanchi, D.; Willis, D.E.; Yudin, D.; Rishal, I.; Rother, F.; Bader, M.; et al. Axonal Transcription Factors Signal Retrogradely in Lesioned Peripheral Nerve. Eur. Mol. Biol. Organ. J. 2012, 31, 1350–1363. [Google Scholar] [CrossRef]
- Frey, E.; Valakh, V.; Karney-grobe, S.; Shi, Y.; Milbrandt, J.; Diantonio, A. An In Vitro Assay to Study Induction of the Regenerative State in Sensory Neurons. Exp. Neurol. 2015, 263, 350–363. [Google Scholar] [CrossRef]
- Ertürk, A.; Hellal, F.; Enes, J.; Bradke, F. Disorganized Microtubules Underlie the Formation of Retraction Bulbs and the Failure of Axonal Regeneration. J. Neurosci. 2007, 27, 9169–9180. [Google Scholar] [CrossRef] [PubMed]
- Kerschensteiner, M.; Schwab, M.E.; Lichtman, J.W.; Misgeld, T. In Vivo Imaging of Axonal Degeneration and Regeneration in the Injured Spinal Cord. Nat. Med. 2005, 11, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Koinuma, S.; Negishi, R.; Nomura, R.; Sato, K.; Kojima, T.; Segi-Nishida, E.; Goitsuka, R.; Iwakura, Y.; Wada, N.; Koriyama, Y.; et al. TC10, a Rho Family GTPase, Is Required for Efficient Axon Regeneration in a Neuron-Autonomous Manner. J. Neurochem. 2021, 157, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Koinuma, S. TC10 as an Essential Molecule in Axon Regeneration through Membrane Supply and Microtubule Stabilization. Neural Regen. Res. 2022, 17, 87–88. [Google Scholar] [CrossRef]
- Teo, C.R.; Casey, P.J.; Rasheed, S.A.K. The GNA13-RhoA Signaling Axis Suppresses Expression of Tumor Protective Kallikreins. Cell. Signal. 2016, 28, 1479–1488. [Google Scholar] [CrossRef]
- Rasheed, S.A.K.; Leong, H.S.; Lakshmanan, M.; Raju, A.; Dadlani, D.; Chong, F.T.; Shannon, N.B.; Rajarethinam, R.; Skanthakumar, T.; Tan, E.Y.; et al. GNA13 Expression Promotes Drug Resistance and Tumor-Initiating Phenotypes in Squamous Cell Cancers. Oncogene 2018, 37, 1340–1353. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, D. Targeting Src Family Kinases in Anti-Cancer Therapies: Turning Promise into Triumph. Trends Pharmacol. Sci. 2012, 33, 122–128. [Google Scholar] [CrossRef]
- Nichols, E.L.; Smith, C.J. Functional Regeneration of the Sensory Root via Axonal Invasion. Cell Rep. 2020, 30, 9–17. [Google Scholar] [CrossRef]
- Robles, E.; Woo, S.; Gomez, T.M. Src-Dependent Tyrosine Phosphorylation at the Tips of Growth Cone Filopodia Promotes Extension. J. Neurosci. 2005, 25, 7669–7681. [Google Scholar] [CrossRef]
- Ferrando, I.M.; Chaerkady, R.; Zhong, J.; Molina, H.; Jacob, H.K.C.; Herbst-Robinson, K.; Dancy, B.M.; Katju, V.; Bose, R.; Zhang, J.; et al. Identification of Targets of C-Src Tyrosine Kinase by Chemical Complementation and Phosphoproteomics. Mol. Cell. Proteom. 2012, 11, 355–369. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Takagawa, K.; Oya, T.; Yang, H.F.; Gao, Z.Y.; Kawaguchi, M.; Ishii, Y.; Sasaoka, T.; Owada, K.; Furuta, I.; et al. Active Src Expression Is Induced after Rat Peripheral Nerve Injury. Glia 2003, 42, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Cho, Y. Experimental Model Systems for Understanding Human Axonal Injury Responses. Int. J. Mol. Sci. 2021, 22, 474. [Google Scholar] [CrossRef]
- Takenawa, T.; Suetsugu, S. The WASP-WAVE Protein Network: Connecting the Membrane to the Cytoskeleton. Nat. Rev. Mol. Cell Biol. 2007, 8, 37–48. [Google Scholar] [CrossRef]
- Verboon, J.M.; Parkhurst, S.M. Rho Family GTPases Bring a Familiar Ring to Cell Wound Repair. Small GTPases 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Meijering, E.; Jacob, M.; Sarria, J.-C.F.; Steiner, P.; Hirling, H.; Unser, M. Design and Validation of a Tool for Neurite Tracing and Analysis in Fluorescence Microscopy Images. Cytom. Part A 2004, 58A, 167–176. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, Y.; Shin, Y.K.; Kim, H.; Choi, Y.Y.; Kang, M.; Kwon, Y.; Cho, Y.; Chi, S.W.; Shin, J.E. βPix Guanine Nucleotide Exchange Factor Regulates Regeneration of Injured Peripheral Axons. Int. J. Mol. Sci. 2023, 24, 14357. https://doi.org/10.3390/ijms241814357
Jeon Y, Shin YK, Kim H, Choi YY, Kang M, Kwon Y, Cho Y, Chi SW, Shin JE. βPix Guanine Nucleotide Exchange Factor Regulates Regeneration of Injured Peripheral Axons. International Journal of Molecular Sciences. 2023; 24(18):14357. https://doi.org/10.3390/ijms241814357
Chicago/Turabian StyleJeon, Yewon, Yoon Kyung Shin, Hwigyeong Kim, Yun Young Choi, Minjae Kang, Younghee Kwon, Yongcheol Cho, Sung Wook Chi, and Jung Eun Shin. 2023. "βPix Guanine Nucleotide Exchange Factor Regulates Regeneration of Injured Peripheral Axons" International Journal of Molecular Sciences 24, no. 18: 14357. https://doi.org/10.3390/ijms241814357
APA StyleJeon, Y., Shin, Y. K., Kim, H., Choi, Y. Y., Kang, M., Kwon, Y., Cho, Y., Chi, S. W., & Shin, J. E. (2023). βPix Guanine Nucleotide Exchange Factor Regulates Regeneration of Injured Peripheral Axons. International Journal of Molecular Sciences, 24(18), 14357. https://doi.org/10.3390/ijms241814357






