The Effect of Plant-Derived Low-Ratio Linoleic Acid/α-Linolenic Acid on Markers of Glucose Controls: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Data Extraction
2.4. Statistical Methods
3. Results and Discussion
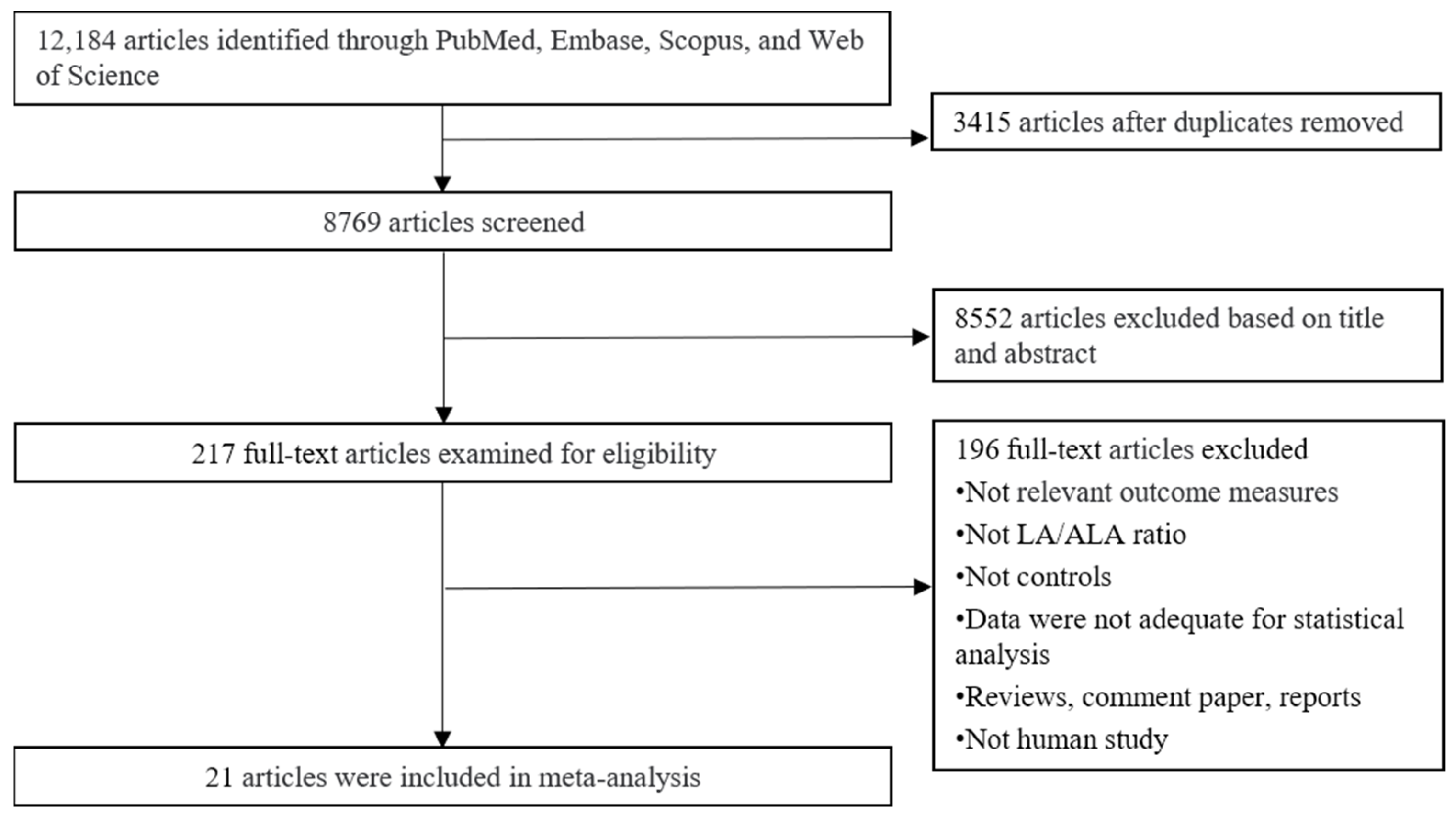
3.1. Study Selection and Description
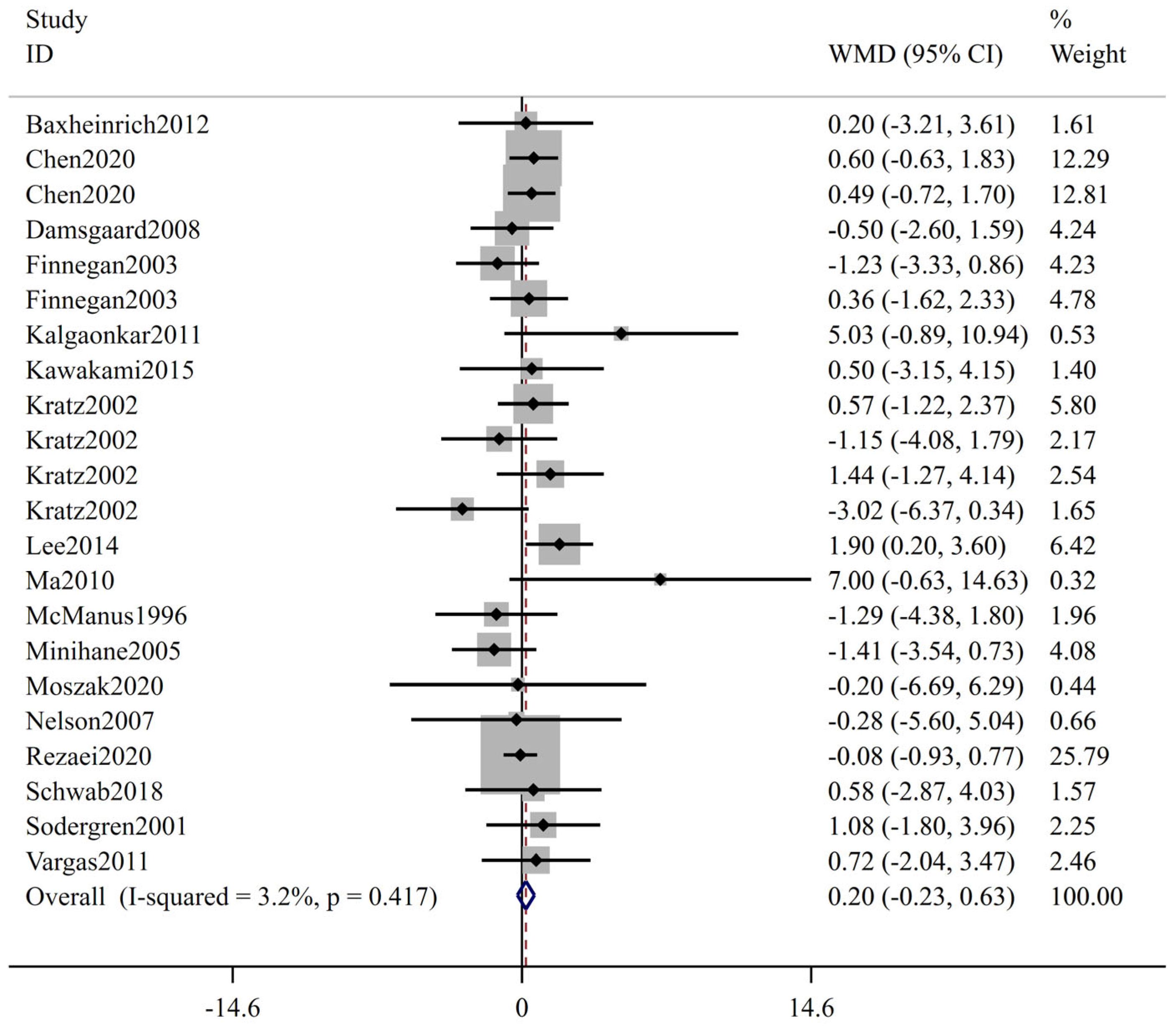
3.2. Meta-Analysis Results
3.3. Sensitivity Analysis, Subgroup Analysis and Meta-Regression
3.4. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LA | Linoleic acid |
| ALA | Alpha-linolenic acid |
| RCTs | Randomized controlled trials |
| WMD | Weighted mean difference |
| CI | Confidence interval |
| FBS | Fasting blood sugar |
| HOMA-IR | Homeostatic model assessment insulin resistance |
| HbA1c | Hemoglobin A1c |
| IDF | International Diabetes Federation |
| T2DM | Type 2 diabetes mellitus |
| CVD | Cardiovascular disease |
| POS | Polycystic ovary syndrome |
| EFA | Essential fatty acids |
| FA | Fatty acids |
| PUFA | Polyunsaturated fatty acid |
| AA | Arachidonic acid |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| CLA | Conjugated linoleic acid |
| BMI | Body mass index |
| SD | Standard deviations |
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Safiri, S.; Karamzad, N.; Kaufman, J.S.; Bell, A.W.; Nejadghaderi, S.A.; Sullman, M.J.M.; Moradi-Lakeh, M.; Collins, G.; Kolahi, A.A. Prevalence, Deaths and Disability-Adjusted-Life-Years (DALYs) Due to Type 2 Diabetes and Its Attributable Risk Factors in 204 Countries and Territories, 1990-2019: Results from the Global Burden of Disease Study 2019. Front. Endocrinol. 2022, 13, 838027. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef]
- Faselis, C.; Katsimardou, A.; Imprialos, K.; Deligkaris, P.; Kallistratos, M.; Dimitriadis, K. Microvascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 117–124. [Google Scholar] [CrossRef]
- Martin, C.L.; Albers, J.W.; Pop-Busui, R.; Grp, D.E.R. Neuropathy and Related Findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes Care 2014, 37, 31–38. [Google Scholar] [CrossRef]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Duran, A.M.; Salto, L.M.; Camara, J.; Basu, A.; Paquien, I.; Beeson, W.L.; Firek, A.; Cordero-MacIntyre, Z.; De Leon, M. Effects of omega-3 polyunsaturated fatty-acid supplementation on neuropathic pain symptoms and sphingosine levels in Mexican-Americans with type 2 diabetes. Diabetes Metab. Syndr. Obes.Targets Ther. 2019, 12, 109–120. [Google Scholar] [CrossRef]
- Huang, L.L.; Zhang, F.J.; Xu, P.; Zhou, Y.J.; Liu, Y.J.; Zhang, H.D.; Tan, X.Z.; Ge, X.X.; Xu, Y.; Guo, M.; et al. Effect of Omega-3 Polyunsaturated Fatty Acids on Cardiovascular Outcomes in Patients with Diabetes: A Meta-analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 629–636. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, L.N.; Wang, D.L.; Yan, N.; Li, C.; Wu, M.; Wang, F.; Mi, B.B.; Chen, F.Y.; Jia, W.R.; et al. Omega-3 polyunsaturated fatty acid biomarkers and risk of type 2 diabetes, cardiovascular disease, cancer, and mortality. Clin. Nutr. 2022, 41, 1798–1807. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; DiNicolantonio, J.J. The importance of a balanced omega-6 to omega-3 ratio in the prevention and management of obesity. Open Heart 2016, 3, e000385. [Google Scholar] [CrossRef]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef]
- Barcelo-Coblijn, G.; Murphy, E.J. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: Benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog. Lipid Res. 2009, 48, 355–374. [Google Scholar] [CrossRef]
- Canetti, L.; Werner, H.; Leikin-Frenkel, A. Linoleic and alpha linolenic acids ameliorate streptozotocin-induced diabetes in mice. Arch. Physiol. Biochem. 2014, 120, 34–39. [Google Scholar] [CrossRef]
- Gomes, P.M.; Hollanda-Miranda, W.R.; Beraldo, R.A.; Castro, A.V.B.; Geloneze, B.; Foss, M.C.; Foss-Freitas, M.C. Supplementation of alpha-linolenic acid improves serum adiponectin levels and insulin sensitivity in patients with type 2 diabetes. Nutrition 2015, 31, 853–857. [Google Scholar] [CrossRef]
- Russell, J.S.; Griffith, T.A.; Peart, J.N.; Headrick, J.P. Cardiomyoblast caveolin expression: Effects of simulated diabetes, alpha-linolenic acid, and cell signaling pathways. Am. J. Physiol. Cell Physiol. 2020, 319, C11–C20. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.J.; Feng, K.; He, C.; Li, P.; Hu, Y.J.; Su, H.; Wan, J.B. Dietary alpha-linolenic acid-rich flaxseed oil prevents against alcoholic hepatic steatosis via ameliorating lipid homeostasis at adipose tissue-liver axis in mice. Sci. Rep. 2016, 6, 26826. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Guo, M.X.; Li, Y.W.; Wang, T.; Ren, Y.; Wang, R.; Jiang, X.; Zhang, X.X.; Tian, J.Y.; Wang, H. alpha-Linolenic acid regulates macrophages via GPR120-NLRP3 inflammasome pathway to ameliorate diabetic rats. J. Funct. Foods 2022, 99, 105348. [Google Scholar] [CrossRef]
- Thakur, G.; Mitra, A.; Pal, K.; Rousseau, D. Effect of flaxseed gum on reduction of blood glucose and cholesterol in type 2 diabetic patients. Int. J. Food Sci. Nutr. 2009, 60, 126–136. [Google Scholar] [CrossRef]
- Morshedzadeh, N.; Rahimlou, M.; Shahrokh, S.; Karimi, S.; Mirmiran, P.; Zali, M.R. The effects of flaxseed supplementation on metabolic syndrome parameters, insulin resistance and inflammation in ulcerative colitis patients: An open-labeled randomized controlled trial. Phytother. Res. 2021, 35, 3781–3791. [Google Scholar] [CrossRef]
- Kalgaonkar, S.; Almario, R.U.; Gurusinghe, D.; Garamendi, E.M.; Buchan, W.; Kim, K.; Karakas, S.E. Differential effects of walnuts vs almonds on improving metabolic and endocrine parameters in PCOS. Eur. J. Clin. Nutr. 2011, 65, 386–393. [Google Scholar] [CrossRef]
- Moszak, M.; Zawada, A.; Juchacz, A.; Grzymislawski, M.; Bogdanski, P. Comparison of the effect of rapeseed oil or amaranth seed oil supplementation on weight loss, body composition, and changes in the metabolic profile of obese patients following 3-week body mass reduction program: A randomized clinical trial. Lipids Health Dis. 2020, 19, 143. [Google Scholar] [CrossRef]
- Lee, T.C.; Ivester, P.; Hester, A.G.; Sergeant, S.; Case, L.D.; Morgan, T.; Kouba, E.O.; Chilton, F.H. The impact of polyunsaturated fatty acid-based dietary supplements on disease biomarkers in a metabolic syndrome/diabetes population. Lipids Health Dis. 2014, 13, 196. [Google Scholar] [CrossRef]
- Javidi, A.; Mozaffari-Khosravi, H.; Nadjarzadeh, A.; Dehghani, A.; Eftekhari, M.H. The effect of flaxseed powder on insulin resistance indices and blood pressure in prediabetic individuals: A randomized controlled clinical trial. J. Res. Med. Sci. 2016, 21, 70. [Google Scholar] [CrossRef]
- Au, M.M.C.; Goff, H.D.; Kisch, J.A.; Coulson, A.; Wright, A.J. Effects of Soy-Soluble Fiber and Flaxseed Gum on the Glycemic and Insulinemic Responses to Glucose Solutions and Dairy Products in Healthy Adult Males. J. Am. Coll. Nutr. 2013, 32, 98–110. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.J.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.T.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Akrami, A.; Nikaein, F.; Babajafari, S.; Faghih, S.; Yarmohammadi, H. Comparison of the effects of flaxseed oil and sunflower seed oil consumption on serum glucose, lipid profile, blood pressure, and lipid peroxidation in patients with metabolic syndrome. J. Clin. Lipidol. 2018, 12, 70–77. [Google Scholar] [CrossRef]
- Baxheinrich, A.; Stratmann, B.; Lee-Barkey, Y.H.; Tschoepe, D.; Wahrburg, U. Effects of a rapeseed oil-enriched hypoenergetic diet with a high content of α-linolenic acid on body weight and cardiovascular risk profile in patients with the metabolic syndrome. Br. J. Nutr. 2012, 108, 682–691. [Google Scholar] [CrossRef]
- Chen, C.G.; Wang, P.; Zhang, Z.Q.; Ye, Y.B.; Zhuo, S.Y.; Zhou, Q.; Chen, Y.M.; Su, Y.X.; Zhang, B. Effects of plant oils with different fatty acid composition on cardiovascular risk factors in moderately hypercholesteremic Chinese adults: A randomized, double-blinded, parallel-designed trial. Food Funct. 2020, 11, 7164–7174. [Google Scholar] [CrossRef]
- Damsgaard, C.T.; Frøkiær, H.; Andersen, A.D.; Lauritzen, L.J.J.o.N. Fish oil in combination with high or low intakes of linoleic acid lowers plasma triacylglycerols but does not affect other cardiovascular risk markers in healthy men. J. Nutr. 2008, 138, 1061–1066. [Google Scholar] [CrossRef]
- Finnegan, Y.E.; Minihane, A.M.; Leigh-Firbank, E.C.; Kew, K.; Meijer, G.W.; Muggli, R.; Calder, P.C.; Williams, C.M. Plant- and marine-derived n-3 polyunsaturated fatty acids have differential effects on fasting and postprandial blood lipid concentrations and on the susceptibility of LDL to oxidative modification in moderately hyperlipidemic subjects. Am. J. Clin. Nutr. 2003, 77, 783–795. [Google Scholar] [CrossRef]
- Griffin, M.D.; Sanders, T.A.; Davies, I.G.; Morgan, L.M.; Millward, D.J.; Lewis, F.; Slaughter, S.; Cooper, J.A.; Miller, G.J.; Griffin, B.A. Effects of altering the ratio of dietary n-6 to n-3 fatty acids on insulin sensitivity, lipoprotein size, and postprandial lipemia in men and postmenopausal women aged 45–70 y: The OPTILIP Study. Am. J. Clin. Nutr. 2006, 84, 1290–1298. [Google Scholar] [CrossRef]
- Kawakami, Y.; Yamanaka-Okumura, H.; Naniwa-Kuroki, Y.; Sakuma, M.; Taketani, Y.; Takeda, E. Flaxseed oil intake reduces serum small dense low-density lipoprotein concentrations in Japanese men: A randomized, double blind, crossover study. Nutr. J. 2015, 14, 39. [Google Scholar] [CrossRef]
- Kontogianni, M.D.; Vlassopoulos, A.; Gatzieva, A.; Farmaki, A.E.; Katsiougiannis, S.; Panagiotakos, D.B.; Kalogeropoulos, N.; Skopouli, F.N. Flaxseed oil does not affect inflammatory markers and lipid profile compared to olive oil, in young, healthy, normal weight adults. Metab. Clin. Exp. 2013, 62, 686–693. [Google Scholar] [CrossRef]
- Kratz, M.; von Eckardstein, A.; Fobker, M.; Buyken, A.; Posny, N.; Schulte, H.; Assmann, G.; Wahrburg, U. The impact of dietary fat composition on serum leptin concentrations in healthy nonobese men and women. J. Clin. Endocrinol. Metab. 2002, 87, 5008–5014. [Google Scholar] [CrossRef]
- Ma, Y.; Njike, V.Y.; Millet, J.; Dutta, S.; Doughty, K.; Treu, J.A.; Katz, D.L. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: A randomized controlled crossover trial. Diabetes Care 2010, 33, 227–232. [Google Scholar] [CrossRef]
- McManus, R.M.; Jumpson, J.; Finegood, D.T.; Clandinin, M.T.; Ryan, E.A. A comparison of the effects of n-3 fatty acids from linseed oil and fish oil in well-controlled type II diabetes. Diabetes Care 1996, 19, 463–467. [Google Scholar] [CrossRef]
- Minihane, A.M.; Brady, L.M.; Lovegrove, S.S.; Lesauvage, S.V.; Williams, C.M.; Lovegrove, J.A. Lack of effect of dietary n-6: n-3 PUFA ratio on plasma lipids and markers of insulin responses in Indian Asians living in the UK. Eur. J. Nutr. 2005, 44, 26–32. [Google Scholar] [CrossRef]
- Nelson, T.L.; Stevens, J.R.; Hickey, M.S. Adiponectin levels are reduced, independent of polymorphisms in the adiponectin gene, after supplementation with alpha-linolenic acid among healthy adults. Metab. Clin. Exp. 2007, 56, 1209–1215. [Google Scholar] [CrossRef]
- Rezaei, S.; Reza Sasani, M.; Akhlaghi, M.; Kohanmoo, A. Flaxseed oil in the context of a weight loss programme ameliorates fatty liver grade in patients with non-alcoholic fatty liver disease: A randomised double-blind controlled trial. Br. J. Nutr. 2020, 123, 994–1002. [Google Scholar] [CrossRef]
- Schwab, U.S.; Lankinen, M.A.; de Mello, V.D.; Manninen, S.M.; Kurl, S.; Pulkki, K.J.; Laaksonen, D.E.; Erkkila, A.T. Camelina sativa oil, but not fatty fish or lean fish, improves serum lipid profile in subjects with impaired glucose metabolism—A randomized controlled trial. Mol. Nutr. Food Res. 2018, 62, 1700503. [Google Scholar] [CrossRef]
- Sodergren, E.; Gustafsson, I.B.; Basu, S.; Nourooz-Zadeh, J.; Nalsen, C.; Turpeinen, A.; Berglund, L.; Vessby, B. A diet containing rapeseed oil-based fats does not increase lipid peroxidation in humans when compared to a diet rich in saturated fatty acids. Eur. J. Clin. Nutr. 2001, 55, 922–931. [Google Scholar] [CrossRef]
- Vargas, M.L.; Almario, R.U.; Buchan, W.; Kim, K.; Karakas, S.E. Metabolic and endocrine effects of long-chain versus essential omega-3 polyunsaturated fatty acids in polycystic ovary syndrome. Metab. Clin. Exp. 2011, 60, 1711–1718. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Z.; Wang, P.; Zhang, B.; Chen, C.; Zhang, C.; Su, Y. EPA plus DHA, but not ALA, Improved Lipids and Inflammation Status in Hypercholesterolemic Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Mol. Nutr. Food Res. 2019, 63, 1801157, Correction in Mol. Nutr. Food Res. 2020, 64, e2070012. [Google Scholar] [CrossRef]
- Naghshi, S.; Aune, D.; Beyene, J.; Mobarak, S.; Asadi, M.; Sadeghi, O. Dietary intake and biomarkers of alpha linolenic acid and risk of all cause, cardiovascular, and cancer mortality: Systematic review and dose-response meta-analysis of cohort studies. BMJ Br. Med. J. 2021, 375, n2213. [Google Scholar] [CrossRef]
- Yue, H.; Qiu, B.; Jia, M.; Liu, W.; Guo, X.-f.; Li, N.; Xu, Z.-x.; Du, F.-l.; Xu, T.; Li, D. Effects of alpha-linolenic acid intake on blood lipid profiles:a systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 61, 2894–2910. [Google Scholar] [CrossRef]
- Chen, C.; Yang, Y.; Yu, X.; Hu, S.; Shao, S. Association between omega-3 fatty acids consumption and the risk of type 2 diabetes: A meta-analysis of cohort studies. J. Diabetes Investig. 2017, 8, 480–488. [Google Scholar] [CrossRef]
- Brown, T.J.; Brainard, J.; Song, F.; Wang, X.; Abdelhamid, A.; Hooper, L.; Ajabnoor, S.; Alabdulghafoor, F.; Brainard, J.; Brown, T.J.; et al. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: Systematic review and meta-analysis of randomised controlled trials. BMJ Br. Med. J. 2019, 366, l4697. [Google Scholar] [CrossRef]
- Hu, M.Y.; Fang, Z.M.; Zhang, T.; Chen, Y. Polyunsaturated fatty acid intake and incidence of type 2 diabetes in adults: A dose response meta-analysis of cohort studies. Diabetol. Metab. Syndr. 2022, 14, 34. [Google Scholar] [CrossRef]
- Taylor, C.G.; Noto, A.D.; Stringer, D.M.; Froese, S.; Malcolmson, L. Dietary milled flaxseed and flaxseed oil improve N-3 fatty acid status and do not affect glycemic control in individuals with well-controlled type 2 diabetes. J. Am. Coll. Nutr. 2010, 29, 72–80. [Google Scholar] [CrossRef]
- Lemay, A.; Dodin, S.; Kadri, N.; Jacques, H.; Forest, J.C. Flaxseed dietary supplement versus hormone replacement therapy in hypercholesterolemic menopausal women. Obstet. Gynecol. 2002, 100, 495–504. [Google Scholar] [CrossRef]
- Jovanovski, E.; Li, D.D.; Ho, H.V.T.; Djedovic, V.; Marques, A.D.R.; Shishtar, E.; Mejia, S.B.; Sievenpiper, J.L.; de Souza, R.J.; Duvnjak, L.; et al. The effect of alpha-linolenic acid on glycemic control in individuals with type 2 diabetes A systematic review and meta-analysis of randomized controlled clinical trials. Medicine 2017, 96, e6531. [Google Scholar] [CrossRef]
- Zheng, J.S.; Li, K.; Huang, T.; Chen, Y.Q.; Xie, H.; Xu, D.F.; Sun, J.Q.; Li, D. Genetic Risk Score of Nine Type 2 Diabetes Risk Variants that Interact with Erythrocyte Phospholipid Alpha-Linolenic Acid for Type 2 Diabetes in Chinese Hans: A Case-Control Study. Nutrients 2017, 9, 376. [Google Scholar] [CrossRef]
- Cho, Y.S.; Go, M.J.; Han, H.R.; Cha, S.H.; Kim, H.T.; Min, H.; Shin, H.D.; Park, C.; Han, B.G.; Cho, N.H.; et al. Association of lipoprotein lipase (LPL) single nucleotide polymorphisms with type 2 diabetes mellitus. Exp. Mol. Med. 2008, 40, 523–532. [Google Scholar] [CrossRef]
- Li, N.; Yue, H.; Jia, M.; Liu, W.; Qiu, B.; Hou, H.; Huang, F.; Xu, T. Effect of low-ratio n-6/n-3 PUFA on blood glucose: A meta-analysis. Food Funct. 2019, 10, 4557–4565. [Google Scholar] [CrossRef]
- Rhee, Y.; Brunt, A. Flaxseed supplementation improved insulin resistance in obese glucose intolerant people: A randomized crossover design. Nutr. J. 2011, 10, 44. [Google Scholar] [CrossRef]

| Reference | Country | Participant Information | Age | BMI | Smoking | No. | M/F | Duration | Design | Low LA/ALA | High LA/ALA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Akrami 2018 [28] | Iran | Metabolic syndrome | 48.6 | NR | Non-smoker | 52 | 33/19 | 7 W | P | 0.14 | 19.1 |
| Baxheinrich 2012 [29] | Netherlands | Metabolic syndrome | 54.1 | 29.8 | Mixed | 163 | 79/84 | 104 W | P | 4.7 | 29.1 |
| Chen 2020 [30] | China | Dyslipidaemia | 54.5 | 23.2 | Mixed | 243 | 92/151 | 48 W | P | 7.1 | 30, 20 |
| Damsgaard 2008 [31] | Denmark | Healthy | 25 | 23.2 | Mixed | 33 | 33/0 | 8 W | P | 4.7 | 7.72 |
| Finnegan 2003 [32] | UK | Dyslipidaemia | 53.7 | 26.1 | Non-smoker | 60 | 35/25 | 24 W | P | 3.6 | 15.5 |
| 54.5 | 26.2 | 59 | 35/24 | 1.4 | 15.5 | ||||||
| Griffin 2006 [33] | UK | Healthy | 59 | 26.3 | Mixed | 97 | 62/35 | 24 W | P | 4.64 | 14 |
| Kalgaonkar 2011 [22] | USA | Polycystic ovary syndrome | 33.5 | 35.2 | Non-smoker | 31 | 0/31 | 6 W | P | 4.62 | 22.06 |
| Kawakami 2015 [34] | Japan | Healthy | 44.5 | 25.1 | Mixed | 15 | 15/0 | 12 W | CO | 1.34 | 9.8 |
| Kontogianni 2013 [35] | Greece | Healthy | 26 | 21.9 | NR | 37 | 8/29 | 6 W | CO | 1.4 | 8.3 |
| Kratz 2002 [36] | Germany | Healthy | 28.9 | 23.8 | Non-smoker | 30 | 30/0 | 4 W | P | 7.5 | 60 |
| 22.8 | 22.1 | 25 | 0/25 | 7.5 | 60 | ||||||
| 28.9 | 23.8 | 30 | 30/0 | 2.56 | 60 | ||||||
| 22.8 | 22.1 | 25 | 0/25 | 2.56 | 60 | ||||||
| Lee 2014 [24] | USA | Type 2 diabetes | 58.6 | 34.5 | Non-smoker | 43 | 18/25 | 8 W | P | 0.95 | 66 |
| Ma 2010 [37] | USA | Type 2 diabetes | 58.1 | 32.5 | Non-smoker | 24 | 10/14 | 8 W | P | 4.48 | 7.75 |
| McManus 1996 [38] | Canada | Type 2 diabetes | 61.8 | 27.8 | NR | 11 | 8/3 | 12 W | CO | 0.25 | 14.45 |
| Minihane 2005 [39] | India | Healthy | 48 | 26 | Non-smoker | 29 | NR | 6 W | P | 9 | 16 |
| Moszak 2020 [23] | Poland | Overweight or obese | 48.7 | 39.6 | Non-smoker | 52 | 20/32 | 3 W | P | 1.88 | 41.5 |
| Nelson 2007 [40] | USA | Overweight or obese subjects | 38.5 | 30.3 | Non-smoker | 57 | 11/46 | 8 W | P | 1.3 | 10.2 |
| Rezaei 2020 [41] | Iran | Non-alcoholic fatty liver | 43.2 | 29.9 | Mixed | 68 | 33/35 | 12 W | P | 0.36 | 228.2 |
| Schwab 2018 [42] | Finland | Type 2 diabetes | 58.9 | 29.2 | NR | 79 | 40/39 | 12 W | P | 1.1 | 4.3 |
| Sodergren 2001 [43] | Sweden | Dyslipidaemia | 50 | 24.5 | Mixed | 19 | 13/6 | 4 W | CO | 3 | 10 |
| Vargas 2011 [44] | USA | Polycystic ovary syndrome | 29.2 | 34.1 | Non-smoker | 34 | 0/34 | 6 W | P | 1.38 | 9 |
| Zhou 2019 [45] | China | Dyslipidaemia | 52.7 | 26 | Mixed | 75 | 39/36 | 12 W | P | 3.8, 2.05 | 16.04 |
| FBS | Insulin | HbA1c | HOMA-IR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup | N | WMD (95% CI) | I2% | N | WMD (95% CI) | I2% | N | WMD (95% CI) | I2% | N | WMD (95% CI) | I2% |
| Low-ratio LA/ALA | ||||||||||||
| ≤1 | 3 | −0.06 (−0.45, 0.33) | 21.9 | 2 | −0.17 (−0.99, 0.65) | 0.0 | ||||||
| 1–5 | 19 | −0.00 (−0.08, 0.07) | 0.0 | 15 | 0.46 (−0.27, 1.18) | 13.3 | 7 | −0.03 (−0.11, 0.05) | 19.6 | 7 | 0.27 (−0.01, 0.54) | 0.0 |
| ≥5 | 5 | 0.01 (−0.11, 0.13) | 0.0 | 4 | 0.24 (−0.47, 0.94) | 0.0 | 2 | 0.02 (−0.15, 0.19) | 0.0 | 3 | 0.04 (−0.15, 0.23) | 0.0 |
| Region | ||||||||||||
| North America | 6 | −0.05 (−0.14, 0.02) | 0.0 | 6 | 1.31 (0.08, 2.54) | 32.0 | 5 | −0.01 (−0.08, 0.07) | 52.3 | 4 | 0.47 (0.10, 0.84) | 0.0 |
| Europe | 13 | −0.01 (−0.09, 0.07) | 0.0 | 11 | −0.08 (−0.85, 0.69) | 0.0 | 4 | −0.01 (−0.13, 0.12) | 0.0 | 3 | 0.00 (−0.42, 0.42) | 25.8 |
| Asia | 8 | 0.00 (−0.12, 0.12) | 0.0 | 5 | 0.12 (−0.46, 0.69) | 0.0 | 4 | 0.02 (−0.15, 0.19) | 0.0 | |||
| Health status | ||||||||||||
| Health | 9 | 0.05 (−0.04, 0.15) | 0.0 | 13 | −0.35 (−1.28, 0.57) | 8.6 | 5 | −0.01 (−0.12, 0.11) | 0.0 | 2 | 0.02 (−0.37, 0.41) | 35.6 |
| Dyslipidaemia | 7 | −0.08 (−0.18, 0.03) | 7.4 | 17 | 0.35 (−0.37, 1.06) | 0.0 | 2 | 0.07 (−0.12, 0.27) | 0.0 | |||
| Type 2 diabetes | 4 | −0.04 (−0.26, 0.18) | 0.2 | 4 | 1.25 (−0.01, 2.60) | 45.3 | 3 | 0.07 (−0.02, 0.17) | 0.0 | 3 | 0.31 (−0.10, 0.71) | 55.4 |
| Overweight or obese | 2 | 0.27 (−0.10, 0.64) | 0.0 | 2 | −0.25 (−4.36, 3.87) | 0.0 | ||||||
| Metabolic syndrome | 3 | −0.18 (−0.52, 0.17) | 0.0 | −0.06 (−0.89, 0.76) | 40.2 | |||||||
| Polycystic ovary syndrome | 2 | 0.09 (−0.20, 0.38) | 0.0 | 4 | 1.49 (−1.01, 3.98) | 3.2 | 2 | −0.12 (−0.23, −0.00) | 0.0 | 2 | 0.42 (−0.26, 1.10) | 0.0 |
| Age | ||||||||||||
| ≤45 | 11 | 0.05 (−0.04, 0.14) | 0.0 | 10 | 0.01 (−0.62, 0.63) | 0.0 | 6 | −0.06 (−0.14, 0.02) | 0.0 | 3 | 0.04 (−0.28, 0.36) | 0.0 |
| >45 | 15 | −0.05 (−0.14, 0.04) | 0.0 | 11 | 0.4 (−0.17, 1.05) | 11.5 | 2 | 0.11 (−0.04, 0.26) | 13.2 | 7 | 0.10 (−0.06, 0.26) | 10.4 |
| BMI | ||||||||||||
| ≤25 | 9 | −0.01 (−0.09, 0.07) | 0.0 | 8 | 0.31 (−0.35, 0.96) | 0.0 | 4 | −0.01 (−0.13, 0.12) | 0.0 | 2 | 0.07 (−0.12, 0.27) | 0.0 |
| 25–30 | 8 | 0.03 (−0.12, 0.18) | 2.1 | 4 | −0.34 (−1.62, 0.95) | 41.1 | 3 | 0.02 (−0.07, 0.14) | 0.0 | 2 | 0.26 (−0.13, 0.65) | 0.0 |
| ≥30 | 9 | 0.02 (−0.13, 0.16) | 0.0 | 10 | −0.23 (−0.41, 0.88) | 2.3 | 3 | −0.04 (−0.14, 0.06) | 72.3 | 7 | 0.03 (−0.22, 0.28) | 27.6 |
| Smoking | ||||||||||||
| Non-smoker | 14 | 0.01 (−0.10, 0.11) | 0.0 | 13 | 0.19 (−0.52, 0.89) | 24.3 | 7 | −0.03 (−0.11, 0.05) | 21.9 | 5 | 0.22 (−0.14, 0.59) | 21.0 |
| Mixed | 9 | −0.02 (−0.12, 0.08) | 0.0 | 6 | 0.21 (−0.35, 0.78) | 0.0 | 4 | 0.06 (−0.10, 0.22) | 0.0 | |||
| NR | 4 | 0.05 (−0.11, 0.20) | 0.0 | 3 | 0.16 (−2.04, 2.37) | 49.9 | 2 | 0.04 (−0.08, 0.15) | 0.0 | 2 | 0.07 (−0.48, 0.63) | 67.1 |
| Duration | ||||||||||||
| <12 W | 15 | 0.01 (−0.07, 0.09) | 0.0 | 13 | 0.38 (−0.37, 1.12) | 32.0 | 7 | −0.03 (−0.10, 0.05) | 9.4 | 6 | 0.26 (−0.06, 0.59) | 5.2 |
| ≥12 W | 12 | −0.02 (−0.12, 0.08) | 0.0 | 9 | 0.11 (−0.42, 0.64) | 0.0 | 2 | 0.04 (−0.08, 0.15) | 0.0 | 5 | 0.04 (−0.12, 0.20) | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Wang, X. The Effect of Plant-Derived Low-Ratio Linoleic Acid/α-Linolenic Acid on Markers of Glucose Controls: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 14383. https://doi.org/10.3390/ijms241814383
Wang Q, Wang X. The Effect of Plant-Derived Low-Ratio Linoleic Acid/α-Linolenic Acid on Markers of Glucose Controls: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2023; 24(18):14383. https://doi.org/10.3390/ijms241814383
Chicago/Turabian StyleWang, Qiong, and Xingguo Wang. 2023. "The Effect of Plant-Derived Low-Ratio Linoleic Acid/α-Linolenic Acid on Markers of Glucose Controls: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 24, no. 18: 14383. https://doi.org/10.3390/ijms241814383
APA StyleWang, Q., & Wang, X. (2023). The Effect of Plant-Derived Low-Ratio Linoleic Acid/α-Linolenic Acid on Markers of Glucose Controls: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 24(18), 14383. https://doi.org/10.3390/ijms241814383








