A Decrease in Maternal Iron Levels Is the Predominant Factor Suppressing Hepcidin during Pregnancy in Mice
Abstract
:1. Introduction
2. Results
2.1. Expression of the Gene Encoding Hepcidin in Pregnant Dams Reflects Maternal Iron Status at E12.5 and E18.5
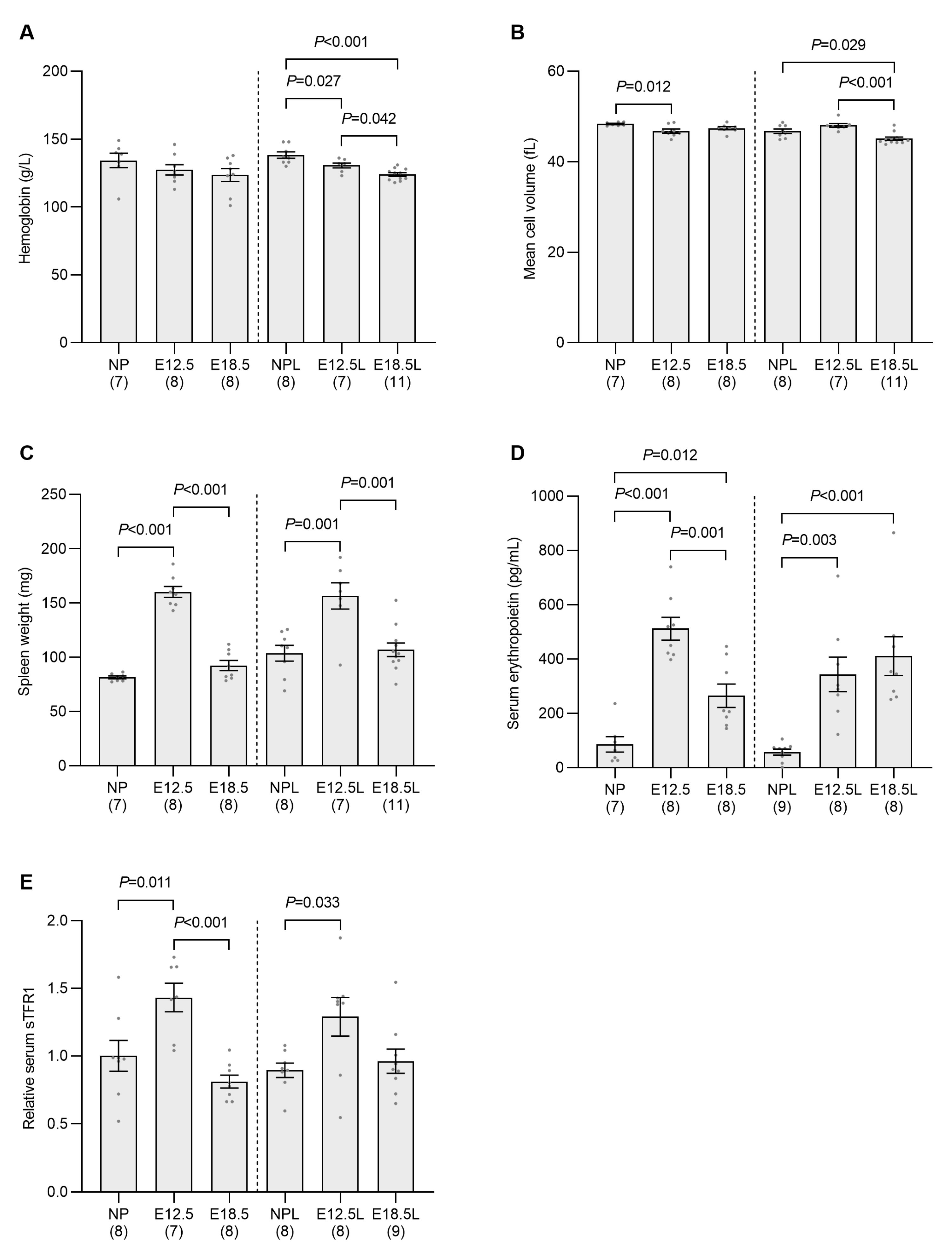
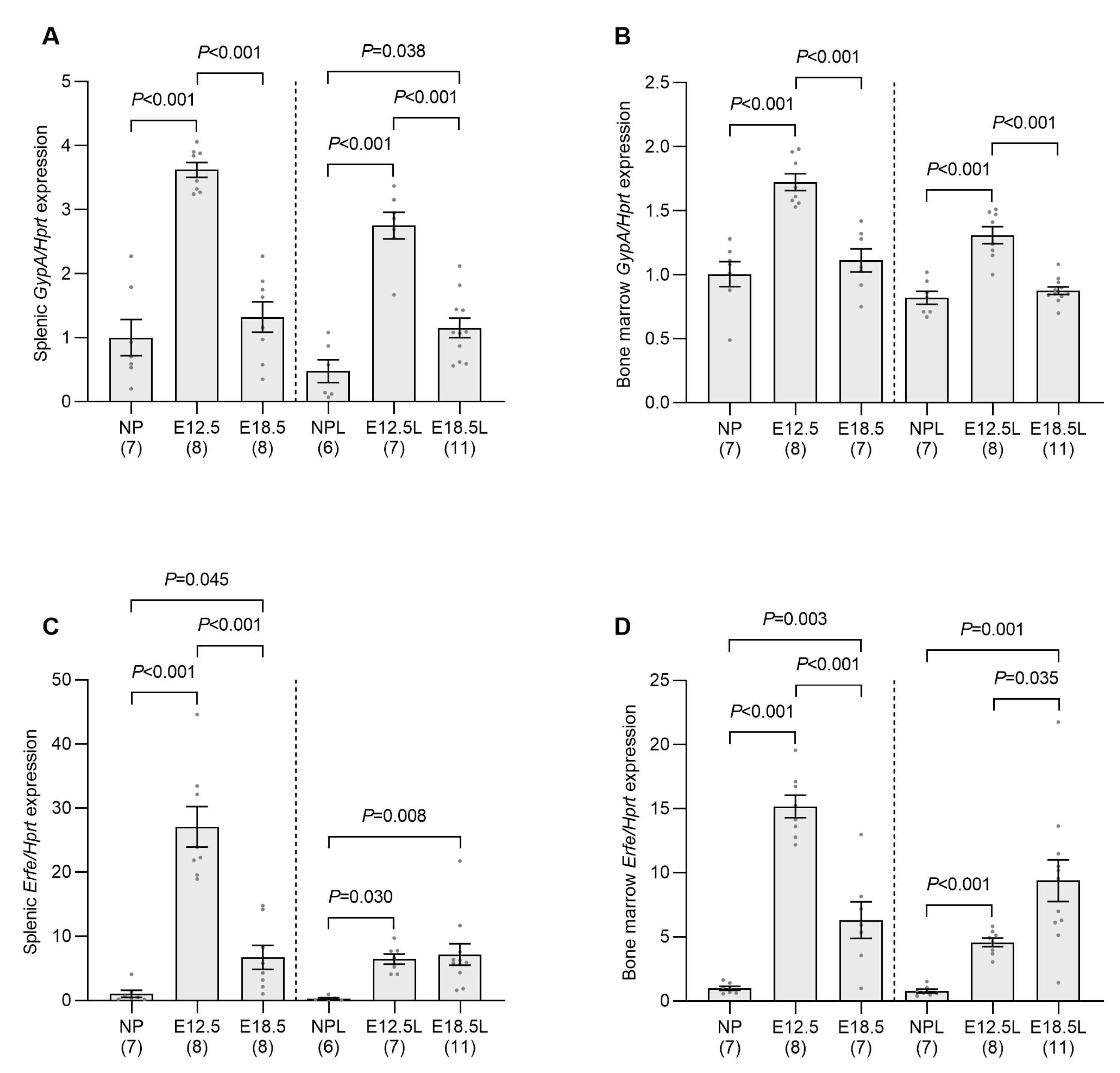
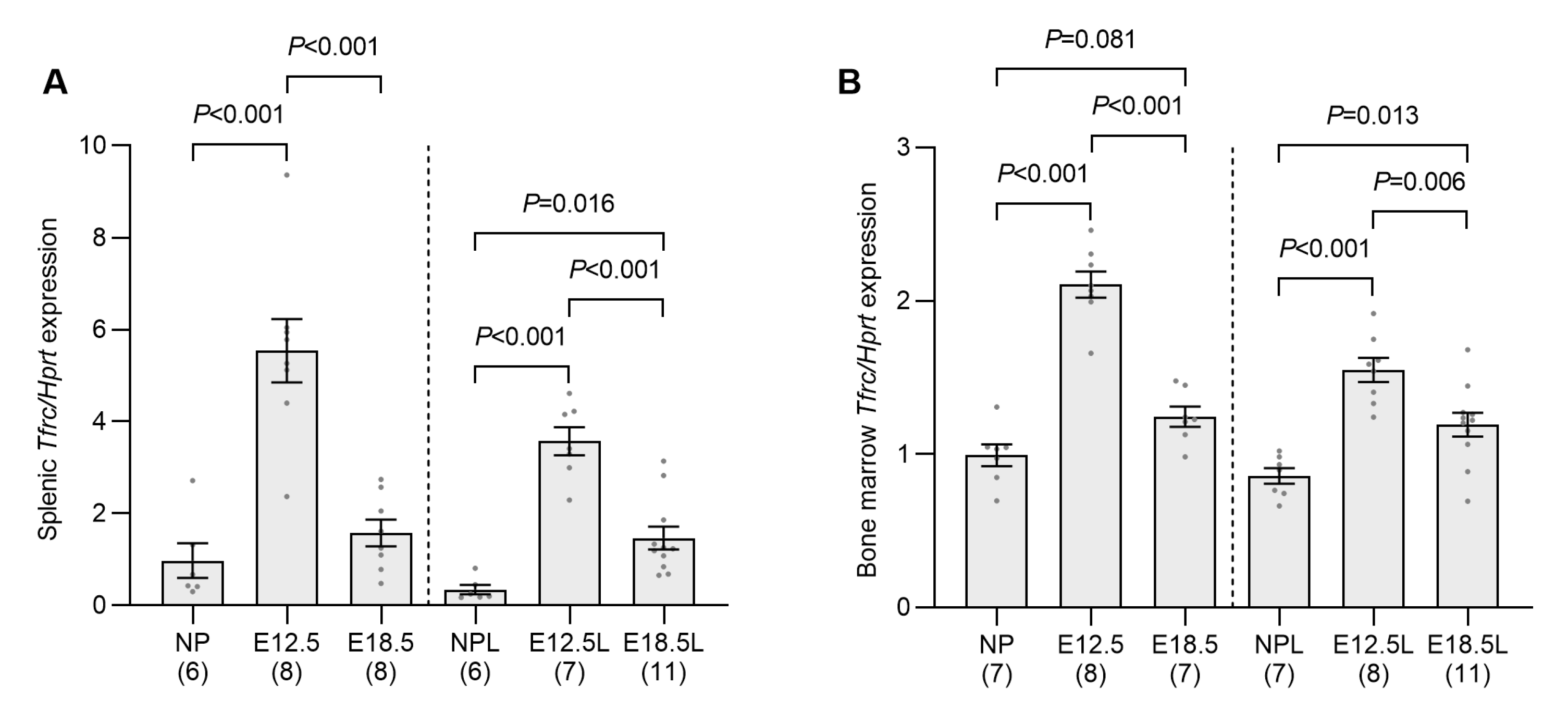
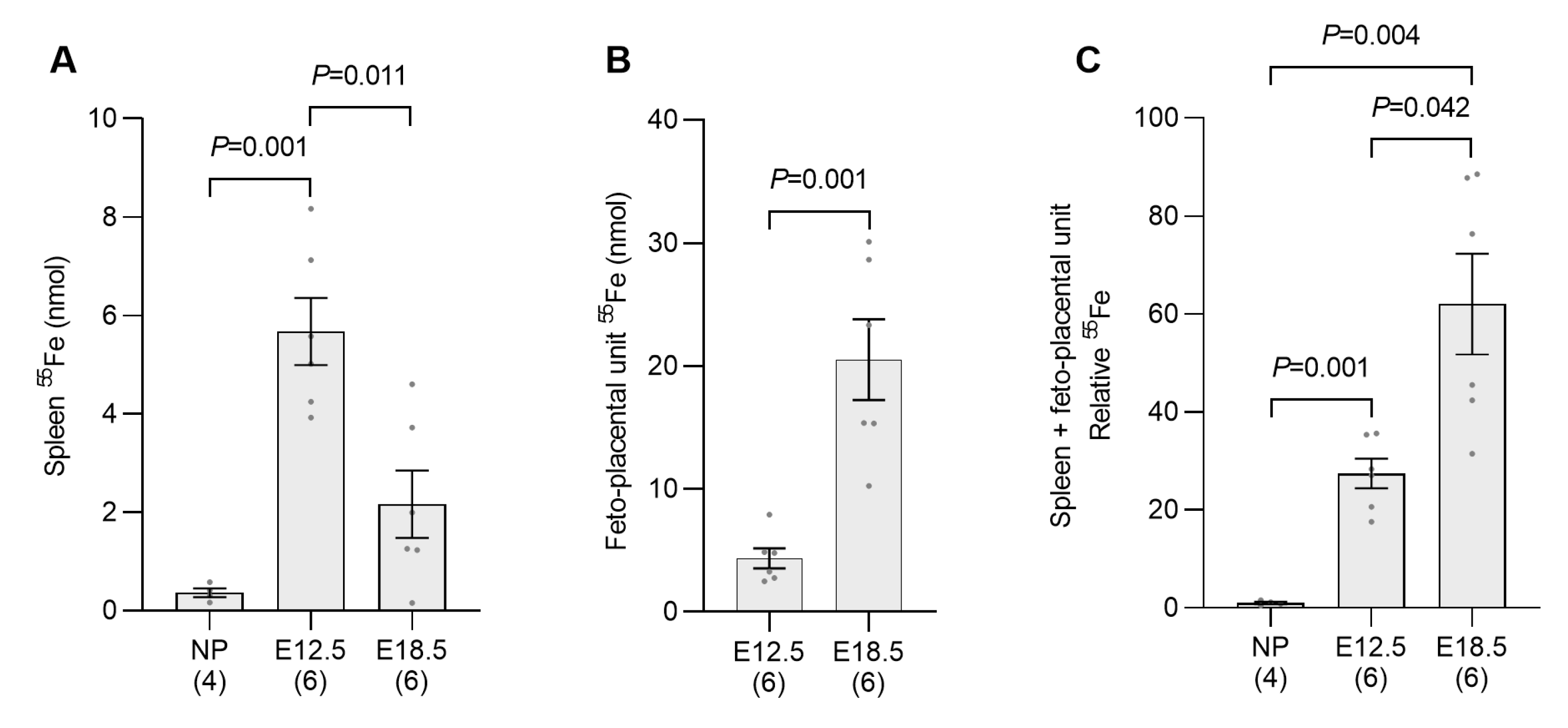
2.2. Erythropoietic Activity Is Increased in the Spleen and Bone Marrow of Pregnant Mice by E12.5
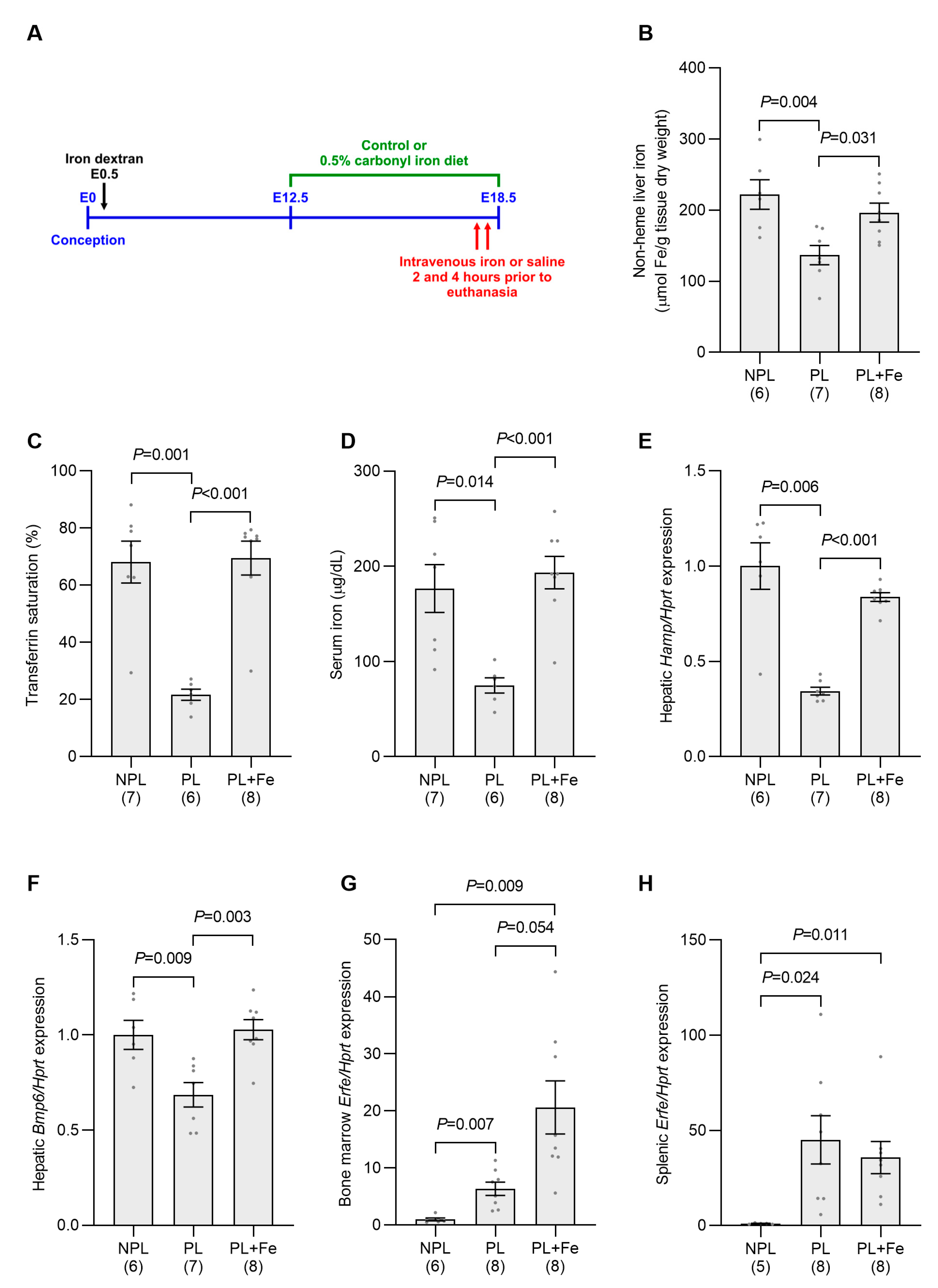
2.3. Increasing Maternal Iron Status Overcomes the Suppression of Maternal Hamp Expression at E18.5
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Tissue Uptake of Radioactive Iron
4.3. Hematological Parameters and Iron Status Markers
4.4. Serum Erythropoietin and sTFR
4.5. RNA Extraction and Gene Expression Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breymann, C. Iron Deficiency Anemia in Pregnancy. Semin. Hematol. 2015, 52, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Georgieff, M.K. Iron deficiency and brain development. Semin. Pediatr. Neurol. 2006, 13, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Georgieff, M.K. Iron in fetal and neonatal nutrition. Semin. Fetal Neonatal. Med. 2007, 12, 54–63. [Google Scholar] [CrossRef]
- Kulik-Rechberger, B.; Kosciesza, A.; Szponar, E.; Domosud, J. Hepcidin and iron status in pregnant women and full-term newborns in first days of life. Ginekol. Pol. 2016, 87, 288–292. [Google Scholar] [CrossRef]
- van Santen, S.; Kroot, J.J.; Zijderveld, G.; Wiegerinck, E.T.; Spaanderman, M.E.; Swinkels, D.W. The iron regulatory hormone hepcidin is decreased in pregnancy: A prospective longitudinal study. Clin. Chem. Lab. Med. 2013, 51, 1395–1401. [Google Scholar] [CrossRef]
- Pigeon, C.; Ilyin, G.; Courselaud, B.; Leroyer, P.; Turlin, B.; Brissot, P.; Loreal, O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J. Biol. Chem. 2001, 276, 7811–7819. [Google Scholar] [CrossRef]
- Abboud, S.; Haile, D.J. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 2000, 275, 19906–19912. [Google Scholar] [CrossRef]
- McKie, A.T.; Marciani, P.; Rolfs, A.; Brennan, K.; Wehr, K.; Barrow, D.; Miret, S.; Bomford, A.; Peters, T.J.; Farzaneh, F.; et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell. 2000, 5, 299–309. [Google Scholar] [CrossRef]
- Donovan, A.; Brownlie, A.; Zhou, Y.; Shepard, J.; Pratt, S.J.; Moynihan, J.; Paw, B.H.; Drejer, A.; Barut, B.; Zapata, A.; et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 2000, 403, 776–781. [Google Scholar] [CrossRef]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017, 106, 1559S–1566S. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.; Chauvet, C.; Viatte, L.; Danan, J.L.; Bigard, X.; Devaux, I.; Beaumont, C.; Kahn, A.; Vaulont, S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J. Clin. Investig. 2002, 110, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Frazer, D.M.; Anderson, G.J. Hepcidin and the hormonal control of iron homeostasis. In Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals; Collins, J.F., Ed.; Elsevier Inc.: London, UK, 2017; pp. 175–186. [Google Scholar]
- Beaton, G.H. Some physiological adjustments relating to nutrition in pregnancy. Can. Med. Assoc. J. 1966, 95, 622–629. [Google Scholar]
- Bothwell, T.H. Iron requirements in pregnancy and strategies to meet them. Am. J. Clin. Nutr. 2000, 72, 257S–264S. [Google Scholar] [CrossRef] [PubMed]
- Sangkhae, V.; Fisher, A.L.; Wong, S.; Koenig, M.D.; Tussing-Humphreys, L.; Chu, A.; Lelic, M.; Ganz, T.; Nemeth, E. Effects of maternal iron status on placental and fetal iron homeostasis. J. Clin. Investig. 2020, 130, 625–640. [Google Scholar] [CrossRef]
- Sangkhae, V.; Fisher, A.L.; Chua, K.J.; Ruchala, P.; Ganz, T.; Nemeth, E. Maternal hepcidin determines embryo iron homeostasis in mice. Blood 2020, 136, 2206–2216. [Google Scholar] [CrossRef]
- Koenig, M.D.; Tussing-Humphreys, L.; Day, J.; Cadwell, B.; Nemeth, E. Hepcidin and iron homeostasis during pregnancy. Nutrients 2014, 6, 3062–3083. [Google Scholar] [CrossRef]
- Ganz, T. The role of hepcidin in fetal iron homeostasis. Blood 2020, 136, 1474–1475. [Google Scholar] [CrossRef]
- Donker, A.E.; van der Staaij, H.; Swinkels, D.W. The critical roles of iron during the journey from fetus to adolescent: Developmental aspects of iron homeostasis. Blood Rev. 2021, 50, 100866. [Google Scholar] [CrossRef]
- Babitt, J.L.; Huang, F.W.; Xia, Y.; Sidis, Y.; Andrews, N.C.; Lin, H.Y. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J. Clin. Investig. 2007, 117, 1933–1939. [Google Scholar] [CrossRef]
- Kautz, L.; Meynard, D.; Monnier, A.; Darnaud, V.; Bouvet, R.; Wang, R.H.; Deng, C.; Vaulont, S.; Mosser, J.; Coppin, H.; et al. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood 2008, 112, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Fruhman, G.J. Blood formation in the pregnant mouse. Blood 1968, 31, 242–248. [Google Scholar] [CrossRef]
- Fowler, J.H.; Nash, D.J. Erythropoiesis in the spleen and bone marrow of the pregnant mouse. Dev. Biol. 1968, 18, 331–353. [Google Scholar] [CrossRef] [PubMed]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 2014, 46, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Mirciov, C.S.G.; Wilkins, S.J.; Hung, G.C.C.; Helman, S.L.; Anderson, G.J.; Frazer, D.M. Circulating iron levels influence the regulation of hepcidin following stimulated erythropoiesis. Haematologica 2018, 103, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Artuso, I.; Pettinato, M.; Nai, A.; Pagani, A.; Sardo, U.; Billore, B.; Lidonnici, M.R.; Bennett, C.; Mandelli, G.; Pasricha, S.R.; et al. Transient decrease of serum iron after acute erythropoietin treatment contributes to hepcidin inhibition by ERFE in mice. Haematologica 2019, 104, e87–e90. [Google Scholar] [CrossRef]
- Wang, S.; He, X.; Wu, Q.; Jiang, L.; Chen, L.; Yu, Y.; Zhang, P.; Huang, X.; Wang, J.; Ju, Z.; et al. Transferrin receptor 1-mediated iron uptake plays an essential role in hematopoiesis. Haematologica 2020, 105, 2071–2082. [Google Scholar] [CrossRef]
- Detivaud, L.; Nemeth, E.; Boudjema, K.; Turlin, B.; Troadec, M.B.; Leroyer, P.; Ropert, M.; Jacquelinet, S.; Courselaud, B.; Ganz, T.; et al. Hepcidin levels in humans are correlated with hepatic iron stores, hemoglobin levels, and hepatic function. Blood 2005, 106, 746–748. [Google Scholar] [CrossRef]
- Jepson, J.H.; Lowenstein, L. Role of erythropoietin and placental lactogen in the control of erythropoiesis during pregnancy. Can. J. Physiol. Pharmacol. 1968, 46, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, J.J.; Dai, G.; Soares, M.J. Pregnancy and lactation modulate maternal splenic growth and development of the erythroid lineage in the rat and mouse. Reprod. Fertil. Dev. 2008, 20, 303–310. [Google Scholar] [CrossRef]
- Maroni, E.S.; de Sousa, M.A. The lymphoid organs during pregnancy in the mouse. A comparison between a syngeneic and an allogeneic mating. Clin. Exp. Immunol. 1973, 13, 107–124. [Google Scholar] [PubMed]
- Frazer, D.M.; Wilkins, S.J.; Darshan, D.; Badrick, A.C.; McLaren, G.D.; Anderson, G.J. Stimulated erythropoiesis with secondary iron loading leads to a decrease in hepcidin despite an increase in bone morphogenetic protein 6 expression. Br. J. Haematol. 2012, 157, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Vokurka, M.; Krijt, J.; Sulc, K.; Necas, E. Hepcidin mRNA levels in mouse liver respond to inhibition of erythropoiesis. Physiol. Res. 2006, 55, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Kautz, L.; Jung, G.; Du, X.; Gabayan, V.; Chapman, J.; Nasoff, M.; Nemeth, E.; Ganz, T. Erythroferrone contributes to hepcidin suppression and iron overload in a mouse model of beta-thalassemia. Blood 2015, 126, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Sangkhae, V.; Yu, V.; Coffey, R.; O’Brien, K.O.; Ganz, T.; Nemeth, E. Erythroferrone contributes to iron mobilization for embryo erythropoiesis in iron-deficient mouse pregnancies. Am. J. Hematol. 2022, 97, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Arezes, J.; Foy, N.; McHugh, K.; Sawant, A.; Quinkert, D.; Terraube, V.; Brinth, A.; Tam, M.; LaVallie, E.R.; Taylor, S.; et al. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood 2018, 132, 1473–1477. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 2015, 15, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Frazer, D.M.; Wilkins, S.J.; Darshan, D.; Mirciov, C.S.G.; Dunn, L.A.; Anderson, G.J. Ferroportin Is Essential for Iron Absorption During Suckling, But Is Hyporesponsive to the Regulatory Hormone Hepcidin. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 410–421. [Google Scholar] [CrossRef]
- Mirciov, C.S.; Wilkins, S.J.; Dunn, L.A.; Anderson, G.J.; Frazer, D.M. Characterization of Putative Erythroid Regulators of Hepcidin in Mouse Models of Anemia. PLoS ONE 2017, 12, e0171054. [Google Scholar] [CrossRef]
- Salojin, K.V.; Cabrera, R.M.; Sun, W.; Chang, W.C.; Lin, C.; Duncan, L.; Platt, K.A.; Read, R.; Vogel, P.; Liu, Q.; et al. A mouse model of hereditary folate malabsorption: Deletion of the PCFT gene leads to systemic folate deficiency. Blood 2011, 117, 4895–4904. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward | Reverse |
|---|---|---|
| Bmp6 | AACAGCTTGCAAGAAGCATGAG | TGGACCAAGGTCTGTACAATGG |
| Erfe | CCAGGCCCCTTTATCCCATC | GTGCTCCAGATGGCTCTCTC |
| GypA | GTGATGGCAGGGATTATCGGA | CACTGTTGTCACCACCCTCA |
| Hamp | CCTGAGCAGCACCACCTATC | TGCAACAGATACCACACTGGG |
| Hprt | ATGATCAGTCAACGGGGGAC | TTGGGGCTGTACTGCTTAAC |
| Smad7 | GGCATTCCTCGGAAGTCAAG | CAGCCTGCAGTTGGTTTGAG |
| Tfrc | TCATGAGGGAAATCAATGATCG | CCAGAGCAGCTTAAATCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helman, S.L.; Wilkins, S.J.; Chan, J.C.J.; Hartel, G.; Wallace, D.F.; Anderson, G.J.; Frazer, D.M. A Decrease in Maternal Iron Levels Is the Predominant Factor Suppressing Hepcidin during Pregnancy in Mice. Int. J. Mol. Sci. 2023, 24, 14379. https://doi.org/10.3390/ijms241814379
Helman SL, Wilkins SJ, Chan JCJ, Hartel G, Wallace DF, Anderson GJ, Frazer DM. A Decrease in Maternal Iron Levels Is the Predominant Factor Suppressing Hepcidin during Pregnancy in Mice. International Journal of Molecular Sciences. 2023; 24(18):14379. https://doi.org/10.3390/ijms241814379
Chicago/Turabian StyleHelman, Sheridan L., Sarah J. Wilkins, Jennifer C. J. Chan, Gunter Hartel, Daniel F. Wallace, Gregory J. Anderson, and David M. Frazer. 2023. "A Decrease in Maternal Iron Levels Is the Predominant Factor Suppressing Hepcidin during Pregnancy in Mice" International Journal of Molecular Sciences 24, no. 18: 14379. https://doi.org/10.3390/ijms241814379
APA StyleHelman, S. L., Wilkins, S. J., Chan, J. C. J., Hartel, G., Wallace, D. F., Anderson, G. J., & Frazer, D. M. (2023). A Decrease in Maternal Iron Levels Is the Predominant Factor Suppressing Hepcidin during Pregnancy in Mice. International Journal of Molecular Sciences, 24(18), 14379. https://doi.org/10.3390/ijms241814379







