Finding Balance in Adversity: Nitrate Signaling as the Key to Plant Growth, Resilience, and Stress Response
Abstract
:1. Introduction
2. Abiotic Stresses and Plant Responses
3. Overview of Nitrate Signaling Pathway
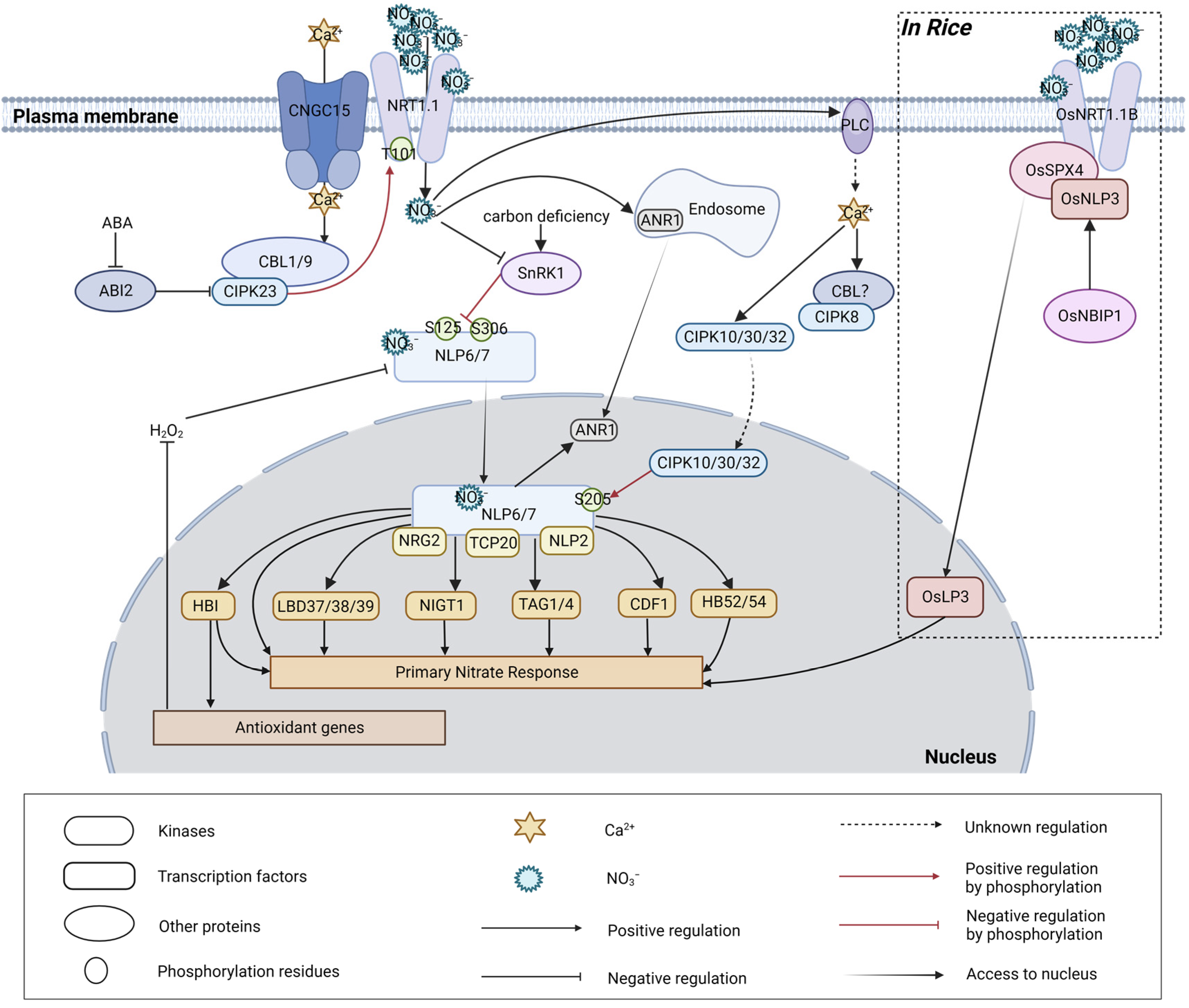
4. Regulations of NRT1.1 under Abiotic Stresses
5. Calcium Sensors as Regulating Hubs in Stress Tolerance
6. Roles of NLP7 in Abiotic Stresses
7. Tissue-Specific Nitrate Signaling in Abiotic Stresses
8. Epigenetic Regulators Integrate Nitrate Signaling and Abiotic Stress Response
| Species | Gene Name | Abiotic Stress | Effects | References |
|---|---|---|---|---|
| Arabidopsis thaliana | NRT1.1 | Drought stress | The nitrate transport activity of NRT1.1 is repressed under drought, leading to smaller stomatal opening. | [44,51,52] |
| Salt stress | The up-regulation of NRT1.1 mediated Cl− uptake under NH4+-aggravated salt stress. | [41] | ||
| Ammonium toxicity | Ammonium toxicity is related to the nitrate-independent signaling function of NRT1.1. Mutation of NRT1.1 enhances ammonium tolerance. | [45] | ||
| Low pH | H+ toxicity induces STOP1 to activate the transcription of NRT1.1, enhancing the coupled proton-driven nitrate uptake, which increases rhizosphere pH and alleviates proton toxicity. | [50] | ||
| Iron deficiency | Transcript level of NRT1.1 is down-regulated by Fe−deficiency stress, which helps plants better adapt to Fe-deficiency stress. | [47] | ||
| STOP1 | Drought stress | STOP1 suppresses drought tolerance by regulating K+ transport. | [111] | |
| SnRK2.2/2.3/2.6 | ABA homeostasis | SnRK2.2/2.3/2.6 kinase proteins phosphorylate NRT1.1 at Ser585 to impair its nitrate transport activity as an effect of stress-response hormone ABA signaling. | [53] | |
| ABI1 | Ammonium toxicity | The inactivation of phosphatase ABI1 under high external ammonium concentrations, in turn, activates CIPK23 for AMT phosphorylation and ammonium transport activity inhibition. | [69] | |
| ABI2 | ABA homeostasis | ABA accumulation would inactivate ABI2 and thereby promote the activity of CBL1-CIPK23 to enhance NRT1.1-mediated nitrate signaling. | [7] | |
| CIPK23 | Drought stress | CIPK23 regulates drought tolerance by combining with CBL1 and CBL9. The complexes can change the ABA sensitivity in guard cells. | [112] | |
| Salt stress | STOP1 regulates salt and drought tolerance by transcriptionally induced CIPK23. | [64] | ||
| Ammonium toxicity | During high external ammonium concentrations, activating CIPK23 for AMTs phosphorylation and ammonium transport activity inhibition. | [69] | ||
| Low-K+ | CIPK23 phosphorylates AKT1 transporters that enhance K+ uptake. | [66] | ||
| CPK10 | Drought stress | CPK10 plays important roles in ABA- and calcium-mediated regulation of stomatal movements in response to drought stress. | [71] | |
| CPK30 | Salt stress | CPK30 was up-regulated in salt-stressed seedlings, suggesting that they might play important roles in salt tolerance. | [72] | |
| CPK32 | ABA homeostasis | CPK32 is able to phosphorylate the ABA-responsive transcription factor ABF4, and CPK32-overexpressing plants showed a hypersensitive phenotype to ABA. | [73] | |
| CML38 | Hypoxia stress | CML38 regulates hypoxia-induced stress granules turnover by autophagy. | [77] | |
| NLP7 | Drought stress | The loss-of-function nlp7 exhibits a nitrogen-deficient phenotype as well as tolerance to drought. | [78] | |
| Salt stress | The nlp7 exhibits a nitrogen-deficient phenotype as well as tolerance to salt. | [78] | ||
| Cold stress | Cold stimulates the nuclear translocation of NLP7 after phosphorylation by CPK28 for cold stress response. | [79] | ||
| SnRK1 | Cold stress | SnRK1 and TOR with ATPase act together to regulate the energy homeostasis under cold stress. | [81] | |
| HBI | ROS homeostasis | Nitrate treatment reduced the accumulation of H2O2, through HBI-mediated ROS homeostasis. | [23] | |
| Triticum aestivum | TaCIPK23 | Drought stress | Wheat and Arabidopsis overexpressing TaCIPK23 showed a higher survival rate under drought conditions with enhanced germination rate. | [65] |
| Zea mays | ZmCHB101 | Osmotic stress | ZmCHB101 affects gene expression by remodeling chromatin states and controls RNAPII occupancies in maize under osmotic stress. | [99] |
| Oryza sativa | OsMADS27 | Salt stress | OsMADS27 directly binds to the promoters of OsHKT1.1 and OsSPL7 to regulate their expression in response to salt stress. | [106] |
9. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.Y.; Cheng, Y.H.; Chen, K.E.; Tsay, Y.F. Nitrate Transport, Signaling, and Use Efficiency. Annu. Rev. Plant Biol. 2018, 69, 85–122. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Lin, S.; Hu, H.; Tsay, Y. CHL1 functions as a nitrate sensor in plants. Cell 2009, 138, 1184–1194. [Google Scholar] [CrossRef]
- Riveras, E.; Alvarez, J.M.; Vidal, E.A.; Oses, C.; Vega, A.; Gutierrez, R.A. The Calcium Ion Is a Second Messenger in the Nitrate Signaling Pathway of Arabidopsis. Plant Physiol. 2015, 169, 1397–1404. [Google Scholar] [CrossRef]
- Wang, X.; Feng, C.; Tian, L.; Hou, C.; Tian, W.; Hu, B.; Zhang, Q.; Ren, Z.; Niu, Q.; Song, J.; et al. A transceptor–channel complex couples nitrate sensing to calcium signaling in Arabidopsis. Mol. Plant 2021, 14, 774–786. [Google Scholar] [CrossRef]
- Léran, S.; Edel, K.H.; Pervent, M.; Hashimoto, K.; Corratgé-Faillie, C.; Offenborn, J.N.; Tillard, P.; Gojon, A.; Kudla, J.; Lacombe, B. Nitrate sensing and uptake in Arabidopsis are enhanced by ABI2, a phosphatase inactivated by the stress hormone abscisic acid. Sci. Signal 2015, 8, ra43. [Google Scholar] [CrossRef]
- Rashid, M.; Bera, S.; Medvinsky, A.B.; Sun, G.Q.; Li, B.L.; Chakraborty, A. Adaptive Regulation of Nitrate Transceptor NRT1.1 in Fluctuating Soil Nitrate Conditions. iScience 2018, 2, 41–50. [Google Scholar] [CrossRef]
- Rashid, M.; Bera, S.; Banerjee, M.; Medvinsky, A.B.; Sun, G.Q.; Li, B.L.; Sljoka, A.; Chakraborty, A. Feedforward Control of Plant Nitrate Transporter NRT1.1 Biphasic Adaptive Activity. Biophys. J. 2020, 118, 898–908. [Google Scholar] [CrossRef]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA perception and signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, Y.; Yu, M.; Su, B.; Gong, W.; Baluška, F.; Komis, G.; Šamaj, J.; Shan, X.; Lin, J. Phosphorylation-Mediated Dynamics of Nitrate Transceptor NRT1.1 Regulate Auxin Flux and Nitrate Signaling in Lateral Root Growth. Plant Physiol. 2019, 181, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Bouguyon, E.; Perrine-Walker, F.; Pervent, M.; Rochette, J.; Cuesta, C.; Benkova, E.; Martiniere, A.; Bach, L.; Krouk, G.; Gojon, A.; et al. Nitrate Controls Root Development through Posttranscriptional Regulation of the NRT1.1/NPF6.3 Transporter/Sensor. Plant Physiol. 2016, 172, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.H.; Niu, Y.J.; Konishi, M.; Wu, Y.; Du, H.; Chung, H.S.; Li, L.; Boudsocq, M.; McCormack, M.; Maekawa, S.; et al. Discovery of nitrate-CPK-NLP signalling in central nutrient-growth networks. Nature 2017, 545, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Han, C.; Wang, J.-G.; Chu, X.; Shi, W.; Yao, L.; Chen, J.; Hao, W.; Deng, Z.; Fan, M.; et al. Regulatory functions of cellular energy sensor SnRK1 for nitrate signalling through NLP7 repression. Nat. Plants 2022, 8, 1094–1107. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.M.; Schinke, A.L.; Brooks, M.D.; Pasquino, A.; Leonelli, L.; Varala, K.; Safi, A.; Krouk, G.; Krapp, A.; Coruzzi, G.M. Transient genome-wide interactions of the master transcription factor NLP7 initiate a rapid nitrogen-response cascade. Nat. Commun. 2020, 11, 1157. [Google Scholar] [CrossRef]
- Marchive, C.; Roudier, F.; Castaings, L.; Bréhaut, V.; Blondet, E.; Colot, V.; Meyer, C.; Krapp, A. Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants. Nat. Commun. 2013, 4, 1713. [Google Scholar] [CrossRef]
- Rubin, G.; Tohge, T.; Matsuda, F.; Saito, K.; Scheible, W.R. Members of the LBD family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis. Plant Cell 2009, 21, 3567–3584. [Google Scholar] [CrossRef]
- Ueda, Y.; Yanagisawa, S. Transcription factor module NLP-NIGT1 fine-tunes NITRATE TRANSPORTER2.1 expression. Plant Physiol. 2023. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, L.; Li, Y.; Zhang, D.; Gao, Y. Plant NIGT1/HRS1/HHO Transcription Factors: Key Regulators with Multiple Roles in Plant Growth, Development, and Stress Responses. Int. J. Mol. Sci. 2021, 22, 8685. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Riveras, E.; Vidal, E.A.; Gras, D.E.; Contreras-López, O.; Tamayo, K.P.; Aceituno, F.; Gómez, I.; Ruffel, S.; Lejay, L.; et al. Systems approach identifies TGA1 and TGA4 transcription factors as important regulatory components of the nitrate response of Arabidopsis thaliana roots. Plant J. 2014, 80, 1–13. [Google Scholar] [CrossRef]
- Varala, K.; Marshall-Colón, A.; Cirrone, J.; Brooks, M.D.; Pasquino, A.V.; Léran, S.; Mittal, S.; Rock, T.M.; Edwards, M.B.; Kim, G.J.; et al. Temporal transcriptional logic of dynamic regulatory networks underlying nitrogen signaling and use in plants. Proc. Natl. Acad. Sci. USA 2018, 115, 6494–6499. [Google Scholar] [CrossRef] [PubMed]
- Ariga, T.; Sakuraba, Y.; Zhuo, M.; Yang, M.; Yanagisawa, S. The Arabidopsis NLP7-HB52/54-VAR2 pathway modulates energy utilization in diverse light and nitrogen conditions. Curr. Biol. 2022, 32, 5344–5353. [Google Scholar] [CrossRef]
- Chu, X.; Wang, J.G.; Li, M.; Zhang, S.; Gao, Y.; Fan, M.; Han, C.; Xiang, F.; Li, G.; Wang, Y.; et al. HBI transcription factor-mediated ROS homeostasis regulates nitrate signal transduction. Plant Cell 2021, 33, 3004–3021. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Bernreiter, A.; Filleur, S.; Abram, B.; Forde, B.G. Overexpressing the ANR1 MADS-box gene in transgenic plants provides new insights into its role in the nitrate regulation of root development. Plant Cell Physiol. 2012, 53, 1003–1016. [Google Scholar] [CrossRef]
- Xu, N.; Wang, R.C.; Zhao, L.F.; Zhang, C.F.; Li, Z.H.; Lei, Z.; Liu, F.; Guan, P.Z.; Chu, Z.H.; Crawford, N.M.; et al. The Arabidopsis NRG2 Protein Mediates Nitrate Signaling and Interacts with and Regulates Key Nitrate Regulators. Plant Cell 2016, 28, 485–504. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.Z.; Ripoll, J.J.; Wang, R.H.; Vuong, L.; Bailey-Steinitz, L.J.; Ye, D.N.; Crawford, N.M. Interacting TCP and NLP transcription factors control plant responses to nitrate availability. Proc. Natl. Acad. Sci. USA 2017, 114, 2419–2424. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.Q.; Li, M.Z.; Zhang, S.J.; Fan, M.; Han, C.; Xiang, F.N.; Li, G.Y.; Wang, Y.; Xiang, C.B.; Wang, J.G.; et al. HBI1-TCP20 interaction positively regulates the CEPs-mediated systemic nitrate acquisition. J. Integr. Plant Biol. 2021, 63, 902–912. [Google Scholar] [CrossRef]
- Guan, P.Z.; Wang, R.C.; Nacry, P.; Breton, G.; Kay, S.A.; Pruneda-Paz, J.L.; Davani, A.; Crawford, N.M. Nitrate foraging by Arabidopsis roots is mediated by the transcription factor TCP20 through the systemic signaling pathway. Proc. Natl. Acad. Sci. USA 2014, 111, 15267–15272. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.H.; Liu, M.H.; Lin, Z.W.; Wang, Z.F.; Chen, B.Q.; Liu, C.; Guo, A.P.; Konishi, M.; Yanagisawa, S.; Wagner, G.; et al. NIN-like protein 7 transcription factor is a plant nitrate sensor. Science 2022, 377, 1419–1425. [Google Scholar] [CrossRef]
- Konishi, M.; Yanagisawa, S. The role of protein-protein interactions mediated by the PB1 domain of NLP transcription factors in nitrate-inducible gene expression. BMC Plant Biol. 2019, 19, 90. [Google Scholar] [CrossRef]
- Konishi, M.; Okitsu, T.; Yanagisawa, S. Nitrate-responsive NIN-like protein transcription factors perform unique and redundant roles in Arabidopsis. J. Exp. Bot. 2021, 72, 5735–5750. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Durand, M.; Brehaut, V.; Hsu, F.C.; Kelemen, Z.; Texier, Y.; Krapp, A.; Tsay, Y.F. Interplay between NIN-LIKE PROTEINs 6 and 7 in nitrate signaling. Plant Physiol. 2023, 192, 3049–3068. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Brehaut, V.; Clement, G.; Kelemen, Z.; Mace, J.; Feil, R.; Duville, G.; Launay-Avon, A.; Roux, C.P.L.; Lunn, J.E.; et al. The Arabidopsis transcription factor NLP2 regulates early nitrate responses and integrates nitrate assimilation with energy and carbon skeleton supply. Plant Cell 2023, 35, 1429–1454. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jiang, Z.; Wang, W.; Qiu, Y.; Zhang, Z.; Liu, Y.; Li, A.; Gao, X.; Liu, L.; Qian, Y.; et al. Nitrate-NRT1.1B-SPX4 cascade integrates nitrogen and phosphorus signalling networks in plants. Nat. Plants 2019, 5, 401–413. [Google Scholar] [CrossRef]
- Hu, B.; Chu, C. Nitrogen-phosphorus interplay: Old story with molecular tale. New Phytol. 2020, 225, 1455–1460. [Google Scholar] [CrossRef]
- Wang, W.; Hu, B.; Li, A.; Chu, C. NRT1.1s in plants: Functions beyond nitrate transport. J. Exp. Bot. 2020, 71, 4373–4379. [Google Scholar] [CrossRef]
- Meng, X.; Yu, X.; Wu, Y.; Kim, D.H.; Nan, N.; Cong, W.; Wang, S.; Liu, B.; Xu, Z.Y. Chromatin Remodeling Protein ZmCHB101 Regulates Nitrate-Responsive Gene Expression in Maize. Front. Plant Sci. 2020, 11, 52. [Google Scholar] [CrossRef]
- Undurraga, S.F.; Ibarra-Henríquez, C.; Fredes, I.; Álvarez, J.M.; Gutiérrez, R.A. Nitrate signaling and early responses in Arabidopsis roots. J. Exp. Bot. 2017, 68, 2541–2551. [Google Scholar] [CrossRef]
- Fang, X.Z.; Tian, W.H.; Liu, X.X.; Lin, X.Y.; Jin, C.W.; Zheng, S.J. Alleviation of proton toxicity by nitrate uptake specifically depends on nitrate transporter 1.1 in Arabidopsis. New Phytol. 2016, 211, 149–158. [Google Scholar] [CrossRef]
- Krouk, G.; Lacombe, B.; Bielach, A.; Perrine-Walker, F.; Malinska, K.; Mounier, E.; Hoyerova, K.; Tillard, P.; Leon, S.; Ljung, K.; et al. Nitrate-Regulated Auxin Transport by NRT1.1 Defines a Mechanism for Nutrient Sensing in Plants. Dev. Cell 2010, 18, 927–937. [Google Scholar] [CrossRef]
- Liu, X.X.; Zhu, Y.X.; Fang, X.Z.; Ye, J.Y.; Du, W.X.; Zhu, Q.Y.; Lin, X.Y.; Jin, C.W. Ammonium aggravates salt stress in plants by entrapping them in a chloride over-accumulation state in an NRT1.1-dependent manner. Sci. Total Environ. 2020, 746, 141244. [Google Scholar] [CrossRef] [PubMed]
- Maghiaoui, A.; Bouguyon, E.; Cuesta, C.; Section, F.P.W.; Alcon, C.; Krouk, G.; Benkova, E.; Nacry, P.; Gojon, A.; Bach, L. The Arabidopsis NRT1.1 transceptor coordinately controls auxin biosynthesis and transport to regulate root branching in response to nitrate. J. Exp. Bot. 2020, 71, 4480–4494. [Google Scholar] [CrossRef] [PubMed]
- Lay-Pruitt, K.S.; Takahashi, H. Integrating N signals and root growth: The role of nitrate transceptor NRT1.1 in auxin-mediated lateral root development. J. Exp. Bot. 2020, 71, 4365–4368. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.O.; Young, J.; Crawford, N.M. The nitrate transporter AtNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in Arabidopsis. Plant Cell 2003, 15, 107–117. [Google Scholar] [CrossRef]
- Hachiya, T.; Noguchi, K. Mutation of NRT1.1 enhances ammonium/low pH-tolerance in Arabiopsis thaliana. Plant Signal. Behav. 2011, 6, 706–708. [Google Scholar] [CrossRef]
- Jian, S.F.; Luo, J.S.; Liao, Q.; Liu, Q.; Guan, C.Y.; Zhang, Z.H. NRT1.1 Regulates Nitrate Allocation and Cadmium Tolerance in Arabidopsis. Front. Plant Sci. 2019, 10, 384. [Google Scholar] [CrossRef]
- Liu, X.Y.; Cui, H.Q.; Li, A.N.; Zhang, M.; Teng, Y.B. The nitrate transporter NRT1.1 is involved in iron deficiency responses in Arabidopsis. J. Plant Nutr. Soil Sci. 2015, 178, 601–608. [Google Scholar] [CrossRef]
- Ye, J.Y.; Zhou, M.; Zhu, Q.Y.; Zhu, Y.X.; Du, W.X.; Liu, X.X.; Jin, C.W. Inhibition of shoot-expressed NRT1.1 improves reutilization of apoplastic iron under iron-deficient conditions. Plant J. 2022, 112, 549–564. [Google Scholar] [CrossRef]
- Fang, X.Z.; Fang, S.Q.; Ye, Z.Q.; Liu, D.; Zhao, K.L.; Jin, C.W. NRT1.1 Dual-Affinity Nitrate Transport/Signalling and its Roles in Plant Abiotic Stress Resistance. Front. Plant Sci. 2021, 12, 715694. [Google Scholar] [CrossRef]
- Ye, J.Y.; Tian, W.H.; Zhou, M.; Zhu, Q.Y.; Du, W.X.; Zhu, Y.X.; Liu, X.X.; Lin, X.Y.; Zheng, S.J.; Jin, C.W. STOP1 activates NRT1.1-mediated nitrate uptake to create a favorable rhizospheric pH for plant adaptation to acidity. Plant Cell 2021, 33, 3658–3674. [Google Scholar] [CrossRef]
- Rolly, N.K.; Yun, B.W. Regulation of Nitrate (NO3) Transporters and Glutamate Synthase-Encoding Genes under Drought Stress in Arabidopsis: The Regulatory Role of AtbZIP62 Transcription Factor. Plants 2021, 10, 2149. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhao, C.; Hu, S.; Zuo, K. Arabidopsis CPK6 regulates drought tolerance under high nitrogen by the phosphorylation of NRT1.1. J. Exp. Bot. 2023. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wang, T.; Ju, C.F.; Deng, J.P.; Zhang, T.Q.; Li, M.J.; Tian, H.; Wang, C. Abscisic acid signaling negatively regulates nitrate uptake via phosphorylation of NRT1.1 by SnRK2s in Arabidopsis. J. Integr. Plant Biol. 2021, 63, 597–610. [Google Scholar] [CrossRef]
- Bouguyon, E.; Brun, F.; Meynard, D.; Kubes, M.; Pervent, M.; Leran, S.; Lacombe, B.; Krouk, G.; Guiderdoni, E.; Zazimalova, E.; et al. Multiple mechanisms of nitrate sensing by Arabidopsis nitrate transceptor NRT1.1. Nat. Plants 2015, 1, 15015. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ding, Y.; Yang, Y.; Song, C.; Wang, B.; Yang, S.; Guo, Y.; Gong, Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021, 63, 53–78. [Google Scholar] [CrossRef]
- Sánchez-Barrena, M.J.; Chaves-Sanjuan, A.; Raddatz, N.; Mendoza, I.; Cortés, Á.; Gago, F.; González-Rubio, J.M.; Benavente, J.L.; Quintero, F.J.; Pardo, J.M.; et al. Recognition and Activation of the Plant AKT1 Potassium Channel by the Kinase CIPK23. Plant Physiol. 2020, 182, 2143–2153. [Google Scholar] [CrossRef]
- Ragel, P.; Ródenas, R.; García-Martín, E.; Andrés, Z.; Villalta, I.; Nieves-Cordones, M.; Rivero, R.M.; Martínez, V.; Pardo, J.M.; Quintero, F.J.; et al. The CBL-Interacting Protein Kinase CIPK23 Regulates HAK5-Mediated High-Affinity K+ Uptake in Arabidopsis Roots. Plant Physiol. 2015, 169, 2863–2873. [Google Scholar] [CrossRef]
- Templalexis, D.; Tsitsekian, D.; Liu, C.; Daras, G.; Šimura, J.; Moschou, P.; Ljung, K.; Hatzopoulos, P.; Rigas, S. Potassium transporter TRH1/KUP4 contributes to distinct auxin-mediated root system architecture responses. Plant Physiol. 2022, 188, 1043–1060. [Google Scholar] [CrossRef]
- Straub, T.; Ludewig, U.; Neuhäuser, B. The Kinase CIPK23 Inhibits Ammonium Transport in Arabidopsis thaliana. Plant Cell 2017, 29, 409–422. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, X.; Yang, A.; Wang, T.; Zhang, W.H. CIPK23 is involved in iron acquisition of Arabidopsis by affecting ferric chelate reductase activity. Plant Sci. 2016, 246, 70–79. [Google Scholar] [CrossRef]
- Dubeaux, G.; Neveu, J.; Zelazny, E.; Vert, G. Metal Sensing by the IRT1 Transporter-Receptor Orchestrates Its Own Degradation and Plant Metal Nutrition. Mol. Cell 2018, 69, 953–964.e955. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Maierhofer, T.; Hashimoto, K.; Xu, X.; Karimi, S.M.; Müller, H.; Geringer, M.A.; Wang, Y.; Kudla, J.; De Smet, I.; et al. The CIPK23 protein kinase represses SLAC1-type anion channels in Arabidopsis guard cells and stimulates stomatal opening. New Phytol. 2023, 238, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fu, D.; Xie, D.; Wang, Z.; Zhao, Y.; Ma, X.; Huang, P.; Ju, C.; Wang, C. CBL1/9-CIPK23-NRAMP1 axis regulates manganese toxicity. New Phytol. 2023, 239, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, A.; Enomoto, T.; Kobayashi, Y.; Watanabe, T.; Iuchi, S.; Kobayashi, M.; Sahoo, L.; Yamamoto, Y.Y.; Koyama, H. Sensitive to Proton Rhizotoxicity1 Regulates Salt and Drought Tolerance of Arabidopsis thaliana through Transcriptional Regulation of CIPK23. Plant Cell Physiol. 2019, 60, 2113–2126. [Google Scholar] [CrossRef]
- Cui, X.Y.; Du, Y.T.; Fu, J.D.; Yu, T.F.; Wang, C.T.; Chen, M.; Chen, J.; Ma, Y.Z.; Xu, Z.S. Wheat CBL-interacting protein kinase 23 positively regulates drought stress and ABA responses. BMC Plant Biol. 2018, 18, 93. [Google Scholar] [CrossRef]
- Ródenas, R.; Vert, G. Regulation of Root Nutrient Transporters by CIPK23: ‘One Kinase to Rule Them All’. Plant Cell Physiol. 2021, 62, 553–563. [Google Scholar] [CrossRef]
- Dong, Q.; Bai, B.; Almutairi, B.O.; Kudla, J. Emerging roles of the CBL-CIPK calcium signaling network as key regulatory hub in plant nutrition. J. Plant Physiol. 2021, 257, 153335. [Google Scholar] [CrossRef]
- Verma, P.; Sanyal, S.K.; Pandey, G.K. Ca2+-CBL-CIPK: A modulator system for efficient nutrient acquisition. Plant Cell Rep. 2021, 40, 2111–2122. [Google Scholar] [CrossRef]
- Ganz, P.; Porras-Murillo, R.; Ijato, T.; Menz, J.; Straub, T.; Stührwohldt, N.; Moradtalab, N.; Ludewig, U.; Neuhäuser, B. Abscisic acid influences ammonium transport via regulation of kinase CIPK23 and ammonium transporters. Plant Physiol. 2022, 190, 1275–1288. [Google Scholar] [CrossRef]
- Dekomah, S.D.; Bi, Z.; Dormatey, R.; Wang, Y.; Haider, F.U.; Sun, C.; Yao, P.; Bai, J. The role of CDPKs in plant development, nutrient and stress signaling. Front. Genet. 2022, 13, 996203. [Google Scholar] [CrossRef]
- Zou, J.-J.; Wei, F.-J.; Wang, C.; Wu, J.-J.; Ratnasekera, D.; Liu, W.-X.; Wu, W.-H. Arabidopsis Calcium-Dependent Protein Kinase CPK10 Functions in Abscisic Acid- and Ca2+-Mediated Stomatal Regulation in Response to Drought Stress. Plant Physiol. 2010, 154, 1232–1243. [Google Scholar] [CrossRef]
- Yang, L.; Jin, Y.; Huang, W.; Sun, Q.; Liu, F.; Huang, X. Full-length transcriptome sequences of ephemeral plant Arabidopsis pumila provides insight into gene expression dynamics during continuous salt stress. BMC Genom. 2018, 19, 717. [Google Scholar] [CrossRef]
- Choi, H.I.; Park, H.J.; Park, J.H.; Kim, S.; Im, M.Y.; Seo, H.H.; Kim, Y.W.; Hwang, I.; Kim, S.Y. Arabidopsis calcium-dependent protein kinase AtCPK32 interacts with ABF4, a transcriptional regulator of abscisic acid-responsive gene expression, and modulates its activity. Plant Physiol. 2005, 139, 1750–1761. [Google Scholar] [CrossRef]
- Qin, D.B.; Liu, M.Y.; Yuan, L.; Zhu, Y.; Li, X.D.; Chen, L.M.; Wang, Y.; Chen, Y.F.; Wu, W.H.; Wang, Y. CALCIUM-DEPENDENT PROTEIN KINASE 32-mediated phosphorylation is essential for the ammonium transport activity of AMT1;1 in Arabidopsis roots. J. Exp. Bot. 2020, 71, 5087–5097. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, J.; Lyu, M.; Kong, X.; Hu, S.; Song, Q.; Zuo, K. CALMODULIN-LIKE-38 and PEP1 RECEPTOR 2 integrate nitrate and brassinosteroid signals to regulate root growth. Plant Physiol. 2021, 187, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- Lokdarshi, A.; Conner, W.C.; McClintock, C.; Li, T.; Roberts, D.M. Arabidopsis CML38, a Calcium Sensor That Localizes to Ribonucleoprotein Complexes under Hypoxia Stress. Plant Physiol. 2016, 170, 1046–1059. [Google Scholar] [CrossRef]
- Field, S.; Conner, W.C.; Roberts, D.M. Arabidopsis CALMODULIN-LIKE 38 Regulates Hypoxia-Induced Autophagy of SUPPRESSOR OF GENE SILENCING 3 Bodies. Front. Plant Sci. 2021, 12, 722940. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.T.; Lee, W.J.; Choi, J.H.; Nguyen, D.T.; Truong, H.A.; Lee, S.A.; Hong, S.W.; Lee, H. The Loss of Function of the NODULE INCEPTION-Like PROTEIN 7 Enhances Salt Stress Tolerance in Arabidopsis Seedlings. Front. Plant Sci. 2022, 12, 743832. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yang, H.; Wu, S.; Fu, D.; Li, M.; Gong, Z.; Yang, S. CPK28-NLP7 module integrates cold-induced Ca2+ signal and transcriptional reprogramming in Arabidopsis. Sci. Adv. 2022, 8, eabn7901. [Google Scholar] [CrossRef]
- Soto-Burgos, J.; Bassham, D.C. SnRK1 activates autophagy via the TOR signaling pathway in Arabidopsis thaliana. PLoS ONE 2017, 12, e0182591. [Google Scholar] [CrossRef]
- Haq, S.I.U.; Shang, J.; Xie, H.; Qiu, Q.S. Roles of TOR signaling in nutrient deprivation and abiotic stress. J. Plant Physiol. 2022, 274, 153716. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- León, J.; Costa-Broseta, Á. Present knowledge and controversies, deficiencies, and misconceptions on nitric oxide synthesis, sensing, and signaling in plants. Plant Cell Environ. 2020, 43, 1–15. [Google Scholar] [CrossRef]
- Castillo, M.C.; Costa-Broseta, Á.; Gayubas, B.; León, J. NIN-like protein7 and PROTEOLYSIS6 functional interaction enhances tolerance to sucrose, ABA, and submergence. Plant Physiol. 2021, 187, 2731–2748. [Google Scholar] [CrossRef]
- Gifford, M.L.; Dean, A.; Gutierrez, R.A.; Coruzzi, G.M.; Birnbaum, K.D. Cell-specific nitrogen responses mediate developmental plasticity. Proc. Natl. Acad. Sci. USA 2008, 105, 803–808. [Google Scholar] [CrossRef]
- Walker, L.; Boddington, C.; Jenkins, D.; Wang, Y.; Grønlund, J.T.; Hulsmans, J.; Kumar, S.; Patel, D.; Moore, J.D.; Carter, A.; et al. Changes in Gene Expression in Space and Time Orchestrate Environmentally Mediated Shaping of Root Architecture. Plant Cell 2017, 29, 2393–2412. [Google Scholar] [CrossRef] [PubMed]
- Contreras-López, O.; Vidal, E.A.; Riveras, E.; Alvarez, J.M.; Moyano, T.C.; Sparks, E.E.; Medina, J.; Pasquino, A.; Benfey, P.N.; Coruzzi, G.M.; et al. Spatiotemporal analysis identifies ABF2 and ABF3 as key hubs of endodermal response to nitrate. Proc. Natl. Acad. Sci. USA 2022, 119, e2107879119. [Google Scholar] [CrossRef] [PubMed]
- Tipper, E.; Leitão, N.; Dangeville, P.; Lawson, D.M.; Charpentier, M. A novel mutant allele of AtCNGC15 reveals a dual function of nuclear calcium release in the root meristem. J. Exp. Bot. 2023, 74, 2572–2584. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.Z.; Liu, X.X.; Zhu, Y.X.; Ye, J.Y.; Jin, C.W. The K+ and NO3− Interaction Mediated by NITRATE TRANSPORTER1.1 Ensures Better Plant Growth under K+-Limiting Conditions. Plant Physiol. 2020, 184, 1900–1916. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lin, S.H.; Cheng, L.H.; Wu, J.J.; Lin, Y.C.; Tsay, Y.F. Potential transceptor AtNRT1.13 modulates shoot architecture and flowering time in a nitrate-dependent manner. Plant Cell 2021, 33, 1492–1505. [Google Scholar] [CrossRef]
- Yan, D.; Easwaran, V.; Chau, V.; Okamoto, M.; Ierullo, M.; Kimura, M.; Endo, A.; Yano, R.; Pasha, A.; Gong, Y.; et al. NIN-like protein 8 is a master regulator of nitrate-promoted seed germination in Arabidopsis. Nat. Commun. 2016, 7, 13179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lang, Z.; Zhu, J.K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Miryeganeh, M. Plants’ Epigenetic Mechanisms and Abiotic Stress. Genes 2021, 12, 1106. [Google Scholar] [CrossRef] [PubMed]
- Widiez, T.; El Kafafi, E.S.; Girin, T.; Berr, A.; Ruffel, S.; Krouk, G.; Vayssières, A.; Shen, W.H.; Coruzzi, G.M.; Gojon, A.; et al. High nitrogen insensitive 9 (HNI9)-mediated systemic repression of root NO3− uptake is associated with changes in histone methylation. Proc. Natl. Acad. Sci. USA 2011, 108, 13329–13334. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Brooks, M.; Yeoh-Wang, J.; McCoy, R.M.; Rock, T.M.; Pasquino, A.; Moon, C.I.; Patrick, R.M.; Tanurdzic, M.; Ruffel, S.; et al. SDG8-Mediated Histone Methylation and RNA Processing Function in the Response to Nitrate Signaling. Plant Physiol. 2020, 182, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Bvindi, C.; Tang, L.; Lee, S.; Patrick, R.M.; Yee, Z.R.; Mengiste, T.; Li, Y. Histone methyltransferases SDG33 and SDG34 regulate organ-specific nitrogen responses in tomato. Front Plant Sci. 2022, 13, 1005077. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.M.; Moyano, T.C.; Zhang, T.; Gras, D.E.; Herrera, F.J.; Araus, V.; O’Brien, J.A.; Carrillo, L.; Medina, J.; Vicente-Carbajosa, J.; et al. Local Changes in Chromatin Accessibility and Transcriptional Networks Underlying the Nitrate Response in Arabidopsis Roots. Mol. Plant 2019, 12, 1545–1560. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, L.; Wu, R.; Meng, X.; Zhang, A.; Li, N.; Xia, Q.; Qi, X.; Pang, J.; Xu, Z.Y.; et al. The Core Subunit of a Chromatin-Remodeling Complex, ZmCHB101, Plays Essential Roles in Maize Growth and Development. Sci. Rep. 2016, 6, 38504. [Google Scholar] [CrossRef]
- Yu, X.; Meng, X.; Liu, Y.; Li, N.; Zhang, A.; Wang, T.J.; Jiang, L.; Pang, J.; Zhao, X.; Qi, X.; et al. The chromatin remodeler ZmCHB101 impacts expression of osmotic stress-responsive genes in maize. Plant Mol. Biol. 2018, 97, 451–465. [Google Scholar] [CrossRef]
- Vidal, E.A.; Araus, V.; Lu, C.; Parry, G.; Green, P.J.; Coruzzi, G.M.; Gutiérrez, R.A. Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 4477–4482. [Google Scholar] [CrossRef]
- Gupta, O.P.; Meena, N.L.; Sharma, I.; Sharma, P. Differential regulation of microRNAs in response to osmotic, salt and cold stresses in wheat. Mol. Biol. Rep. 2014, 41, 4623–4629. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Bian, H.; Zeng, Z.; Hou, N.; Shi, B.; Wang, J.; Zhu, M.; Han, N. miR393-Mediated Auxin Signaling Regulation is Involved in Root Elongation Inhibition in Response to Toxic Aluminum Stress in Barley. Plant Cell Physiol. 2017, 58, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhu, H.; Li, N.; Batley, J.; Wang, Y. The miR393-Target Module Regulates Plant Development and Responses to Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2022, 23, 9477. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, H.; Hamera, S.; Chen, X.; Fang, R. miR444a has multiple functions in the rice nitrate-signaling pathway. Plant J. 2014, 78, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Pachamuthu, K.; Hari Sundar, V.; Narjala, A.; Singh, R.R.; Das, S.; Avik Pal, H.C.Y.; Shivaprasad, P.V. Nitrate-dependent regulation of miR444-OsMADS27 signalling cascade controls root development in rice. J. Exp. Bot. 2022, 73, 3511–3530. [Google Scholar] [CrossRef] [PubMed]
- Alfatih, A.; Zhang, J.; Song, Y.; Jan, S.U.; Zhang, Z.S.; Xia, J.Q.; Zhang, Z.Y.; Nazish, T.; Wu, J.; Zhao, P.X.; et al. Nitrate-responsive OsMADS27 promotes salt tolerance in rice. Plant Commun. 2023, 4, 100458. [Google Scholar] [CrossRef]
- Vidal, E.A.; Moyano, T.C.; Krouk, G.; Katari, M.S.; Tanurdzic, M.; McCombie, W.R.; Coruzzi, G.M.; Gutiérrez, R.A. Integrated RNA-seq and sRNA-seq analysis identifies novel nitrate-responsive genes in Arabidopsis thaliana roots. BMC Genom. 2013, 14, 701. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, J.T. Nitrate/ammonium-responsive microRNA-mRNA regulatory networks affect root system architecture in Populus × canescens. BMC Plant Biol. 2022, 22, 96. [Google Scholar] [CrossRef]
- Liu, F.; Xu, Y.; Chang, K.; Li, S.; Liu, Z.; Qi, S.; Jia, J.; Zhang, M.; Crawford, N.M.; Wang, Y. The long noncoding RNA T5120 regulates nitrate response and assimilation in Arabidopsis. New Phytol. 2019, 224, 117–131. [Google Scholar] [CrossRef]
- Zhao, Z.; Zang, S.; Zou, W.; Pan, Y.B.; Yao, W.; You, C.; Que, Y. Long Non-Coding RNAs: New Players in Plants. Int. J. Mol. Sci. 2022, 23, 9301. [Google Scholar] [CrossRef]
- Sadhukhan, A.; Kobayashi, Y.; Iuchi, S.; Koyama, H. Synergistic and antagonistic pleiotropy of STOP1 in stress tolerance. Trends Plant Sci. 2021, 26, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Cheong, Y.H.; Pandey, G.K.; Grant, J.J.; Batistic, O.; Li, L.; Kim, B.G.; Lee, S.C.; Kudla, J.; Luan, S. Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J. 2007, 52, 223–239. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Qin, D.; Zheng, Y.; Wang, Y. Finding Balance in Adversity: Nitrate Signaling as the Key to Plant Growth, Resilience, and Stress Response. Int. J. Mol. Sci. 2023, 24, 14406. https://doi.org/10.3390/ijms241914406
Jia Y, Qin D, Zheng Y, Wang Y. Finding Balance in Adversity: Nitrate Signaling as the Key to Plant Growth, Resilience, and Stress Response. International Journal of Molecular Sciences. 2023; 24(19):14406. https://doi.org/10.3390/ijms241914406
Chicago/Turabian StyleJia, Yancong, Debin Qin, Yulu Zheng, and Yang Wang. 2023. "Finding Balance in Adversity: Nitrate Signaling as the Key to Plant Growth, Resilience, and Stress Response" International Journal of Molecular Sciences 24, no. 19: 14406. https://doi.org/10.3390/ijms241914406





