Resistant and Susceptible Pinus thunbergii ParL. Show Highly Divergent Patterns of Differentially Expressed Genes during the Process of Infection by Bursaphelenchus xylophilus
Abstract
1. Introduction
2. Results
2.1. DEGs Vary in Response to PWN Inoculation of Susceptible and Resistant P. thunbergii
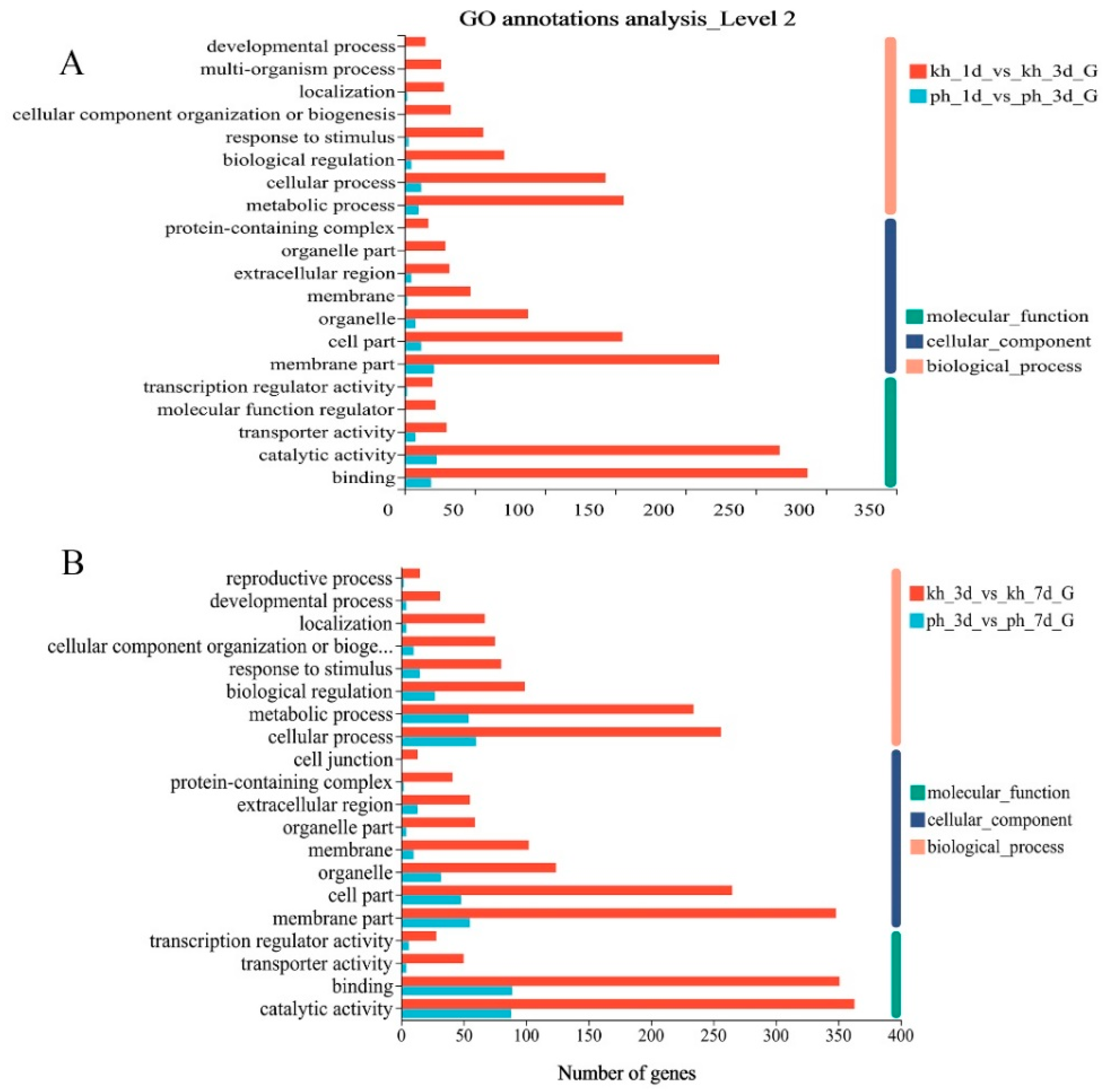
2.2. Functional Annotation and Enrichment Analysis of DEGs
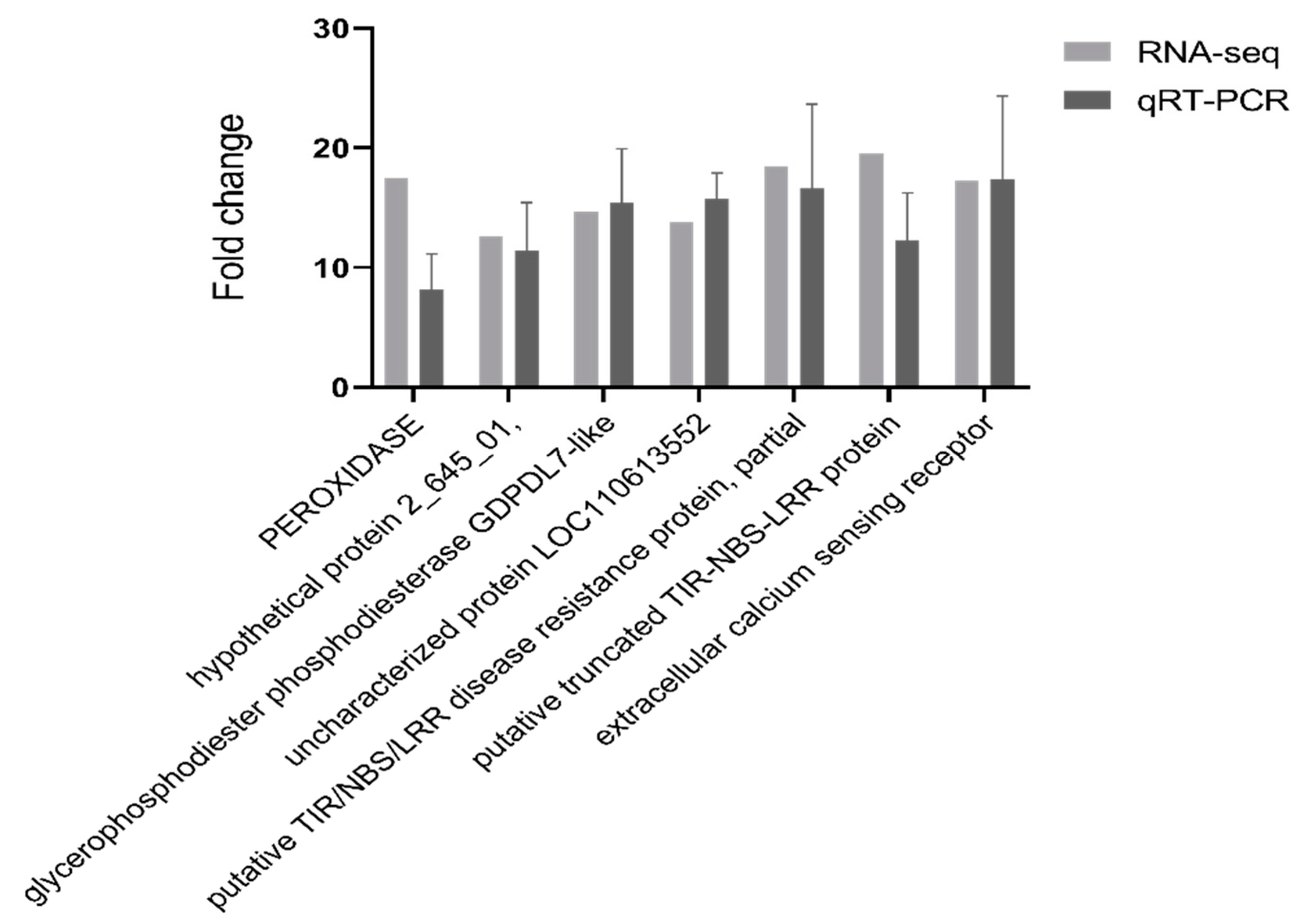
2.3. The qPCR Validation
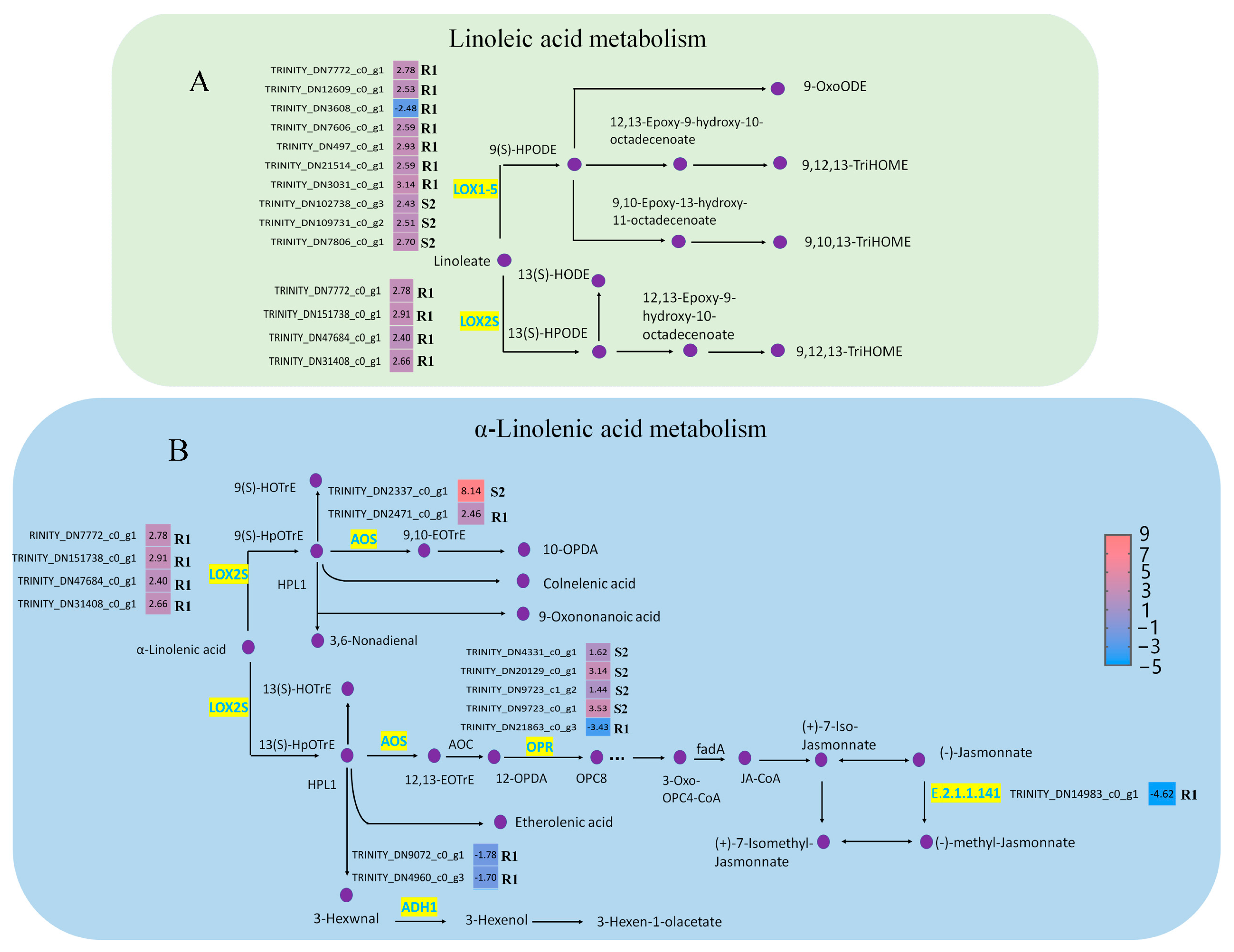
2.4. The Alpha-Linolenic Acid and Linoleic Acid Metabolism Were Involved in the Response to PWN
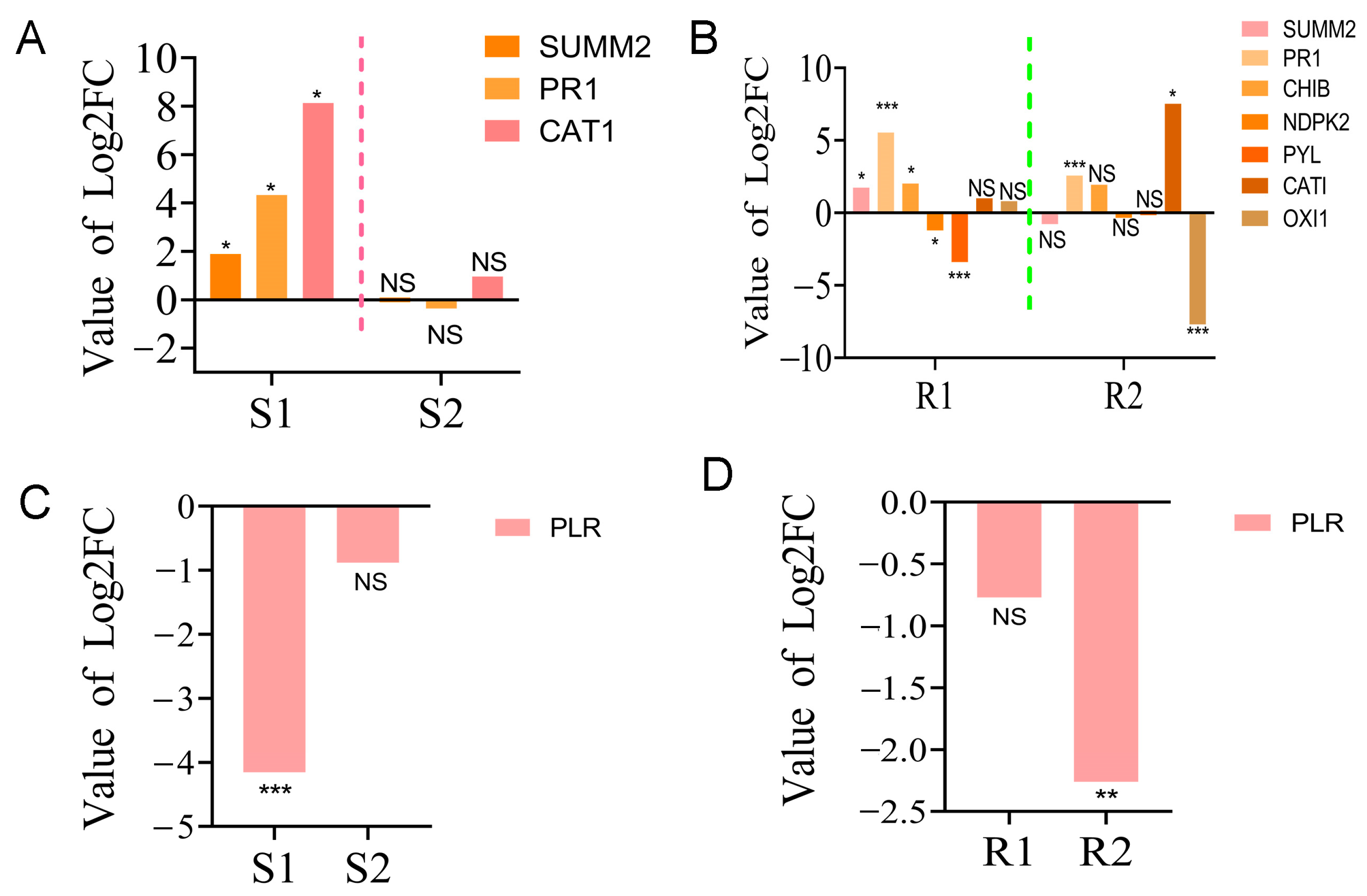
2.5. Regulation of the “MAPK Signaling Pathway—Plant” and “Biosynthesis of Various Secondary Metabolites—Part 2” Contributed to PWN Resistance
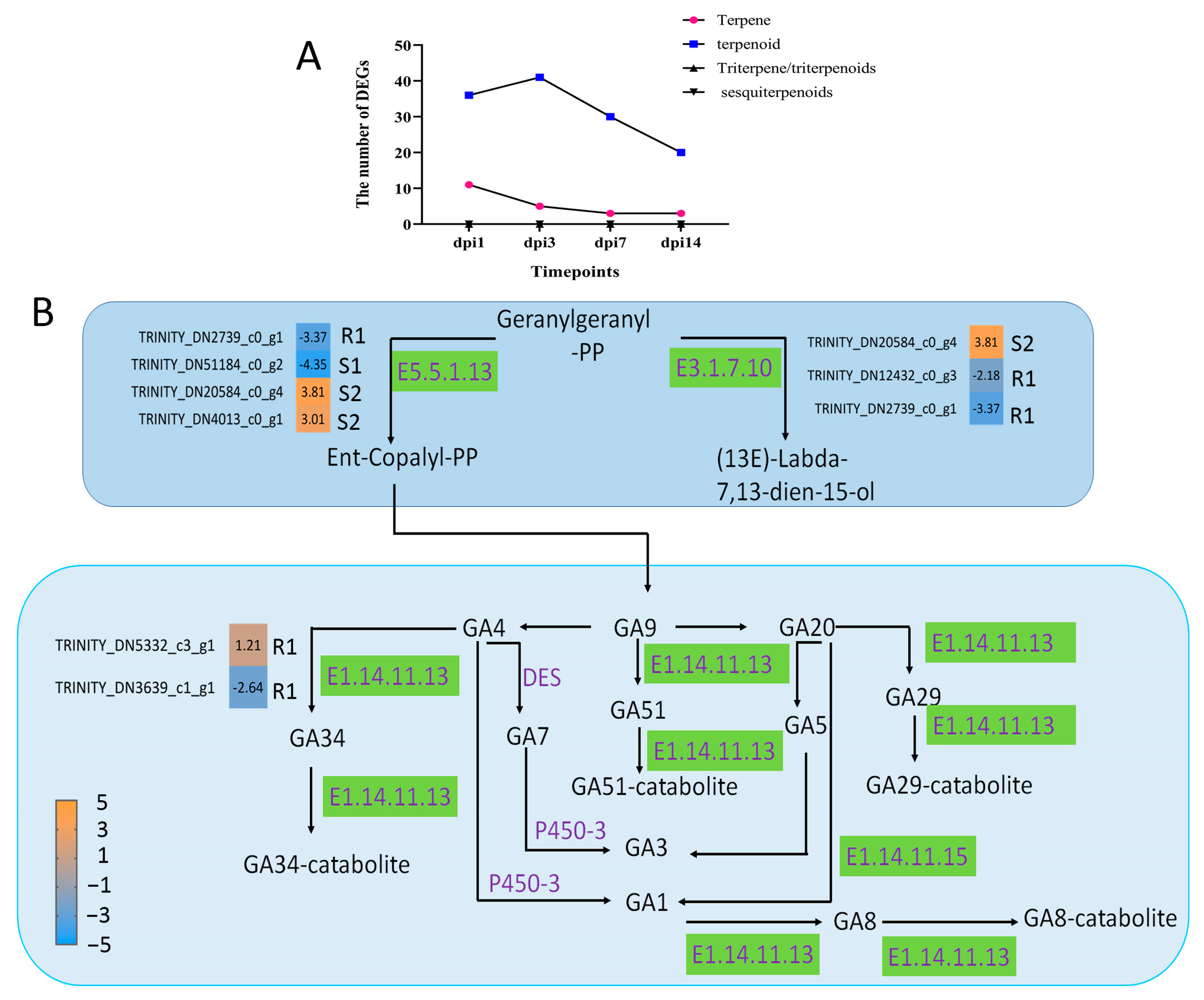
2.6. Characterization of Gene Expression Related to Terpenoids Biosynthesis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Pine Wood Nematode Inoculation and Sampling
4.3. RNA Isolation Procedure and Quantification
4.4. Quantitative Real-Time PCR Analysis
4.5. De Novo Assembly and Annotation
4.6. Differential Expression Analysis and Functional Enrichment
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maruyama, T.E.; Hosoi, Y. Post-maturation treatment improves and synchronizes somatic embryo germination of three species of Japanese pines. Plant Cell Tissue Organ Cult. 2012, 110, 45–52. [Google Scholar] [CrossRef]
- Ho, U.H.; Song, S.R.; Pak, H.S.; Kim, K.; Ho, T.S.; Ju, I.Y. Genetic evidence of stable northward extension of Pinus thunbergii Parl. forests in the Democratic People’s Republic of Korea. Genet. Resour. Crop Evol. 2022, 69, 2105–2114. [Google Scholar] [CrossRef]
- Choi, J.; Cha, D.; Kim, D.S.; Lee, S. Review of Japanese Pine Bast Scale, Matsucoccus matsumurae (Kuwana) (Coccomorpha: Matsucoccidae), Occurring on Japanese Black Pine (Pinus thunbergii Parl.) and Japanese Red Pine (P. densiflora Siebold & Zucc.) from Korea. Forests 2019, 10, 639. [Google Scholar]
- Zhang, P.; Wen, Y.; Wang, L.; Zhang, H.; Wang, G.G.; Wu, T. Leaf Structural Carbohydrate Decreased for Pinus thunbergii along Coast–Inland Gradients. Forests 2020, 11, 449. [Google Scholar] [CrossRef]
- Todo, C.; Yamase, K.; Ikeno, H.; Tanikawa, T.; Ohashi, M.; Hirano, Y. Maximum rooting depth of Pinus thunbergii Parl. estimated with depth at the center point of rotation in a tree-pulling experiment in a coastal forest in Japan. Forests 2022, 13, 1506. [Google Scholar] [CrossRef]
- Ding, X.L.; Lin, S.X.; Zhao, R.; Ye, J. First report of needle blight on Pinus thunbergii Parl. caused by Fusarium proliferatum in China. Plant Dis. 2022, 106, 2989. [Google Scholar] [CrossRef]
- Qi, Y.; Duan, C.; Ren, L.; Wu, H. Growth dynamics of galls and chemical defence response of Pinus thunbergii Parl. to the pine needle gall midge, Thecodiplosis japonensis Uchida & Inouye (Diptera: Cecidomyiidae). Sci. Rep. 2020, 10, 12289. [Google Scholar]
- Zhang, X.; Xing, J.; Zhu, X.; Zhang, B.; Liu, C.; Hong, L.; Liu, Y.; Chen, Y.; Wen, Z. Diversity and community structure of ectomycorrhizal fungi in Pinus thunbergii coastal forests bordering the Yellow Sea of China. Braz. J. Microbiol. 2021, 52, 801–809. [Google Scholar] [CrossRef]
- Wang, X.; Wu, X.; Wen, T.; Feng, Y.Q.; Zhang, Y. Transcriptomic analysis reveals differentially expressed genes associated with pine wood nematode resistance in resistant P. thunbergii. Tree Physiol. 2023, 43, 995–1008. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, T.Y.; Wu, X.Q.; Hu, L.; Qiu, Y.; Rui, L. The Bursaphelenchus xylophilus effector BxML1 targets the cyclophilin protein (CyP) to promote parasitism and virulence in pine. BMC Plant Biol. 2022, 22, 216. [Google Scholar] [CrossRef]
- Yano, M. Investigation on the cause of pine mortality in Nagasaki. Prefect. Sanrinkoho 1913, 4, 1–14. [Google Scholar]
- Mamiya, Y. History of pine wilt disease in Japan. J. Nematol. 1988, 20, 219–226. [Google Scholar] [PubMed]
- Togashi, K.; Shigesada, N. Spread of the pinewood nematode vectored by the Japanese pine sawyer: Modeling and analytical approaches. Popul. Ecol. 2006, 48, 271–283. [Google Scholar] [CrossRef]
- Abelleira, A.; Picoaga, A.; Mansilla, J.P.; Aguin, O. Detection of Bursaphelenchus Xylophilus, causal agent of pine wilt diseaseon Pinus pinaster in northwestern Spain. Plant Dis. 2011, 96, 770–780. [Google Scholar]
- Shin HLee, H.; Woo, K.S.; Noh, E.W.; Koo, Y.B.; Lee, K.J. Identification of genes upregulated by pinewood nematode inoculation in Japanese red pine. Tree Physiol. 2009, 29, 411–421. [Google Scholar] [CrossRef]
- Hirao, T.; Fukatsu, E.; Watanabe, A. Characterization of resistance to pine wood nematode infection in Pinus thunbergii using suppression subtractive hybridization. BMC Plant Biol. 2012, 12, 207–212. [Google Scholar] [CrossRef]
- Lee, J.P.; Sekhon, S.S.; Kim, J.H.; Kim, S.C.; Cho, B.K.; Ahn, J.Y.; Kim, Y.H. The Pine Wood Nematode Bursaphelenchus xylophilus and Molecular Diagnostic Methods. Mol. Cell Toxicol. 2020, 17, 1–13. [Google Scholar] [CrossRef]
- Kong, Q.Q.; Ding, X.L.; Chen, Y.F.; Ye, J. Comparison of Morphological Indexes and the Pathogenicity of Bursaphelenchus xylophilus in Northern and Southern China. Forests 2021, 12, 310. [Google Scholar] [CrossRef]
- Ikegami, M.; Jenkins Thomas, A.R. Estimate global risks of a forest disease under current and future climates using species distribution model and simple thermal model-pine wilt disease as a model case. Forest Ecol. Manag. 2018, 409, 343–352. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Matsunaga, K.; Watanabe, A. Influence of temperature on pine wilt disease progression in Pinus thunbergii seedlings. Eur. J. Plant Pathol. 2020, 156, 581–590. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Ding, X.L.; Wang, L.; Wang, X.; Chen, F. The detection of pine wilt disease: A literature review. Int. J. Mol. Sci. 2022, 23, 10797. [Google Scholar] [CrossRef]
- Lim, W.; Choi, K.; Cho, W.; Chang, B.; Ko, D.W. Efficient dead pine tree detecting method in the forest damaged by pine wood nematode (Bursaphelenchus xylophilus) through utilizing unmanned aerial vehicles and deep learning-based object detection techniques. For. Sci. Technol. 2022, 18, 36–43. [Google Scholar] [CrossRef]
- Hussain, T.; Aslam, A.; Ozair, M.; Tasneem, F.; Gomez-Aguilar, J.F. Dynamical aspects of pine wilt disease and control measures. Chaos Solitons Fractals 2021, 145, 110764. [Google Scholar] [CrossRef]
- Hirao, T.; Matsunaga, K.; Hirakawa, H.; Shirasawa, k.; Isoda, K.; Mishima, K.; Tamura, M.; Watanabe, A. Construction of genetic linkage map and identification of a novel major locus for resistance to pine wood nematode in Japanese black pine (Pinus thunbergii). BMC Plant Biol. 2019, 19, 424. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y. Breeding project on resistance to the pine-wood nematode, An outline of the research and the achievement of the project for ten years. Bull. For. Tree Breed. Inst. 1989, 7, 1–84. [Google Scholar]
- Toda, T. Studies on the breeding for resistance to the pine wilt disease in Pinus densiflora and P. thunbergii. Bull For. Tree Breed Cent. 2004, 20, 83–217. [Google Scholar]
- Wu, X.Q.; Zhang, Y.; Chen, W.S. Resistance and histopathological observation of wilt-resistant Pinus thunbergia families from Japan to Bursaphelenchus xylophilus. Acta Phytopathol. Sin. 2008, 38, 44–50. [Google Scholar]
- Sun, T.; Wang, Y.; Zhu, L.; Wu, X.; Ye, J. Plant regeneration by somatic embryogenesis in Pinus thunbergii resistant to the pine wood nematode. Can. J. Forest Res. 2019, 49, 1604–1612. [Google Scholar] [CrossRef]
- Parker, J. Early Leads to Mechanisms of Plant Cultivar-Specific Disease Resistance. Plant Cell 2019, 31, 1410–1411. [Google Scholar] [CrossRef]
- Jacob, P.; Hige, J.; Dangl, J.L. Is localized acquired resistance the mechanism for effector-triggered disease resistance in plants? Nat. Plants 2023, 9, 1184–1190. [Google Scholar]
- Gaspar, D.; Trindade, C.; Usié, A.; Meireles, B.; Barbosa, P.; Fortes, A.M.; Pesquita, C.; Costa, R.L.; Ramos, A.M. Expression Profiling in Pinus pinaster in response to infection with the pine wood nematode Bursaphelenchus xylophilus. Forests 2017, 8, 279. [Google Scholar] [CrossRef]
- Neale, D.B.; Savolainen, O. Association genetics of complex traits in conifers. Trends Plant Sci. 2004, 9, 325–330. [Google Scholar] [CrossRef]
- Guevara, M.A.; Soto, A.; Collada, C.; Plomion, C.; Savolainen, O.; Neale, D.B.; Gonzalez-Martinez, S.C.; Cervera, M.T. Genomics applied to the study of adaptation in pine species. Invest. Agrar. Sist. Recur. For. 2005, 14, 292–306. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Parchman, T.L.; Geist, K.S.; Grahnen, J.A.; Benkman, C.W.; Buerkle, C.A. Transcriptome sequencing in an ecologically important tree species: Assembly, annotation, and marker discovery. BMC Genom. 2010, 11, 180. [Google Scholar] [CrossRef]
- Lee, I.H.; Han, H.; Koh, Y.; Kim, I.s.; Lee, S.W.; Shim, D. Comparative transcriptome analysis of Pinus densiflora following inoculation with pathogenic (Bursaphelenchus xylophilus) or non-pathogenic nematodes (B. thailandae). Sci. Rep. 2018, 9, 12180. [Google Scholar] [CrossRef] [PubMed]
- Modesto, I.; Sterck, L.; Arbona, V.; Gomaz-Cadenas, A.; Carrasquinho, I.; Van de Peer, Y. Insights into the mechanisms implicated in Pinus pinaster resistance to pine wood nematode. Front. Plant Sci. 2021, 12, 690857. [Google Scholar] [CrossRef]
- Liu, Q.; Wei, Y.; Xu, L.; Hao, Y.; Chen, X.; Zhou, Z. Transcriptomic profiling reveals differentially expressed genes associated with pine wood nematode resistance in masson pine (Pinus massoniana Lamb.). Sci. Rep. 2018, 7, 4693. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Gutiérrez, M.; Villar, L.; Díaz, R. Virulence of seven pathogenic Bursaphelenchus xylophilus isolates in Pinus pinaster and Pinus radiata seedlings and its relation with multiplication. For. Pathol. 2021, 51, e12677. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Y.; Wu, X.; Ye, J.; Cheng, F. Promoting the application of Pinus thunbergii Parl. to enhance the growth and survival rates of post-germination somatic plantlets. BMC Plant Biol. 2023, 23, 195. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Wang, Y.; Li, C.; Zhang, X.; Liu, H.; Jiang, C.; Bian, Z.; Xu, R. Deletion of all three MAP kinase genes results in severe defects in stress responses and pathogenesis in Fusarium graminearum. Stress Biol. 2022, 2, 6. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, S.; Xu, Q.L.; Xiao, J. Transcriptome responses of grafted Citrus sinensis plants to inoculation with the arbuscular mycorrhizal fungus Glomus versiforme. Trees. 2016, 30, 1073–1082. [Google Scholar] [CrossRef]
- Fernandes, L.B.; Ghag, S.B. Molecular insights into the jasmonate signaling and associated defense responses against wilt caused by Fusarium oxysporum. Plant Physiol. Biochem. 2022, 174, 22–34. [Google Scholar] [CrossRef]
- Fu, J.; Ren, R.; Jin, S.; Fang, R.; Wen, Z.; Yang, M.; Wang, X.; Lu, G.; Yang, Y.; Qi, J. Overexpression of a putative 12-oxophytodienoate reductase gene, EpOPR1, enhances acetylshikonin production in Echium plantagineum. Vitr. Cell. Dev. Biol. -Plant 2022, 58, 311–320. [Google Scholar] [CrossRef]
- Modesto, I.; Mendes, A.; Carrasquinho, I.; Miguel, C.M. Molecular defense response of pine trees (Pinus spp.) to the Parasitic Nematode Bursaphelenchus xylophilus. Cells 2022, 11, 3208. [Google Scholar] [CrossRef] [PubMed]
- Pyo, Y.; Moon, H.; Nugroho, A.B.D.; Yang, S.W.; Jung, I.L.; Kim, D.H. Transcriptome analysis revealed that jasmonic acid biosynthesis/signaling is involved in plant response to Strontium stress. Ecotoxicol. Environ. Saf. 2022, 237, 113552. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pang, J.; Zhang, F.; Sun, L.; Yang, L.; Siddique, K.H.M. Transcriptomic and metabolomics-based analysis of key biological pathways reveals the role of lipid metabolism in response to salt stress in the root system of Brassica napus. Plant Growth Regul. 2022, 97, 127–141. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Langer, S.; Carrasquinho, I.; Bergstrom, E.; Larson, T.; Thomas-Oates, J.; Antonio, C. Pinus pinaster Early Hormonal Defense Responses to Pinewood Nematode (Bursaphelenchus xylophilus) Infection. Metabolites 2021, 11, 227. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Ohta, H.; Okawa, K.; Iwamatsu, A.; Shimada, H.; Masuda, T.; Takamiya, K. Cloning of chlorophyllase, the key enzyme in chlorophyll degradation: Finding of a lipase motif and the induction by methyl jasmonate. Proc. Natl. Acad. Sci. USA 1999, 96, 15362–15367. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, Y.; Tang, W.; Chen, J.; Ge, Y.; Li, J. Concentration-dependent impacts of exogenous methyl jasmonate (MeJA) on chlorophyll degradation of apple fruit during ripening. Postharvest Biol. Technol. 2023, 203, 112398. [Google Scholar] [CrossRef]
- Völz, R.; Harris, W.; Hirt, H.; Lee, Y.H. ROS homeostasis mediated by MPK4 and SUMM2 determines synergid cell death. Nat. Commun. 2022, 13, 1746. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Minaker, S.; Roth, C.; Huang, S.; Hieter, P.; Liper, V.; Wiermer, M.; Li, X. An E4 ligase facilitates polyubiquitination of plant immune receptor resistance proteins in Arabidopsis. Plant Cell 2014, 26, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Liang, C.; Wang, S.; Hou, Y.; Gao, L.; Liu, L.; He, W.; Ma, W.; Mo, B.; Chen, X. The disease resistance protein SNC1 represses the biogenesis of microRNAs and phased siRNAs. Nat. Commun. 2018, 9, 5080. [Google Scholar] [CrossRef]
- Nitta, Y.; Qiu, Y.; Yaghmaiean, H.; Zhang, Q.; Huang, J.; Adams, K.; Zhang, Y. MEKK2 inhibits activation of MAP kinases in Arabidopsis. Plant J. 2020, 103, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef]
- Shine, M.B.; Xiao, X.; Kachroo, P.; Kachroo, A. Signaling mechanisms underlying systemic acquired resistance to microbial pathogens. Plant Sci. 2019, 279, 81–86. [Google Scholar] [CrossRef]
- Mukhtar, M.S.; McCormack, M.E.; Argueso, C.T.; Pajerowska-Mukhtar, K.M. Pathogen Tactics to Manipulate Plant Cell Death. Curr. Biol. 2016, 26, 608–619. [Google Scholar] [CrossRef]
- Pitsili, E.; Phukan, U.J.; Coll, N.S. Cell Death in Plant Immunity. Cold Spring Harb. Perspect. Biol. 2020, 12, a036483. [Google Scholar] [CrossRef]
- Seo, J.S.; Diloknawarit, P.; Park, B.S.; Chua, N.H. ELF18-induced long noncoding RNA 1 evicts fibrillarin from mediator subunit to enhance ATHOGENESIS-RELATED GENE 1 (PR1) Expression. New Phytol. 2019, 221, 2067–2079. [Google Scholar] [CrossRef]
- Breen, S.; Williams, S.J.; Winterberg, B.; Kobe, b.; Solomon, P.S. Wheat PR-1 proteins are targeted by necrotrophic pathogen effector proteins. Plant J. 2016, 88, 13–25. [Google Scholar] [CrossRef]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Xiong, D.; Schneiter, R.; Tian, C. The function of plant PR1 and other members of the CAP protein superfamily in plant–pathogen interactions. Mol. Plant Pathol. 2023, 24, 651–668. [Google Scholar] [CrossRef]
- Wu, Y.; Xing, D.; Ma, G.; Dai, X.; Gao, L.; Xia, T. A variable loop involved in the substrate selectivity of pinoresinol/lariciresinol reductase from Camellia sinensis. Phytochemistry 2019, 62, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Chattopadhyay, D. Lignin: The Building Block of Defense Responses to Stress in Plants. J. Plant Growth Regul. 2023. [CrossRef]
- Ott, D.S.; Yanchuk, A.D.; Huber, D.P.W.; Wallin, K.F. Genetic variation of lodgepole pine, Pinus contorta var. latifolia, chemical and physical defenses that affect mountain pine beetle, Dendroctonus ponderosae, attack and tree mortality. J. Chem. Ecol. 2011, 37, 1002–1012. [Google Scholar] [CrossRef]
- Clark, E.L.; Huber, D.P.W.; Carroll, A.L. The legacy of attack: Implications of high phloem resin monoterpene levels in lodgepole pine following mass attack by mountain pine beetle, Dendroctonus ponderosae Hopkins. Environ. Entomol. 2012, 42, 392–398. [Google Scholar] [CrossRef]
- Trapp, S.; Croteau, R. Defensive resin biosynthesis in conifers. Annu. Rev. Plant Biol. 2001, 52, 689–724. [Google Scholar] [CrossRef]
- Martin, D.M.; Bohlmann, J. Molecular biochemistry and genomics of terpenoid defenses in conifers. Rec. Adv. Phytochem. 2005, 39, 29–56. [Google Scholar]
- Tanaka, N.; Yoshida, S.; Takagi, H.; Terauchi, R.; Shimizu, A.; Fujiwara, T. Evidence for rice heading date 16 contribution to yield increase under low-nutrient conditions. Soil Sci. Plant Nutr. 2019, 65, 589–597. [Google Scholar] [CrossRef]
- Prisic, S.; Xu, M.; Wilderman, P.R. Rice Contains Two Disparate ent-Copalyl Diphosphate Synthases with Distinct Metabolic Functions. Plant Physiol. 2004, 36, 4228–4236. [Google Scholar] [CrossRef]

| Host of PWN | 1 d vs. 3 d | 3 d vs. 7d | ||
|---|---|---|---|---|
| Pathway Description | p-Value | Pathway Description | p-Value | |
| Susceptible P. thunbergii | MAPK signaling pathway—plant | 0.0053 | alpha-Linolenic acid metabolism | 0.0064 |
| Biosynthesis of various secondary metabolites—part 2 | 0.0239 | Linoleic acid metabolism | 0.0153 | |
| Resistant P. thunbergii | MAPK signaling pathway—plant | p < 0.001 | Cutin, suberine and wax biosynthesis | p < 0.001 |
| Linoleic acid metabolism | p < 0.001 | MAPK signaling pathway—plant | p < 0.001 | |
| Plant–pathogen interaction | p < 0.001 | Plant–pathogen interaction | p < 0.001 | |
| Plant hormone signal transduction | p < 0.001 | Photosynthesis—antenna proteins | p < 0.001 | |
| alpha-Linolenic acid metabolism | p < 0.001 | Phenylpropanoid biosynthesis | p < 0.001 | |
| Flavonoid biosynthesis | p < 0.001 | Biosynthesis of various secondary metabolites—part 2 | p < 0.001 | |
| Diterpenoid biosynthesis | 0.005 | Photosynthesis | 0.015 | |
| Protein processing in endoplasmic reticulum | 0.018 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.; Rahman, M.U.; Wu, X.; Ye, J. Resistant and Susceptible Pinus thunbergii ParL. Show Highly Divergent Patterns of Differentially Expressed Genes during the Process of Infection by Bursaphelenchus xylophilus. Int. J. Mol. Sci. 2023, 24, 14376. https://doi.org/10.3390/ijms241814376
Sun T, Rahman MU, Wu X, Ye J. Resistant and Susceptible Pinus thunbergii ParL. Show Highly Divergent Patterns of Differentially Expressed Genes during the Process of Infection by Bursaphelenchus xylophilus. International Journal of Molecular Sciences. 2023; 24(18):14376. https://doi.org/10.3390/ijms241814376
Chicago/Turabian StyleSun, Tingyu, Mati Ur Rahman, Xiaoqin Wu, and Jianren Ye. 2023. "Resistant and Susceptible Pinus thunbergii ParL. Show Highly Divergent Patterns of Differentially Expressed Genes during the Process of Infection by Bursaphelenchus xylophilus" International Journal of Molecular Sciences 24, no. 18: 14376. https://doi.org/10.3390/ijms241814376
APA StyleSun, T., Rahman, M. U., Wu, X., & Ye, J. (2023). Resistant and Susceptible Pinus thunbergii ParL. Show Highly Divergent Patterns of Differentially Expressed Genes during the Process of Infection by Bursaphelenchus xylophilus. International Journal of Molecular Sciences, 24(18), 14376. https://doi.org/10.3390/ijms241814376






