Hepatocellular Carcinoma Prevention in the Era of Hepatitis C Elimination
Abstract
:1. Introduction
2. Hepatitis C Elimination, where Do We Stand?
3. Mechanisms of Liver Cell Carcinogenesis by HCV
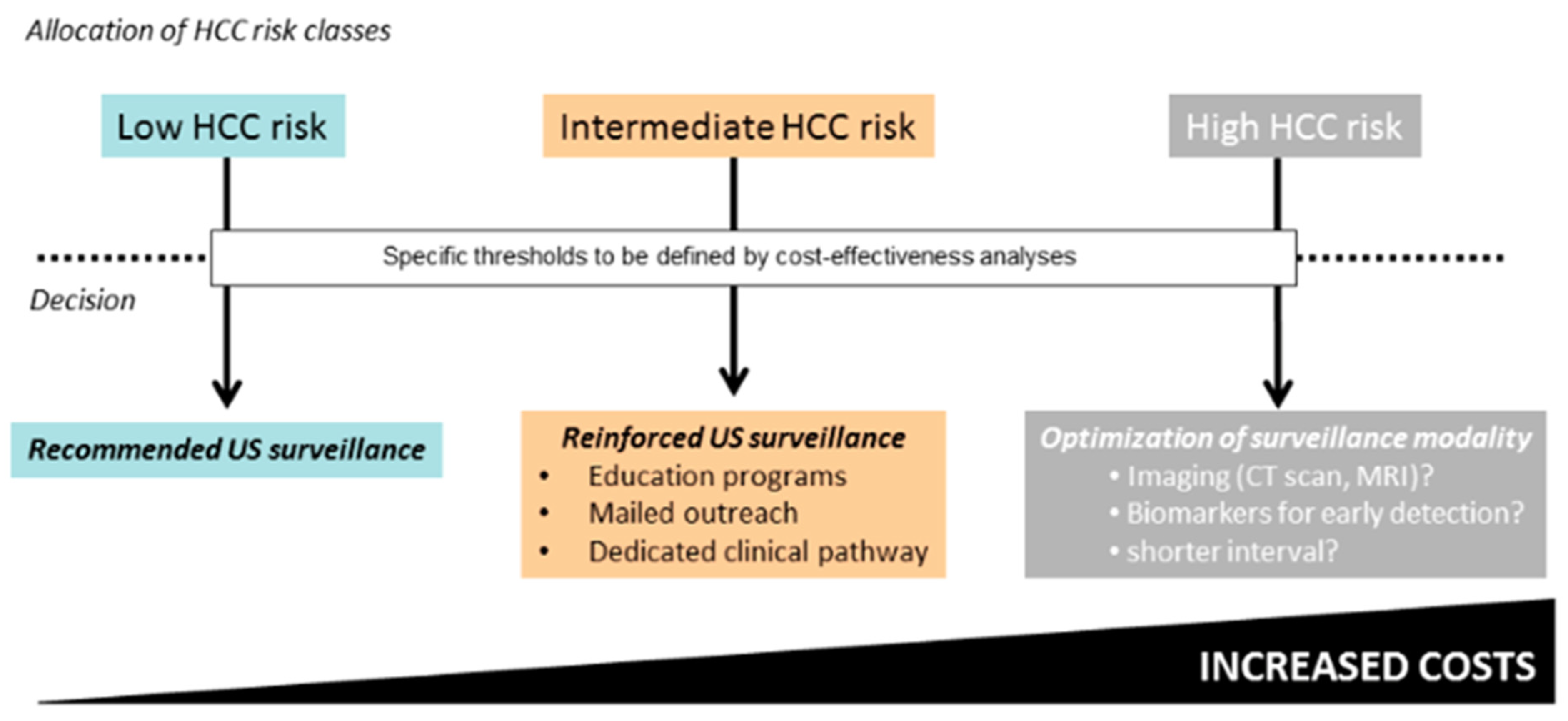
4. Recognition of People Living with HCV at Risk of HCC
5. Recommended Strategies of HCC Surveillance
6. Overcoming the Underuse and Low Diagnostic Accuracy of Currently Available Screening Tests
7. The Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| aMAP | HCC risk calculator: age, sex, albumin, T bilirubin, platelets |
| AFP | Alpha Fetoprotein |
| AFP-L3 | Alpha Fetoprotein Lecitin-3 |
| Akt | serine/threonine protein kinase family member |
| APRI | Aspartate aminotransferase to platelet ratio index |
| ARFI | Acoustic Radiation Force Impulse US scan |
| APASL | Asia Pacific Association for the Study of the Liver |
| AASLD | American Association for the Study of the Liver |
| Chat-GPT | Chat Generative Pre-trained Transformer |
| CT scan | Computed tomography scan |
| COVID-19 | Coronavirus Disease 2019 |
| CEUS | Contrast-Enhanced Ultrasonography |
| DAAs | Direct antiviral agents |
| DCP | Des-gamma-carboxy prothrombin |
| DNA | Deoxyribonucleic acid |
| EASL | European Association for the Study of the Liver |
| GALAD | HCC risk calculator: gender × age × log alpha-fetoprotein × des-gamma-carboxy prothrombin |
| GHSS | Global Health Sector Strategy |
| HBV | Hepatitis B virus |
| HCC | Hepatocellular carcinoma |
| HCV | Hepatitis C virus |
| IL-6 | Interleukin 6 |
| ILCA | International Liver Cancer Association |
| kPa | kilo Pascal |
| LI-RADS | Liver Imaging Reporting and Data System |
| MAPK | Mitogen-activated protein kinase |
| MRI | Magnetic resonance imaging |
| METAVIR | Meta-analysis of histological data in viral hepatitis |
| MIR-155 | MicroRNA-155 |
| mTOR | Mammalian Target of Rapamycin |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NS5A | Non-Structural 5 A PI3K Phospho Inositide 3-Kinases |
| PWID | People who inject drugs |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| SNIP | Single nucleotide polymorphism |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| SVR | Sustained virological response |
| TGF-alfa | Tumor Growth Factor alfa |
| WHO | World Health Organization |
| Wnt | Wingless-related integration site |
References
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level. JAMA Oncol. 2017, 3, 1683. [Google Scholar] [PubMed]
- The Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: A modelling study. Lancet Gastroenterol. Hepatol. 2022, 7, 396–415. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections. 2021. Available online: https://www.who.int/publications/i/item/9789240027077 (accessed on 15 July 2021).
- Tan, D.J.H.; Setiawan, V.W.; Ng, C.H.; Lim, V.H.; Muthiah, M.D.; Tan, E.X.; Dan, Y.Y.; Roberts, L.R.; Loomba, R.; Huang, D.Q. Global burden of liver cancer in males and females: Changing etiological basis and the growing contribution of NASH. Hepatology 2023, 77, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and prediction to 2040. J. Hepatol. 2022, 6, 1598–1606. [Google Scholar] [CrossRef]
- WHO. Global Health Sector Strategies on viral hepatitis, 2016–2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Lazarus, J.V.; Pericàs, J.M.; Picchio, C.A.; Cernosa, J.; Hoekstra, M.; Luhmann, N.; Maticic, M.; Read, P.; Robinson, E.M.; Dillon, J.F.; et al. We know DAAs work, so now what? Simplifying models of care to enhance the hepatitis C cascade. J. Intern. Med. 2019, 286, 503–525. [Google Scholar] [CrossRef]
- Europe’s Beating Cancer Plan: A New EU Approach to Prevention, Treatment and Care. 2021. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_342 (accessed on 12 September 2023).
- Karlsen, T.H.; Sheron, N.; Zelber-Sagi, S.; Carrieri, P.; Dusheiko, G.G.; Bugianesi, E.; Pryke, R.; Hutchinson, S.J.; Sangro, B.; Martin, N.K.; et al. The EASL-Lancer Liver Commission: Protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet 2022, 399, 61–116. [Google Scholar] [CrossRef]
- Allaire, M.; Bruix, J.; Korenjak, M.; Manes, S.; Maravic, Z.; Reeves, H.; Salem, R.; Sangro, B.; Sherman, M. What to do about hepatocellular carcinoma: Recommendations for health authorities from the International Liver Cancer Association. J. Hepathol. Rep. 2022, 4, 100578. [Google Scholar] [CrossRef]
- Hamdane, N.; Jühling, F.; Crouchet, E.; El Saghire, H.; Thumann, C.; Oudot, M.A.; Bandiera, S.; Saviano, A.; Ponsolles, C.; Roca Suarez, A.A.R.; et al. HCV-induced epigenetic changes associated with liver cancer risk persist after sustained virologic response. Gastroenterology 2019, 156, 2313–2329.e7. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 2017, 153, 996–1005. [Google Scholar] [CrossRef]
- Butt, A.A.; Yan, P.; Shaikh, O.S.; Lo Re, V., III; Abou-Samra, A.-B.; Sherman, K.E. Treatment of HCV reduces viral hepatitis-associated liver-related mortality in patients: An ERCHIVES study. J. Hepatol. 2020, 73, 277–284. [Google Scholar] [CrossRef]
- Chen, Q.; Ayer, T.; Adee, M.G.; Wang, X.; Kanwal, F.; Chhatwal, J. Assessment of incidence of and surveillance burden for hepatocellular carcinoma among patients with hepatitis c in the era of direct-acting antiviral agents. JAMA Netw. Open 2020, 3, e2021173. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Lockart, I.; Yeo, M.G.H.; Hajarizadeh, B.; Dore, G.J.; Danta, M. HCC incidence after hepatitis C cure among patients with advanced fibrosis or cirrhosis: A meta-analysis. Hepatology 2022, 76, 139–154. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Health Sector Strategies on, Respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections for the Period 2022–2030; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Gamkrelidze, I.; Pawlotsky, J.; Europe’s, J.V.; Feld, J.J.; Zeuzem, S.; Bao, Y.; dos Santos, A.G.P.; Gonzalez, Y.S.; Razavi, H. Progress towards hepatitis C virus elimination in high-income countries: An updated analysis. Liver Int. 2021, 41, 456–463. [Google Scholar] [CrossRef]
- Blach, S.; Kondili, L.A.; Aghemo, A.; Cai, Z.; Dugan, E.; Estes, C.; Gamkrelidze, I.; Ma, S.; Pawlotsky, J.-M.; Razavi-Shearer, D.; et al. Impact of COVID-19 on global HCV elimination efforts. J. Hepatol. 2021, 74, 31–36. [Google Scholar] [CrossRef]
- Lazarus, J.A.; Safreed-Harmon, K.; Thurz, M.; Dillon, J.F.; El-Sayed, M.H.; Elsharkawy, A.M.; Hatzakis, A.; Jadoul, M.; Prestileo, T.; Razavi, H.; et al. The micro-elimination approach to eliminating hepatitis C: Strategic and operational considertaions. Sem. Liver Dis. 2018, 38, 181–192. [Google Scholar]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2022, 7, 6. [Google Scholar] [CrossRef]
- Bandiera, S.; Bian, B.; Hoshida, Y.; Baumert, T.F.; Zeise, M.B. Chronic hepatitis C virus infection and pathogenesis of hepatocellular carcinoma. Curr. Opin. Virol. 2016, 20, 99–105. [Google Scholar] [CrossRef]
- D’Souza, S.; Lau, K.C.; Coffin, C.S.; Patel, T.R. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J. Gastroenterol. 2020, 26, 5759–5783. [Google Scholar] [CrossRef]
- Wölfl, M.; Rutebemberwa, A.; Mosbruger, T.; Mao, Q.; Li, H.-M.; Netski, D.; Ray, S.; Pardoll, D.; Sidney, J.; Sette, A.; et al. Hepatitis C virus immune escape via exploitation of a hole in the T cell repertoire. J. Immunol. 2008, 181, 6435–6444. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liao, Z.-X.; Ping, J.; Xu, D.; Wang, H. Molecular mechanism of hepatic stellate cell activation and antifibrotic therapeutic strategies. J. Gastroenterol. 2008, 43, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Goto, T.; Hirotsu, Y.; Moriyama, M.; Omata, M. Molecular mechanisms driving progression of liver cirrhosis towards hepatocellular carcinoma in chronic hepatitis B and C infections: A review. Int. J. Mol. Sci. 2019, 20, 1358. [Google Scholar] [CrossRef]
- Quan, H.; Zhou, F.; Nie, D.; Chen, Q.; Cai, X.; Shan, X.; Zhou, Z.; Chen, K.; Huang, A.; Li, S.; et al. Hepatitis C virus core protein epigenetically silences SFRP1 and enhances HCC aggressiveness by inducing epithelial–mesenchymal transition. Oncogene 2013, 33, 2826–2835. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Xu, C.; Yang, P.; Lin, Z.; Wu, W.; Zhang, W.; Ding, J.; Ding, R.; Zhang, X.; Dou, K. Molecular pathogenesis: Connections between viral hepatitis-induced and non-alcoholic steatohepatitis-induced hepatocellular carcinoma. Front. Immunol. 2022, 13, 984728. [Google Scholar] [CrossRef]
- Braconi, C.; Valeri, N.; Gasparini, P.; Huang, N.; Taccioli, C.; Nuovo, G.; Suzuki, T.; Croce, C.M.; Patel, P. Hepatitis C virus proteins modulate MicroRNA expression and chemosensitivity in malignant hepatocytes. Clin. Cancer Res. 2010, 16, 957–966. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, W.; Cheng, N.; Wang, K.; Li, B.; Jiang, X.; Sun, S. Hepatitis C virus-induced up-regulation of microRNA-155 promotes hepatocarcinogenesis by activating Wnt signaling. Hepatology 2012, 56, 1631–1640. [Google Scholar] [CrossRef]
- Kouromalis, E.; Tsomidis, I.; Voumvouraki, A. Pathogenesis of hepatocellular carcinoma: The interplay of apoptosis and autophagy. Biomedicines 2023, 11, 1166. [Google Scholar] [CrossRef]
- Lida, N.; Mizukoshi, E.; Yamashita, T.; Yutani, M.; Seishima, J.; Wang, Z.; Arai, K.; Okada, H.; Yamashita, T.; Sakai, Y.; et al. Chronic liver disease enables gut Enterococcus faecalis colonization to promote liver carcinogenesis. Nat. Cancer 2021, 2, 1039–1054. [Google Scholar]
- Singal, A.G.; Zhang, E.; Narasimman, M.; Rich, N.E.; Waljee, A.K.; Hoshida, Y.; Yang, J.D.; Reig, M.; Cabibbo, G.; Nahon, P.; et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: A meta-analysis. J. Hepatol. 2022, 77, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Beste, L.A.; Green, P.K.; Singal, A.G.; Tapper, E.B.; Waljee, A.K.; Sterling, R.K.; Feld, J.J.; Kaplan, D.E.; Taddei, T.H.; et al. Increased risk for hepatocellular carcinoma persists up to 10 years after HCV Eradication in patients with baseline cirrhosis or high FIB-4 scores. Gastroenterology 2019, 157, 1264–1278.e4. [Google Scholar] [CrossRef]
- Calvaruso, V.; Cabibbo, G.; Cacciola, I.; Petta, S.; Madonia, S.; Bellia, A.; Tinè, F.; Distefano, M.; Licata, A.; Giannitrapani, L. Incidence of hepatocellular carcinoma in patients with HCV-associated cirrhosis treated with direct-acting antiviral agents. Gastroenterology 2018, 155, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Degasperi, E.; D’ambrosio, R.; Iavarone, M.; Sangiovanni, A.; Aghemo, A.; Soffredini, R.; Borghi, M.; Lunghi, G.; Colombo, M.; Lampertico, P. Factors associated with increased risk of de novo or recurrent hepatocellular carcinoma in patients with cirrhosis treated with direct-acting antivirals for HCV infection. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2019, 17, 1183–1191.e7. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef] [PubMed]
- AASLD-IDSA HCV Guidance Panel. Hepatitis C guidance 2018 update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin. Infect. Dis. 2018, 67, 1477–1492. [Google Scholar]
- Ioannou, G.N. HCC surveillance after SVR in patients with F3/F4 fibrosis. J. Hepatol. 2021, 74, 458–465. [Google Scholar] [CrossRef]
- Kim, N.J.; Vutien, P.; Berry, K.; Ioannou, G.N. Hepatocellular carcinoma risk declines but remains high enough for screening in the first 7 years after hepatitis c virus cure with direct acting antivirals in patients with cirrhosis or high fibrosis-4 score. Gastroenterology 2022, 163, 1104–1106.e3. [Google Scholar] [CrossRef]
- Nahon, P.; Bamba-Funck, J.; Layese, R.; Trépo, E.; Zucman-Rossi, J.; Cagnot, C.; Ganne-Carrié, N.; Chaffaut, C.; Guyot, E.; Ziol, M.; et al. Integrating genetic variants into clinical models for hepatocellular carcinoma risk stratification in cirrhosis. J. Hepatol. 2022, 78, 584–595. [Google Scholar] [CrossRef]
- Huntley, C.; Torr, B.; Sud, A.; Rowlands, C.F.; Way, R.; Snape, K.; Hanson, H.; Swanton, C.; Broggio, J.; Lucassen, A.; et al. Utility of polygenic risk scores in UK cancer screening: A modelling analysis. Lancet Oncol. 2023, 24, 658–668. [Google Scholar] [CrossRef]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Metha, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.; Kulik, L.; Agopian, V.; Marrero, J.A. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 10. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.-L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawla, Y.K.; Shiina, S. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef]
- Johnson, P.J.; Sarah, J.; Pirrie, S.J.; Trevor, F.; Cox, T.F.; Berhane, S.; Teng, M.; Palmer, D.; Morse, J.; Hull, D.; et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 144–153. [Google Scholar] [CrossRef]
- Berhane, S.; Toyoda, H.; Tada, T.; Kumada, T.; Kagebayashi, C.; Satomura, S.; Schweitzer, N.; Vogel, A.; Manns, M.P.; Benckert, J.; et al. Role of the GALAD and BALAD-2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin. Gastroenterol. Hepatol. 2016, 14, 875–886. [Google Scholar] [CrossRef]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: A meta-analysis. Gastroenterology 2018, 154, 1706–1718. [Google Scholar] [CrossRef]
- Santi, V.; Trevisani, F.; Gramenzi, A.; Grignaschi, A.; Mirici-Cappa, F.; Del Poggio, P.; Di Nolfo, M.A.; Benvegnù, L.; Farinati, F.; Zoli, M.; et al. Semi-annual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J. Hepatol. 2010, 53, 291–297. [Google Scholar] [CrossRef]
- Trinchet, J.C.; Chaffaut, C.; Bourcier, V.; Degos, F.; Henrion, J.; Fontaine, H.; Roulot, D.; Mallat, A.; Hillaire, S.; Cales, P.; et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: A randomized trial comparing 3- and 6-month periodicies. Hepatology 2011, 54, 1987–1997. [Google Scholar] [CrossRef]
- Mueller, P.P.; Chen, Q.; Ayer, T.; Nemutlu, G.S.; Hajjar, A.; Bethea, E.D.; Peters, M.L.B.; Lee, B.P.; Janjua, N.Z.; Kanwal, F.; et al. Duration and cost-effectiveness of hepatocellular carcinoma surveillance in hepatitis C patients after viral eradication. J. Hepatol. 2022, 77, 55–62. [Google Scholar] [CrossRef]
- Cabibbo, G.; Celsa, C.; Calvaruso, V.; Petta, S.; Cacciola, I.; Cannavò, M.R.; Madonia, S.; Rossi, M.; Magro, B.; Rini, F.; et al. Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J. Hepatol. 2019, 71, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Reddy, S.; Patel, H.R.A.; Villarreal, D.; Khan, A.; Liu, Y.; Cerda, V.; Rich, N.E.; Murphy, C.C.; Tiro, J.A.; et al. Multicenter randomized clinical trial of a mailed outreach strategy for hepatocellular carcinoma surveillance. Clin. Gastroenterol. Hepatol. 2022, 20, 2818–2825. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Lee, J.M.; Lee, D.H.; Joo, I.; Jeon, J.H.; Ahn, S.J.; Kim, S.-T.; Cho, E.J.; Yu, S.J.; Kim, Y.J.; et al. A comparison of biannual two-phase low-dose liver CT and US for HCC surveillance in a group at high risk of HCC development. Liver Cancer 2020, 9, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; An, J.; Lim, Y.-S.; Han, S.; Lee, J.-Y.; Byun, J.H.; Won, H.J.; Lee, S.J.; Lee, H.C.; Lee, Y.S. MRI with liver-specific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma. JAMA Oncol. 2017, 3, 456–463. [Google Scholar] [CrossRef]
- Gupta, P.; Soundararjan, R.; Patel, A.; Kumar, M.P.; Sharma, V.; Kaira, N. Abbreviated MRI for hepatocellular carcinoma screening: A systematic review and meta-analysis. J. Hepatol. 2021, 75, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Huo, E.J.; Weinstein, S.; Santos, C.; Monto, A.; Corvera, C.U.; Yee, J.; Hope, T.A. Evaluation of an abbreviated screening MRI protocol for patients at risk for hepatocellular carcinoma. Abdom. Radiol. 2018, 43, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, T.; Masaki, N.; Parikh, N.D.; Lane, B.F.; Feng, M. Mendiratta-lala multicenter validation of abbreviated MRI for detecting early-stage hepatocellular carcinoma. Radiology 2023, 307, e220917. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Roberts, L.R.; Engel-Nitz, N.M.; Bancroft, T.; Ozbay, A.B.; Singal, A.G. Gaps in hepatocellular carcinoma surveillance in a United States cohort of insured patients with cirrhosis. Curr. Med. Res. Opin. 2022, 38, 2163–2173. [Google Scholar] [CrossRef]
- Parikh, N.D.; Tayob, N.; Singal, A.G. Blood-based biomarkers for hepatocellular carcinoma screening: Approaching the end of the ultrasound era? J. Hepatol. 2023, 78, 207–216. [Google Scholar] [CrossRef]
- Haug, C.J.; Drazen, J.M. Artificial intelligence and machine learning in clinical medicine, 2023. N. Engl. J. Med. 2023, 388, 1201–1208. [Google Scholar] [CrossRef]
- Nahon, P.; Quang, E.V.; Ganne-Carre, N. Stratification of hepatocellular carcinoma risk following HCV eradication or HBV. Control J. Clin. Med. 2021, 10, 353. [Google Scholar] [CrossRef]
- Yeo, Y.H.; Samaan, J.S.; Ng, W.H.; Ting, P.-S.; Trivedi, H.; Vipani, A.; Ayoub, W.; Yang, J.D.; Liran, O.; Spiegel, B.; et al. Assessing the performance of ChatGPT in answering questions regarding cirrhosis and hepatocellular carcinoma. Clin. Mol. Hepatol. 2023. ahead of print. [Google Scholar] [CrossRef]
- Page, K.; Melia, M.T.; Veenhuis, R.; Winter, M.; Rousseau, K.E.; Massacesi, G.; Osburn, W.O.; Forman, M.; Thomas, E.; Thornton, K.; et al. Randomized trial of vaccine regimen to prevent chronic HCV infection. N. Engl. J. Med. 2021, 384, 541–549. [Google Scholar] [CrossRef] [PubMed]
| Progress in Reducing Hepatitis-Related Mortality Has been Made between 2015 and 2019 |
|---|
| Ten-times increase in HCV treatment from 2015 |
| 98% of HBV infections and 87% of HCV infections have still not been treated |
| Only 10% of HBV-infected and 21% of HCV-infected persons even know their diagnosis |
| In Africa and South East Asia, diagnosis and treatment rates lag far behind 2030 targets |
| Current funding stands at less than 10% of the estimated USD 6 billion a year being required to meet hepatitis B and C elimination goals |
| Recommendation | EASL 2018 | AASLD 2023 | APASL 2017 |
|---|---|---|---|
| Screening interval | Six months | Six months | Six months |
| Imaging | US | US, contrast | US |
| enhanced MR/CT when | |||
| US visualization limited | |||
| Serum AFP | No | Yes | Yes |
| Liver biopsy | Non-cirrhotics | Non-cirrhotics | Unsolved >/= 1 cm |
| Unsolved nodules | |||
| LI-RADs 4 and 5 in trials | |||
| Diagnosis of HCC | CT/MRI/CEUS | CT/MRI | CT/MRI/CEUS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarus, J.V.; Picchio, C.A.; Colombo, M. Hepatocellular Carcinoma Prevention in the Era of Hepatitis C Elimination. Int. J. Mol. Sci. 2023, 24, 14404. https://doi.org/10.3390/ijms241814404
Lazarus JV, Picchio CA, Colombo M. Hepatocellular Carcinoma Prevention in the Era of Hepatitis C Elimination. International Journal of Molecular Sciences. 2023; 24(18):14404. https://doi.org/10.3390/ijms241814404
Chicago/Turabian StyleLazarus, Jeffrey V., Camila A. Picchio, and Massimo Colombo. 2023. "Hepatocellular Carcinoma Prevention in the Era of Hepatitis C Elimination" International Journal of Molecular Sciences 24, no. 18: 14404. https://doi.org/10.3390/ijms241814404
APA StyleLazarus, J. V., Picchio, C. A., & Colombo, M. (2023). Hepatocellular Carcinoma Prevention in the Era of Hepatitis C Elimination. International Journal of Molecular Sciences, 24(18), 14404. https://doi.org/10.3390/ijms241814404






